Abstract
Galectins are a family of animal lectins comprising 15 members in vertebrates. These proteins are involved in many biological processes including epithelial homeostasis and tumor progression by displaying intracellular and extracellular activities. Hence Galectins can be found either in the cytoplasm or the nucleus, associated with membranes or in the extracellular matrix. Current studies aim at understanding the roles of Galectins in cell-cell and cell-matrix adhesion, cellular polarity and motility. This review discusses recent progress in defining the specificities and mechanisms of action of Galectins as cell regulators in epithelial cells. Physiological, cellular and molecular aspects of Galectin specificities will be treated successively.
Keywords: galectins, epithelia, glycosylation, cell junctions, polarity, migration, adhesion
Introduction
To date, 15 members of the Galectin family have been identified in vertebrates. Interestingly, Galectin-like genes were identified from early in evolution in invertebrate, protists and even in fish and pig viruses. Among the multiple animal lectin families identified so far, Galectins are distinctly characterized by a conserved Carbohydrate Recognition Domain (CRD), a common affinity for β-galactosides, no divalent cation requirement for binding, a shared primary structure motif, and a unique structural fold.1
The 15 mammalian Galectins share a highly conserved CRD that appeared during evolution concommitantly with the emergence of multi-cellular organisms.2 Structurally, the CRD is a globular region with a jellyroll topology containing 2 β sheets of 5 and 6 strands, with a ligand-binding groove possessing key features that define its specificity. On the basis of their molecular architecture, Galectins have been classified into three main types: (i) “prototype” monomeric Galectins, comprising a single polypeptide chain that is able to dimerize (Galectin-1, -2, -5, -7, -10, -11, -13 -14, and -15); (ii) “tandem repeat-type” Galectins, composed of a single polypeptide chain presenting two CRDs connected by a linker peptide (Galectin-4, -6, -8, -9, and -12); and (iii) the “chimera-type” Galectin-3, that consists of one C-terminal CRD linked to an N-terminal domain.3 Strikingly, the CRD domain has been reported to be involved in protein-protein interactions. Galectin-3 is also involved in protein-protein interactions.4
Although Galectins share common affinity for galactose, important differences in glycan binding preferences have been reported between the different members of the family.5,6 Indeed, they recognize basic β-galactosides while having selective preferences for complex glycan structures depending on the glycan structural architecture (either branched, repeated and substituted glycans).5 Thus, they are capable of recognizing various and complex carbohydrates structures generated during the chain elongation process. As a consequence, each Galectin displays exquisite glycan binding properties.5
These proteins exhibit a dynamic distribution. They bind to different ligands located in cytoplasmic and nuclear compartments, or even in extracellular spaces. Multiple functions have been proposed for these proteins such as modulation of signaling pathways, regulation of RNA splicing, intracellular control of apoptotic signaling, control of the endocytic machinery, and trafficking.4,7 Their secretion pathway remains poorly understood as these proteins lack signal sequences for transport into the endoplasmic reticulum (ER) and are not glycosylated, indicating that they do not pass through the ER-Golgi network.1,8
In contrast to transmembrane CLRs (C-type lectin receptors) and siglecs (sialic acid-binding Ig superfamily lectins), Galectins are soluble proteins that function in the extracellular and intracellular compartments by interacting with a myriad of membrane-associated cell surface glycosylated ligands.9 This perimembranous localization has suggested important roles for Galectins as modulators of cell adhesion, migration and signaling. Each member of the Galectin family has a unique distribution pattern. While some Galectins (e.g., Galectin-1 and -3) are widely expressed in different tissues and cells of various species, other family members have a more restricted tissue localization and compartmentalization: e.g., Galectin-2 is restricted to digestive epithelia10 and Galectin-7 is preferentially found in stratified epithelia such as the epidermis.11
In the present review, we discuss the roles of Galectins in several biological processes of the epithelial barrier. We notably focus on cell polarization, adhesion, migration and proliferation.
Galectins and Epithelia
Epithelia are robust tissues that support the structure of embryos and organs and serve as effective barriers against environmental insults and pathogens. An important property of epithelia is therefore their capacity to assemble into a physical barrier separating tissues. Hence, epithelia form sheets of cells organized either in a monolayer as in the gut or in multilayers as in the epidermis. These vital functions require tight association between cells through the assembly of junctions such as desmosomes, adherens and tight junctions that mechanically stabilize the tissue. Beyond this crucial function, many cell junctions are also involved in the regulation of cell polarity, differentiation, proliferation and motility.
The epidermis is a highly specialized epithelium that has evolved to protect the organism from dehydration and to provide a barrier against harmful environmental influences, such as UV, temperature and microbes. Indeed, the function of the epidermal barrier is ensured by controlled cell-cell and cell-basal lamina adhesion sites. The epidermis is an excellent model system in cell biology to study the dynamics of cell junctions and (re-) polarization during tissue regeneration. Cell-cell and cell-matrix contacts are normally involved in the modulation of cellular polarity and motility during tissue formation. Furthermore loss of adhesive function is implicated in several disease states including tumor progression, inflammation and cystic development in branching epithelia such as kidney tubules.12 Participation of Galectins in cell migration during wound healing has been reported. Indeed, this model has been used to study the role of Galectin-7 in migration during skin repair as discussed later.13 Extracellular Galectin-1 and Galectin-3 bind molecules involved in cell-adhesion such as integrins, laminin and fibronectin.14
Interestingly, interactions between Galectin-3 and N-cadherin has recently been described in a mouse mammary tumor cell line.15 Indeed, Galectin-3 has been shown to accumulate at cell-cell junctions with N-cadherin and the raft marker ganglioside GM1, suggesting that Galectin-3 might regulate the dynamics of N-cadherin and raft components at cell-cell junctions and play a complementary role to p120-catenin in the regulation of junction stability.15
Galectins and Epithelial Homeostasis
Several Galectins have been proposed to be involved in the regulation of cell proliferation or cell death in response to stress, such as for instance tissue injury.
The role of Galectin-3 in tissue homeostasis has been documented in diverse cell types. How Galectin-3 regulates cell proliferation is not clearly demonstrated. However, it has been shown that it indirectly modulates cell cycle progression, by repressing cyclin-E and -A and inducing cyclin-D1 in BT549 breast cancer cells.16 For the induction of cycline-D1, Galectin-3 has been shown to enhance cyclin-D1 promoter activity in the same human breast epithelial cell line.17 Moreover, Galectin-3 has an anti-apoptotic activity that has been well documented in various cell types,7 possibly through its interaction with the anti-apoptotic protein Bcl-2, as observed in vitro.18 Finally, Galectin-3 binds to the oncogene protein Kras and increases its activation.19
In the brain, it has been demonstrated that Galectin-3 and IGF-1 (Insulin Growth factor-1) are upregulated and co-expressed in a subset of activated/proliferating microglial cells after a stroke.20 In Galectin-3 null mutant mice, disruption of the Galectin-3 gene significantly alters microglia activation, it induces a 4-fold decrease in microglia proliferation and a 2-fold increase in the number of apoptotic neurons.20 These results suggest that Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. In folic acid-induced acute kidney injury, utilization of a Galectin-3 inhibitor such as modified citrus pectin (MCP) reduced renal cell proliferation but did not affect apoptosis,21 again suggesting that Galectin-3 induces cell proliferation in response to tissue injury.
