Abstract
When the apicolateral border of epithelial cells is compared with a polygon, its sides correspond to the apical junctional complex, where cell adhesion molecules assemble from the plasma membranes of two adjacent cells. On the other hand, its vertices correspond to tricellular contacts, where the corners of three cells meet. Vertebrate tricellular contacts have specialized structures of tight junctions, termed tricellular tight junctions (tTJs). tTJs were identified by electron microscopic observations more than 40 years ago, but have been largely forgotten in epithelial cell biology since then. The identification of tricellulin and angulin family proteins as tTJ-associated membrane proteins has enabled us to study tTJs in terms of not only the paracellular barrier function but also unknown characteristics of epithelial cell corners via molecular biological approaches.
Keywords: tight junctions, tricellular tight junctions, tricellulin, angulin family, epithelial cells
Introduction
Tight junctions (TJs) are one mode of vertebrate epithelial cell-cell junctions that restrict free diffusion of solutes through the paracellular pathway.1-3 The functional unit of TJs is the TJ strand, which is a fibril-like structure formed by the assembly of claudin and tight junction-associated MARVEL protein (TAMP) family membrane proteins from both of the adjacent plasma membranes.4-6 A network of TJ strands circumscribes each cell, such that most of the intercellular spaces within the cellular sheet are continuously sealed by TJs. However, TJs cannot practically seal some exceptional regions, namely tricellular contacts (TCs), where the corners of three polygonal epithelial cells meet. It is not simple to understand how the extracellular space between three plasma membranes at TCs is plugged, because TJs are zipper-like structures formed between two plasma membranes. To seal the extracellular space at TCs and achieve a strict barrier function of the cellular sheet, TCs contain specialized structures of TJs, namely tricellular junctions or tricellular TJs (tTJs).7 In this article, we summarize recent progress on the molecular dissection of tTJs.
Morphology of tTJs
TJs were first described on the basis of ultrathin section electron microscopy as focal fusions of two adjacent plasma membranes at the junctional complex.8 Later, Staehelin and colleagues and others observed the surface view of the corresponding structure by freeze-fracture replica electron microscopy as a meshwork of fibrils, namely TJ strands, in the form of a band along the whole length of the upper lateral border of epithelial cells.9,10 The ultrastructure of tTJs was reported in these initial studies as a modification of the TJ fibrillar mesh at TCs. Based on further detailed observations, Staehelin7 presented a detailed structural model of tTJs that has remained accurate until today (Fig. 1). At a TC, the most apical sealing elements of horizontal TJs formed between each pair of the three cells extend to the center of the TC. These three sealing elements then turn and extend in the basal direction and are nearly attached to each other to form vertical strands (arrowheads in Figure 1A and B). As a result, a very long and narrow tube (~1 µm in length and 10 nm in diameter) is formed at the extracellular space between three vertical strands, and this tubular structure is thought to reduce free diffusion of solutes for provision of a sufficient paracellular barrier.7 It would be natural to consider that the vertical strands of tTJs are mainly composed of claudins because they appear to be a simple extension of the most apical TJ strands. Plaque proteins of TJs may be also incorporated into tTJs similar to that for bicellular TJs. Consistent with this speculation, immunofluorescence staining of ZO-1, for example, often shows strong signals at TCs in addition to bicellular TJs. Despite the fascinating structures in electron micrographs, little research was reported for tTJs in epithelial cell biology until tricellulin was identified as their first molecular component.11
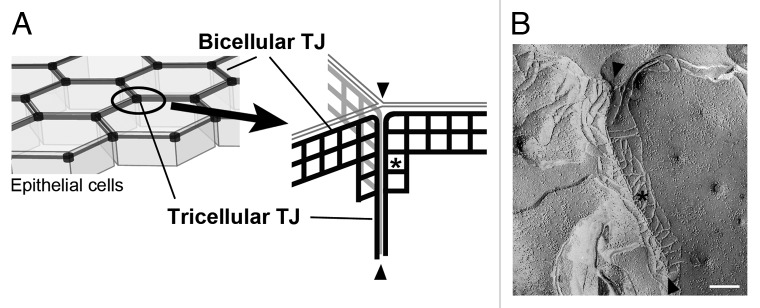
Figure 1. (A) Structural model of tTJs. See the text for detail. (B) Freeze-fracture electron micrograph of MDCK cells. Bar: 200 nm.
