Abstract
Objectives:
Afzal is an illegally sold smokeless tobacco product (STP) commonly used by youths and teenagers in Oman. The aim of this study was to analyse the composition of Afzal, also commonly known as sweekah, as it is believed to contain many carcinogens and toxic components. In particular, Afzal’s heavy metal content includes cadmium (Cd), chromium (Cr), lead (Pb) and nickel (Ni).
Methods:
This study was conducted between March and June 2013. Three samples of Afzal were first dried and then ground to form a homogenous powder. The powder was digested prior to the heavy metal analysis by inductively coupled plasma-mass spectrometry (ICP-MS).
Results:
Afzal was shown to have high levels of all heavy metals except for Ni and Pb, which were detected in quantities below acceptable international limits. The concentrations of the tested metals were 15.75 μg/g, 1.85 μg/g, 1.62 μg/g and 1.57 μg/g for Cr, Cd, Pb and Ni, respectively. The estimated daily intake of heavy metals from Afzal was below the maximum permissible limit accepted by the Food and Agriculture Organization and World Health Organization, except for Cr and Ni which were found to be dangerously elevated when compared with international standards.
Conclusion:
The results of this study showed that Afzal contains a number of heavy metals that may cause health problems. Therefore, urgent regulation of the illegal sale of Afzal is needed at the national level in Oman along with a campaign to address public health education and awareness of Afzal and its health risks.
Keywords: Smokeless Tobaccos, Heavy Metals, Spectrum Analysis, Oman
Advances in Knowledge
- This study analysed the composition of Afzal, a smokeless tobacco product common in Oman. It revealed high levels of certain heavy metals in Afzal, including chromium, cadmium, lead and nickel.
Application to Patient Care
- The results of this study will help raise awareness within the Omani community, especially among young people, about the danger of using Afzal.
Smokeless tobacco products (STPs) have a complex range of ingredients, from those containing only tobacco to others which include various chemicals and additives. Since 1970, global tobacco companies have competed to manufacture different forms, flavours and packages of tobacco products so as to attract users.1 The use of STPs has been associated with various health complications. Several studies have reported cases of different cancers, mainly oral and oesophageal, associated with the use of STPs.2–5 Other health risks that have been correlated with the use of STPs include hypertension, cardiovascular diseases, diabetes, compromised platelet function and oxidative stress.6–9 Moreover, the use of STPs has been associated with a tendency towards alcohol consumption and lower physical activity levels, and it may also be a risk factor leading to smoking.10,11
In addition to these risks, the World Health Organization (WHO) International Agency for Research in Cancer (IARC) has classified STPs as a Grou One carcinogen, i.e. substances that are definitely carcinogenic to humans.12 Substances in Group Two A are probably carcinogenic to humans and substances in Group Two B are possibly carcinogenic.12 Toxic heavy metal s are found in tobacco leaves and in processed tobacco products like cigarettes and STPs; this is most probably due to the addition of ash, used as a binding factor for the other agents, and lime, used to alkalinise the product.13 In addition, these metals, which occur in polluted air, are absorbed from the soil.14 There are a variety of toxic metals found in STPs but the main health concerns arise from cadmium (Cd), nickel (Ni), chromium (Cr) and lead (Pb). The first three have been declared by the IARC as a Group One carcinogen, and the fourth one as a Group Two A probable human carcinogen.15
Cd is very toxic to bones, the nervous system and kidneys, and can accumulate in the lenses of the eyes and cause cataracts.14 A high intake of Cd can compete with zinc for biological binding sites, which affects mostly the kidney and, to a lesser extent, the reproductive system.16 Pb can accumulate in bones and cause toxic developmental effects. In adults, Pb is known to induce renal tumors and increase blood pressure and the risk of cardiovascular diseases. Moreover, the brain is one of the organs most affected by this metal; Pb exposure has been found to correlate with reduced cognitive development, decreased intelligence quotient levels and poor learning outcomes in children.17,18 Ni, along with most of the metals mentioned previously, can cause inflammatory responses; in most cases, this results in allergic contact dermatitis inflammations.19 Ni also causes oral allergic contact sensitisation.20 The chronic irritation-induced inflammation of epithelial tissue has long been associated with the risk of neoplastic changes.21,22 Pb, Cd and arsenic are toxic at much lower levels than Ni.16 The analysis of heavy metals such as Cd and Pb in STPs is an important area of study since these elements are non-biodegradable, have long biological half-lives and have the tendency to accumulate in different organs of the body, leading to unwanted toxic effects.23 Hexavalent chromium (Cr VI) is also considered a Group One carcinogen and has been identified in cigarette smoke and ash; in contrast, low levels of chromium oxide (Cr III) are required nutritionally and, if lacking, cause people to experience immune system sensitisation.24 A WHO study on tobacco product regulation suggested that the Cr in tobacco is oxidised as Cr III.14
Out of more than 2,500 carcinogenic chemical substances, 28 have been identified in the STP snuff. However, the chemical composition of tobacco changes from year to year due to any given plant’s growth factors and geographical location, as well as the different processing methods such as curing, fermentation, mixing, packing and storage.12 Different components lead to different health risks. The most dangerous components in STPs are nicotine (due to its addictive nature); carcinogenic tobacco-specific nitrosamines; nitrosodimethyamine; polycyclic aromatic hydrocarbons like benzo(a) pyrene, and toxic heavy metals. The latter is the target of this study.
