Abstract
We investigated the role of Toll-like receptor (TLR) 2 in maintaining the integrity of the airway epithelial barrier using the human bronchial epithelial cell line Calu-3. Activation of TLR2 by its ligands, Pam3CysSK4 and Peptidoglycan showed a concentration dependent increase in epithelial barrier function, as measured by transepithelial electrical resistance (TEER). This was confirmed by a decrease in paracellular flux of fluorescein sodium. This TLR2 induced increase in TEER was significantly reduced by pretreatment with polyclonal anti-human TLR2-neutralizing antibody. TLR2 stimulation in Calu-3 cell monolayers resulted in an increased expression of the tight junction proteins claudin-1 and ZO-1, and a decreased expression of occludin, at both the mRNA and protein levels. A pseudosubstrate inhibitor to PKCζ significantly prevented the TLR2 mediated increase in barrier function. It also prevented the increase in claudin-1 in a concentration dependent manner up to 1 µM. TLR2 stimulation led to an increase in phosphorylation of atypical PKC ζ, which was prevented by the pseudosubstrate inhibitor in a concentration dependent manner. Taken together, our observations support a model whereby increased tight junction barrier function induced by activation of TLR2 occurs through increased expression of claudin-1, and through modulation of PKC ζ activity.
Keywords: Calu-3, tight junctions, toll-like receptor, airway inflammation, barrier function, epithelial permeability, protein kinase C
Introduction
The upper airways serve as an important physical and immunological barrier against inhaled antigens and allergens. As a first line of defense against invading airborne pathogens, bronchial epithelial cells are important contributors to innate mucosal immunity.1 This vital barrier function is enhanced by the formation of apical intercellular junctional complexes between bronchial epithelial cells. The dynamic regulation of tight junctions, which are the most apical junctions in the junctional complex, is essential to many physiological processes, as their disruption leads to drastic changes in paracellular permeability, a feature of many pathologic conditions of the respiratory tract, such as cystic fibrosis.2,3
Tight junctions (TJ) are composed of transmembrane proteins including occludin, claudins, junctional adhesion molecule (JAM), and cytoplasmic plaque proteins, including the zonula occludens (ZO) proteins (ZO-1, ZO-2 and ZO-3), cingulin, and polarity complex proteins.4-7
Toll-like receptors (TLRs) are a family of receptors that monitor the presence of pathogens by their ability to identify microbial structural patterns. Studies have reported that bronchial epithelial cells express functional TLRs 1–6 and TLR9.8 Of these, TLR4 is found to be expressed basolaterally, whereas TLR2 is found primarily at the apical surface of the airway epithelial cells, and is recruited to the cell surface in response to the presence of pathogenic bacteria.9
Despite the presence of TLRs at the apical surface, bronchial epithelial cells are functionally hyporesponsive to TLR2 ligands. However, Gram-negative bacteria and respiratory syncytial virus (RSV), which are ligands of TLR4 and TLR3, respectively, provoke elevated responsiveness.1 Although the molecular basis of microbial recognition by TLR2 receptors is unknown, its downstream functions are found to involve protein kinase C (PKC).10 PKC is a super-family of 11 isozymes, which are involved in the regulation of epithelial barrier function through tight junctions. The PKC isoforms are classified as conventional (α, β1, β2, γ), novel (ᵟ, ε, η, µ, θ), and atypical (ζ, ι/λ) isoforms, based on their structure and cofactor regulation.11 Interestingly, atypical protein kinases C have also been implicated in the regulation of the assembly of tight junctions in polarized epithelial cells.12
Since TJs play a key role in epithelial protection and signaling at the airway epithelial cellular level,13,14 in this study, we investigated the role of TLR2 activation in modulating airway barrier function, and the possible molecular mechanisms through which TLR2 activation operates. We used the Calu-3 cell-line as a model system, an established airway epithelial in vitro model that exhibits many morphological features of the airway epithelium like cilia, production of mucus, metabolic pathways, and transport systems.15,16 Our results show a clear role of TLR2 in promoting enhancement of barrier function through the selective increase of expression of specific TJ proteins, and the involvement of the PKC-ζ isoform in this process.