Tricellulin
Tricellulin belongs to the tetramembrane-spanning TAMP family, which contains occludin, tricellulin, and marveld311,12 (Fig. 2). Tricellulin was identified as a tTJ-associated membrane protein during screening of genes that were suppressed in cultured mouse mammary epithelial cells by overexpression of the Snail transcription suppressor, which is involved in epithelial-mesenchymal transition.11 Tricellulin was also cloned as a causative gene for a recessive nonsyndromic familial deafness, DFNB49.13,14 In immunofluorescence staining, tricellulin concentration at TCs is observed ubiquitously in various epithelial cells in tissues, with additional localization at bicellular cell-cell contacts in some epithelial cells. Immunolabeling combined with freeze-fracture replica electron microscopy revealed that tricellulin is concentrated at the vertical strands of tTJs.11 The C-terminal ~130 amino acids in the cytoplasmic domain of tricellulin are highly conserved with those of occludin, which associates with ZO-1, a plaque protein of TJs. However, the binding of tricellulin to ZO-1 remains controversial, with one group reporting that it does bind to ZO-1,13 and another group reporting that it does not.11 Among TAMP family proteins, tricellulin is co-precipitated with marveld3, but not with occludin, in the lysate from Caco-2 epithelial cells.12
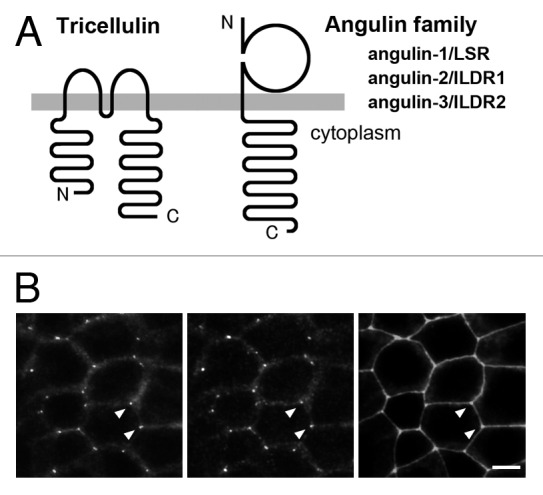
Figure 2. Tricellulin and angulin family proteins. (A) Membrane-spanning model of tricellulin and angulins. (B) Triple immunofluorescence staining images of EpH4 mouse mammary epithelial cells with antibodies against tricellulin (left), angulin-1/LSR (middle), and ZO-1 (right). Bar: 10 μm.
RNAi-mediated suppression of tricellulin in cultured mouse EpH4 mammary epithelial cells leads to a reduction in transepithelial electrical resistance (TER) and an increase in paracellular flux. Additionally, tricellulin knockdown cells show an abnormal pattern of occludin localization around TC regions during cell–cell junction formation in immunofluorescence staining.11 These observations indicate the involvement of tricellulin in tTJ formation and epithelial barrier function.
Tricellulin appears to modify the mode of assembly of claudin-based TJ strands. When tricellulin was expressed in claudin-1-overexpressing mouse L fibroblasts, in which claudin-1-based TJ-like structures were reconstituted, tricellulin and claudin-1 were colocalized and the pattern of TJ strands was drastically changed, in that they formed a mesh by their end-to-side attachments and their average lengths became shorter.15,16 This property of tricellulin may reflect the appearance of tTJs shown in Figure 1, where short horizontal TJ strands (asterisk) are connected to the vertical strands (arrowheads) in freeze-fracture replica electron microscopy.7
Very recently, Riazuddin and colleagues reported tricellulin gene knock-in mice that mimic a human tricellulin mutation of DFNB49, namely a nonsense mutation that generates a deletion of ~60 amino acids at the C-terminal cytoplasmic region.17 In these mice, mutant tricellulin was not concentrated at TCs and the morphology of tTJs formed by hair cells and supporting cells in the inner ear was impaired. Specifically, the vertical strands of tTJs were detached and the number of short horizontal strands was decreased. After birth, these mice suffered from deafness with degeneration of hair cells.