In the last two decades, STPs have started to gain popularity in Oman, especially among young people. Afzal is considered to be a snuff tobacco, or a type of moist STP. It is prohibited by law in Oman, yet it is still sold. Afzal is used by applying a pinch of the product between the lips and the upper or lower gums.12 Users then sucks the juice from the Afzal for varying periods of time, often up to 30 min, and subsequently spit out the rest. Its composition varies due to the differences in the unmonitored and unstandardised manufacturing processes and the various additives that are mixed in with the dried tobacco [Figure 1].
Figure 1 A & B:
(A) A small plastic bag of Afzal ready to be sold after other additives have been mixed in; (B) large package of loose Afzal.
Afzal has gained popularity in Oman because of its cheap price and easy availability as well as the lack of awareness about its dangers. To the best of the authors’ knowledge, no study has been previously conducted analysing the heavy metal content of Afzal in Oman; thus, this report is the first of its kind. The objectives of the study were to analyse four potential toxic heavy metals (Cd, Pb, Ni and Cr) in Omani Afzal and compare the results with an international standard.
Methods
This study analysed the heavy metal content of Afzal between March and June 2013. A single sample of Afzal weighing 4 kg was purchased personally by the investigators from one source in order to maintain uniformity. The sample was labelled with the date of purchase and the product was kept refrigerated at 4 °C in plastic bags until analysis.
Three samples of Afzal were dried to determine their moisture content using the method developed by the Centers for Disease Control and Prevention Federal Register in 2009.25 Subsequently, 15 g samples of Afzal were measured using a weighing moisture dish and placed uncovered in an oven at 99 ± 1 °C for three hours. Samples were then removed from the oven, covered and cooled to room temperature in a desiccator for approximately 30 min to prevent the reabsorption of moisture from the air. After drying, the samples were ground to form a homogenous powder. Thereafter, the samples were digested prior to heavy metal analysis The elemental analysis of the heavy metal content of the Afzal was performed using an Aurora M90™ inductively coupled plasma-mass spectrometry (ICP-MS) system, Software Version V3.0 b797, Firmware Version 1.87 (Bruker Corp., Billerica, Massachusetts, USA) coupled with manual injection of the samples. Tuning solution standards were used to calibrate the ICP-MS system. All solutions were prepared with analytical reagent grade chemicals. Deionised water was used exclusively throughout the study by using the Milli-Q Integral Water Purification System (Millipore Corp., Bedford, Massachusetts, USA). Reagent blank determinations were used to apply corrections to the instrument readings. Blank and standard solutions were prepared in a similar acid matrix.