In response to intestinal epithelial wound healing in vitro, binding of Galectin-2 and Galectin-4 to epithelial cells have been shown to promote cell proliferation by increasing cyclin B1 expression.22 On the other hand, in the same study, the authors showed that exogenously added Galectin-1 inhibited cell cycling and significantly induced apoptosis of epithelial cells.22
The response to UVB (Ultra Violet B) irradiation has been studied in the skin of Galectin-7 null mutant mice.13 Indeed, the authors showed that after sunburn, the apoptotic response is prematurely triggered and lasts for a longer period of time in the absence of Galectin-7. A burst of proliferation is also observed in Galectin-7 null mutant mice whereas the number of dividing cells increased moderately and regularly in wild type mice. Moreover, hyperproliferation has also been described after epidermal tail injury in Galectin-7 null mutant mice.13 Thus, Galectin-7 plays a critical role in the epidermal response to environmental injury by modulating keratinocyte apoptosis and proliferation.
Galectins and Cell Migration During Tissue Repair
Collective cell migration is the most widely accepted model of cell migration during wound healing, and by analogy during tumor invasion or metastasis (note that conversely, isolated cell movement after epithelial to mesenchymal transition is also debated).23 This concerted displacement is tightly regulated by dynamic cell-cell contacts and tissue cohesion allowing the disturbed tissue to move as a whole. Indeed, either physical and biochemical - i.e., intracellular signaling - constraints are reported to be crucial for such cell movement in different biological conditions including development, tissue remodeling and repair but also during cancer progression.24,25 Cells of endothelial or epithelial origin have been mostly studied to dissect collective cell migration both in vivo and in vitro. Galectins can contribute to cell migration after tissue injury, mainly by participating in cell adhesion or cell-cell contacts.
A recent study in rat corneal epithelial cells highlighted a role of Galectin-3 in adhesion and migration.26 Indeed, exogenous Galectin-3 promotes adhesion of corneal epithelial cells onto the collagen IV substrate by directly binding to collagen IV in vitro. It also enhances wound healing in corneal explants.26 In mammary carcinoma cell lines, a lattice of Galectin-3 and GlcNAc-transferase V modified proteins might stabilize focal adhesions and promote signaling, disassembly and translocation at these membrane structures.27 This action is mostly mediated by activation of α5β1 integrin. At cell–cell junctions of these epithelial tumor cells, Galectin-3 modulates the mobility of N-cadherin and raft localization of signaling proteins.15 Moreover, this lectin is localized in lipid raft domains of membrane ruffles, in lamellipodia in epithelial cells28 and in stimulated dendritic cells where it regulates cell motility.28,29 Another study has also shown a role for Galectin-8 as a modulator of cell adhesion through the binding of integrins.30 Galectins can influence cell adhesion by direct contact to integrins or by modulated adhesion protein expression. In squamous cell carcinoma, Galectin-1 was shown to promote collective cell migration by enhancing the expression of α2β5 integrins.31
As mentioned above, Galectin-7 is expressed predominantly in stratified epithelia and particularly in the skin. Early studies suggested that Galectin-7 could intervene in the process of wound-healing: Galectin-7 is overexpressed in wounded cornea,32 and the addition of recombinant protein accelerates the speed of healing in cell cultures.33
Thanks to the characterization of Galectin-7-deficient mice, it was later shown that Galectin-7 is required for the maintenance of epidermal homeostasis and during wound healing. These results are in accordance with several lines of evidence suggesting that Galectin-7 might play a role in epithelial tissue response to environmental stimuli. Altered polarization of cortactin in Galectin-7-defective leader keratinocytes was associated with delayed wound closure.13 Furthermore, careful electron microscopy examination revealed weakened adherens junctions correlated with E-cadherin mis-association. Interestingly, similar observations were made after epidermal overexpression of Galectin-7 in transgenic mice. A correlation was also reported between abnormal expression levels of Galectin-7 in basal keratinocytes and delayed wound healing (Gendronneau, et al., submitted). These observations suggest that an optimal amount of Galectin-7 is required for proper wound healing.
Cellular Dynamics of Galectins
Cellular distribution of Galectins
As mentioned above, Galectins are found in different cell compartments, either intracellularly in the cytoplasm or the nucleus or both, associated with mitochondrial membranes and even in vesicles, or extracellularly, either at the membrane or associated with the matrix. These localizations vary widely from one cell/tissue type to the other and from one state of cell activation/differentiation to the other.
The intracellular pool of Galectins is mostly detected in the cytoplasm, but a substantial fraction is also observed in the nucleus. Localization of Galectin-3 in the plasma membrane, in the cytoplasm but also within the nucleus has been documented by immunofluorescent staining and biochemical approaches.34 Indeed targeting signals that are recognized by importins for nuclear localization and exportin-1 for nuclear export may regulate the distribution of Galectins between these compartments. Intriguingly, in these compartments the intracellular binding partners of Galectins are mostly unglycosylated with the exception of cytokeratins.4 During cell differentiation of human colonic epithelia, cancer progression in the colon, in the prostate or in thyroid cancer, the cytosolic vs. nuclear distribution varies dramatically,35 suggesting that shuttle mechanisms are involved in carrying lectin into and out of the nucleus. Several Galectins are found in the nucleus but the mechanism of nuclear shuttling has been evidenced only for Galectin-3. Since interacting partners and underlying pathways for nuclear trafficking of Galectin-3 have been discussed elsewhere in more detail,36 we will focus here on Galectins in the secretory pathway.
Galectins are secreted by an unconventional secretory pathway
The various members of the Galectin family lack signal sequences and are collectively classified among the proteins secreted by the so-called non classical pathway of secretion which has remained poorly documented.37-39 Galectins are synthesized on free ribosomes and exhibit no signal sequence. Thus, they bypass the classical biosynthetic ER-Golgi pathway. However, they are detected in carrier vesicles and endosomal compartments, and can be secreted into the extracellular medium. Yet, how are Galectins secreted across the plasma membrane and do they join the classical secretion pathway? Are these two events interdependent? Indeed in intestinal and renal epithelial cells, Galectin-3 release could be blocked at an incubation temperature of 20 °C and by microtubule disruption with nocodazole.40 This indicates that intracellular trafficking events might be linked to the apical secretion of Galectin-3.
It has been described that Galectins could translocate directly across the membrane bilayer. According to data published by Lukyanov et al., Galectin-3 can spontaneously penetrate the lipid bilayer of liposomes by direct interaction with membrane lipids.41 Translocation of Galectin-1 on the other hand requires a molecular machinery composed of integral and peripheral membrane proteins.42 The hypothesis of the involvement of ABC transporters is unlikely, since Lindstedt and coworkers have shown that treatment of MDCK cells with ABC transporter inhibitors, methylamine or verapamil, does not affect Galectin-3 secretion.43 Galectin-1 was secreted by non-classical secretion.43 This mechanism does not depend on the multidrug resistance homolog ABC transporter Ste6p.