Angulin Family Proteins
Lipolysis-stimulated lipoprotein receptor (LSR), which was originally cloned as a candidate for a lipoprotein receptor,18 was identified as a tTJ-associated protein during screening by localization-based expression cloning.19 LSR is a type I transmembrane protein of 575 amino acids (in mice) containing an immunoglobulin-like domain (Fig. 2). LSR is localized at the vertical strands of tTJs similar to tricellulin in immuno-freeze-fracture replica electron microscopy. LSR is expressed in various epithelial tissues and a large amount of LSR is localized at the basolateral membrane in addition to tTJs in some epithelial cells such as hepatocytes and intestinal epithelial cells.19 Two LSR-related proteins, immunoglobulin-like domain-containing receptor (ILDR)120 and ILDR2/Lisch-Like,21 are also localized at TCs, and probably at tTJs although this has not yet been demonstrated by immunoelectron microscopy.22 Their expressions in mouse tissues are mostly complementary, although LSR and ILDR1 are redundantly expressed in some tissues. Given their common characteristics in the localization of TCs, LSR, ILDR1, and ILDR2 are proposed to be designated angulin family proteins (angulin-1/LSR, angulin-2/ILDR1, and angulin-3/ILDR2) from the Latin word ‘angulus’, meaning corner.22
The functions of angulins have been analyzed using EpH4 mouse mammary epithelial cells, which only express angulin-1/LSR among the angulin family members. In angulin-1/LSR knockdown EpH4 cells, the TER of the cellular sheet is reduced and the flux of paracellular markers is increased, demonstrating that angulin-1/LSR is required for the full barrier function of epithelial cells. Importantly, the TC localization of tricellulin is impaired in angulin-1/LSR knockdown cells, whereas the angulin-1/LSR localization is not affected in tricellulin knockdown cells, suggesting that angulin-1/LSR recruits tricellulin to tTJs.19 When angulin-1, angulin-2/ILDR1, and angulin-3/ILDR2 were individually introduced into angulin-1/LSR knockdown EpH4 cells, any angulin restored TC localization of tricellulin.22 In this experiment, angulin-1/LSR and angulin-2/ILDR1 recovered the epithelial barrier function of the cellular sheet well, but angulin-3/ILDR2 achieved only slight recovery.22 These findings imply that the functions of the angulin family proteins vary and that the TC localization of tricellulin may not be sufficient for the full barrier function of the epithelial cellular sheet. In addition, it is of interest whether each angulin provides different properties of tTJs to each epithelial type. Regarding the recruitment of tricellulin by the angulin family proteins, several lines of evidence indicate that an interaction between the cytoplasmic domain of angulins and the C-terminal cytoplasmic domain of tricellulin is needed, although their direct binding has not been demonstrated.19,22 When co-expressed in HEK293T cells, co-precipitation of angulins not only with tricellulin but also with occludin was observed.19,22
Contribution of tTJs to Epithelial Barrier Function
The ideal approach to clarify the role of tTJs in the epithelial barrier function is loss-of-function studies of tTJ-specific proteins. A problem is that tricellulin and angulin family proteins may also influence the barrier function of bicellular TJs. Indeed, tricellulin is localized at bicellular contacts in some epithelial tissues and tricellulin knockdown in mouse EpH4 epithelial cells affects the immunolocalization of occludin at bicellular TJs.11 Although the tricellulin gene knock-in mice mimicking DFNB49 show clear disruption of the ultrastructure of tTJs with fairly normal bicellular TJs in the inner ear,17 we should keep in mind the possibility that the loss-of-function of tricellulin and angulins may also affect bicellular TJs. RNAi-mediated knockdown of tricellulin and angulin-1/LSR in EpH4 cells led to reduced TER and increased paracellular flux, but their TER values still remained at ~500 Ω ·cm2.11,19,22 The tricellulin gene knock-in mice suffer from hearing loss, but grow up with only slight morphological abnormalities in limited tissues.17 Given these observations, tTJs may not be essential, but are required for epithelial cellular sheets that need a strict barrier function.