Three samples of 0.5 g of powdered Afzal were mixed with 20 ml of 56% nitric acid of trace metal grade (Merck KGaA, Darmstadt, Germany) for 30 min. Then 4 mL of hydrochloric acid was added to each digestion vessel and the mixture was heated on a hot plate (Bibby Scientific Ltd., Staffordshire, UK) at 200 °C for 1–2 min and then allowed to cool for 30 min. The heating-cooling-reheating process was repeated until a clear solution was obtained. The contents of each digestion vessel were diluted with deionised water to obtain a final volume of 10 mL. This was then filtered with Whatman® Grade #42 filter paper (General Electric Health Care, Pittsburgh, Pennsylvania, USA) and diluted to a volume of 50 mL with 2% nitric acid. This was subsequently injected into the ICP-MS. All of the digestion processes took place in a well-ventilated fume hood and the researchers wore gloves and masks at all times when handling the acids.
In order to estimate their content within the Afzal product, five different concentrations of Cd, Pb, Ni and Cr solutions were prepared at 5, 10, 20, 30 and 50 parts per billion (ppb) for each metal standard using multi-element calibration standards (High-Purity Standards, North Charleston, South Carolina, USA). The Afzal samples and the standard solutions of Cr, Cd, Pb and Ni were aspirated into the ICP-MS system using the auto-sampler. The instrumental operating conditions for the ICP-MS system were as follows: the power was 1.40 kw; the cool gas flow rate was 18 L/min; the nebuliser gas flow was 1 L/min; the auxiliary gas flow rate was 1.8 L/min; the condenser temperature was 3 °C; the voltage was −12.00 V, −160.00 V and −388.00 V for extract lens 1, 2 and 3, respectively; the pole bias was 0.00 V; the fringe bias was −2.80 V and the pump rate was 5 rpm. The main run setup included peak jumping, with 100 sweeps, at a dwell time of 10,000 μsec and with a stabilisation delay of 60 sec. Five replicates were made and the sampler cone identification and skimmer cone identification colour rendering index was off with a flow of 0 mL/min. There was external drift correction.
The minimum daily intake Afzal was estimated at 1–2 g; this was based on findings that the most common dosage of snuff or moist STPs is a pinch-sized amount.26 Thus, the daily intake of heavy metals was calculated as follows: daily intake (μg/day) = metal concentration in a pinch-sized doze of Afzal × number of times a pinch-sized dose of Afzal was taken. This calculation assumed that users took a minimum of a single average pinch-sized dose (2 g) per day of Afzal. The average daily consumption of any STP in Oman is unknown; therefore, the lowest probable daily consumption of Afzal (2 g/day) was selected in calculating the lowest daily intake of metals.
Through 10 analyses of a blank sample and the application of the Health Canada Official Test Method T-306,27 the limit of method detection and limits of detection and quantitation of the ICP-MS system were calculated. Other calculations using the same method were used to determine the concentration of metals in the samples on a wet weight or “as received” basis as Afzal usually contains some moisture. The moisture content on this basis was 52% as determined by the following formula: analyte “as received” (μg/g) = dry matter analyte (μg/g) × 1− (% moisture/100).
The toxic heavy metal contents of Afzal were then compared to the snus (moist powder tobacco) quality standards used by GOTHIATEK® (Swedish Match, Stockholm, Sweden).28
Appropriate quality-assurance procedures and precautions were taken to enhance the reliability of the results. Samples were carefully handled to avoid cross-contamination. Glassware was properly cleaned and all reagents used were of analytical grade. Blank samples of deionised water were run to calculate the limits of detection and limits of quantification. Blank procedural reagent samples were also used to subtract the results of all tested metal standards and samples injected into the ICP-MS system. A calibration curve for each tested metal was constructed by using five different concentrations of the metal standard from the detection limit up to 5, 10, 20, 30 and 50 μg/g.
Excel spreadsheet software Version 2007 (Microsoft Corp., Redmond, Washington, USA) and the ICP-MS instrumental software were used for the statistical analysis. This study involved a chemical analysis of Afzal and hence did not require ethical approval.
Results
The method used for the ICP-MS analysis of heavy metals is a validated method applied by the Central Analytical & Applied Research Unit in the College of Science at Sultan Qaboos University, Oman. All analytical values of samples and metal standards obtained showed good precision and repeatability as reflected in the value of relative standard deviation percentage (RSD%), which was 8.14% [Table 1]. For the same analyte level, the Association of Official Analytical Chemists sets the maximum acceptable value of RSD% at 11%.29 To further strengthen the reliability of the method and based on the calibration curves constructed for each metal standard, the R-value to determine linearity in all cases was 1 >R >0.99.
