Summary
There has been a resurgence of interest in the neutrophil’s role in autoimmune disease. Classically considered an early responder that dies at the site of inflammation, new findings using live imaging of embryonic zebrafish and other modalities suggest that neutrophils can reverse migrate away from sites of inflammation. These “inflammation sensitized” neutrophils, as well as the neutrophil extracellular traps (NETs) and other products made by neutrophils in general, may have many implications for autoimmunity. Here we review what is known about the role of neutrophils in three different autoimmune diseases: rheumatoid arthritis, systemic lupus erythematosus, and small vessel vasculitis. We then highlight recent findings related to several cytoskeletal regulators that guide neutrophil recruitment including Lyn, Rac2, and SHIP. Finally we discuss how our improved understanding of the molecules that control neutrophil chemotaxis may impact our knowledge of autoimmunity.
Keywords: Neutrophil, Migration, Autoimmune, Lyn, Rac2, SHIP
Neutrophils are classically known as critical first responders in an immune response. They are generated in the bone marrow, but circulate in the bloodstream until they are attracted into the tissue by numerous different types of molecules (1). In the tissue, activated neutrophils produce reactive oxygen species and release cytoplasmic granules filled with molecules like alarmins and anti-microbial peptides (2), which results in the killing of pathogens as well as significant host cell death and tissue damage. Neutrophils also can die in a process called NETosis in which chromatin is extruded as part of a nuclear extracellular trap, or NET (2). These NETs contain anti-microbial proteins and are important for the innate immune response against pathogens, but they have also been implicated in autoimmunity, as discussed below.
Neutrophils have many immediate effects mediated by their antimicrobial products and reactive oxygen species, but they also have more long-lasting effects related to the cytokines that they secrete and their influence on other immune cells. They can recruit macrophages to sites of inflammation, where the macrophages have the potential to perform many pro- and anti-inflammatory activities, in part, dependent on signals from neutrophils (3). Neutrophils also have been shown to carry antigens from sites of inflammation to lymph nodes in mice (4) and have been reported to have an average lifespan greater than five days, much longer than the previously generally accepted lifespan of less than one day (5). What these longer-lived and/or “inflammation sensitized” neutrophils might be doing is not known. However, there is evidence that neutrophils can alter the long-lasting adaptive immune response through interactions with B and T cells. For example, neutrophils colonize an area near the splenic marginal zone where they can expand the production of antibodies made by B lineage cells (6). Further, they can present antigens to T cells (7) and appear, in different studies, to be able to activate (8) and/or repress (9) T cell activity. Thus neutrophils may be playing a complex role in regulating adaptive immunity.
All of these neutrophil effects depend on neutrophils arriving at sites of inflammation and they have a very specific mechanism for doing so (10). First they are captured by selectins on the vessel wall leading to intraluminal crawling, then rolling, and then firm adhesion via β2 integrins (CD11/CD18) and chemokines. The next step is neutrophil extravasation through the vessel wall, either between endothelial cells or through an individual endothelial cell, in a process called diapedesis. Finally, they migrate through the basement membrane, perivascular region, and into the tissue. There is a large literature about cytoskeletal control of neutrophil motility that is derived largely from in vitro studies (11–14). We are interested in inflammatory disease, and have therefore developed and studied the zebrafish system, which allows us to look at cytoskeletal regulatory pathways in the context of in vivo neutrophil movements.
Zebrafish are an excellent model system for imaging cell movement since embryonic zebrafish are transparent, allowing an observer to track cells in vivo using time-lapse imaging. One of our early observations in zebrafish is neutrophil reverse migration. Using a transgenic zebrafish whose neutrophils express GFP, time-lapse imaging showed that neutrophils not only migrate to a wound (a type of sterile inflammation), but also away from the wound back to the vasculature to resolve the local inflammatory response (15). This reverse migration may be how neutrophils carry antigens to the lymph nodes in mice. Using transgenic zebrafish whose neutrophils express a photoconvertible fluorescent reporter, Dendra2 (16), individual migrating neutrophils can be photolabeled and then tracked as they migrate elsewhere in the living fish. Using this model, we found that neutrophils migrate to and from the wound repeatedly and then disperse throughout the body as the wound heals (16). Thus, in addition to wound resolution, this process leads to the dissemination of “wound sensitized” neutrophils. Similar events may be occurring in mammals during inflammation, although the role of the “inflammation sensitized” neutrophils is unknown. However, it is interesting to hypothesize how these experienced neutrophils might influence other immune cells. In this review we focus on how live imaging of neutrophil movements in zebrafish has shed light on the roles of several important cytoskeletal regulatory molecules and what the implications may be for neutrophil migration and human autoimmunity.
Neutrophils in Autoimmune Diseases
Rheumatoid arthritis
Rheumatoid arthritis is an inflammatory, destructive arthritis that affects about 1% of people. It is an autoimmune disease characterized by antibodies against citrullinated proteins. However, the innate immune system and inflammatory cytokines like TNFα and IL-6 play a large role. A hallmark of rheumatoid arthritis is the transformation of the normal synovial lining, which surrounds the joint and synovial fluid, into a tumor-like pannus composed of activated synovial fibroblasts and immune cells. The pannus grows and invades into the cartilage and bone of the joint causing joint deformity and destruction (17). Neutrophils are the primary cell type found in the synovial fluid of joints affected by rheumatoid arthritis as well as at the junction of the pannus and cartilage, where invasion occurs (1). Neutrophils are thought to be critical for disease pathogenesis since a lack of neutrophils blocks the development of two different models of the innate, effector arm of rheumatoid arthritis, the K/BxN (18) and collagen antibody induced arthritis (19) models.
Neutrophils likely contribute in many ways to rheumatoid arthritis. Neutrophils from patients with rheumatoid arthritis are typically more activated (20) with high baseline intracellular reactive oxygen species production compared to control neutrophils. Further, rheumatoid neutrophils appear to be primed even in patients in remission (21). These activated neutrophils likely cause tissue destruction, leading to joint damage as well as immune response amplification. In addition to, or possibly due to, their destructive effects, neutrophils may be involved in increasing the vasopermeability of the joint (22) to allow antibodies to enter and deposit in the joint (23). Antibody deposition, particularly as part of immune complexes, is thought to be a major contributor to joint inflammation. Neutrophils may also contribute to autoantibody formation since netting neutrophils, i.e. neutrophils making NETs, can be found in rheumatoid synovium. These NETs display some of the citrullinated antigens that are known to be targets of the pathologic anti-citrullinated protein antibodies (ACPAs) in rheumatoid arthritis (24). Thus, as shown in Figure 1, there may be a positive feedback loop in rheumatoid arthritis where ACPA immune complexes accelerate inflammation, which attracts neutrophils, which present citrullinated proteins to be incorporated into immune complexes, which amplify inflammation. Neutrophils and their NETs also enhance synovial fibroblast cytokine production (24), chemotaxis, matrix metalloproteinase production and invasiveness (25) as well as affect B cell behavior through release of B lymphocyte stimulator upon activation with TNFα in the rheumatoid joint (26).
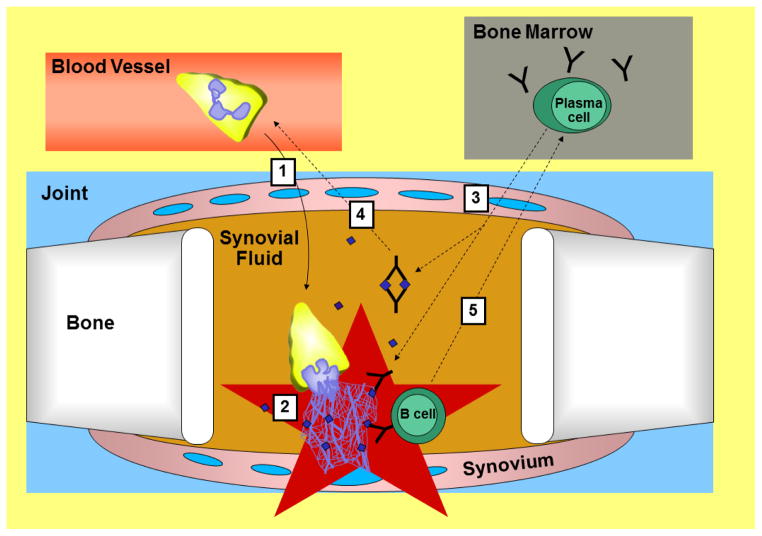
Figure 1. Model for how neutrophils may contribute to rheumatoid arthritis.
This model is numbered, but which step is actually first in disease is not clear. 1. Neutrophils enter the joint space. They (2) become activated and (3) increase synovial permeability to allow antibodies to enter the joint, including anti-citrullinated protein antibodies (ACPAs). 2. Activated neutrophils can die by NETosis releasing their NETs, which are studded with citrullinated proteins (purple diamonds). ACPAs bind to these proteins increasing inflammation. 4. Immune complexes composed of ACPAs and citrullinated proteins attract more neutrophils to the joint amplifying inflammation. 5. Also, naïve and/or memory B cells, which are attracted to inflammation and have B cell receptors that recognize citrullinated proteins, become activated upon recognizing citrullinated antigens in NETs and ultimately home to the bone marrow where they reside as ACPA secreting plasma cells. Those plasma cells make more ACPAs, which enter the joint (3) to amplify inflammation.