Another possible mechanism for Galectin export from the cytosol to the extracellular matrix is ectocytosis by membrane blebbing. In mouse muscle cells, Galectin-1 is packed into vesicles, that “bud off” at the plasma membrane.44,45 This phenomenon has also been reported for fusion proteins of Galectin-3 in COS cells. Furthermore, this study demonstrated that the N-terminal domain of Galectin-3 is sufficient to direct membrane translocation.46
In addition, several studies reported the presence of Galectins in exosomes, i.e., internal vesicles of multivesicular bodies that are released upon exocytic fusion with the plasma membrane.47 In dendritic cells, a proteomic approach showed that Galectin-3 is a component of purified exosomes.48 Analyses performed in nasopharyngeal carcinoma cells also linked Galectin-9 exocytosis to exosomes.49 These data suggest that the exosomal pathway could provide an alternative route for Galectin secretion into the extracellular milieu. However a precise trafficking mechanism of lectins through this particular pathway still remains to be determined.
However, the secretion of Galectins must probably be a highly regulated process, since the same Galectin can initiate different cellular responses at distinct locations. For example, cytosolic Galectin-3 of T cells blocks cell death, while extracellular Galectin-3 induces the death of T cells and thymocytes.50 The translocation of Galectin-3 to the mitochondrial membranes depends on the presence of synexin, and knockdown of this phospholipid-binding protein blocks the translocation process and abolishes Galectin-3-mediated anti-apoptotic activities.51 Second, only a relatively small proportion of Galectins is secreted into the extracellular milieu.8 This regulation may change during cell differentiation. Galectin-3 is secreted predominantly from the apical cell surface of polarized epithelial MDCK cells, with relatively low efficiency.40,52 However, when MDCK cell cysts are grown within 3D-collagen gels, larger amounts of Galectin-3 are secreted from the basolateral side.53 The varying quantities of Galectin-3 secreted from the apical or the basolateral domain under these defined exogenous conditions suggest that distinct trafficking mechanisms might be involved for Galectin secretion.
Interestingly, a recent study showed that arrest of caspase-1 activation led to inhibition of unconventional secretion of various cytoplasmic proteins including Galectins-1 and -3 in keratinocytes. The authors proposed the constitution of a hetero-oligomeric complex of caspase-1, an unidentified caspase-1 substrate and other unconventional secretory proteins.54 Remarkably, among the proteins secreted in a caspase-1-dependent pathway, several are involved in inflammation, cytoprotection and tissue repair. The authors underscored the striking observation that the activation of caspase-1 by various environmental stresses appeared to be coupled to the release of several proteins including Galectins that are involved in cell and tissue repair. This observation is consistent with the predominant expression of caspase-1 in inflammatory and epithelial cells,55 that are principally involved in cellular defense mechanisms. The interplay between extracellular and intracellular pools of Galectins may well add some complexity to the functions of Galectins.
Furthermore, several caspase-1 substrates have been identified, of which some are involved in the regulation of the cytoskeleton and its dynamics.56 Possible candidates are implicated in the dynamics of microtubules or of the actin cytoskeleton. Others may participate in exocytosis and membrane protein mobility or in the regulation of membrane composition. All these proteins may be involved in vesicular trafficking and may thus be components of a complex machinery that mediates unconventional secretion. Indeed further studies are needed to uncover the molecular and cellular mechanisms of this secretion pathway.
Galectins as regulators of protein and vesicular transport
To make the picture even more complex, intracellular Galectins can directly modulate protein transport per se. Epithelial cells carry out vectorial transport that requires polarized distribution of transporters and receptors to apical or basolateral membrane domains. Numerous studies have indicated that both O- and N-glycans attached to the extracellular domains of membrane proteins are important for the apical location of these proteins.57 There is now some evidence of the importance of glycoprotein clustering in membrane microdomains and of putative roles of glycan-binding proteins in apical distribution of N-glycosylated proteins. Three Galectins, Galectin-3, -4 and -9, have been described as key molecular components of polarized molecular transport to the apical compartment of epithelial cells.58-61 Delacour et al. demonstrated that Galectin-3 interacts with newly synthesized gp114 and p75NTR and thus sorts apical cargos within the biosynthetic pathway of these receptors. It thus seems that Galectins, even when added exogenously to the cell, can modulate forward transport and sorting of newly synthesized glycoproteins to the apical cell surface.62 Galectin-4 interacts with sulphatides in lipid raft microdomains and modulates the transport pathway to the apical cell surface of the HT29 enterocytic cell line.58 In kidney epithelial cells, Galectin-9 can interfere with apicobasal polarity by binding the Forssman glycosphingolipid.60 Interestingly, in renal epithelial cells Galectin-9 seems to be involved in the transport of the non-raft sialomucin endolyn.61 Furthermore, Galectin-3,63 -464 and -960 have been identified in rab11-positive recycling endosomes.65 Thus, recycling endosomes are candidate compartments for the assembly of internalized Galectins with newly synthesized cargo molecules.
The extracellular pool of Galectins is observed on some but not all cell types, and even could be observed on very specific domains. For instance, Galectin-3 is associated with lipid raft microdomains at the plasma membranes, and particularly on focal adhesions in migrating cells. Galectins are proposed to form a lattice with various membrane glycoconjugates among which integrins are the most studied. For example, Galectin-1 binds to α7β1,66 Galectin-3 to α1β1,67 and Galectin-8 to α3β1 and α6β168 as well as to αM74. Galectin-3 promotes the raft-dependent endocytosis of integrins and also localizes to clathrin-independent carriers (CLICs) possibly playing a role in raft-dependent endocytosis.69,70 Galectin-3 has also been reported to be endocytosed in recycling endosomes, and associated binding to caveolin-1 and flotillin-1 has been documented.71 In non-polarized breast cancer cells, Galectin-3 is endocytosed via a caveolae-like, raft-dependent pathway.69 Here, endocytosis of Galectin-3 is coupled to integrin-uptake and plays a role in cell-matrix interaction. In migrating epithelial cells, Galectin-3 is even secreted either at cell-cell contacts or at the lamellipodial front of the leader cell during re-epithelialization of corneal wounds.28,33
Once outside the cell, Galectins also bind glycoproteins in the extracellular matrix, such as laminin, fibronectin, hensin and elastin.14,72 Altogether Galectins (Galectin-1, -3, -4, -8, -9) have been localized to lipid raft microdomains and shown to interact with GM1 ganglioside and other glycosphingolipids.29,58,64,73-77
To date, however, and in spite of several studies, there is no clear information about the precise dynamics of Galectins in the intracellular compartment. This may depend on the cell type used, as well as the differentiation level of the cells. A detailed study of the dynamics in real-time of Galectins in a specific epithelial cell type is required, as well as global view of the intra- and extra-cellular pathway followed by Galectins ; these elements are still sadly lacking.
Galectins and Signal Transduction
By their binding to various cellular ligands, Galectins are also involved in several signal transduction pathways, thus modulating multiple biological functions.