Table 1:
Relative standard deviation percentages of metal standards and Afzal samples
| Metal | International standard | Afzal | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 at 5 ppb conc. | 2 at 10 ppb conc. | 3 at 20 ppb conc. | 4 at 30 ppb conc. | 5 at 50 ppb conc. | Sample 1 | Sample 2 | Sample 3 | |
| Cr | 1.13 | 0.67 | 0.42 | 0.93 | 0.58 | 1.19 | 1.88 | 1.04 |
| Ni | 2.46 | 2.50 | 0.74 | 1.29 | 1.47 | 3.78 | 3.35 | 3.74 |
| Cd | 0.85 | 1.87 | 1.28 | 1.09 | 1.03 | 8.13 | 1.78 | 8.14 |
| Pb | 2.83 | 1.31 | 0.65 | 0.53 | 1.34 | 1.62 | 1.28 | 1.24 |
ppb = parts per billion; conc. = concentration; Cr = chromium; Ni = nickel; Cd = cadmium; Pb = lead.
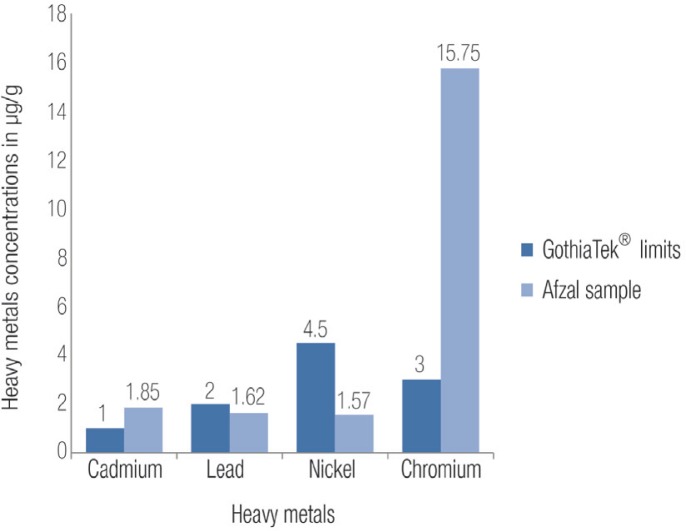
The concentrations of the heavy metals (Cr, Ni, Cd and Pb) in 1 g of Afzal are listed in Table 2 as the mean values of the three samples of Afzal and their corresponding standard deviations. The average concentrations in μg/g of the heavy metals in the three Afzal samples were compared with the international GOTHIATEK® standard, as shown in Figure 2.28
Table 2:
The concentrations of heavy metals in the tested Afzal in μg/g
| Cr | Ni | Cd | Pb | |
|---|---|---|---|---|
| Sample 1 | 16.38 | 1.77 | 1.75 | 1.61 |
| Sample 2 | 15.85 | 1.57 | 1.95 | 1.7 |
| Sample 3 | 15.02 | 1.38 | 1.85 | 1.56 |
| Mean ± SD on a dry wt. basis | 15.75 ± 0.69 | 1.57 ± 0.20 | 1.85 ± 0.10 | 1.62 ± 0.07 |
| Wet wt.basis* | 7.56 | 0.75 | 0.89 | 0.78 |
| MDL | 0.0594 | 0.0586 | 0.059 | 0.0584 |
| QDL | 0.1782 | 0.1758 | 0.177 | 0.1752 |
Cr = chromium; Ni = nickel; Cd = cadmium; Pb = lead; SD = standard deviation; wt. = weight; MDL = method detection limit in μg/mL; QDL = quantitation detection limit in μg/mL.
Weight of sample “as received”.