In order to exert many of their pathologic effects, neutrophils need to enter the joint and there are many factors that contribute to this process. There is a role for P-selectin, E-selectin, CD18 (the β2 integrin), CXCR2, IL-6, Duffy antigen receptor for chemokines, and CXCL5 (27, 28). Moreover, recruitment may be biphasic and related to the presence of immune complexes. Immune complexes lead to activation of the complement cascade with production of C5a and can bind to Fcγ receptors. Activation of neutrophils via the C5a receptor leads to the release of leukotriene B(4) which attracts neutrophils to the joint early in disease. Later, engagement of Fcγ receptors on neutrophils leads to IL-1β release and subsequent neutrophil-attracting chemokine production, which promotes chronic inflammation (29). Neutrophil migration to the joint appears to be a critical point in disease pathogenesis since many of the treatments for rheumatoid arthritis decrease recruitment of neutrophils into the inflamed joint including leflunomide, methotrexate (30), methylprednisolone (31) and anti-TNFα agents (32).
It is interesting that neutrophils are such a large presence in the chronically inflamed joints in rheumatoid arthritis since they are classically thought of as early responders and short-lived. It is not known how much of the neutrophil presence is due to continued recruitment versus long-lived cells. However, there is some data suggesting that neutrophils in rheumatoid arthritis may be longer lived. For example, synovial fluid from rheumatoid arthritis patients inhibits both spontaneous and immune complex-mediated neutrophil apoptosis (33). This effect is thought to be mediated by adenosine, but IL-17, TNFα, and granulocyte-macrophage colony-stimulating factor may also contribute to increasing the lifespan of the neutrophils (34). The lifespan of neutrophils may be important to disease pathogenesis since methotrexate blocks the neutrophil longevity seen in rheumatoid arthritis (35).
Thus, the neutrophil is a critical cell in rheumatoid arthritis with many neutrophils recruited to the inflamed joint with a longer life and a more activated phenotype.
Small Vessel Vasculitis
Vasculitis is a life-threatening disorder caused by inflammation of the blood vessels. There is a subset of vasculitis called small vessel vasculitis that includes granulomatosis with polyangiitis (previous Wegener’s granulomatosis), microscopic polyangiitis, and Churg-Strauss disease. In all of these disorders, there is inflammation of the small vessels primarily in the lungs and kidneys although vessels can also be affected in the skin, upper airways, peripheral nerves, brain, and heart depending on the specific small vessel vasculitis. Although not detected in every case of small vessel vasculitis, anti-neutrophil cytoplasmic antibodies (ANCAs) can be found in the majority of patients. These antibodies target myeloperoxidase (MPO) and proteinase 3 (PR3), both components of the primary granules in neutrophils (1), and may also target other unknown antigens. Thus, in small vessel vasculitis, neutrophils are targets of the auto-antibodies, intricately linking them to the pathophysiology of disease.
ANCAs are thought to drive small vessel vasculitis. In support of this theory, an intravenous infusion of anti-MPO antibodies can drive a glomerular nephritis in mice that is histologically similar to human small vessel vasculitis (36). However, the data is not as clear in humans since small vessel vasculitis can occur without detectible ANCAs and ANCA levels do not always correlate with disease. Regardless, ANCAs, through binding to Fc receptors and recognizing targets with their antigen-binding domains (37), do have many pathologic effects on neutrophils. ANCAs stimulate neutrophils resulting in degranulation, production of oxygen radicals (38) and IL-1β (39), and NET formation (40). ANCAs also increase the chemotactic response of neutrophils (41) and increase their ability to adhere to endothelial cells in a manner dependent on β2 integrins and CXCR2 (42, 43). Thus neutrophils appear to be both activated by ANCAs and stimulated by them to migrate into the vessels where they can cause destruction as illustrated in Figure 2.
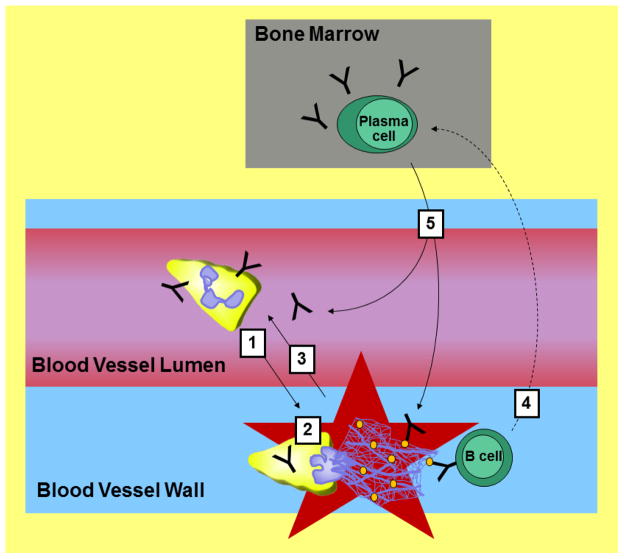
Figure 2. Model for how neutrophils may contribute to small vessel vasculitis.
1. Peripheral blood neutrophils are activated by circulating anti-neutrophil cytoplasmic antibodies (ANCAs) and stimulated by them to enter the vessels. 2. In the vessel wall, activated neutrophils can degranulate and generate free radicals to cause damage and undergo NETosis. The NETs contain myeloperoxidase (MPO) and proteinase 3 (PR3), which are represented as orange ovals and are targets of ANCAs. ANCA immune complexes and damage by neutrophils increases inflammation and (3) attracts more neutrophils. 4. Naïve and/or memory B cells, which are attracted to the vessel inflammation and have B cell receptors that recognize MPO, PR3, and potentially other antigens, become activated and ultimately differentiate into plasma cells. The plasma cells migrate to the bone marrow and continue to secrete ANCAs, which (5) enter the bloodstream to promote disease progression.
In addition to a role for ANCAs, neutrophil recruitment into the vessels in vasculitis appears to involve CXCL8 and IL-1β (44). Neutrophil recruitment is key for pathology since the necrosis of blood vessels in small vessel vasculitis is thought to be due to neutrophil infiltration and activation (45). Damage could be occurring through any number of the cytotoxic neutrophil products. Further, NETs are present in the inflamed kidneys in vasculitis and display PR3 and MPO, providing a target for ANCA binding. (40). Thus, a feedback loop of inflammation may be occurring where ANCAs bind to the netting neutrophils causing further inflammation and the PR3 and MPO displayed on the neutrophils in the inflammatory milieu may be inducing further ANCA production as shown in Figure 2.
In human disease the data for neutrophil involvement is primarily correlative or derived from in vitro experiments, but there is evidence for a critical pathologic role for neutrophils in vasculitis in rodent models. In mouse models of small vessel vasculitis, neutrophils are detected at sites of glomerular necrosis and depletion of neutrophils completely blocks disease (46). A separate model of lung disease has been made where infusion of TNFα-primed neutrophils and ANCAs together cause increased pulmonary endothelial permeability and lung edema that requires reactive oxygen species and neutrophil elastase (47). Neutrophil myeloperoxidase can alter endothelial cell function and close contact of neutrophils via integrins can transfer MPO to endothelial cells (48). Further, migration of neutrophils into the vessels is likely critical for disease since a synthetic retinoic acid receptor agonist, ameliorates a murine model of vasculitis (induced by Candida albicans) through the suppression of neutrophil migration and activation (49).
Thus, similar to rheumatoid arthritis, neutrophils are present in the sites of inflammation in vasculitis and likely contribute to disease.
Lupus
Lupus is a systemic autoimmune disease that presents with a constellation of symptoms that can be different for each individual. Some of the more severe manifestations of lupus include lupus nephritis, lupus cerebritis, and lupus vasculitis, but many other manifestations can occur including pericarditis, pleuritis, skin rashes, cytopenias, hair loss, and oral ulcers. Indeed, almost any organ system can become affected in lupus making this an amorphous and unpredictable disorder. Like rheumatoid arthritis and small vessel vasculitis, patients with lupus have autoantibodies, classically anti-nuclear antibodies in addition to others.
The role of neutrophils in lupus may be different than in rheumatoid arthritis and small vessel vasculitis. In both rheumatoid arthritis and vasculitis, neutrophils are thought to migrate to the joint or blood vessel and create local inflammation and damage. However, in lupus, a more diffuse systemic disease, pathology due to neutrophils may be more complex and involve more indirect effects (2). For example, there is evidence for increased activation of neutrophils in rheumatoid arthritis, but in lupus the data are mixed. Neutrophils from lupus patients have been shown to have decreased phagocytosis, chemotaxis, and oxidative burst in response to IL-8 (50) and neutropenia is often seen in lupus. In contrast, others have reported that neutrophils in lupus are more activated intravascularly (51). One possible explanation for the conflicting data about lupus neutrophils is the presence of a subset of neutrophil-like cells in lupus patients called low density granulocytes, or LDGs, which have enhanced NETosis, increased ability to kill endothelial cells, and increased ability to stimulate plasmacytoid dendritic cells to secrete type I interferon (52), one of the major cytokines involved in lupus. Perhaps, these hyperactive LDGs are distinct from the hypo-activated neutrophils seen in some studies and the LDGs are the main contributors to lupus nephritis, accelerated atherosclerosis, or other manifestations of lupus (53). Alternatively, there is a large range of clinical presentations of lupus and the diverse findings with neutrophils may reflect differences in underlying pathogenesis.