Results
TLR2 ligands enhance tight junction barrier function in Calu-3 cell monolayers
To investigate the effect of TLR2 ligands on the TJ barrier function of human bronchial epithelial cells, polarized Calu-3 cells were treated with 20 or 50 µg/ml of Pam3CysK4 (P3C) and peptidoglycan (PGN), and successively examined for changes in TEER and paracellular permeation of fluorescein sodium. As shown in Figure 1A, P3C at concentrations of 20 and 50 µg/ml induced an increase in TEER at 20.30 h (126% ± 7% and 165% ± 13%, respectively). As shown in Figure 1B, PGN at concentrations of 20 and 50 µg/ml induced an increase in TEER at 25 h (122% ± 4% and 153% ± 11%, respectively). Thus, both bacterial ligands induced an increase in TEER in a dose dependent manner. Stimulation with PGN led to an increase after 20.30 h, and with P3C after 25 h. A similar variation in duration of biological activity was noticed between P3C and PGN in intestinal epithelial cells. This may be explained by the changes encountered by these ligands due to the environmental conditions such as oxidation, de-esterfication and micelle formation.17,18
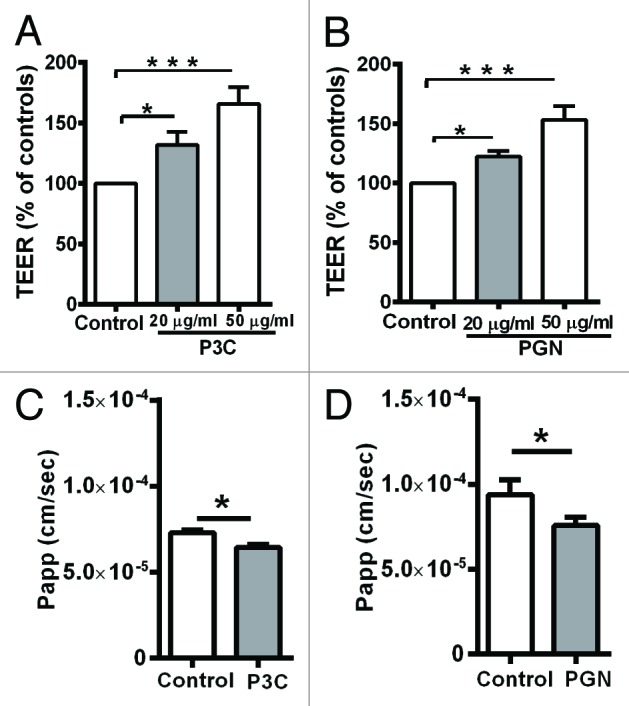
Figure 1. Barrier function measured as TEER (A, B) in Calu-3 cell monolayers treated with bacterial ligands. A, P3C and B, PGN significantly increased the TEER of Calu-3 cells in a dose dependent manner. All TEER values are expressed as percentage relative to control (the baseline value was > 520 Ω* cm2). Values represent mean ± SD *P < 0.05, **P < 0.01, ***P < 0.001. The data were analyzed using one-way analysis of variance (ANOVA). All the experiments were performed atleast 3 times during different days with number of replicates (n = 4). Paracellular flux of fluorescein sodium (C, D) after treatment with TLR2 ligands. C, P3C and D, PGN significantly decreased the flux of fluorescein sodium compared with the control. The data shown are representative figures of at least 3 independent experiments with number of replicates (n = 3). The data are shown as means ± SD *P < 0.05 as determined by the students t test.
The paracellular flux of fluorescein sodium was measured between 24.30 h and 25.30 h after treatment with 20 µg/ml P3C. The apparent permeability (Papp) of the control group was measured as 7.27 * 10−5cm/s, treatment with P3C decreased the apparent permeability to 6.42 * 10−5 cm/s (Fig. 1C). The decrease in paracellular flux after treatment with P3C was significant as compared with the control group (P < 0.05). For PGN, the flux was measured between 20 and 21 h. The apparent permeability (Papp) of the control group was measured as 9.37 * 10−5cm/s, treatment with PGN significantly decreased the apparent permeability to 7.56 * 10−5 cm/s (Fig. 1D) (P < 0.05).