Extracellular Galectins interact with glycosylated receptors or integrins. Integrins possess many N‐glycosylation sites, and several Galectins have been shown to behave as binding partners of integrins through their CRDs, thus suggesting that Galectin–integrin interactions can affect integrin‐mediated signal transduction under certain circumstances.78 Levy et al. showed that cross-linking of integrins to Galectin‐8 can activate integrin‐mediated signaling cascades such as the extracellular signal‐regulated kinase (ERK) 1/2, phosphatidylinositol 3‐kinase (PI3K), Akt, p70 S6 kinase, and consequently trigger a distinct pattern of cytoskeletal organization compared with fibronectin.79 Moreover, a study has recently demonstrated a role for Galectin-1 in lung cancer metastasis through its interplay with integrin α6β4 and the Notch1/Jagged2 signaling pathways.80 More generally, Galectins-1, -4, -8 and -9 interact with glycolipids and may be involved in the organization of the lipid raft signaling plateform.
Focal adhesions are complex signaling domains where interactions of Galectins with various receptors regulate integrin-dependent signaling to Rho GTPases, and other downstream effectors. Furthermore, Galectin-8 has recently been involved in cytoskeleton remodeling through Rho signaling.81 Interestingly Galectin-3 promotes fibronectin-dependent activation of α5β1 integrin and focal adhesion signaling, microfilament remodeling and fibronectin fibrillogenesis. In tumor cells, extracellular Galectin-3 and intracellular tyrosine-phosphorylated caveolin-1 (pY14Cav) have been shown to be required, but not necessarily sufficient, for activation of Src kinases, resulting in the stabilization of focal adhesion components and promotes focal adhesion signaling and disassembly.27 Moreover, Galectins -1, -2, -3 and -8 regulate cell adhesion. It is noteworthy that recruitment of integrins to the lattice by Galectin-3, but not Galectin-1 or Galectin-8, stimulates lamellipodia formation and promotes cell adhesion and migration in epithelial cells.77
In 2004, extracellular Galectin‐3 was shown to be able to bind to the carbohydrate structures / motifs of EGF and TGF‐β receptors, that are modified by the specific Golgi enzyme, β1,6‐N‐acetylglucosaminyltransferase V (GnT‐V or Mgat5).82 Soon after, it was demonstrated that Galectin-3 is a key regulator in the Wnt/β-catenin signaling pathway.83 Successive studies later highlighted the role of Galectin-3 in this pathway in several cancers of epithelial origin such as colorectal cancer,84,85 pancreatic and gastric cancer cells,86,87 breast cancer and tongue cancer.88-90
It should be noted that intracellular Galectins also participate in several signaling cascades and can consequently induce different biological processes such as cell proliferation, senescence, survival, or death, depending on the cellular context. Hence, Galectin‐1 was the first Galectin reported to directly binding to H‐Ras (G12V), which is the constitutively active GTP‐bound H‐Ras mutant and regulate Ras signaling as an escort protein.91 This observation has been reinforced by studies showing that Galectin-1 is mostly involved in Ras activation92 and AP-1 pathway.93 Galectin-3 was later shown to take part in the JAK/STAT pathway94,95 and in the K-ras, Raf, EGFR and ERK pathways in breast carcinoma and pancreatic cancer cells.19,96,97
Moreover, Galectins have been involved in TNF-related signaling98-100 and in signaling of the immune system notably in TCR and NF-κB signaling. These phenomena have been well reviewed and we shall not dwell on them.9,78
Concluding Remarks and Futures Directions: Galectin Therapeutic Potential
As Galectins are involved in many biological processes such as cellular communication, cellular adhesion, inflammation, regulation of immune homeostasis, differentiation and apoptosis, they represent a potential target to modulate pathological processes.101 Nevertheless, since Galectins can bind multiple ligands with varying specificity, their use as therapeutic agent has to be critically evaluated in view of putative adverse effects.
Increasing evidence suggests roles played by Galectins in modulating immune response and inflammation. Thus, Galectins appear to be a potential target in immune diseases. Recently, they were shown to possess significant immuno-regulatory activities, such as in cell differentiation, tissue organization, and regulation of immune homeostasis. For instance, Galectin-3 is involved in many aspects of asthma,102 such as eosinophil recruitment, airway remodeling, development of a Th2 phenotype as well as increased expression of inflammatory mediators.103,104 This role in inflammation suggests Galectin-3 as a potential anti-inflammatory target.
Another member of the Galectin family, Galectin-9, has been shown to promote allograft tolerance in mice when combined with rapamycin.105 Indeed, a recent study shows that combined treatment of Galectin-9 and rapamycin promotes allograft tolerance that is associated with reduced Th1 and Th2 responses. Addition of rapamycin is required for tolerance as rapamycin inhibits pro-inflammatory effects of Galectin-9 on dendritic cells.
Most examples of Galectins related to pathological situations have mainly come from cancers where Galectins seem to play an important role in different steps of tumor formation and progression such as metastasis, immune escape or angiogenesis.72 Therefore, several strategies had been developed to impair Galectin functions in cancer progression.106 Galectin-7 is differentially expressed in different types of cancer but its functions in cancer initiation and progression still remains to be investigated. For example, patients with gastric cancer revealed significantly low expression levels of Galectin-7 in malignant tissues compared with matched normal tissues, suggesting a tumor suppressor function of Galectin-7.107 However, it has been shown that high levels of Galectin-7 correlate with aggressive subtypes of breast cancer,108 indicating that Galectin-7 had a bivalent function in cancer cells. Further studies are necessary to investigate whether Galectin-7 could be a valid target to stop tumor progression. Inhibitors of Galectins, and in particular inhibitors of Galectin-7, have been developed109,110 their use could help to elucidate the role of Galectin-7 in cancer.
The use of peptides inhibiting Galectin-3 has been shown to suppress rolling and stable adhesion of carcinoma cells to endothelial cells in vitro suggesting a potent anti-metastatic treatment.111 Moreover, nude mice fed with modified citrus pectin, a Galectin-3 inhibitor, show significantly reduced tumor growth of human breast carcinoma cell lines, angiogenesis, and spontaneous metastasis in vivo,112 however MCP specificity for Galectin-3 needs to be confirmed.
Members of the Galectin family (Galectins-1, -3, -9) are expressed by endothelial cells and provide a target for tumor-type independent treatment strategies.113 For instance, Rabinovich and colleagues demonstrated that Galectin-1 is involved in a compensatory angiogenic pathway that limits the efficiency of anti-angiogenic treatments.114 In anti-VEGF-A (Vascular Endothelial Growth Factor-A) antibodies treatment, endothelial cells associated with refractory tumors exhibit a differential N-glycan expression profile compared with endothelial cells associated with sensitive tumors. This particular glycophenotype increases the affinity of Galectin-1 for the VEGFR2 receptor on endothelial cells and activates the VEGF-like signaling in a VEGF-independent manner, thus leading to resistance to anti-VEFG-A treatment. Thijssen and collegues showed that Galectin-1 in the endothelium can be targeted in vivo by the angiogenesis inhibitor anginex for therapeutic applications.115 A combination of the anti-VEGF-A treatment with anginex or anti-Galectin-1 treatment should be considered to improve anti-angiogenic treatment. Injection of the Galectin-1 disaccharide inhibitor has also been shown to promote immune responses and thus increase survival of tumor-challenged mice when combined with vaccine immunotherapy.116 In conclusion, Galectin-1 is a good target in cancer therapy as it plays a role in angiogenesis and tumor immunity.