Neutrophil NETs have also attracted significant attention in lupus. Lupus neutrophils have been shown to have increased NET formation (54) and impaired NET breakdown (55). NETs can activate plasmacytoid dendritic cells to secrete type I interferon (54). Further, netting neutrophils can infiltrate tissues and cause endothelial damage in lupus (52). Similar to small vessel vasculitis and rheumatoid arthritis, NETs have been hypothesized to be a source of autoantigens in lupus (2), but there is contradictory data for this theory. In lupus-prone mice deficient in the NADPH oxidase, Nox2, neutrophils cannot make NETs and the mice have worsened lupus instead of the predicted improved lupus (56).
Despite the fact that some neutrophil effects in lupus may be indirect, neutrophils do migrate to sites of inflammation and there is some literature on the regulators of this recruitment. In a strain of lupus prone mice, neutrophils have higher levels of CXCR4, kidneys have high levels of CXCL12 and high numbers of infiltrating CXCR4 expressing cells, and a peptide antagonist of CXCR4 improves glomerulonephritis and survival (57), suggesting that kidney damaging neutrophils depend on the CXCR4/CXCL12 axis for recruitment. This study also suggests that lupus neutrophils, through elevated levels of CXCR4 may have enhanced recruitment to inflamed sites. In contrast to this study, others have shown that in a mouse model of peritonitis involving thioglycolate, two different strains of lupus mice showed normal recruitment of neutrophils to the peritoneum, but delayed resolution of the neutrophil infiltrate (58). Other factors are also at play. For example, IL-8 may be important in recruiting neutrophils to the inflamed kidney since there are high levels of IL-8 in the urine in patients with lupus nephritis (59). However, as mentioned above, neutrophils in lupus have been observed to have impaired migration towards IL-8. VCAM-1 is also elevated in the urine of patients with active lupus glomerulonephritis (60) and neutrophils from patients with active lupus express higher levels of the β2 integrin CD11b/CD18 (51), suggesting that integrin signaling is important for neutrophil recruitment in lupus.
Thus, there are many conflicting reports regarding the phenotype and functions of neutrophils in lupus, however, the bulk of the evidence supports a pathologic role, the details of which are still poorly defined.
Behcet’s Disease
Although it is more of an autoinflammatory disorder than an autoimmune disorder, Behcet’s disease is worth mentioning since neutrophils are found in the inflammatory lesions (61) and there is some interesting literature regarding their phenotype and Behcet’s treatment. The classic symptoms of Behcet’s disease are oral and genital ulcers but patients also have at least some of the following symptoms: pathergy, a variety of skin rashes, eye inflammation, arthritis, vascular thrombosis, and vasculitis. Indeed Behcet’s may be caused by inflammation of the blood vessels.
Neutrophils have been evaluated in active Behcet’s disease and have increased in vivo migration compared to normal controls and patients whose Behcet’s is in remission. However, no differences were seen in reactive oxygen species production or adhesion (62). Further, sera from Behcet’s patients increase neutrophil migration, a phenotype that can be inhibited by colchicine (63). Treatment of Behcet’s often involves colchicine, a microtubule inhibitor, which will be discussed below.
Neutrophil Studies in Zebrafish and Implications for Human Autoimmune Disease
As discussed above, neutrophils play prominent roles in autoimmunity and their migratory function is often critical for their pathogenic roles. However it is challenging to study neutrophil motility in humans, or even mice. Moreover, observations from in vitro migration studies do not always parallel what occurs in three dimensions in vivo. Thus studies in zebrafish may enable us to better understand neutrophil motility with implications for autoimmunity.
One of the major recent observations is that following recruitment to a wound, neutrophils can reverse migrate away from the wound to distant locations in the zebrafish (15), as discussed in detail above. This observation could impact our understanding of the pathophysiology of autoimmunity since reverse migrated neutrophils might influence progression of disease. For example, neutrophils might reverse migrate away from areas of inflammation like the rheumatoid joint or inflamed blood vessel. Those neutrophils could migrate to lymphoid organs to present antigens from the inflamed site to T cells or B cells and contribute to the diversification of the auto-antibody repertoire. Perhaps, even subclinical inflammation, in the joint for example, could lead to neutrophil recruitment. Some neutrophils become activated and die by NETosis and expose the citrullinated antigens that become targets for ACPAs. Other neutrophils may reverse migrate to the lymph node to diversify the antibody response. Indeed, it has been shown that the ACPA repertoire expands in pre-clinical rheumatoid arthritis even before symptoms develop (64). In these scenarios, long-lived, inflammation-sensitized neutrophils would be pathologic, but the opposite might also be true with previously educated neutrophils acting as a tolerizing force for T cells. At this time, all of the ways in which neutrophils affect autoimmunity are unknown. Using the zebrafish model to answer questions regarding the regulators of cytoskeletal dynamics in neutrophil movements, we hope to better understand inflammation and autoimmunity. Below, we discuss some of our recent findings and how these observations may contribute to improving our understanding of autoimmune disease.
Rac2 in Neutrophils
Rac2 is a member of the Rho family of guanosine triphosphatases (Rho GTPases) whose expression is restricted to the hematopoietic lineage (65). Rho GTPases are a group of small signaling molecules that activate various cellular signaling pathways leading to events such as actin polymerization and the formation of a reduced NADPH oxidase complex (66). In leukocytes, Rho GTPases participate in the tightly controlled process of transendothelial migration as well as chemotaxis in response to immune system stimulation. Rho GTPases integrate signals from integrins and chemokine receptors and then propagate these signals through the reorganization of the actin cytoskeleton into a motile structure (67).
Among all Rho GTPases, in vitro studies reveal a specific role for Rac2 in neutrophil chemotaxis (68). Following fMLP stimulation of neutrophils, Rac2 appears to be a key factor for actin polarization and assembly of a pseudopod. Moreover, Rac2 deficient mice display mild neutrophilia and defective host defense (69) suggesting a defect in neutrophil transendothelial migration and chemotaxis. However, how Rac2 regulates neutrophil directed migration in vivo during inflammation was not known. Taking advantage of zebrafish to investigate neutrophil migration in vivo (70), we generated two transgenic zebrafish lines that specifically express Rac2 and Rac2 D57N point mutation in neutrophils (71). The Rac2 D57N mutant corresponds to an identified mutation in two infants suffering from a new type of leukocyte adhesion deficiency (LAD), LAD IV (72). We had previously shown that neutrophils from those patients have impaired polarization and directed migration in vitro (73). Using zebrafish, we were then able to confirm the impaired polarization and directed migration phenotype of Rac2 D57N neutrophils in vivo as well as show that Rac2 not only drives actin architecture but is also required for proper activation of phosphoinositide 3-kinase (PI(3)K). This finding is of particular interest because PI(3)K was previously considered to be an upstream regulator of Rac2 (74), and thus these findings suggest a more complex feedback interaction as shown in Figure 3. Rac2 D57N mutants also exhibit increased release of neutrophils from hematopoietic tissue into the vasculature mainly due to a disruption of the SDF1-CXCR4 neutrophil retention signal in hematopoietic tissue (75). Therefore, using zebrafish and photoconversion cell fate mapping, this disease model helped to explain how the specific D57N mutation seen in patients was responsible for the observed neutrophilia.
Figure 3. Model for signaling involving SFKs, Rac2, SHIP, and PI(3)K in neutrophil recruitment.
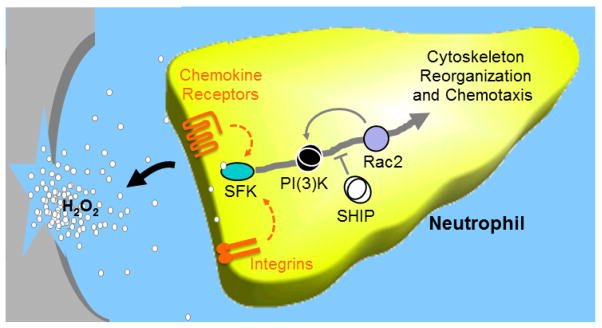
Upon tissue injury or infection, neutrophils are the first responders of the innate immune response. They are attracted to sites of inflammation by various stimuli including binding of integrin ligands, chemokines, or hydrogen peroxide (H2O2), which can lead to signaling through Src family kinases (SFKs). In the case of a wound, H2O2 is released by damaged epithelial cells and oxidizes a cysteine in the SFK family member, Lyn, which thus acts as a H2O2 gradient sensor. Oxidation of that cysteine leads to increased kinase activity of Lyn. Once activated, Lyn can drive the cytoskeleton reorganization that ultimately leads to neutrophil chemotaxis through the activation of PI(3)K which induces Rac2. Interestingly, Rac2 can also activate PI(3)K in what may be a positive feedback loop enhancing migration. Neutrophil chemotaxis is inhibited by SHIP via regulation of PI(3)K signaling.