In order to confirm the specificity of TLR2 signaling in the upregulation of TJ-associated barrier function caused by TLR2 stimulation, a polyclonal TLR2-neutralizing antibody was used to block the TLR2 receptor. Treatment with isogenic IgG (rat IgG) control had no effect on the barrier function (Fig. 2A, B). Treatment with PGN and P3C on isogenic IgG control pretreated monolayers resulted in a significant increase in TEER when compared with isogenic IgG control treated monolayers (Fig. 2A, B). Pretreatment of monolayers with TLR2-neutralizing antibody significantly (P < 0.01) reduced TEER when compared with monolayers treated with TLR2 ligands PGN and P3C (Fig. 2A, B). These observations indicated that the effects of PGN and P3C are specifically mediated by the TLR2 receptor.
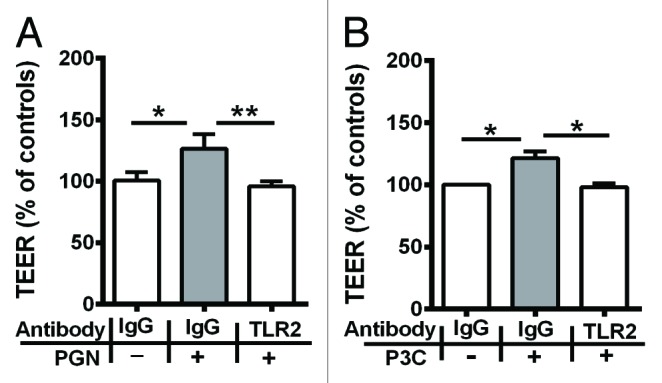
Figure 2. Pretreatment with TLR2-neutralizing antibody significantly reduced the increase in TJ-associated barrier function by A, PGN and B, P3C. All TEER values are expressed as percentage relative to control (the baseline value was > 520 Ω*cm2). Values represent mean ± SD *P < 0.05, **P < 0.01, ***P < 0.001. The data were analyzed using one-way analysis of variance (ANOVA). All the experiments were performed atleast 3 times during different days with number of replicates (n = 3).
Increased expression and junctional accumulation of tight junction proteins after TLR2 stimulation
Next, we evaluated the effect of TLR2 stimulation on the expression and localization of TJ proteins. In Calu-3 cells, RT-PCR analysis revealed an upregulation of claudin-1 and ZO-1 mRNA by about 200-fold and 30-fold increase, respectively, when compared with the control (Fig. 3A). In contrast, the expression of claudin-2 and occludin was decreased about 50-fold and 70-fold, respectively (Fig. 3A). Immunofluoresence analysis showed that treatment with P3C leads to an increase in the junctional labeling of claudin-1 and ZO-1, a decrease in occludin labeling and no effect on cingulin labeling (Fig. 3B). Western blotting analysis for claudin-1, ZO-1 and occludin showed an increase in the levels of claudin-1 and ZO-1 while a decrease in occludin compared with control. (Fig. 4B and C).
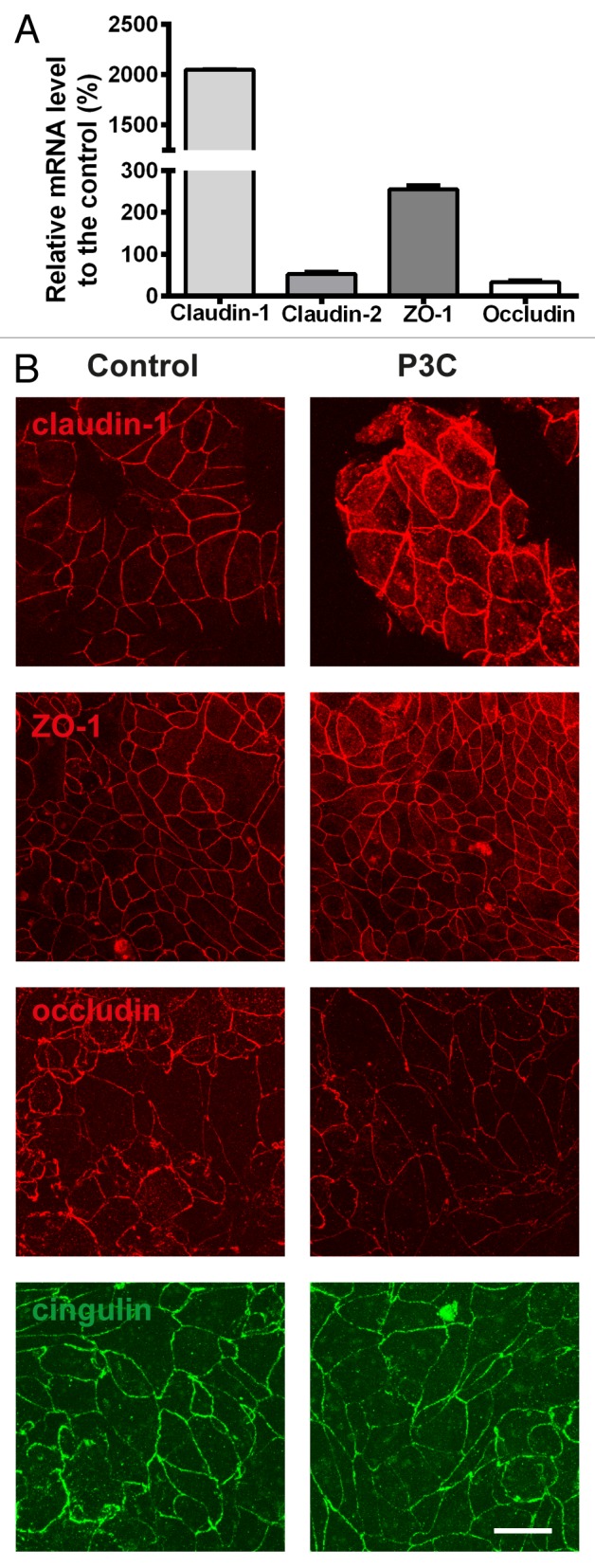
Figure 3. RT-PCR (A) for claudin-1 and 2, ZO-1, occludin in Calu-3 cells after treatment with P3C. Upregulation of mRNAs of claudin-1, ZO-1 but not claudin-2 and occludin is observed. (B) Immunofluoresence for claudin-1, ZO-1, occludin and cingulin in Calu-3 cell monolayers treated in the absence or presence of P3C. Junctional labeling of claudin-1 and ZO-1 increased, occludin decreased, while cingulin remained unchanged. All the experiments were performed three times with the number of replicates (n = 3).
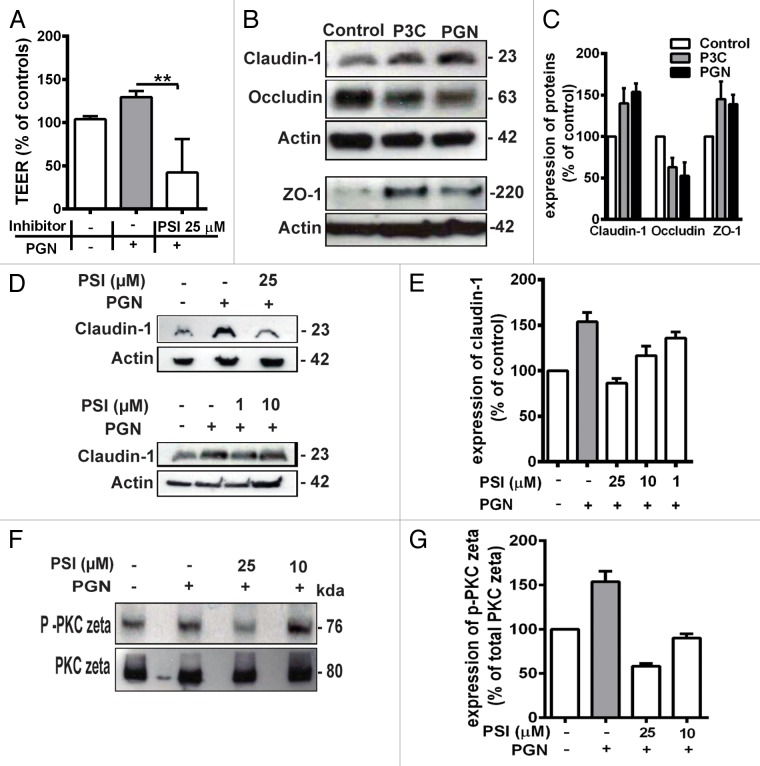