As Galectins exhibit a broad range of biological functions, various expression patterns and subcellular localizations, they represent a potential target in multiple pathologies.
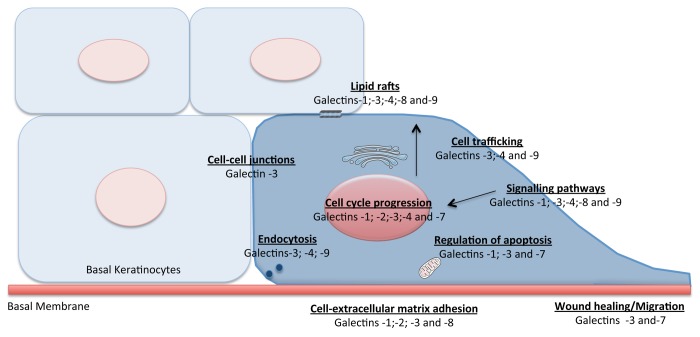
NOTE: Figure 1 is not cited in the text. Please add an in-text citation or delete the figure.
Figure 1.Physiological functions of galectins in epithelial cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Anne-Lise Haenni for helpful discussions and english corrections. This work was supported by GEFLUC (groupement des entreprises françaises contre le cancer), LNCC (ligue contre le cancer, comité de Paris) and ARC (association contre le cancer) grants.
References
- 1.Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–8. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DNW. Galectinomics: finding themes in complexity. Biochim Biophys Acta. 2002;1572:209–31. doi: 10.1016/S0304-4165(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 3.Hirabayashi J, Kasai K. The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology. 1993;3:297–304. doi: 10.1093/glycob/3.4.297. [DOI] [PubMed] [Google Scholar]
- 4.Wang JL, Gray RM, Haudek KC, Patterson RJ. Nucleocytoplasmic lectins. Biochim Biophys Acta. 2004;1673:75–93. doi: 10.1016/j.bbagen.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232–54. doi: 10.1016/S0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 6.Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283:10109–23. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F-T, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–73. doi: 10.1016/S0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 8.Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–85. doi: 10.1016/S0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 9.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–52. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 10.Oka T, Murakami S, Arata Y, Hirabayashi J, Kasai K, Wada Y, Futai M. Identification and cloning of rat galectin-2: expression is predominantly in epithelial cells of the stomach. Arch Biochem Biophys. 1999;361:195–201. doi: 10.1006/abbi.1998.0968. [DOI] [PubMed] [Google Scholar]
- 11.Magnaldo T, Fowlis D, Darmon M. Galectin-7, a marker of all types of stratified epithelia. Differentiation. 1998;63:159–68. doi: 10.1046/j.1432-0436.1998.6330159.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–57. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 13.Gendronneau G, Sidhu SS, Delacour D, Dang T, Calonne C, Houzelstein D, Magnaldo T, Poirier F. Galectin-7 in the control of epidermal homeostasis after injury. Mol Biol Cell. 2008;19:5541–9. doi: 10.1091/mbc.E08-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes RC. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667–76. doi: 10.1016/S0300-9084(01)01289-5. [DOI] [PubMed] [Google Scholar]
- 15.Boscher C, Zheng YZ, Lakshminarayan R, Johannes L, Dennis JW, Foster LJ, Nabi IR. Galectin-3 protein regulates mobility of N-cadherin and GM1 ganglioside at cell-cell junctions of mammary carcinoma cells. J Biol Chem. 2012;287:32940–52. doi: 10.1074/jbc.M112.353334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HR, Lin HM, Biliran H, Raz A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 1999;59:4148–54. [PubMed] [Google Scholar]
- 17.Lin H-M, Pestell RG, Raz A, Kim H-RC. Galectin-3 enhances cyclin D(1) promoter activity through SP1 and a cAMP-responsive element in human breast epithelial cells. Oncogene. 2002;21:8001–10. doi: 10.1038/sj.onc.1205820. [DOI] [PubMed] [Google Scholar]
- 18.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996;93:6737–42. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elad-Sfadia G, Haklai R, Balan E, Kloog Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:34922–30. doi: 10.1074/jbc.M312697200. [DOI] [PubMed] [Google Scholar]
- 20.Lalancette-Hébert M, Swarup V, Beaulieu JM, Bohacek I, Abdelhamid E, Weng YC, Sato S, Kriz J. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J Neurosci. 2012;32:10383–95. doi: 10.1523/JNEUROSCI.1498-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolatsi-Joannou M, Price KL, Winyard PJ, Long DA. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011;6:e18683. doi: 10.1371/journal.pone.0018683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paclik D, Lohse K, Wiedenmann B, Dignass AU, Sturm A. Galectin-2 and -4, but not galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism. Inflamm Bowel Dis. 2008;14:1366–72. doi: 10.1002/ibd.20499. [DOI] [PubMed] [Google Scholar]
- 23.Rørth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–29. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- 24.Rørth P. Fellow travellers: emergent properties of collective cell migration. EMBO Rep. 2012;13:984–91. doi: 10.1038/embor.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vedula SRK, Ravasio A, Lim CT, Ladoux B. Collective cell migration: a mechanistic perspective. Physiology (Bethesda) 2013;28:370–9. doi: 10.1152/physiol.00033.2013. [DOI] [PubMed] [Google Scholar]
- 26.Yabuta C, Yano F, Fujii A, Shearer TR, Azuma M. Galectin-3 enhances epithelial cell adhesion and wound healing in rat cornea. Ophthalmic Res. 2014;51:96–103. doi: 10.1159/000355846. [DOI] [PubMed] [Google Scholar]
- 27.Goetz JG, Joshi B, Lajoie P, Strugnell SS, Scudamore T, Kojic LD, Nabi IR. Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine-phosphorylated caveolin-1. J Cell Biol. 2008;180:1261–75. doi: 10.1083/jcb.200709019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saravanan C, Liu F-T, Gipson IK, Panjwani N. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on α3β1 integrin. J Cell Sci. 2009;122:3684–93. doi: 10.1242/jcs.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu DK, Chernyavsky AI, Chen HY, Yu L, Grando SA, Liu FT. Endogenous galectin-3 is localized in membrane lipid rafts and regulates migration of dendritic cells. J Invest Dermatol. 2009;129:573–83. doi: 10.1038/jid.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zick Y, Eisenstein M, Goren RA, Hadari YR, Levy Y, Ronen D. Role of galectin-8 as a modulator of cell adhesion and cell growth. Glycoconj J. 2004;19:517–26. doi: 10.1023/B:GLYC.0000014081.55445.af. [DOI] [PubMed] [Google Scholar]
- 31.Rizqiawan A, Tobiume K, Okui G, Yamamoto K, Shigeishi H, Ono S, Shimasue H, Takechi M, Higashikawa K, Kamata N. Autocrine galectin-1 promotes collective cell migration of squamous cell carcinoma cells through up-regulation of distinct integrins. Biochem Biophys Res Commun. 2013;441:904–10. doi: 10.1016/j.bbrc.2013.10.152. [DOI] [PubMed] [Google Scholar]