Thus, Rac2 is a critical molecule for neutrophil motility. As touched upon above (76–79) inhibitory mutations in Rac2 lead to a severe human neutrophil immunodeficiency syndrome with neutrophils displaying defects such as disorganized actin polymerization, defective oxidative burst, and abnormal chemotaxis depending on the specific mutation. Thus, it is clear that when Rac2 is impaired, immunity is impaired as well. However, decreased immune response due to deficient or defective Rac2 perhaps could be capitalized upon in order to treat autoimmune disease. If Rac2 could be partially inhibited or temporarily inhibited, there could be benefits in autoimmune diseases like rheumatoid arthritis or vasculitis. In support of this idea, in a murine model of infective arthritis, mice deficient in both Rac1 and Rac2 had reduced arthritis and reduced synovial infiltration of neutrophils in the acute phase, but arthritis was more severe in the chronic phase with decreased pathogen clearance from the joint (80). It seems likely that the more severe chronic arthritis was related to poor pathogen clearance, so if one were to extrapolate to an autoimmune arthritis like rheumatoid arthritis, where there is no pathogen to clear, inhibition of Rac1 and Rac2 might be beneficial leading to decreased neutrophil infiltration and improved arthritis. Further, inhibition of Rac1 reduces paw swelling in collagen induce arthritis, a model of rheumatoid arthritis (81). Therefore, the Rac GTPases may be potential therapeutic targets in rheumatoid arthritis.
The involvement of Rac2 in neutrophil migration in vivo also suggests that Rac2 might be abnormally regulated in autoimmune disorders. Indeed, one of the three major RAC2 haplogroups in humans is associated with multiple sclerosis and Crohn’s disease, two chronic inflammatory disorders (82). Thus, Rac2 may be important in diseases that involve recruitment of neutrophils to an area of inflammation like the joint in rheumatoid arthritis or the vessels in vasculitis, potentially making it a target for treatment.
Lyn in Neutrophils
Src family kinases (SFKs) are signalling proteins that have long been recognized to regulate critical cellular processes such as leukocyte proliferation, survival and migration (83). One essential role of SFKs in the inflammatory response is regulation of neutrophil activation and recruitment to inflamed tissue (84, 85). SFKs are activated in response to stimulation of a variety of cell surface receptors such as tumour necrosis factor receptor (TNFR) (86), P-selectin glycoprotein ligand 1 (PSGL-1) (85), interleukin receptors, integrin receptors and chemotactic sensitive G protein-coupled receptors (87). Once activated, SFKs contribute to both chemotactic recruitment and endothelium adhesion of neutrophils (84). Whereas SFK involvement in selectin-mediated neutrophil adhesion is widely accepted, the precise role of SFKs in chemotactic movement and recruitment is still under debate. Indeed, some studies claim that neutrophil recruitment to liver (88, 89), lung (90), peritoneal cavity (85), skin and inflamed organs (91) requires the activity of SFKs while others (92, 93) argue for a SFK independent recruitment of neutrophil into the peritoneal cavity and skin. However, these apparent discrepant observations may be due to differences in which SFK was evaluated or which modality was used to trigger inflammation.
Among SFKs, emerging evidence suggests that Lyn is the main contributor to endothelial adhesion (86, 94) and directed migration of neutrophils (95). Like all SFKs, Lyn is composed of several domains: an N-terminal unique domain, SH3 and SH2 domains, and a C-terminal kinase domain (83, 96). At the N-terminus, Lyn is myristoylated and palmitoylated causing its association with the plasma membrane. The SH3 and SH2 domains mediate interactions with proline-rich motifs and with phosphotyrosine-containing sequences, respectively (97). Lyn is in an inactive conformation involving an intramolecular interaction between a phosphotyrosine residue and the SH2 domain. Upon dephosphorylation of Y508, Lyn adopts an open conformation in which the activation loop tyrosine (Y397 in humans) is phosphorylated and the kinase is fully activated (83). A lysine at position 275 is required for full enzymatic activity of the C-terminal kinase domain (96).
SDF1-CXCR4 can trigger the Lyn/PI(3)K signalling pathway in the neutrophil-like HL-60 cell line and SDF1 induced cell migration is defective in Lyn deficient bone marrow cells (98). Lyn then has many downstream effects. A recent report from He et al. depicts a Lyn dependent mechanism that governs neutrophil adhesion in chemotaxis gradients (99). This pathway depends upon Gi-protein-mediated Lyn activation and leading edge recruitment. Next, Lyn relieves the inhibition of and promotes the recruitment of the CrkL-C3G (an adaptor protein-guanine exchange factor protein) complex at the leading edge, which leads to localized activation of the small GTPase Rap1 and its downstream target, β2 integrin (CD18). Ultimately, β2 integrins mediate interactions with fibrinogen in the extracellular matrix of endothelial cells and initiates cell migration. In neutrophils, Lyn also controls Rac2 dependent chemotaxis through ELMO-Dock2 (adaptor protein-guanine exchange factor protein) complex activation in CXCR8 stimulated cells (100) and Lyn is known to activate the MAP kinase ERK in human neutrophils (101), which represents a critical factor in neutrophil migration (102). Lyn can also drive neutrophil chemoattraction via the PI(3)K signalling pathway (103). However, others studies call into question the role of Lyn in neutrophil migration. Pereira et al. have shown that neutrophils from Lyn deficient mice display a hyper-adhesive integrin dependent phenotype (104) and Moscai et al. demonstrate that Lyn deficient neutrophils have normal migration in vitro and in vivo (93). These opposing observations highlight the need to investigate more precisely the role of Lyn in the regulation of neutrophil chemotaxis in vivo.
Despite the conflicting findings, these studies raise the possibility that Lyn could be a key component in neutrophil inflammation. It had recently been shown that H2O2 mediates rapid recruitment of neutrophils to a wound (Niethammer et al., 2009) and reactive oxygen intermediates (ROIs) can increase Lyn kinase activity (86, 105). Thus, we speculated that Lyn could be the neutrophil’s detector molecule for the H2O2 gradient formed after tissue injury. We took advantage of an ROI-insensitive Lyn, due to C245A and C487A mutations (106), to explore the possibility that these particular cysteines could be key factors for Lyn activation following tissue injury and subsequent H2O2 release in vivo. We generated a transgenic zebrafish carrying these cysteine mutations in neutrophils and tested if neutrophils with ROI-insensitive Lyn could reach inflammatory sites (107). We discovered that, in zebrafish, cysteine 466 of Lyn (corresponding to human C487) is targeted by H2O2 oxidation and this event is necessary for neutrophil recruitment to wounded tissue, showing for the first time in vivo that a SFK could act as a redox sensor and drive directional migration through a H2O2 gradient (107). This finding sets the stage to determine the molecular mechanism that supports Lyn-mediated directed migration. Lyn could link SDF1-CXCR4 activation to the Rac2 signalling pathway in zebrafish haematopoietic tissue to regulate neutrophil retention and release into the circulation. Further Lyn could regulate neutrophil polarization and migration through a PI(3)K/Rac/Rho and/or a MAPK/ERK dependant signalling pathway. The ways in which Lyn could interact with Rac and PI(3)K and other molecules to regulate migration is outlined in Figure 3.
How Lyn might contribute to autoimmune disease is more complex than how Rac2 might be involved. However Lyn and other Src family kinases are thought to be important and are a focus of drug development (89). There are clear relationships between Lyn in B cells and lupus. For example, Lyn-deficient mice have reduced B cell tolerance and a lupus-like glomerulonephritis (108). Also lupus patients have decreased Lyn expression and translocation in B cells (109). However, for neutrophils in autoimmune disease, a role for Lyn has not been described. One could hypothesize that Lyn might act as a redox sensor in neutrophils in autoimmune disease similar to wound healing. That said, reactive oxygen species may not play the same role in wound recruitment as in inflamed joint recruitment. H2O2 attracts neutrophils to the wound in zebrafish (110). In contrast, treatment with exogenous H2O2 or superoxide dismutase was associated with decreased neutrophilic infiltrate and increased neutrophil apoptosis secondary to PI(3)K/Akt activation in a murine model of inflammatory arthritis (111). Further, mice deficient in gp91phox, a NADPH oxidase complex, had unaltered neutrophil recruitment to the inflamed joint but delayed resolution (111). These data are somewhat difficult to interpret. It is possible that the treatment with H2O2 or superoxide dismutase was supraphysiologic and simply resulted in the observed neutrophil death. Also, there may be other sources of reactive oxygen species in the inflamed joint besides those produced by gp91phox resulting in the unimpaired neutrophil recruitment in the absence of gp91phox. Alternatively the role of H2O2 may be different in zebrafish wound response and mammalian arthritis. Lyn may act as a redox sensor in both situations, but with different outcomes. Much additional work is needed in this area, but the clear role for Lyn in mediating neutrophil recruitment in zebrafish identifies it as a target for future investigations in human autoimmunity.