Figure 4. (A) Pretreatment with 25 µM PKC ζ pseudosubstrate inhibitor (PSI) significantly prevented the increase in TEER induced by PGN. All TEER values are expressed as percentage relative to control (the baseline value was > 520 Ω*cm2). Values represent mean ± SD *P < 0.05, **P < 0.01, ***P < 0.001. The data were analyzed using one-way analysis of variance (ANOVA). All the experiments were performed atleast 3 times during different days with number of replicates (n = 4). Western blotting (B) for claudin-1, occludin and ZO-1. Upregulation of claudin-1 and ZO-1 was observed in Calu-3 cells after treatment with P3C and PGN. Occludin was decreased. The images are representative of 3 independent experiments. β–actin was used as a lane loading control. The number of replicates is (n = 2). (C) the corresponding expression levels of claudin-1, occluding and ZO-1 are shown as bar graphs. (D) western blotting for claudin-1. The PSI inhibited the claudin-1 expression induced by PGN treatment in a concentration dependent manner (25 µM, 10 µM and 1 µM). E, the corresponding expression levels of claudin-1 are shown as bar graphs. (F) western blotting for phospho-PKC ζ in Calu-3 cell monolayers treated with PGN. Stimulation by PGN increased phosphorylation of PKC ζ in the treated cells which was inhibited by pretreatment with PSI 25 µM and 10 μM. Expression of phospho-PKC ζ and total PKC ζ was examined using western-blot analyses of total cell lysates. The images are representative of 3 independent experiments. The number of replicates is (n = 2). (G) the corresponding expression levels of phospho-PKC ζ are shown as relative to total PKC ζ expression.
TLR2 dependent increase in bronchial epithelial barrier function is mediated by atypical PKC ζ
We investigated the pathways involved in the TLR2-dependent increase in bronchial epithelial barrier function by focusing on PKC, which has been shown to be involved in TLR2 signaling.17 To investigate the role of PKC isoform ζ, we used the PKC ζ pseudosubstrate inhibitor (PSI) which can act as an specific antagonist to PKC isoform ζ. Treatment with PSI (25 µM) significantly prevented the increase in TEER induced by TLR2 stimulation (Fig. 4A). Importantly, immunoblotting analysis showed that pretreatment with PSI inhibitor prevented the PGN induced increase in claudin-1 protein levels in a concentration dependent manner up to 1 μM concentration (Fig. 4D and E), indicating that PKC ζ mediates the increase in claudin-1 expression.
Finally, we asked whether treatment with PGN modified the phosphorylation level of atypical isoform PKC ζ, which is known to affect PKC ζ activity.19,20 As shown in Figure 4E, the relative phosphorylation of PKC ζ was increased by about 1.5-fold following PGN treatment, this effect was reduced by the PSI in a concentration dependent manner at 25 and 10 µM (Fig. 4F,G). It is therefore concluded that TLR2-dependent increase in bronchial epithelial barrier function occurs through modulation of the function of the atypical PKC isoform ζ.
Discussion