- 32.Cao Z, Said N, Wu HK, Kuwabara I, Liu FT, Panjwani N. Galectin-7 as a potential mediator of corneal epithelial cell migration. Arch Ophthalmol. 2003;121:82–6. doi: 10.1001/archopht.121.1.82. [DOI] [PubMed] [Google Scholar]
- 33.Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, Hsu DK, Kuwabara I, Liu FT, Panjwani N. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002;277:42299–305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- 34.Moutsatsos IK, Davis JM, Wang JL. Endogenous lectins from cultured cells: subcellular localization of carbohydrate-binding protein 35 in 3T3 fibroblasts. J Cell Biol. 1986;102:477–83. doi: 10.1083/jcb.102.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotz MM, Andrews CW, Jr., Korzelius CA, Lee EC, Steele GD, Jr., Clarke A, Mercurio AM. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci U S A. 1993;90:3466–70. doi: 10.1073/pnas.90.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funasaka T, Raz A, Nangia-Makker P. Nuclear transport of galectin-3 and its therapeutic implications. Semin Cancer Biol. 2014 doi: 10.1016/j.semcancer.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem. 2003;270:2109–19. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 38.Levine T, Rabouille C. Endoplasmic reticulum: one continuous network compartmentalized by extrinsic cues. Curr Opin Cell Biol. 2005;17:362–8. doi: 10.1016/j.ceb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Delacour D, Koch A, Jacob R. The role of galectins in protein trafficking. Traffic. 2009;10:1405–13. doi: 10.1111/j.1600-0854.2009.00960.x. [DOI] [PubMed] [Google Scholar]
- 40.Lindstedt R, Apodaca G, Barondes SH, Mostov KE, Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem. 1993;268:11750–7. [PubMed] [Google Scholar]
- 41.Lukyanov P, Furtak V, Ochieng J. Galectin-3 interacts with membrane lipids and penetrates the lipid bilayer. Biochem Biophys Res Commun. 2005;338:1031–6. doi: 10.1016/j.bbrc.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 42.Schäfer T, Zentgraf H, Zehe C, Brügger B, Bernhagen J, Nickel W. Unconventional secretion of fibroblast growth factor 2 is mediated by direct translocation across the plasma membrane of mammalian cells. J Biol Chem. 2004;279:6244–51. doi: 10.1074/jbc.M310500200. [DOI] [PubMed] [Google Scholar]
- 43.Cleves AE, Cooper DN, Barondes SH, Kelly RB. A new pathway for protein export in Saccharomyces cerevisiae. J Cell Biol. 1996;133:1017–26. doi: 10.1083/jcb.133.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper DN, Barondes SH. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol. 1990;110:1681–91. doi: 10.1083/jcb.110.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison FL, Wilson TJ. The 14 kDa beta-galactoside binding lectin in myoblast and myotube cultures: localization by confocal microscopy. J Cell Sci. 1992;101:635–46. doi: 10.1242/jcs.101.3.635. [DOI] [PubMed] [Google Scholar]
- 46.Mehul B, Hughes RC. Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J Cell Sci. 1997;110:1169–78. doi: 10.1242/jcs.110.10.1169. [DOI] [PubMed] [Google Scholar]
- 47.Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–30. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 48.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 49.Keryer-Bibens C, Pioche-Durieu C, Villemant C, Souquère S, Nishi N, Hirashima M, Middeldorp J, Busson P. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006;6:283. doi: 10.1186/1471-2407-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–89. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 51.Yu F, Finley RL, Jr., Raz A, Kim H-RC. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J Biol Chem. 2002;277:15819–27. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- 52.Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol. 2002;168:1813–22. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- 53.Bao Q, Hughes RC. Galectin-3 and polarized growth within collagen gels of wild-type and ricin-resistant MDCK renal epithelial cells. Glycobiology. 1999;9:489–95. doi: 10.1093/glycob/9.5.489. [DOI] [PubMed] [Google Scholar]
- 54.Keller M, Rüegg A, Werner S, Beer H-D. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–31. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 55.Lin XY, Choi MS, Porter AG. Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolysaccharide and interferon-gamma. J Biol Chem. 2000;275:39920–6. doi: 10.1074/jbc.M007255200. [DOI] [PubMed] [Google Scholar]
- 56.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282:36321–9. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 57.Vagin O, Kraut JA, Sachs G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am J Physiol Renal Physiol. 2009;296:F459–69. doi: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delacour D, Gouyer V, Zanetta JP, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, André S, et al. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol. 2005;169:491–501. doi: 10.1083/jcb.200407073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delacour D, Cramm-Behrens CI, Drobecq H, Le Bivic A, Naim HY, Jacob R. Requirement for galectin-3 in apical protein sorting. Curr Biol. 2006;16:408–14. doi: 10.1016/j.cub.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 60.Mishra R, Grzybek M, Niki T, Hirashima M, Simons K. Galectin-9 trafficking regulates apical-basal polarity in Madin-Darby canine kidney epithelial cells. Proc Natl Acad Sci U S A. 2010;107:17633–8. doi: 10.1073/pnas.1012424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mo D, Costa SA, Ihrke G, Youker RT, Pastor-Soler N, Hughey RP, Weisz OA. Sialylation of N-linked glycans mediates apical delivery of endolyn in MDCK cells via a galectin-9-dependent mechanism. Mol Biol Cell. 2012;23:3636–46. doi: 10.1091/mbc.E12-04-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Straube T, von Mach T, Hönig E, Greb C, Schneider D, Jacob R. pH-dependent recycling of galectin-3 at the apical membrane of epithelial cells. Traffic. 2013;14:1014–27. doi: 10.1111/tra.12086. [DOI] [PubMed] [Google Scholar]