Krug et al.23 proposed that tTJs are the pathways for macromolecules. In their study using MDCK II cells, exogenous expression of a small amount of tricellulin, which was only localized at tTJs, increased the barrier against macromolecules but not against ions, whereas expression of a large amount of tricellulin, which was localized at both bicellular TJs and tTJs, increased the barrier against both macromolecules and ions. This group further reported that the increased macromolecule permeation at the tTJs of intestinal epithelial cells by the treatment of caprate, a 10-carbon medium chain fatty acid clinically employed as a drug absorption-enhancing agent, correlated with the loss of TC localization of tricellulin.24 Tricellulin removal by caprate was also observed in the fish gill epithelium.25 It would be interesting to determine whether loss of tricellulin at TCs by caprate treatment is accompanied by abnormal localization of angulins.
In Vivo Function of tTJ-Associated Proteins
The DFNB49-mimicking tricellulin gene knock-in mice suffer from hearing loss with degeneration of hair cells.17 In addition to DFNB49, it was reported that autosomal recessive mutations in the ILDR1 gene cause the familial nonsyndromic deafness DFNB42.26 Given that angulins recruit tricellulin, the cause of the deafness may be interpreted as impairment of the angulin-tricellulin system in the inner ear.22 Angulin-2/ILDR1 is expressed in the inner ear including the organ of Corti and is localized at TCs. Although angulin-1/LSR is also expressed in the inner ear, only angulin-2/ILDR1 among the angulin family members was detected at TCs around hair cells in immunofluorescence staining.22 When mutant proteins of angulin-2/ILDR1 reported in DFNB42 patients were expressed in angulin-1/LSR knockdown (and other angulin-negative) EpH4 epithelial cells, the angulin-2/ILDR1 mutant proteins were unable to localize at TCs or could only localize at TCs inefficiently compared with the wild-type proteins.22 Furthermore, when four DFNB49-associated mutant proteins of human tricellulin, containing frameshift or nonsense mutations in the part of the tricellulin gene encoding the C-terminal cytoplasmic domain, were expressed in angulin-1/LSR-tricellulin double-negative EpH4 cells transfected with human angulin-2/ILDR1, none of them were recruited to TCs by angulin-2/ILDR1.22 In addition to hearing loss, the tricellulin knock-in mice show some histological abnormalities in some tissues such as the salivary gland, stomach, and heart.17 Further examination of these mice as well as tricellulin-deficient mice would help to clarify the in vivo functions of tricellulin and tTJs.
It has been reported that angulin family proteins have various roles in metabolism. LSR binds to apolipoprotein B/E in the presence of free fatty acids and is thought to be involved in the clearance of triglyceride-rich lipoproteins.18 LSR gene knockout mice (homozygotes) show embryonic lethality between days 12.5–15.5 of gestation with a much smaller liver than that of their littermates,27 while LSR heterozygote mice show delayed postprandial lipid clearance compared with wild-type mice.28 In the intestine, Ildr1 mRNA is highly expressed in discrete enteroendocrine cells, which express cholecystokinin (CCK), a classic gastrointestinal hormone.29 Upon fatty acid administration, a postprandial increase in plasma CCK was observed in wild-type mice, but not in Ildr1 knockout mice, suggesting that angulin-2/ILDR1 is involved in the signaling for CCK secretion.29 Positional cloning identified the mouse Ildr2 gene as a candidate modifier of susceptibility to type-2 diabetes.21 It remains unknown whether these functions of angulin family proteins in metabolism are caused by impairment of tTJ functions.