Barrier function of epithelial tissues is modulated by a large number of physiological and pathological signals and stimuli, including pathogenic organisms and their molecular components.21 Toll-like receptor 2 is implicated in microbial recognition, and studies on human intestinal epithelial cells and epidermal keratinocytes have shown that TLR2 activation enhances tight junction barrier function.17,22 In intestinal epithelial cells, TLR2 activation was associated with activation of PKC, an increase in TEER, an apical redistribution of ZO-1, although no changes in the expression or localization of claudin-1 and occludin were observed.17 In epidermal keratinocytes the increase in barrier function upon stimulation of TLR2 by peptidoglycan correlated with a increased association of atypical protein kinase CZ/I with occludin, but no changes in the levels of ZO-1, claudin-1, claudin-4 and occludin were reported.22 In a more recent study, however, an increased expression of claudin-1, ZO-1 and other TJ proteins was observed in keratinocytes after activation of TLR2.23 In our present study, ligand-induced TLR2 activation significantly increased the barrier integrity of the Calu-3 human bronchial epithelial cells, and this enhancement in barrier function was associated with an upregulation in expression levels of TJ proteins ZO-1 and claudin-1, mediated by atypical protein kinase C ζ. Our results provide a mechanistically plausible molecular pathway to explain the increase in barrier function, considering the known role of claudin-1 as a major determinant of “tightness” of the paracellular seal. Indeed, in kidney epithelial cells the expression of claudin-1, in the absence of the leaky claudin-2, is a hallmark of the “tight” TJ of distal tubule cells.24 In addition, knockout of claudin-1 in mice leads to early post-natal death, due to disruption of the epidermal barrier, and water loss through the skin.25 In lung cancers, claudin-1 expression differentiates squamous carcinomas, where claudin-1 is upregulated, from adenocarcinomas, where it is downregulated.26 Concerning the observed increase in ZO-1 expression, although ZO-1 by itself is not directly implicated in regulating the TJ barrier to ions, it is involved in controlling the barrier permeability to large solutes and, redundantly with other ZO proteins, it forms the scaffold upon which claudins polymerize in the membrane, to form TJ pores for ion permeation.27-29 In contrast, neither cingulin nor occludin have been shown to play a major role in the structural assembly and regulation of the TJ barrier in knockout model systems.30-32
In a more recent study by Rezaee et al.21 it was observed that P3C had no effect on TEER in 16HBE cells after 24 h. In Calu-3 cells increase in barrier function induced by P3C stimulation of TLR2 in bronchial epithelial cells is an effect that starts at 24.30 h, reaching a maximum by 25 h and declining slowly from around 120% at 25 h to about 105% at 27.30 h. Hence it is probable that the effect might have been left unnoticed in the study published by Rezaee et al. In case of PGN, the effect starts at 20 h, reaching a maximum by 21 h and declining slowly from around 120% to about 105% by 24.30 h.