- 63.Schneider D, Greb C, Koch A, Straube T, Elli A, Delacour D, Jacob R. Trafficking of galectin-3 through endosomal organelles of polarized and non-polarized cells. Eur J Cell Biol. 2010;89:788–98. doi: 10.1016/j.ejcb.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Stechly L, Morelle W, Dessein AF, André S, Grard G, Trinel D, Dejonghe MJ, Leteurtre E, Drobecq H, Trugnan G, et al. Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic. 2009;10:438–50. doi: 10.1111/j.1600-0854.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 65.Lepur A, Salomonsson E, Nilsson UJ, Leffler H. Ligand induced galectin-3 protein self-association. J Biol Chem. 2012;287:21751–6. doi: 10.1074/jbc.C112.358002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu M, Wang W, Song WK, Cooper DN, Kaufman SJ. Selective modulation of the interaction of alpha 7 beta 1 integrin with fibronectin and laminin by L-14 lectin during skeletal muscle differentiation. J Cell Sci. 1994;107:175–81. doi: 10.1242/jcs.107.1.175. [DOI] [PubMed] [Google Scholar]
- 67.Ochieng J, Leite-Browning ML, Warfield P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem Biophys Res Commun. 1998;246:788–91. doi: 10.1006/bbrc.1998.8708. [DOI] [PubMed] [Google Scholar]
- 68.Levy Y, Arbel-Goren R, Hadari YR, Eshhar S, Ronen D, Elhanany E, Geiger B, Zick Y. Galectin-8 functions as a matricellular modulator of cell adhesion. J Biol Chem. 2001;276:31285–95. doi: 10.1074/jbc.M100340200. [DOI] [PubMed] [Google Scholar]
- 69.Furtak V, Hatcher F, Ochieng J. Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells. Biochem Biophys Res Commun. 2001;289:845–50. doi: 10.1006/bbrc.2001.6064. [DOI] [PubMed] [Google Scholar]
- 70.Howes MT, Kirkham M, Riches J, Cortese K, Walser PJ, Simpson F, Hill MM, Jones A, Lundmark R, Lindsay MR, et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J Cell Biol. 2010;190:675–91. doi: 10.1083/jcb.201002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Osterhues A, Liebmann S, Schmid M, Buk D, Huss R, Graeve L, Zeindl-Eberhart E. Stem cells and experimental leukemia can be distinguished by lipid raft protein composition. Stem Cells Dev. 2006;15:677–86. doi: 10.1089/scd.2006.15.677. [DOI] [PubMed] [Google Scholar]
- 72.Liu F-T, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 73.Danielsen EM, van Deurs B. Galectin-4 and small intestinal brush border enzymes form clusters. Mol Biol Cell. 1997;8:2241–51. doi: 10.1091/mbc.8.11.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanikawa R, Tanikawa T, Okada Y, Nakano K, Hirashima M, Yamauchi A, Hosokawa R, Tanaka Y. Interaction of galectin-9 with lipid rafts induces osteoblast proliferation through the c-Src/ERK signaling pathway. J Bone Miner Res. 2008;23:278–86. doi: 10.1359/jbmr.071008. [DOI] [PubMed] [Google Scholar]
- 75.Braccia A, Villani M, Immerdal L, Niels-Christiansen LL, Nystrøm BT, Hansen GH, Danielsen EM. Microvillar membrane microdomains exist at physiological temperature. Role of galectin-4 as lipid raft stabilizer revealed by “superrafts”. J Biol Chem. 2003;278:15679–84. doi: 10.1074/jbc.M211228200. [DOI] [PubMed] [Google Scholar]
- 76.Ideo H, Seko A, Ishizuka I, Yamashita K. The N-terminal carbohydrate recognition domain of galectin-8 recognizes specific glycosphingolipids with high affinity. Glycobiology. 2003;13:713–23. doi: 10.1093/glycob/cwg094. [DOI] [PubMed] [Google Scholar]
- 77.Boscher C, Dennis JW, Nabi IR. Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol. 2011;23:383–92. doi: 10.1016/j.ceb.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 78.Nakahara S, Raz A. On the role of galectins in signal transduction. Methods Enzymol. 2006;417:273–89. doi: 10.1016/S0076-6879(06)17019-6. [DOI] [PubMed] [Google Scholar]
- 79.Levy Y, Ronen D, Bershadsky AD, Zick Y. Sustained induction of ERK, protein kinase B, and p70 S6 kinase regulates cell spreading and formation of F-actin microspikes upon ligation of integrins by galectin-8, a mammalian lectin. J Biol Chem. 2003;278:14533–42. doi: 10.1074/jbc.M207380200. [DOI] [PubMed] [Google Scholar]
- 80.Hsu Y-L, Wu CY, Hung JY, Lin YS, Huang MS, Kuo PL. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin α6β4 and Notch1/Jagged2 signaling pathway. Carcinogenesis. 2013;34:1370–81. doi: 10.1093/carcin/bgt040. [DOI] [PubMed] [Google Scholar]
- 81.Diskin S, Chen WS, Cao Z, Gyawali S, Gong H, Soza A, González A, Panjwani N. Galectin-8 promotes cytoskeletal rearrangement in trabecular meshwork cells through activation of Rho signaling. PLoS One. 2012;7:e44400. doi: 10.1371/journal.pone.0044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–4. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 83.Shimura T, Takenaka Y, Fukumori T, Tsutsumi S, Okada K, Hogan V, Kikuchi A, Kuwano H, Raz A. Implication of galectin-3 in Wnt signaling. Cancer Res. 2005;65:3535–7. doi: 10.1158/0008-5472.CAN-05-0104. [DOI] [PubMed] [Google Scholar]
- 84.Shi Y, He B, Kuchenbecker KM, You L, Xu Z, Mikami I, Yagui-Beltran A, Clement G, Lin YC, Okamoto J, et al. Inhibition of Wnt-2 and galectin-3 synergistically destabilizes beta-catenin and induces apoptosis in human colorectal cancer cells. Int J Cancer. 2007;121:1175–81. doi: 10.1002/ijc.22848. [DOI] [PubMed] [Google Scholar]
- 85.Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, Hung MC, Bresalier RS. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009;69:1343–9. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobayashi T, Shimura T, Yajima T, Kubo N, Araki K, Tsutsumi S, Suzuki H, Kuwano H, Raz A. Transient gene silencing of galectin-3 suppresses pancreatic cancer cell migration and invasion through degradation of β-catenin. Int J Cancer. 2011;129:2775–86. doi: 10.1002/ijc.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim S-J, Shin JY, Cheong TC, Choi IJ, Lee YS, Park SH, Chun KH. Galectin-3 germline variant at position 191 enhances nuclear accumulation and activation of β-catenin in gastric cancer. Clin Exp Metastasis. 2011;28:743–50. doi: 10.1007/s10585-011-9406-8. [DOI] [PubMed] [Google Scholar]
- 88.Dakeng S, Duangmano S, Jiratchariyakul W, U-Pratya Y, Bögler O, Patmasiriwat P. Inhibition of Wnt signaling by cucurbitacin B in breast cancer cells: reduction of Wnt-associated proteins and reduced translocation of galectin-3-mediated β-catenin to the nucleus. J Cell Biochem. 2012;113:49–60. doi: 10.1002/jcb.23326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang D, Chen ZG, Liu SH, Dong ZQ, Dalin M, Bao SS, Hu YW, Wei FC. Galectin-3 gene silencing inhibits migration and invasion of human tongue cancer cells in vitro via downregulating β-catenin. Acta Pharmacol Sin. 2013;34:176–84. doi: 10.1038/aps.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L-P, Chen S-W, Zhuang S-M, Li H, Song M. Galectin-3 accelerates the progression of oral tongue squamous cell carcinoma via a Wnt/β-catenin-dependent pathway. Pathol Oncol Res. 2013;19:461–74. doi: 10.1007/s12253-013-9603-7. [DOI] [PubMed] [Google Scholar]