Expression of tricellulin and Angulins in Non-Epithelial Cells
To date, tTJs have not been described in endothelial cells. However, immunofluorescence staining revealed that tricellulin was localized at TCs in cultured brain endothelial cells.30 In vivo, tricellulin and angulin-1/LSR were expressed and concentrated at TCs in brain and retinal endothelial cells, which generate the blood-brain barrier and inner blood-retinal barrier, respectively, but not in the endothelial cells of many other tissues.31 These observations suggest the existence of tTJs in endothelial cells that form strong endothelial barriers, although a final demonstration is needed by freeze-fracture replica electron microscopy. Kubo et al.32 reported that tricellulin was concentrated at TCs between epidermal keratinocytes and Langerhans cells, when Langerhans cells expressing tricellulin extended their projections through keratinocyte TJs for antigen uptake. These observations suggest that tTJs can form between different cell types, namely epithelial cells and immune cells. Tricellulin is also expressed in other non-epithelial cells, including glial cells in the brain30 and immune cells of the monocyte/macrophage lineage and microglia.33 In mouse myelinating Schwann cells, tricellulin is concentrated at autotypic TJs of myelin.34
Tricellular Junctions in Invertebrates
The epithelial permeability barriers in most invertebrate species are formed by septate junctions (SJs), another type of cell-cell junction, as the functional counterparts of vertebrate TJs.35 Similar to TJs, SJs are formed between two adjacent epithelial cells and cannot simply seal TCs. SJs in insects are categorized into two types: pleated SJs in ectoderm-derived epithelial cells and smooth SJs in endoderm-derived epithelial cells.35 Electron microscopic observations have shown that specialized structures, namely tricellular junctions, are formed at TCs attached to both types of SJs.36-38 There are long channels with diaphragms at their TCs along the depth of epithelial cells and they are proposed to work as anchors for SJ strands. These structures may also function as a permeability barrier at TCs as a plug. To date, Gliotactin (Gli) is the only known protein that is highly concentrated into tricellular junctions in invertebrates. Gli is a transmembrane protein containing a cholinesterase-like domain. It was originally identified as a gene product expressed in glia in the fruit fly Drosophila by enhancer trap screening and was shown to be required for formation of the peripheral blood-nerve barrier.39 Later, Gli was also identified in a genetic screening to find genes required for the paracellular barrier of the salivary glands in Drosophila.40 Interestingly, detailed localization studies revealed that Gli was concentrated into TCs in ectoderm-derived epithelial cells with pleated SJs, including glial cells.41 In Gli mutants of Drosophila, pleated SJ structures were still present, but not compacted into clusters, and pleated SJ marker proteins were mislocalized, being more diffusely distributed compared with wild-type flies.41 Gli is associated indirectly with Discs large (Dlg), a cytoplasmic component of SJs. In Dlg mutant flies, not only the TC localization but also the plasma membrane localization of Gli was hampered.42 The cytoplasmic domain of Gli contains two tyrosine phosphorylation sites.43 Phosphorylation of these residues appears to be required for endocytosis and lysosome degradation, which regulate the correct level and localization of Gli.43 The Gli-like membrane proteins in mammals comprise four neuroligins, which are involved in synaptic function by acting as adhesion molecules.44 To date, there have been no reports about TC localization of neuroligins in epithelial tissues.
Future Perspectives
Information regarding the molecular architecture and physiological function of tTJs is still fragmentary. The present model for tTJ formation by the angulin-tricellulin system is shown in Figure 3. In this model, angulins assemble at TCs according to an unknown positional cue. Tricellulin is then recruited to TCs through an interaction with angulins. Tricellulin draws claudin-based TJs to TCs potentially by interacting with claudins within the plasma membrane to modify the properties of TJ strands to generate short horizontal strands at tTJs. Regarding the process of tTJ formation, the mechanism of angulin localization at TCs is intriguing. There may be a required order for the protein-protein interactions that finally determine the angulin localization, and the search for the most upstream signal should reach into an unknown physicochemical nature of cell vertices. Further analyses of the functions of tricellulin and angulin family proteins as well as the identification of their interacting molecules are needed to describe the full picture of tTJs.