A similar variation in duration of biological activity was noticed when comparing the effect of P3C and PGN in intestinal epithelial cells. This may explained by the changes encountered by these ligands due to the environmental conditions such as oxidation, de-esterfication and micelle formation.17,18
The Calu-3 cells used here as a model system is a cell line derived from lung adenocarcinoma, which may not perfectly reflect the expression or function of human bronchial epithelial cells in vivo. However, Calu-3 cell line has been established as a reliable pulmonary epithelial model over several years now. The cell line has been characterized to display an mRNA and protein profile, typical of the lung epithelium.23
We also showed that in Calu-3 cells, TLR2 stimulation resulted in increased phosphorylation of atypical PKC ζ, indicating that TLR2 stimulation activates PKC ζ by means of phosphorylation. TLR2 stimulation by bacterial lipopeptides has been shown to activate atypical PKC ζ and its association with RhoA. Inhibition of this isoform of protein kinase resulted in decreased NF-κB activation and p65/RelA trans activation, indicating that atypical PKC ζ and partially RhoA might mediate the transcription of TJ proteins via the NF-κB activation pathway.33
In conclusion, activation of TLR2 of bronchial epithelial cells may help improve the barrier function, which is compromised in many airway inflammatory diseases. Fragile epithelia and increased epithelial barrier permeability is commonly seen in airway inflammatory conditions.34 In asthma, disruption of airway epithelial TJs by aeroallergens is an important cause for bronchial hyper-reponsiveness as the impairment of barrier function allows an easy access to the airway wall and there by to immune and inflammatory cells.35
The specific targeting of TLR2 ligands could hold promise for the treatment of inflammatory airway diseases. The in vitro airway epithelial model used in the current study is representative of the healthy epithelium and hence further studies should be directed to investigate the potential of TLR2 activation in enhancing epithelial barrier function under inflammatory conditions.
Materials and Methods
Cell culture
Calu-3 cells (used at passage numbers (PN) 29–38) were obtained from ATCC (Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, GlutamaxTM-1, Invitrogen, Saint Aubin, France), containing 10% fetal calf serum (FCS), 100 U/ml of penicillin and 100 U/ml of streptomycin (PAN® Biotech, GmbH, Aidenbach, Germany). The cells were cultured at 37οC under a humidified atmosphere of 5% CO2 in air.