Figure 2:
The different concentrations of heavy metals in the tested Afzal sample versus the Swedish GOTHIATEK® limits for snus (moist powder tobacco).28
When participants were informally questioned about their Afzal use, most stated that they consumed a minimum of five doses per day; however, use patterns are also dependent on the level of addiction and the needs of the user. Considering the assumption that the average pinch-sized dose of Afzal is 2 g, the minimum estimated daily intake of heavy metals in Afzal is shown in Table 3. These were compared with the permissible daily limits set by the WHO and FAO.30
Table 3:
Permissible daily intake limits for the human consumption of the heavy metals according to the Food and Agriculture Organization and World Health Organization and the estimated minimum daily intake of metals in single dose of the tested Afzal
| Metal | Permissible daily intake limit* in μg/kg/day | Permissible daily intake limit for a 60 kg individual in μg/day | Estimated daily intake of metals in a Single dose† of tested Afzal per day in μg | |
|---|---|---|---|---|
| Dry wt. | Wet wt. | |||
| Pb | 5.0 | 300.0 | 3.24 | 1.56 |
| Cd | 0.4–2.0 | 60.0 | 3.70 | 1.78 |
| Cr | 0.1 | 6.0 | 31.50 | 15.12 |
| Ni | 0.2 | 12.0 | 3.1 | 1.50 |
wt. = weight; Pb = lead; Cd = cadmium; Cr = chromium; Ni = nickel
Source: Joint Food and Agriculture Organization and World Health Organization Expert Committee. Evaluation of certain food additives and contaminants.30
For this calculation, a pinch-sized single dose of Afzal was considered to be 2 g.
Discussion
Afzal consumption in Oman has increased in the last two decades, especially among young people. This STP contains several heavy metals such as Cr, Cd, Pb and Ni. Some of these heavy metals are carcinogenic.26 Many studies have reported the presence of heavy metals in various types of STPs, such as snuff and the Alaskan iqmik,31 Indian smokeless tobacco32 and Pakistani STPs.14,33 To the best of the authors’ knowledge, this is the first analysis of its kind in Oman or among other Arabian Gulf countries.
The results of this study provide very useful information about the concentrations of four heavy metals in Afzal. A comparison of the contents of different STPs from various countries may vary widely due to the heterogeneity of the tobacco plants, different processing techniques and storage conditions, and the additives used. However, the results of an analysis can give an idea of the most dangerous ingredients present in one particular brand of STP.
From the heavy metals analysis, it was found that the Cr and Cd levels in the Afzal sample exceeded the GOTHIATEK® standards. In contrast, the concentrations of Ni and Pb were lower than the maximum levels indicated as acceptable by the standard. The order in which the elements occurred from greatest to least was as follows: Cr (15.75 μg/g), Ni (1.57 μg/g), Pb (1.62 μg/g) and Cd (1.85 μg/g). In a similar study of moist snuff and Alaskan iqmik conducted in the USA, the order in which the elements occurred from greatest to least was as follows: Ni (2.28 μg/g), Cr (2.04 μg/g), Cd (1.40 μg/g) and Pb (0.45 μg/g).31 It seems that the STPs in this study from the USA have lower levels of heavy metals than both the Omani Afzal sample of the current study and Pakistani STPs in a similar study.33 Tese types of variations are to be expected due to the aforementioned plant heterogeneity or variations in processing criteria around the world.
The results of the current study can be used to formulate an idea about the indirect intake of those metals by the STP users. This was done by estimating their daily intake and then assessing whether the indirect intake of heavy metals was consistent with the permissible and acceptable daily levels set by the WHO and the FAO.30
Estimating the minimum levels of heavy metals in one dose of the selected Afzal sample can help people better understand the possible health risks associated with using Afzal, particularly in doses greater than the estimated intake. The estimated minimum daily intake of the selected heavy metals in this sample of Afzal was below the permissible levels set by the FAO and WHO, except for Cr which was recorded in concentrations above the maximum permissible limit.30 Moreover, the levels of Cr found in the sample exceeded the maximum limits set by the Swedish GOTHIATEK® standards.28 Unfortunately, the actual quantity of heavy metals ingested by a user may be higher than the minimum daily intake estimated in this study, due to variations in the blend of Afzal as well as the user’s individual preferences. A single dose of Afzal cannot be assigned a standard weight of 1–2 g like the tea-bag shaped Swedish snus; each dose depends on the user’s level of addiction. In addition, the rate of extraction of the metals from the STPs by the saliva will vary according to the individual. However, the moist form of the product fortunately has a lower metal content than that found in the dry samples analysed.