SHIP in Neutrophils
SH2-domain-containing inositol 5-phosphatase (SHIP) is a 5′ inositol polyphosphatase that balances PI(3)K activity by converting PI(3,4,5)P3 and PI(1,2,3,5)P4 into PI(3,4)P3 and PI(1,2,3)P4 respectively. Related to this specific enzymatic function, SHIP is at the nexus of this intracellular signaling pathway and its activation is considered to be the termination of PI(3)K mediated signaling (112). In innate immune cells, PI(3)K and SHIP act in concert to control cell polarization in chemotaxis gradients (113). PI(3)Ks generate the intracellular second messengers PI(3,4,5)P3 and PI(3,4)P2, which are critical regulators of a wide variety of cellular processes including cell migration (114). More specifically, PI(3)K regulates forward protrusion of the leading edge of a migrating cell by activating Rac through a Rac Guanine Exchange Factor (74). PI(3)K is considered to be a central regulator of gradient sensing during chemotaxis of neutrophils in vitro (115). However little is known about how polarized cell migration is regulated in three-dimensional (3D) tissue environments in vivo because few systems are amenable to high resolution imaging. Recent advances uncoupled two roles for PI(3)K in regulating neutrophil migration in live zebrafish: a Rac-mediated protrusion at the leading edge and a Rho-dependent tail contraction (116). This two-tiered regulation of motility by PI(3)K is indispensable to coordinate F-actin anteroposterior polarization and subsequent directed migration of neutrophils toward injured tissue. Given the importance of SHIP in regulating PI(3)K activity, we focused further studies on the role of SHIP in neutrophil behaviour in live zebrafish.
Whereas PI(3)K-mediated chemotaxis is well understood, the role of SHIP is less studied. SHIP deficient mice show robust leukocyte infiltration into the lung and their bone marrow-derived mast cells display increased PIP3 levels and Akt activation, suggesting a suppressor role of SHIP in inflammation (117). Contradicting these observations, an in vitro study showed that SHIP1-null neutrophils have impaired polarization and motility (118). These divergent observations required deeper investigation to clarify the impact of SHIP on PIP3 subcellular distribution and the effect on neutrophil response to acute inflammatory signals. To this end, we investigated the role of SHIP using high resolution real time imaging in live zebrafish. In contrast to PI(3)K, whose activation is concentrated at the leading edge of motile neutrophils, our findings suggest that SHIP is active both at the leading edge and at the tail, possibly to avoid any rear Akt activation and consequently favour uni-directionality in migratory neutrophils (119). We also show that SHIP acts as a repressor of inflammation since higher numbers of neutrophils accumulate at a wound when SHIP is downregulated using a morpholino. Further, the increased recruitment of neutrophils in SHIP-deficient zebrafish could be reversed by treatment with a PI(3)Kγ inhibitor, suggesting that SHIP restricts neutrophil attraction via PI(3)K. These observations in live zebrafish are consistent with the mouse studies noted above (117) and not the in vitro studies, suggesting that (1) SHIP limits neutrophil motility by modulating PI(3)K signalling and that (2) the 3D environment is critical for the physiologic regulation of the phosphatidyl inositol signalling pathway.
Of interest, as mentioned above, Lyn can regulate Rac2 dependent chemotaxis through ELMO-Dock2 and Lyn can mediate neutrophil chemoattraction via PI(3)K. Lyn can also drive both pro- and anti-chemotaxis signals and consequently repress or activate SHIP (120). Thus, these molecules may all intersect in controlling neutrophil migration as shown in Figure 3.
There are several ways in which SHIP may be important for autoimmune disease. There is the most evidence for a role of SHIP in rheumatoid arthritis. SHIP-1 levels are reduced in monocyte lineage cells in the synovium in rheumatoid arthritis compared to osteoarthritis, a degenerative arthritis. Interestingly, the microRNA miR-155 is upregulated in synovial fluid macrophages and in the synovial membrane and miR-155 downregulates expression of SHIP-1 and causes increased production of TNFα. Further, mice that lack miR-155 are resistant to collagen-induced arthritis with reduced autoantibody production and joint inflammation (121). Thus, these studies suggest that reduced SHIP-1 activity, at least in macrophages, may play a role in rheumatoid arthritis by leading to increased TNFα and likely other effects. Given our findings regarding SHIP in limiting neutrophil motility, the lack of miRNA-155 in mice (and thus upregulation of SHIP) may lead to reduced arthritis due to poor neutrophil recruitment as well as reduced macrophage related inflammation. Thus, novel treatments for rheumatoid arthritis could involve activators of SHIP. There have been small molecule activators of SHIP1 generated which stimulate SHIP-1 activity in macrophages and mast cells and have protective effects in endotoxemia and anaphylaxis (122). Thus, although the effects are not specific for neutrophils or arthritis, these activators may be beneficial in autoimmune diseases in which neutrophils play a role.
As noted above, SHIP is a negative regulator of PI(3)K activity and PI(3)K has numerous roles in neutrophils. Further, hyperactivation of PI(3)K signaling predisposes to autoimmunity (123). The reverse may also be true with reductions in PI(3)K function reducing inflammation, specifically due to reduced neutrophil function. For example, in the K/BxN serum transfer model of inflammatory arthritis, loss of PI(3)Kδ or PI(3)Kγ reduces arthritis and correlates with decreased neutrophil migration into tissue in response to leukotriene B(4) (124). Also, a PI(3)Kγ inhibitor ameliorates collagen-induced arthritis and reduces neutrophil infiltration into the joint (125). Thus molecules inhibiting PI(3)K or activating SHIP may be novel therapeutic strategies in rheumatoid arthritis.
In vasculitis, there are no studies involving SHIP, but there is evidence that PI(3)K plays a critical role. For example, neutrophil activation by ANCAs leads to PI(3)K activation (126). Further, bone marrow from PI(3)Kγ deficient mice protected wild-type mice from the development of glomerulonephritis in a mouse model vasculitis in which MPO deficient mice are immunized with MPO (127). Also, a PI(3)Kγ inhibitor was able to suppress glomerulonephritis when wild type bone marrow was transplanted in this model, likely due to reduced ANCA induced superoxide production, degranulation and GM-CSF induced migration of neutrophils (127).
Thus, activation of SHIP and inhibition of PI(3)K could improve both rheumatoid arthritis and vasculitis. There is less known about the role of SHIP and PI(3)K in neutrophils in lupus, making this disease even more in need of research into PI(3)K and SHIP function.
Microtubules in Neutrophils
Microtubules have essential roles during directed cell migration. They play an important role in focal adhesion turnover (128), cell polarity, and regulation of actin-generated force (129) – three critical components of cell migration. Microtubules had been shown to inhibit neutrophil random motility in vitro, but are required for directional migration (130, 131). We found that upon depolymerizing microtubules in live zebrafish with nocodozole, neutrophils exhibit impaired directional migration to a wound (132). Microtubule depolymerization activates RhoA in vitro (130) and both microtubule polymerization and depolymerization appear to activate Rac in vitro (132, 133). Further, microtubule depolymerization inhibits PI(3)K activation at the leading edge of neutrophils in zebrafish (132). Thus the assembly status of microtubules regulates Rac and PI(3)K activity, which contributes to directional motility.
The role of microtubules in inflammatory disease is most obvious in Behcet’s disease. As mentioned above, colchicine, a microtubule polymerization inhibitor, is first line treatment for Behcet’s and can reduce disease flares. Neutrophils from patients with active Behcet’s have more microtubules than controls (134) with altered distribution (135), perhaps consistent with why Behcet’s is responsive to colchicine. Since colchicine inhibits microtubule assembly and since microtubule disassembly reduces recruitment of neutrophils to wounds in zebrafish, colchicine may be acting in Bechet’s by limiting neutrophil recruitment to inflamed areas. However, colchicine may have other effects such as reducing the microtubule-regulated endocytosis of inflammatory mediators into neutrophils and thus reducing neutrophil activation (136).
Colchicine is generally not used as a treatment in lupus, vasculitis, or rheumatoid arthritis, but there have been no large studies investigating its use. Thus, there could be an unidentified role for colchicine in these disorders. Alternatively, it is possible that there is a unique role for colchicine in Behcet’s disease for reasons that are not completely clear. Perhaps abnormalities in microtubules in Behcet’s leads to the effectiveness of colchicine. However, colchicine is also effective in gout, an acute inflammatory arthritis due to monosodium urate crystals, where no microtubule abnormalities have been reported. Another possibility is that perhaps neutrophil recruitment or activation of Rac or PI(3)K signaling occurs in a microtubule-independent way in some autoimmune diseases, and thus colchicine would not be effective. More studies are needed in this area to clarify the role of microtubules and colchicine in autoimmunity.
Conclusion
There is a clear role for neutrophils in autoimmunity although the extent of neutrophil involvement and various ways that neutrophils contribute to disease are still being uncovered. By exploring the cytoskeletal regulators of neutrophil migration in zebrafish in vivo, we hope to shed light on how neutrophils impact human autoimmunity.