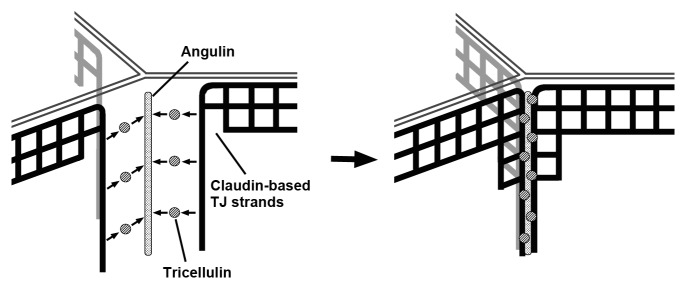
Figure 3. Model of tTJ formation. According to a signaling pathway downstream of an unknown positional cue for cell vertices, angulin family proteins are localized at TCs. Angulins then recruit tricellulin to TCs. Tricellulin draws claudin-based TJ strands and induces their meshwork pattern for tTJ formation.
As described earlier, tricellulin knock-in mice expressing a C-terminal cytoplasmic region-truncated tricellulin that lacks the ability to locate at TCs are viable with hearing loss,17 while angulin-1/LSR-deficient mice show embryonic lethality.27 If the role of angulin-1/LSR, which is expressed in many, but not all, epithelial cells, is only to recruit tricellulin to TCs, the phenotype of these mice must be no more than that of tricellulin knock-in mice. Regarding the lethality of angulin-1/LSR-deficient mice, it should be carefully examined in future whether angulin-1/LSR acts at tTJs with an unknown function in addition to tricellulin recruitment or whether it has totally different functions such as lipid uptake. Generation and analyses of angulin-1/LSR conditional knockout mice and tricellulin-deficient mice will be of help to address these issues.
Potential roles of tTJs other than epithelial barrier function against solutes are also of interest. Vertices of epithelial cells have been paid attention as the points supporting the tensile force of actomyosin along cell-cell junctions. Additionally, TCs are used as windows for protrusions of cells just beneath epithelial cellular sheets to sense the outer environment.32,45 tTJs may be involved in these phenomena directly or indirectly. Examining the unknown characteristics of cell vertices is a new research theme in epithelial cell biology. Angulin family proteins and tricellulin are good markers to label cell vertices and may be involved the behavior of cell vertices in addition to their epithelial barrier function.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Hiroyuki Sasaki for providing an electron micrograph. This work is supported by the Funding Program for Next Generation World Leading Researchers (NEXT Program) from the Japan Society for the Promotion of Science (LS084).
References
- 1.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–76. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–8. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–29. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 4.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–50. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Günzel D, Fromm M. Claudins and other tight junction proteins. Compr Physiol. 2012;2:1819–52. doi: 10.1002/cphy.c110045. [DOI] [PubMed] [Google Scholar]
- 7.Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13:763–86. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staehelin LA, Mukherjee TM, Williams AW. Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma. 1969;67:165–84. doi: 10.1007/BF01248737. [DOI] [PubMed] [Google Scholar]
- 10.Friend DS, Gilula NB. Variations in tight and gap junctions in mammalian tissues. J Cell Biol. 1972;53:758–76. doi: 10.1083/jcb.53.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–45. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200–13. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riazuddin S, Ahmed ZM, Fanning AS, Lagziel A, Kitajiri S, Ramzan K, Khan SN, Chattaraj P, Friedman PL, Anderson JM, et al. Tricellulin is a tight-junction protein necessary for hearing. Am J Hum Genet. 2006;79:1040–51. doi: 10.1086/510022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Šafka Brožková D, Laštůvková J, Štěpánková H, Krůtová M, Trková M, Myška P, Seeman P. DFNB49 is an important cause of non-syndromic deafness in Czech Roma patients but not in the general Czech population. Clin Genet. 2012;82:579–82. doi: 10.1111/j.1399-0004.2011.01817.x. [DOI] [PubMed] [Google Scholar]