Nevertheless, the frequent use of this brand of STP allows the accumulation of nondegradable and dangerous elements in the user’s body.31 Furthermore, excluding other potential sources of daily heavy metal intake such as from environmental and dietary sources, frequent use of Afzal may put users at risk of exposure to combined metals. Heavy metals have potential toxic and carcinogenic effects and some of them can cause severe health problems, even in trace amounts.16
Conclusion
This study gives a better understanding of the levels of selected heavy metals in Afzal. The targeted metals were Cr, Ni, Cd and Pb. The results showed levels of Cr and Cd in Afzal above the international limits, while the concentrations of Ni and Pb were lower than the maximum permissible limits. Unfortunately, the estimated daily intake of the tested metals exceeded the allowable safe limits recommended by the FAO and the WHO, rendering users at high risk of being poisoned or developing other undesirable side-effects. It is therefore recommended that the sale of illegal STPs such as Afzal be urgently regulated in Oman. Furthermore, it is important that education programmes aimed at the Omani youth be implemented to raise awareness of the health risks and dangers of using this product.
References
- 1.World Health Organization The World Health Report 2002; Reducing risks, promoting healthy life. From: www.who.int/whr/2002/en/ Accessed: May 2014. [DOI] [PubMed]
- 2.Wasnik KS, Ughade SN, Zodpey SP, Inqole DL. Tobacco consumption practices and risk of oro-pharyngeal cancer: A case control study in central India. Southeast Asian J Trop Med Public Health. 1998;29:827–34. [PubMed] [Google Scholar]
- 3.Merchant A, Husain SS, Hosain M, Fikree FF, Pitiphat W, Siddiqui AR, et al. Paan without tobacco: An independent risk factor for oral cancer. Int J Cancer. 2000;86:128–31. doi: 10.1002/(SICI)1097-0215(20000401)86:1<128::AID-IJC20>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 4.Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2008;9:667–75. doi: 10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- 5.Khan AM, Khan MS, ul Haq N. Oral snuff and carcinoma oesophagus. Gomal J Med Sci. 2009;7:58–61. [Google Scholar]
- 6.Bolinder G, de Faire U. Ambulatory 24-h blood pressure monitoring in healthy, middle-aged smokeless tobacco users, smokers, and nontobacco users. Am J Hypertens. 1998;11:1153–63. doi: 10.1016/S0895-7061(98)00137-X. [DOI] [PubMed] [Google Scholar]
- 7.Persson PG, Carlsson S, Svanström L, Ostenson CG, Efendic S, Grill V. Cigarette smoking, oral moist snuff use and glucose intolerance. J Intern Med. 2000;248:103–10. doi: 10.1046/j.1365-2796.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 8.Yildiz D, Liu YS, Ercal N, Armstrong DW. Comparison of pure nicotine and smokeless tobacco extract-induced toxicities and oxidative stress. Arch Environ Contam Toxicol. 1999;37:434–9. doi: 10.1007/s002449900537. [DOI] [PubMed] [Google Scholar]
- 9.Stegmayr B, Johansson I, Huhtasaari F, Moser U, Asplund K. Use of smokeless tobacco and cigarettes: Effects on plasma levels of antioxidant vitamins. Int J Vitam Nutr Res. 1993;63:195–200. [PubMed] [Google Scholar]
- 10.Eliasson M, Lundblad D, Hägg E. Cardiovascular risk factors in young snuff-users and cigarette smokers. J Intern Med. 1991;230:17–22. doi: 10.1111/j.1365-796.1991.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 11.Hu FB, Hedeker D, Flay BR, Sussman S, Day LE, Siddiqui O. The patterns and predictors of smokeless tobacco onset among urban public school teenagers. Am J Prev Med. 1996;12:22–8. [PubMed] [Google Scholar]
- 12.World Health Organization International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 89 Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. From: www.monographs.iarc.fr/ENG/recentpub/mono89.pdf Accessed: Nov 2013.
- 13.Zakiullah U, Saeed M, Muhammad N, Khan SA, Gul F, Khuda F, et al. Assessment of potential toxicity of a smokeless tobacco product (naswar) available on the Pakistani market. Tob Control. 2012;21:396–401. doi: 10.1136/tc.2010.042630. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Report on the Scientific Basis of Tobacco Product Regulation: Fourth Report of a WHO Study Group. From: www.whqlibdoc.who.int/publications/2012/9789241209670_eng.pdf Accessed: Nov 2013. [Google Scholar]
- 15.World Health Organization International Agency for Research on Cancer Agents Classified by the IARC Monographs, Volumes 1–109. From: www.monographs.iarc.fr/ENG/Classification/ClassificationsGroupOrder.pdf Accessed: May 2014.