TLR2 ligands
TLR2 agonists Pam3CSK4 (P3C) and peptidoglycan (PGN) were purchased from Invivogen (Toulouse, France). The lipopetides were resuspended in endotoxin free water and stored at -20 °C as aliquots of 200 μg/ml for further dilution. The bioactivity of lipopeptide TLR2 agonists is highly dependent on the dilution protocol employed. There might be a loss of activity of these lipopeptides when diluted in protein- and serum-free buffers. This is due to the formation of large aggregates of lipopeptides when not solubilized properly.24
Measurement of transepithelial electrical resistance
Transepithelial electrical resistance (TEER) of the cell monolayers was measured using an EVOM® voltohmmeter (World Precision Instruments, Aston, UK) equipped with STX-2 chopstick electrodes (WPI, Sarasota, FL, USA). Calu-3 cells of an initial seeding density of 5*105cells/cm2, on collagen-coated Transwell® permeable supports in cell culture inserts (0.4 μm pore size, 6.5 mm inside diameter, Corning Costar, Acton, MA, USA). The medium in the apical chamber was aspirated after 24 h and the Calu-3 cell monolayers were grown at an air-liquid interface. The basolateral medium was changed every two days. Polarized Calu-3 cell monolayers grown over a time period of 14 d were used for the experiments. Measurements were made as described in.36 All TEER values are expressed as percentage relative to control (the baseline value was 520 Ω* cm2). Values represent mean ± SD *P < 0.05, **P < 0.01, ***P < 0.001. The data were analyzed using one-way analysis of variance (ANOVA). All the experiments were performed at least 3 times during different days with a number of replicates equal to four (n = 4).
Determination of paracellular permeability
To determine paracellular permeability, the cells were cultured on 6.5 mm Transwell, 0.4 μm pore size filters and then fluorescein sodium (molecular weight: 330 Da) at a concentration of 50 µM in culture medium was added to the apical chamber.37 The permeation of fluorescein sodium (NaF) (Fluka, Buchs, Switzerland) through control or stimulated cell monolayers was determined by measuring the fluoresence of the solution in the basolateral compartment at 458/528 nm (exicitation/emission wavelengths) using a fluorometer (FLx 800, BioTek Instruments Inc., Winooski, VT, USA).
Apparent permeability of Fluorescein sodium
The apparent permeability of NaF was calculated using equation 1:
| (1) |
where C0 is the initial concentration (mmol/ml) of NaF in the donor compartment, A (cm2) is the surface area of the cell layers (0.33 cm2 for 24 well plate inserts) and dQ/dt (mmol/s) is the appearance rate of NaF in the receiver compartment.
Inactivation of TLR2
To investigate the effect of TLR2 blockade on PGN (Invivogen) induced increase in barrier function, the Calu-3 cell monolayers were pretreated with rat polyclonal anti-human TLR2 antibody (10 μg/ml) or rat polyclonal IgG (Invivogen) as isogenic control for 2 h before PGN stimulation. After 20.30 h of co-incubation TEER measurements were taken.
Inhibitor
PKC inhibitor PKC-ζ pseudosubstrate myristoylated from Invitrogen (CA, USA), was used at different concentrations. The TLR2 ligands were tested after 1 h of pre-incubation with the inhibitor.
RNA isolation and RT-PCR analysis
Total RNA from Calu-3 cells was isolated with RNeasy mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol, with the DNase step. cDNA synthesis was achieved with 1 µg RNA using the iScript cDNA synthesis kit (Biorad, Reinach, Switzerland).
To quantify the transcript levels, SYBR-Green-based qRT-PCR (Applied Biosystems, Rotkreuz, Switzerland) and a 96-channel iCycler optical system (Biorad) were used. SYBR-Green-based reaction of 25 µl contained 1x SYBR-Green mastermix (Biorad), a single gene-specific primer set (forward and reverse 2.5 µM), 20 ng of cDNA and 10 nM of fluorescein calibration dye (Biorad) and 50% volume of PCR master mix. Each reaction was done in triplicate.
Primer sets for ZO-1, OCLN, CLDN-1, and CLDN-2 (human species) purchased from Microsynth (Balgach, Switzerland) had the following sequences; OCLN (sense 5′-CAGGA ACCGA GAGCC AGGT-3′ and antisense 5′-ATAAA CCAAT CTGCT GCGTC CTA-3), ZO-1 (5′- CAGCC GGTCA CGATC TCCT -3′ and antisense 5′- TCCGG AGACT GCCAT TGC -3′) CLDN-1 (5′-CTGCC CCAGT GGAGG ATTTA-3′ and antisense 5′-CATGG CCTGG GCGGT 3′) CLDN-2 (5′-TCCC TGGCC TGCAT TATCTC-3′ and antisense 5′-CTTTG GCTCG GGATT CCTG-3′).