Acknowledgments
Support for this work is provided by NIH R01 GM074827 to AH.
Footnotes
The authors have no financial and personal relationships that could be viewed as presenting a potential conflict of interest.
References
- 1.Nemeth T, Mocsai A. The role of neutrophils in autoimmune diseases. Immunol Lett. 2012;143:9–19. doi: 10.1016/j.imlet.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MJ. Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol. 2011;7:691–699. doi: 10.1038/nrrheum.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 4.Maletto BA, et al. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood. 2006;108:3094–3102. doi: 10.1182/blood-2006-04-016659. [DOI] [PubMed] [Google Scholar]
- 5.Pillay J, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 6.Puga I, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nature immunology. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostanin DV, et al. Acquisition of antigen-presenting functions by neutrophils isolated from mice with chronic colitis. Journal of immunology. 2012;188:1491–1502. doi: 10.4049/jimmunol.1102296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan SO, Johnson JL, Cobb BA. Neutrophils Confer T Cell Resistance to Myeloid-Derived Suppressor Cell-Mediated Suppression To Promote Chronic Inflammation. Journal of immunology. 2013 doi: 10.4049/jimmunol.1203404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pillay J, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. The Journal of clinical investigation. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pick R, Brechtefeld D, Walzog B. Intraluminal crawling versus interstitial neutrophil migration during inflammation. Molecular immunology. 2013;55:70–75. doi: 10.1016/j.molimm.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Developmental cell. 2009;17:310–322. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Yuan SY, Shen Q, Rigor RR, Wu MH. Neutrophil transmigration, focal adhesion kinase and endothelial barrier function. Microvascular research. 2012;83:82–88. doi: 10.1016/j.mvr.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Affolter M, Weijer CJ. Signaling to cytoskeletal dynamics during chemotaxis. Developmental cell. 2005;9:19–34. doi: 10.1016/j.devcel.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Niedergang F, Chavrier P. Regulation of phagocytosis by Rho GTPases. Current topics in microbiology and immunology. 2005;291:43–60. doi: 10.1007/3-540-27511-8_4. [DOI] [PubMed] [Google Scholar]
- 15.Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. Journal of leukocyte biology. 2006;80:1281–1288. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- 16.Yoo SK, Huttenlocher A. Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. J Leukoc Biol. 2011;89:661–667. doi: 10.1189/jlb.1010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pap T, Muller-Ladner U, Gay RE, Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000;2:361–367. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. Journal of immunology. 2001;167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka D, Kagari T, Doi H, Shimozato T. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis. Immunology. 2006;119:195–202. doi: 10.1111/j.1365-2567.2006.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capsoni F, et al. Effect of adalimumab on neutrophil function in patients with rheumatoid arthritis. Arthritis research & therapy. 2005;7:R250–255. doi: 10.1186/ar1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cedergren J, Forslund T, Sundqvist T, Skogh T. Intracellular oxidative activation in synovial fluid neutrophils from patients with rheumatoid arthritis but not from other arthritis patients. The Journal of rheumatology. 2007;34:2162–2170. [PubMed] [Google Scholar]
- 22.Binstadt BA, et al. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nature immunology. 2006;7:284–292. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 23.Wipke BT, Wang Z, Nagengast W, Reichert DE, Allen PM. Staging the initiation of autoantibody-induced arthritis: a critical role for immune complexes. Journal of immunology. 2004;172:7694–7702. doi: 10.4049/jimmunol.172.12.7694. [DOI] [PubMed] [Google Scholar]
- 24.Khandpur R, et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Science translational medicine. 2013;5:178ra140. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang CH, et al. Expression of CD147 (EMMPRIN) on neutrophils in rheumatoid arthritis enhances chemotaxis, matrix metalloproteinase production and invasiveness of synoviocytes. Journal of cellular and molecular medicine. 2011;15:850–860. doi: 10.1111/j.1582-4934.2010.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assi LK, et al. Tumor necrosis factor alpha activates release of B lymphocyte stimulator by neutrophils infiltrating the rheumatoid joint. Arthritis and rheumatism. 2007;56:1776–1786. doi: 10.1002/art.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith E, et al. Duffy antigen receptor for chemokines and CXCL5 are essential for the recruitment of neutrophils in a multicellular model of rheumatoid arthritis synovium. Arthritis and rheumatism. 2008;58:1968–1973. doi: 10.1002/art.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lally F, et al. A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synovium. Arthritis and rheumatism. 2005;52:3460–3469. doi: 10.1002/art.21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadik CD, Kim ND, Iwakura Y, Luster AD. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcgammaR signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3177–3185. doi: 10.1073/pnas.1213797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraan MC, de Koster BM, Elferink JG, Post WJ, Breedveld FC, Tak PP. Inhibition of neutrophil migration soon after initiation of treatment with leflunomide or methotrexate in patients with rheumatoid arthritis: findings in a prospective, randomized, double-blind clinical trial in fifteen patients. Arthritis and rheumatism. 2000;43:1488–1495. doi: 10.1002/1529-0131(200007)43:7<1488::AID-ANR11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 31.Youssef PP, et al. Neutrophil trafficking into inflamed joints in patients with rheumatoid arthritis, and the effects of methylprednisolone. Arthritis and rheumatism. 1996;39:216–225. doi: 10.1002/art.1780390207. [DOI] [PubMed] [Google Scholar]
- 32.den Broeder AA, Wanten GJ, Oyen WJ, Naber T, van Riel PL, Barrera P. Neutrophil migration and production of reactive oxygen species during treatment with a fully human anti-tumor necrosis factor-alpha monoclonal antibody in patients with rheumatoid arthritis. The Journal of rheumatology. 2003;30:232–237. [PubMed] [Google Scholar]
- 33.Ottonello L, et al. Delayed neutrophil apoptosis induced by synovial fluid in rheumatoid arthritis: role of cytokines, estrogens, and adenosine. Annals of the New York Academy of Sciences. 2002;966:226–231. doi: 10.1111/j.1749-6632.2002.tb04219.x. [DOI] [PubMed] [Google Scholar]
- 34.Parsonage G, et al. Prolonged, granulocyte-macrophage colony-stimulating factor-dependent, neutrophil survival following rheumatoid synovial fibroblast activation by IL-17 and TNFalpha. Arthritis research & therapy. 2008;10:R47. doi: 10.1186/ar2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinmann P, et al. Delayed neutrophil apoptosis in very early rheumatoid arthritis patients is abrogated by methotrexate therapy. Clinical and experimental rheumatology. 2007;25:885–887. [PubMed] [Google Scholar]
- 36.Xiao H, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. The Journal of clinical investigation. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kettritz R. How anti-neutrophil cytoplasmic autoantibodies activate neutrophils. Clinical and experimental immunology. 2012;169:220–228. doi: 10.1111/j.1365-2249.2012.04615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks CJ, King WJ, Radford DJ, Adu D, McGrath M, Savage CO. IL-1 beta production by human polymorphonuclear leucocytes stimulated by anti-neutrophil cytoplasmic autoantibodies: relevance to systemic vasculitis. Clinical and experimental immunology. 1996;106:273–279. doi: 10.1046/j.1365-2249.1996.d01-835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kessenbrock K, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nature medicine. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keogan MT, Esnault VL, Green AJ, Lockwood CM, Brown DL. Activation of normal neutrophils by anti-neutrophil cytoplasm antibodies. Clin Exp Immunol. 1992;90:228–234. doi: 10.1111/j.1365-2249.1992.tb07934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keogan MT, Rifkin I, Ronda N, Lockwood CM, Brown DL. Anti-neutrophil cytoplasm antibodies (ANCA) increase neutrophil adhesion to cultured human endothelium. Adv Exp Med Biol. 1993;336:115–119. doi: 10.1007/978-1-4757-9182-2_19. [DOI] [PubMed] [Google Scholar]
- 43.Calderwood JW, Williams JM, Morgan MD, Nash GB, Savage CO. ANCA induces beta2 integrin and CXC chemokine-dependent neutrophil-endothelial cell interactions that mimic those of highly cytokine-activated endothelium. Journal of leukocyte biology. 2005;77:33–43. doi: 10.1189/jlb.0104054. [DOI] [PubMed] [Google Scholar]