- 91.Paz A, Haklai R, Elad-Sfadia G, Ballan E, Kloog Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene. 2001;20:7486–93. doi: 10.1038/sj.onc.1204950. [DOI] [PubMed] [Google Scholar]
- 92.Elad-Sfadia G, Haklai R, Ballan E, Gabius H-J, Kloog Y. Galectin-1 augments Ras activation and diverts Ras signals to Raf-1 at the expense of phosphoinositide 3-kinase. J Biol Chem. 2002;277:37169–75. doi: 10.1074/jbc.M205698200. [DOI] [PubMed] [Google Scholar]
- 93.Rabinovich GA, Alonso CR, Sotomayor CE, Durand S, Bocco JL, Riera CM. Molecular mechanisms implicated in galectin-1-induced apoptosis: activation of the AP-1 transcription factor and downregulation of Bcl-2. Cell Death Differ. 2000;7:747–53. doi: 10.1038/sj.cdd.4400708. [DOI] [PubMed] [Google Scholar]
- 94.Jeon S-B, Yoon HJ, Chang CY, Koh HS, Jeon SH, Park EJ. Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J Immunol. 2010;185:7037–46. doi: 10.4049/jimmunol.1000154. [DOI] [PubMed] [Google Scholar]
- 95.Koopmans SM, Bot FJ, Schouten HC, Janssen J, van Marion AM. The involvement of Galectins in the modulation of the JAK/STAT pathway in myeloproliferative neoplasia. Am J Blood Res. 2012;2:119–27. [PMC free article] [PubMed] [Google Scholar]
- 96.Shalom-Feuerstein R, Cooks T, Raz A, Kloog Y. Galectin-3 regulates a molecular switch from N-Ras to K-Ras usage in human breast carcinoma cells. Cancer Res. 2005;65:7292–300. doi: 10.1158/0008-5472.CAN-05-0775. [DOI] [PubMed] [Google Scholar]
- 97.Merlin J, Stechly L, de Beaucé S, Monté D, Leteurtre E, van Seuningen I, Huet G, Pigny P. Galectin-3 regulates MUC1 and EGFR cellular distribution and EGFR downstream pathways in pancreatic cancer cells. Oncogene. 2011;30:2514–25. doi: 10.1038/onc.2010.631. [DOI] [PubMed] [Google Scholar]
- 98.Mazurek N, Byrd JC, Sun Y, Hafley M, Ramirez K, Burks J, Bresalier RS. Cell-surface galectin-3 confers resistance to TRAIL by impeding trafficking of death receptors in metastatic colon adenocarcinoma cells. Cell Death Differ. 2012;19:523–33. doi: 10.1038/cdd.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mazurek N, Sun YJ, Liu KF, Gilcrease MZ, Schober W, Nangia-Makker P, Raz A, Bresalier RS. Phosphorylated galectin-3 mediates tumor necrosis factor-related apoptosis-inducing ligand signaling by regulating phosphatase and tensin homologue deleted on chromosome 10 in human breast carcinoma cells. J Biol Chem. 2007;282:21337–48. doi: 10.1074/jbc.M608810200. [DOI] [PubMed] [Google Scholar]
- 100.Oka N, Nakahara S, Takenaka Y, Fukumori T, Hogan V, Kanayama HO, Yanagawa T, Raz A. Galectin-3 inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer Res. 2005;65:7546–53. doi: 10.1158/0008-5472.CAN-05-1197. [DOI] [PubMed] [Google Scholar]
- 101.Yang R-Y, Rabinovich GA, Liu F-T. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 102.Gao P, Simpson JL, Zhang J, Gibson PG. Galectin-3: its role in asthma and potential as an anti-inflammatory target. Respir Res. 2013;14:136. doi: 10.1186/1465-9921-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zuberi RI, Hsu DK, Kalayci O, Chen HY, Sheldon HK, Yu L, Apgar JR, Kawakami T, Lilly CM, Liu FT. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am J Pathol. 2004;165:2045–53. doi: 10.1016/S0002-9440(10)63255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saegusa J, Hsu DK, Chen HY, Yu L, Fermin A, Fung MA, Liu FT. Galectin-3 is critical for the development of the allergic inflammatory response in a mouse model of atopic dermatitis. Am J Pathol. 2009;174:922–31. doi: 10.2353/ajpath.2009.080500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai L, Zhou H, Fang Z, Yuan J, Niki T, Hirashima M, He W, Chen ZK. Galectin-9 in combination with rapamycin induces cardiac allograft tolerance in mice. Transplantation. 2013;96:379–86. doi: 10.1097/TP.0b013e31829b07b5. [DOI] [PubMed] [Google Scholar]
- 106.Ingrassia L, Camby I, Lefranc F, Mathieu V, Nshimyumukiza P, Darro F, Kiss R. Anti-galectin compounds as potential anti-cancer drugs. Curr Med Chem. 2006;13:3513–27. doi: 10.2174/092986706779026219. [DOI] [PubMed] [Google Scholar]
- 107.Kim S-J, Hwang J-A, Ro JY, Lee Y-S, Chun K-H. Galectin-7 is epigenetically-regulated tumor suppressor in gastric cancer. Oncotarget. 2013;4:1461–71. doi: 10.18632/oncotarget.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Demers M, Rose AA, Grosset AA, Biron-Pain K, Gaboury L, Siegel PM, St-Pierre Y. Overexpression of galectin-7, a myoepithelial cell marker, enhances spontaneous metastasis of breast cancer cells. Am J Pathol. 2010;176:3023–31. doi: 10.2353/ajpath.2010.090876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Masuyer G, Jabeen T, Öberg CT, Leffler H, Nilsson UJ, Acharya KR. Inhibition mechanism of human galectin-7 by a novel galactose-benzylphosphate inhibitor. FEBS J. 2012;279:193–202. doi: 10.1111/j.1742-4658.2011.08414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cumpstey I, Carlsson S, Leffler H, Nilsson UJ. Synthesis of a phenyl thio-beta-D-galactopyranoside library from 1,5-difluoro-2,4-dinitrobenzene: discovery of efficient and selective monosaccharide inhibitors of galectin-7. Org Biomol Chem. 2005;3:1922–32. doi: 10.1039/b502354h. [DOI] [PubMed] [Google Scholar]
- 111.Zou J, Glinsky VV, Landon LA, Matthews L, Deutscher SL. Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancer cell adhesion. Carcinogenesis. 2005;26:309–18. doi: 10.1093/carcin/bgh329. [DOI] [PubMed] [Google Scholar]
- 112.Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94:1854–62. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- 113.Thijssen VLJL, Poirier F, Baum LG, Griffioen AW. Galectins in the tumor endothelium: opportunities for combined cancer therapy. Blood. 2007;110:2819–27. doi: 10.1182/blood-2007-03-077792. [DOI] [PubMed] [Google Scholar]
- 114.Croci DO, Cerliani JP, Dalotto-Moreno T, Méndez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, Toscano MA, Caramelo JJ, García-Vallejo JJ, Ouyang J, et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–58. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 115.Thijssen VLJL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci U S A. 2006;103:15975–80. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stannard KA, Collins PM, Ito K, Sullivan EM, Scott SA, Gabutero E, Darren Grice I, Low P, Nilsson UJ, Leffler H, et al. Galectin inhibitory disaccharides promote tumour immunity in a breast cancer model. Cancer Lett. 2010;299:95–110. doi: 10.1016/j.canlet.2010.08.005. [DOI] [PubMed] [Google Scholar]