- 16.García-Rico L, Leyva-Perez J, Jara-Marini ME. Content and daily intake of copper, zinc, lead, cadmium, and mercury from dietary supplements in Mexico. Food Chem Toxicol. 2007;45:1599–605. doi: 10.1016/j.fct.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Kauffman JF, Westenberger BJ, Robertson JD, Guthrie J, Jacobs A, Cummins SK. Lead in pharmaceutical products and dietary supplements. Regul Toxicol Pharmacol. 2007;48:128–34. doi: 10.1016/j.yrtph.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Ang HH. Lead contamination in Eugenia dyeriana herbal preparations from different commercial sources in Malaysia. Food Chem Toxicol. 2008;46:1969–75. doi: 10.1016/j.fct.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Linneberg A, Nielsen NH, Menné T, Madsen F, Jørgensen T. Smoking might be a risk factor for contact allergy. J Allergy Clin Immunol. 2003;111:980–4. doi: 10.1067/mai.2003.1394. [DOI] [PubMed] [Google Scholar]
- 20.De Rossi SS, Greenberg MS. Intraoral contact allergy: A literature review and case reports. J Am Dent Assoc. 1998;129:1435–41. doi: 10.14219/jada.archive.1998.0078. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: How hot is the link? Biochem Pharmacol. 2006;72:1605–21. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 22.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–82. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 24.Sógor C, Gáspár A, Posta J. Flame atomic absorption spectrometric determination of total chromium and Cr(VI) in cigarette ash and smoke using flow injection/hydraulic high-pressure sample introduction. Microchem J. 1998;58:251–5. doi: 10.1006/mchj.1997.1552. [DOI] [Google Scholar]
- 25.Centers for Disease Control and Prevention Laboratory protocol to measure the quantity of nicotine contained in smokeless tobacco products manufactured, imported, or packaged in the United States. Federal Register. 2009;62:24116–19. [PubMed] [Google Scholar]
- 26.European Network for Smoking Prevention (ENSP) Status Report on Oral Tobacco. From: www.ensp.org/node/812 Accessed: May 2014.
- 27.Health Canada Official Methods for the Collection of Data on Toxic Constituents in Chewing Tobacco and Snuff : Official Method T-306 Determination of Ni, Pb, Cd, Cr, As, Se and Hg in Whole Tobacco. From: www.hc-sc.gc.ca/hc-ps/pubs/tobac-tabac/rc/index-eng.php Accessed: May 2014.
- 28. Swedish Match. GOTHIATEK® Standard: Product Requirements. From: www.swedishmatch.com/en/Snus-and-health/GOTHIATEK/GOTHIATEK-standard/ Accessed: May 2014.
- 29.González AG, Herrador MA. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. Trends Analyt Chem. 2007;26:227–38. doi: 10.1016/j.trac.2007.01.009. [DOI] [Google Scholar]
- 30.Joint Food and Agriculture Organization and World Health Organization Expert Committee Evaluation of certain food additives and contaminants. From: www.whqlibdoc.who.int/trs/WHO_TRS_922.pdf Accessed: May 2014.
- 31.Pappas RS, Stanfill SB, Watson CH, Ashley DL. Analysis of toxic metals in commercial moist snuff and Alaskan iqmik. J Anal Toxicol. 2008;32:281–91. doi: 10.1093/jat/32.4.281. [DOI] [PubMed] [Google Scholar]
- 32.Dhaware D, Deshpande A, Khandekar RN, Chowgule R. Determination of toxic metals in Indian smokeless tobacco products. Sci World J. 2009;9:1140–7. doi: 10.1100/tsw.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musharraf SG, Shoaib M, Siddiqui AJ, Najam-Ul-Haq M, Ahmed A. Quantitative analysis of some important metals and metalloids in tobacco products by inductively coupled plasma-mass spectrometry (ICP-MS) Chem Cent J. 2012;6:56. doi: 10.1186/1752-153X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]