- 44.Richter AG, Perkins GD, Chavda A, Sapey E, Harper L, Thickett DR. Neutrophil chemotaxis in granulomatosis with polyangiitis (Wegener’s) and idiopathic pulmonary fibrosis. Eur Respir J. 2011;38:1081–1088. doi: 10.1183/09031936.00161910. [DOI] [PubMed] [Google Scholar]
- 45.Kallenberg CG. Pathogenesis of ANCA-associated vasculitides. Annals of the rheumatic diseases. 2011;70 (Suppl 1):i59–63. doi: 10.1136/ard.2010.138024. [DOI] [PubMed] [Google Scholar]
- 46.Xiao H, et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. The American journal of pathology. 2005;167:39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hattar K, et al. c-ANCA-induced neutrophil-mediated lung injury: a model of acute Wegener’s granulomatosis. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2010;36:187–195. doi: 10.1183/09031936.00143308. [DOI] [PubMed] [Google Scholar]
- 48.Jerke U, Rolle S, Purfuerst B, Luft FC, Nauseef WM, Kettritz R. beta2 integrin-mediated cell-cell contact transfers active myeloperoxidase from neutrophils to endothelial cells. The Journal of biological chemistry. 2013 doi: 10.1074/jbc.M112.434613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyabe C, et al. Am80, a retinoic acid receptor agonist, ameliorates murine vasculitis through the suppression of neutrophil migration and activation. Arthritis Rheum. 2013;65:503–512. doi: 10.1002/art.37784. [DOI] [PubMed] [Google Scholar]
- 50.de la Fuente H, Richaud-Patin Y, Jakez-Ocampo J, Gonzalez-Amaro R, Llorente L. Innate immune mechanisms in the pathogenesis of systemic lupus erythematosus (SLE) Immunol Lett. 2001;77:175–180. doi: 10.1016/s0165-2478(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 51.Molad Y, Buyon J, Anderson DC, Abramson SB, Cronstein BN. Intravascular neutrophil activation in systemic lupus erythematosus (SLE): dissociation between increased expression of CD11b/CD18 and diminished expression of L-selectin on neutrophils from patients with active SLE. Clin Immunol Immunopathol. 1994;71:281–286. doi: 10.1006/clin.1994.1087. [DOI] [PubMed] [Google Scholar]
- 52.Villanueva E, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knight JS, Kaplan MJ. Lupus neutrophils: ‘NET’ gain in understanding lupus pathogenesis. Curr Opin Rheumatol. 2012;24:441–450. doi: 10.1097/BOR.0b013e3283546703. [DOI] [PubMed] [Google Scholar]
- 54.Lande R, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hakkim A, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell AM, Kashgarian M, Shlomchik MJ. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med. 2012;4:157ra141. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang A, et al. CXCR4/CXCL12 hyperexpression plays a pivotal role in the pathogenesis of lupus. J Immunol. 2009;182:4448–4458. doi: 10.4049/jimmunol.0801920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Potter PK, Cortes-Hernandez J, Quartier P, Botto M, Walport MJ. Lupus-prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. J Immunol. 2003;170:3223–3232. doi: 10.4049/jimmunol.170.6.3223. [DOI] [PubMed] [Google Scholar]
- 59.Wada T, et al. Detection of urinary interleukin-8 in glomerular diseases. Kidney Int. 1994;46:455–460. doi: 10.1038/ki.1994.293. [DOI] [PubMed] [Google Scholar]
- 60.Singh S, et al. Urine VCAM-1 as a marker of renal pathology activity index in lupus nephritis. Arthritis Res Ther. 2012;14:R164. doi: 10.1186/ar3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pineton de Chambrun M, Wechsler B, Geri G, Cacoub P, Saadoun D. New insights into the pathogenesis of Behcet’s disease. Autoimmun Rev. 2012;11:687–698. doi: 10.1016/j.autrev.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 62.Carletto A, et al. Changes of neutrophil migration without modification of in vitro metabolism and adhesion in Behcet’s disease. J Rheumatol. 1997;24:1332–1336. [PubMed] [Google Scholar]
- 63.Jorizzo JL, et al. Behcet’s syndrome: immune regulation, circulating immune complexes, neutrophil migration, and colchicine therapy. J Am Acad Dermatol. 1984;10:205–214. doi: 10.1016/s0190-9622(84)70024-7. [DOI] [PubMed] [Google Scholar]
- 64.Sokolove J, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Courjal F, Chuchana P, Theillet C, Fort P. Structure and Chromosomal Assignment to 22q12 and 17qter of the ras-Related Rac2 and Rac3 Human Genes. Genomics. 1997;44:242–246. doi: 10.1006/geno.1997.4871. [DOI] [PubMed] [Google Scholar]
- 66.Wittchen ES, van Buul JD, Burridge K, Worthylake RA. Trading spaces: Rap, Rac, and Rho as architects of transendothelial migration. Current Opinion in Hematology. 2005;12:14–21. doi: 10.1097/01.moh.0000147892.83713.a7. [DOI] [PubMed] [Google Scholar]
- 67.Sun CX, Magalhães MAO, Glogauer M. Rac1 and Rac2 differentially regulate actin free barbed end formation downstream of the fMLP receptor. The Journal of Cell Biology. 2007;179:239–245. doi: 10.1083/jcb.200705122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pestonjamasp KN, et al. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814–2820. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts AW, et al. Deficiency of the Hematopoietic Cell-Specific Rho Family GTPase Rac2 Is Characterized by Abnormalities in Neutrophil Function and Host Defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 70.Mathias J, Walters K, Huttenlocher A. Neutrophil Motility In Vivo Using Zebrafish. In: Jin T, Hereld D, editors. Chemotaxis. Humana Press; 2009. pp. 151–166. [DOI] [PubMed] [Google Scholar]
- 71.Deng Q, Yoo SK, Cavnar PJ, Green JM, Huttenlocher A. Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Developmental cell. 2011;21:735–745. doi: 10.1016/j.devcel.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pai S-Y, Kim C, Williams DA. Rac GTPases in human diseases. Disease Markers. 2010;29:177–187. doi: 10.3233/DMA-2010-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berthier E, Surfus J, Verbsky J, Huttenlocher A, Beebe D. An arrayed high-content chemotaxis assay for patient diagnosis. Integrative Biology. 2010;2:630–638. doi: 10.1039/c0ib00030b. [DOI] [PubMed] [Google Scholar]
- 74.Nishikimi A, et al. Sequential Regulation of DOCK2 Dynamics by Two Phospholipids During Neutrophil Chemotaxis. Science. 2009;324:384–387. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116:2803–2811. doi: 10.1182/blood-2010-03-276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Accetta D, et al. Human phagocyte defect caused by a Rac2 mutation detected by means of neonatal screening for T-cell lymphopenia. The Journal of allergy and clinical immunology. 2011;127:535–538. e531–532. doi: 10.1016/j.jaci.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 77.Williams DA, et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood. 2000;96:1646–1654. [PubMed] [Google Scholar]
- 78.Ambruso DR, et al. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4654–4659. doi: 10.1073/pnas.080074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kurkchubasche AG, Panepinto JA, Tracy TF, Jr, Thurman GW, Ambruso DR. Clinical features of a human Rac2 mutation: a complex neutrophil dysfunction disease. The Journal of pediatrics. 2001;139:141–147. doi: 10.1067/mpd.2001.114718. [DOI] [PubMed] [Google Scholar]
- 80.Zhang X, Glogauer M, Zhu F, Kim TH, Chiu B, Inman RD. Innate immunity and arthritis: neutrophil Rac and toll-like receptor 4 expression define outcomes in infection-triggered arthritis. Arthritis and rheumatism. 2005;52:1297–1304. doi: 10.1002/art.20984. [DOI] [PubMed] [Google Scholar]
- 81.Firestein GS. ‘Rac’-ing upstream to treat rheumatoid arthritis. Arthritis research & therapy. 2010;12:109. doi: 10.1186/ar2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sironi M, et al. An evolutionary analysis of RAC2 identifies haplotypes associated with human autoimmune diseases. Molecular biology and evolution. 2011;28:3319–3329. doi: 10.1093/molbev/msr164. [DOI] [PubMed] [Google Scholar]
- 83.Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 84.Baruzzi A, Caveggion E, Berton G. Regulation of phagocyte migration and recruitment by Src-family kinases. Cell Mol Life Sci. 2008;65:2175–2190. doi: 10.1007/s00018-008-8005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zarbock A, Ley K. Protein tyrosine kinases in neutrophil activation and recruitment. Archives of Biochemistry and Biophysics. 2011;510:112–119. doi: 10.1016/j.abb.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 86.Yan SR, Berton G. Regulation of Src family tyrosine kinase activities in adherent human neutrophils. Evidence that reactive oxygen intermediates produced by adherent neutrophils increase the activity of the p58c-fgr and p53/56lyn tyrosine kinases. J Biol Chem. 1996;271:23464–23471. doi: 10.1074/jbc.271.38.23464. [DOI] [PubMed] [Google Scholar]
- 87.Thomas SM, Brugge JS. CELLULAR FUNCTIONS REGULATED BY SRC FAMILY KINASES. Annual Review of Cell and Developmental Biology. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 88.Meng F, Lowell CA. A beta 1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. Embo J. 1998;17:4391–4403. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schenone S, Manetti F, Botta M. Synthetic SRC-kinase domain inhibitors and their structural requirements. Anti-cancer agents in medicinal chemistry. 2007;7:660–680. doi: 10.2174/187152007784111269. [DOI] [PubMed] [Google Scholar]