- 15.Ikenouchi J, Sasaki H, Tsukita S, Furuse M, Tsukita S. Loss of occludin affects tricellular localization of tricellulin. Mol Biol Cell. 2008;19:4687–93. doi: 10.1091/mbc.E08-05-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cording J, Berg J, Käding N, Bellmann C, Tscheik C, Westphal JK, Milatz S, Günzel D, Wolburg H, Piontek J, et al. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J Cell Sci. 2013;126:554–64. doi: 10.1242/jcs.114306. [DOI] [PubMed] [Google Scholar]
- 17.Nayak G, Lee SI, Yousaf R, Edelmann SE, Trincot C, Van Itallie CM, Sinha GP, Rafeeq M, Jones SM, Belyantseva IA, et al. Tricellulin deficiency affects tight junction architecture and cochlear hair cells. J Clin Invest. 2013;123:4036–49. doi: 10.1172/JCI69031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yen FT, Masson M, Clossais-Besnard N, André P, Grosset JM, Bougueleret L, Dumas JB, Guerassimenko O, Bihain BE. Molecular cloning of a lipolysis-stimulated remnant receptor expressed in the liver. J Biol Chem. 1999;274:13390–8. doi: 10.1074/jbc.274.19.13390. [DOI] [PubMed] [Google Scholar]
- 19.Masuda S, Oda Y, Sasaki H, Ikenouchi J, Higashi T, Akashi M, Nishi E, Furuse M. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci. 2011;124:548–55. doi: 10.1242/jcs.072058. [DOI] [PubMed] [Google Scholar]
- 20.Hauge H, Patzke S, Delabie J, Aasheim HC. Characterization of a novel immunoglobulin-like domain containing receptor. Biochem Biophys Res Commun. 2004;323:970–8. doi: 10.1016/j.bbrc.2004.08.188. [DOI] [PubMed] [Google Scholar]
- 21.Dokmanovic-Chouinard M, Chung WK, Chevre JC, Watson E, Yonan J, Wiegand B, Bromberg Y, Wakae N, Wright CV, Overton J, et al. Positional cloning of “Lisch-Like”, a candidate modifier of susceptibility to type 2 diabetes in mice. PLoS Genet. 2008;4:e1000137. doi: 10.1371/journal.pgen.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashi T, Tokuda S, Kitajiri S, Masuda S, Nakamura H, Oda Y, Furuse M. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2--tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J Cell Sci. 2013;126:966–77. doi: 10.1242/jcs.116442. [DOI] [PubMed] [Google Scholar]
- 23.Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–24. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krug SM, Amasheh M, Dittmann I, Christoffel I, Fromm M, Amasheh S. Sodium caprate as an enhancer of macromolecule permeation across tricellular tight junctions of intestinal cells. Biomaterials. 2013;34:275–82. doi: 10.1016/j.biomaterials.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 25.Kolosov D, Kelly SP. A role for tricellulin in the regulation of gill epithelium permeability. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1139–48. doi: 10.1152/ajpregu.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borck G, Ur Rehman A, Lee K, Pogoda HM, Kakar N, von Ameln S, Grillet N, Hildebrand MS, Ahmed ZM, Nürnberg G, et al. Loss-of-function mutations of ILDR1 cause autosomal-recessive hearing impairment DFNB42. Am J Hum Genet. 2011;88:127–37. doi: 10.1016/j.ajhg.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesli S, Javorschi S, Bérard AM, Landry M, Priddle H, Kivlichan D, Smith AJ, Yen FT, Bihain BE, Darmon M. Distribution of the lipolysis stimulated receptor in adult and embryonic murine tissues and lethality of LSR-/- embryos at 12.5 to 14.5 days of gestation. Eur J Biochem. 2004;271:3103–14. doi: 10.1111/j.1432-1033.2004.04223.x. [DOI] [PubMed] [Google Scholar]
- 28.Yen FT, Roitel O, Bonnard L, Notet V, Pratte D, Stenger C, Magueur E, Bihain BE. Lipolysis stimulated lipoprotein receptor: a novel molecular link between hyperlipidemia, weight gain, and atherosclerosis in mice. J Biol Chem. 2008;283:25650–9. doi: 10.1074/jbc.M801027200. [DOI] [PubMed] [Google Scholar]