- 90.Ernst M, et al. Constitutive Activation of the Src Family Kinase Hck Results in Spontaneous Pulmonary Inflammation and an Enhanced Innate Immune Response. The Journal of Experimental Medicine. 2002;196:589–604. doi: 10.1084/jem.20020873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas RM, et al. C-Terminal Src Kinase Controls Acute Inflammation and Granulocyte Adhesion. Immunity. 2004;20:181–191. doi: 10.1016/s1074-7613(04)00023-8. [DOI] [PubMed] [Google Scholar]
- 92.Scapini P, et al. CXCL1/Macrophage Inflammatory Protein-2-Induced Angiogenesis In Vivo Is Mediated by Neutrophil-Derived Vascular Endothelial Growth Factor-A. The Journal of Immunology. 2004;172:5034–5040. doi: 10.4049/jimmunol.172.8.5034. [DOI] [PubMed] [Google Scholar]
- 93.Mócsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk Is Required for Integrin Signaling in Neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 94.Yago T, et al. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaL beta2-mediated slow leukocyte rolling. Blood. 2010;116:485–494. doi: 10.1182/blood-2009-12-259556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gaudry M, Gilbert C, Barabe F, Poubelle PE, Naccache PH. Activation of Lyn is a common element of the stimulation of human neutrophils by soluble and particulate agonists. Blood. 1995;86:3567–3574. [PubMed] [Google Scholar]
- 96.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 97.Rickles RJ, et al. Identification of Src, Fyn, Lyn, PI3K and Abl SH3 domain ligands using phage display libraries. Embo J. 1994;13:5598–5604. doi: 10.1002/j.1460-2075.1994.tb06897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ptasznik A, et al. Crosstalk between BCR/ABL oncoprotein and CXCR4 signaling through a Src family kinase in human leukemia cells. J Exp Med. 2002;196:667–678. doi: 10.1084/jem.20020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He Y, et al. The non-receptor tyrosine kinase Lyn controls neutrophil adhesion by recruiting the CrkL–C3G complex and activating Rap1 at the leading edge. Journal of Cell Science. 2011;124:2153–2164. doi: 10.1242/jcs.078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sai J, Raman D, Liu Y, Wikswo J, Richmond A. Parallel phosphatidylinositol 3-kinase (PI3K)-dependent and Src-dependent pathways lead to CXCL8-mediated Rac2 activation and chemotaxis. J Biol Chem. 2008;283:26538–26547. doi: 10.1074/jbc.M805611200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei S, et al. IL-2 induces the association of IL-2Rbeta, lyn, and MAP kinase ERK-1 in human neutrophils. Immunobiology. 2000;202:363–382. doi: 10.1016/s0171-2985(00)80040-6. [DOI] [PubMed] [Google Scholar]
- 102.Aomatsu K, et al. Toll-like receptor agonists stimulate human neutrophil migration via activation of mitogen-activated protein kinases. Immunology. 2008;123:171–180. doi: 10.1111/j.1365-2567.2007.02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ptasznik A, Prossnitz ER, Yoshikawa D, Smrcka A, Traynor-Kaplan AE, Bokoch GM. A tyrosine kinase signaling pathway accounts for the majority of phosphatidylinositol 3,4,5-trisphosphate formation in chemoattractant-stimulated human neutrophils. J Biol Chem. 1996;271:25204–25207. doi: 10.1074/jbc.271.41.25204. [DOI] [PubMed] [Google Scholar]
- 104.Pereira S, Lowell C. The Lyn Tyrosine Kinase Negatively Regulates Neutrophil Integrin Signaling. The Journal of Immunology. 2003;171:1319–1327. doi: 10.4049/jimmunol.171.3.1319. [DOI] [PubMed] [Google Scholar]
- 105.Giannoni E, Taddei ML, Chiarugi P. Src redox regulation: Again in the front line. Free Radical Biology and Medicine. 2010;49:516–527. doi: 10.1016/j.freeradbiomed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 106.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular Reactive Oxygen Species Activate Src Tyrosine Kinase during Cell Adhesion and Anchorage-Dependent Cell Growth. Molecular and Cellular Biology. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hibbs ML, et al. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 109.Flores-Borja F, Kabouridis PS, Jury EC, Isenberg DA, Mageed RA. Decreased Lyn expression and translocation to lipid raft signaling domains in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:3955–3965. doi: 10.1002/art.21416. [DOI] [PubMed] [Google Scholar]
- 110.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lopes F, et al. Resolution of neutrophilic inflammation by H2O2 in antigen-induced arthritis. Arthritis Rheum. 2011;63:2651–2660. doi: 10.1002/art.30448. [DOI] [PubMed] [Google Scholar]
- 112.Kerr WG. Inhibitor and activator: dual functions for SHIP in immunity and cancer. Annals of the New York Academy of Sciences. 2011;1217:1–17. doi: 10.1111/j.1749-6632.2010.05869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stephens L, Milne L, Hawkins P. Moving towards a Better Understanding of Chemotaxis. Current Biology. 2008;18:R485–R494. doi: 10.1016/j.cub.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 114.Gambardella L, Vermeren S. Molecular players in neutrophil chemotaxis—focus on PI3K and small GTPases. Journal of Leukocyte Biology. 2013 doi: 10.1189/jlb.1112564. [DOI] [PubMed] [Google Scholar]
- 115.Sasaki T, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 116.Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18:226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Helgason CD, et al. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes & Development. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nishio M, et al. Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nat Cell Biol. 2007;9:36–44. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- 119.Lam PY, Yoo SK, Green JM, Huttenlocher A. The SH2-domain-containing inositol 5-phosphatase (SHIP) limits the motility of neutrophils and their recruitment to wounds in zebrafish. J Cell Sci. 2012;125:4973–4978. doi: 10.1242/jcs.106625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu Jing W, et al. Receptor-like Tyrosine Phosphatases CD45 and CD148 Have Distinct Functions in Chemoattractant-Mediated Neutrophil Migration and Response to S. aureus. Immunity. 2011;35:757–769. doi: 10.1016/j.immuni.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kurowska-Stolarska M, et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci U S A. 2011;108:11193–11198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ong CJ, et al. Small-molecule agonists of SHIP1 inhibit the phosphoinositide 3-kinase pathway in hematopoietic cells. Blood. 2007;110:1942–1949. doi: 10.1182/blood-2007-03-079699. [DOI] [PubMed] [Google Scholar]
- 123.Banham-Hall E, Clatworthy MR, Okkenhaug K. The Therapeutic Potential for PI3K Inhibitors in Autoimmune Rheumatic Diseases. Open Rheumatol J. 2012;6:245–258. doi: 10.2174/1874312901206010245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Randis TM, Puri KD, Zhou H, Diacovo TG. Role of PI3Kdelta and PI3Kgamma in inflammatory arthritis and tissue localization of neutrophils. European journal of immunology. 2008;38:1215–1224. doi: 10.1002/eji.200838266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Camps M, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 126.Ben-Smith A, Dove SK, Martin A, Wakelam MJ, Savage CO. Antineutrophil cytoplasm autoantibodies from patients with systemic vasculitis activate neutrophils through distinct signaling cascades: comparison with conventional Fcgamma receptor ligation. Blood. 2001;98:1448–1455. doi: 10.1182/blood.v98.5.1448. [DOI] [PubMed] [Google Scholar]
- 127.Schreiber A, et al. Phosphoinositol 3-kinase-gamma mediates antineutrophil cytoplasmic autoantibody-induced glomerulonephritis. Kidney Int. 2010;77:118–128. doi: 10.1038/ki.2009.420. [DOI] [PubMed] [Google Scholar]
- 128.Stehbens S, Wittmann T. Targeting and transport: how microtubules control focal adhesion dynamics. J Cell Biol. 2012;198:481–489. doi: 10.1083/jcb.201206050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kaverina I, Straube A. Regulation of cell migration by dynamic microtubules. Semin Cell Dev Biol. 2011;22:968–974. doi: 10.1016/j.semcdb.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Niggli V. Microtubule-disruption-induced and chemotactic-peptide-induced migration of human neutrophils: implications for differential sets of signalling pathways. J Cell Sci. 2003;116:813–822. doi: 10.1242/jcs.00306. [DOI] [PubMed] [Google Scholar]
- 131.Xu J, Wang F, Van Keymeulen A, Rentel M, Bourne HR. Neutrophil microtubules suppress polarity and enhance directional migration. Proc Natl Acad Sci U S A. 2005;102:6884–6889. doi: 10.1073/pnas.0502106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoo SK, Lam PY, Eichelberg MR, Zasadil L, Bement WM, Huttenlocher A. The role of microtubules in neutrophil polarity and migration in live zebrafish. J Cell Sci. 2012;125:5702–5710. doi: 10.1242/jcs.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- 134.Saga T, Matsuda H. Microtubules in neutrophils of patients with Behcet’s disease. Jpn J Ophthalmol. 1985;29:31–36. [PubMed] [Google Scholar]
- 135.Saga T, Matsuda H, Aso S. Microtubules in neutrophils of patients with Behcet’s disease--immunofluorescence microscopic study. Jpn J Ophthalmol. 1987;31:315–323. [PubMed] [Google Scholar]
- 136.Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep. 2008;10:218–227. doi: 10.1007/s11926-008-0036-3. [DOI] [PubMed] [Google Scholar]