- 29.Chandra R, Wang Y, Shahid RA, Vigna SR, Freedman NJ, Liddle RA. Immunoglobulin-like domain containing receptor 1 mediates fat-stimulated cholecystokinin secretion. J Clin Invest. 2013;123:3343–52. doi: 10.1172/JCI68587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariano C, Palmela I, Pereira P, Fernandes A, Falcão AS, Cardoso FL, Vaz AR, Campos AR, Gonçalves-Ferreira A, Kim KS, et al. Tricellulin expression in brain endothelial and neural cells. Cell Tissue Res. 2013;351:397–407. doi: 10.1007/s00441-012-1529-y. [DOI] [PubMed] [Google Scholar]
- 31.Iwamoto N, Higashi T, Furuse M. Localization of angulin-1/LSR and tricellulin at tricellular contacts of brain and retinal endothelial cells in vivo. Cell Struct Funct. 2014;39:1–8. doi: 10.1247/csf.13015. [DOI] [PubMed] [Google Scholar]
- 32.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 2009;206:2937–46. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariano C, Silva SL, Pereira P, Fernandes A, Brites D, Brito MA. Evidence of tricellulin expression by immune cells, particularly microglia. Biochem Biophys Res Commun. 2011;409:799–802. doi: 10.1016/j.bbrc.2011.05.093. [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi S, Ninomiya T, Tatsumi H, Sawada N, Kojima T. Tricellulin is expressed in autotypic tight junctions of peripheral myelinating Schwann cells. J Histochem Cytochem. 2010;58:1067–73. doi: 10.1369/jhc.2010.956326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lane NJ, Dallai R, Martinucci G, Burighel P. Electron microscopic structure and evolution of epithelial janctions., in: S. Citi (Ed.), Molecular Mechanisms of Epithelial Cell Junctions: From Development to Disease, 1994, pp. 23-43. [Google Scholar]
- 36.Fristrom DK. Septate junctions in imaginal disks of Drosophila: a model for the redistribution of septa during cell rearrangement. J Cell Biol. 1982;94:77–87. doi: 10.1083/jcb.94.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noirot-Timothée C, Graf F, Noirot C. The specialization of septate junctions in regions of tricellular junctions. II. Pleated septate junctions. J Ultrastruct Res. 1982;78:152–65. doi: 10.1016/S0022-5320(82)80020-8. [DOI] [PubMed] [Google Scholar]
- 38.Graf F, Noirot-Timothée C, Noirot C. The specialization of septate junctions in regions of tricellular junctions. I. Smooth septate junctions (=continuous junctions) J Ultrastruct Res. 1982;78:136–51. doi: 10.1016/S0022-5320(82)80019-1. [DOI] [PubMed] [Google Scholar]
- 39.Auld VJ, Fetter RD, Broadie K, Goodman CS. Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood-nerve barrier in Drosophila. Cell. 1995;81:757–67. doi: 10.1016/0092-8674(95)90537-5. [DOI] [PubMed] [Google Scholar]
- 40.Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J Cell Biol. 2003;161:979–89. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulte J, Tepass U, Auld VJ. Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila. J Cell Biol. 2003;161:991–1000. doi: 10.1083/jcb.200303192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulte J, Charish K, Que J, Ravn S, MacKinnon C, Auld VJ. Gliotactin and Discs large form a protein complex at the tricellular junction of polarized epithelial cells in Drosophila. J Cell Sci. 2006;119:4391–401. doi: 10.1242/jcs.03208. [DOI] [PubMed] [Google Scholar]
- 43.Padash-Barmchi M, Browne K, Sturgeon K, Jusiak B, Auld VJ. Control of Gliotactin localization and levels by tyrosine phosphorylation and endocytosis is necessary for survival of polarized epithelia. J Cell Sci. 2010;123:4052–62. doi: 10.1242/jcs.066605. [DOI] [PubMed] [Google Scholar]
- 44.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell. 2008;135:1108–17. doi: 10.1016/j.cell.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