Each reaction was done in triplicate on a 96-channel iCycler optical system (BioRad). The housekeeping actin gene was used as internal control. The cycling conditions were as follows: one cycle at 95 °C for 10 min, followed by 40 cycles of PCR amplification, each at 95 °C for 10 s and 60 °C for 45 s. The iCycler MyiQ software v5.0 (Biorad) was used to analyze the real-time fluorescence signal. For each sample, a threshold cycle (Ct) was determined, using the exponential growth phase and the base line signal from fluorescence vs. cycle number plots. Cts were normalized to the level of expression of the actin gene.
Immunofluorescence
Immunofluorescent labeling of cells was performed by rinsing cells with cold phosphate buffered saline (PBS) pH 7.4 (PAN® Biotech GmbH), followed by fixing for 10 min in cold methanol at -20 °C, washing with PBS, and incubating with the primary antibody (1 h, room temperature). This was followed by an additional washing step with PBS, incubation with the secondary antibody (30 min, 37 °C), washing, and mounting using Vectashield® with DAPI (Vector Laboratories, Servion, Switzerland). The following primary antibodies were used: rabbit anti-ZO-1 (Cell signaling, Leiden, Netherlands), mouse anti-occludin (33–1500, InvitrogenAG, Basel, Switzerland), rabbit anti-claudin-1 (71–7800, Invitrogen), mouse anti-claudin-2 (32–5600, Invitrogen), rabbit anti-cingulin.38 Secondary donkey anti-rabbit or anti-mouse antibodies labeled with Cy3 (Jackson Laboratories, ME, USA) were used at a dilution of 1:300. Images were taken using a confocal laser scanning microscope (Zeiss 510 Meta, Zeiss, CA, USA).
Western blot analysis
The cells were tested in the absence or presence of TLR2 ligands after having reached 80% confluency. The cells were collected after treatment with PGN and P3C after 21 and 25 h, respectively. Cells were detached by means of a cell scraper and total-cell lysates were prepared for analysis. The denatured proteins were separated by electrophoresis on 10% SDS-polyacrylamide gels and transferred electrophoretically onto a nitro cellulose membrane (Biorad) (20V, 20mins, 1Amp). The membranes were blocked using 5% non-fat milk and 0.05% Tween 20 in Tris-buffered saline at room temperature for 1 h and immunoblotted overnight with anti-phospho-PKCζ (Santa Cruz Biotechnology, Heidelberg, Germany), anti-claudin-1 (Invitrogen), anti-ZO-1, anti-occludin, and anti-actin antibodies (Sigma-Aldrich) and anti-cingulin38 at the dilutions recommended by the manufacturers at 4 °C. The membranes were incubated for 1h at room temperature with horse-radish peroxidase labeled anti-mouse (Sigma-Aldrich) or anti-rabbit IgG (Merck KGaA). Detection was performed using enhanced chemiluminescent western blotting system (Applichem, Darmstadt, Germany). To confirm equal loading, immunoblots were probed with anti-actin or anti-pkc ζ (Santa Cruz Biotechnology). All experiments were repeated at least 3 times; representative blots are shown for each experiment.
Statistical analysis
Data are reported as mean ± standard deviation (S.D). Analyses of multiple groups were assessed using one way ANOVA followed by Tukey’s post-hoc test. P < 0.05 was considered as statistically significant. Significance is denoted as *P < 0.05, **P < 0.01, ***P < 0.001.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- TLR
toll-like receptor
- ZO
zonula occludens
- PKC
protein kinase C
- TEER
transepithelial electrical resistance
- TJ
tight junctions
- JAM
junctional adhesion molecule
- ASIP
atypical protein kinase C- interacting protein
- PGN
peptidoglycan
- P3C
Pam3CysSerLys4
- RT-PCR
reverse transcription-polymerase chain reaction
- RSV
respiratory syncytial virus
- PBS
phosphate buffered saline
- NaF
fluorescein sodium
- PSI
PKC ζ pseudosubstrate inhibitor
References
- 1.Mayer AK, Muehmer M, Mages J, Gueinzius K, Hess C, Heeg K, Bals R, Lang R, Dalpke AH. Differential recognition of TLR-dependent microbial ligands in human bronchial epithelial cells. J Immunol. 2007;178:3134–42. doi: 10.4049/jimmunol.178.5.3134. [DOI] [PubMed] [Google Scholar]
- 2.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218–34. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh SV, Hopkins AM, Nusrat A. Modulation of tight junction structure and function by cytokines. Adv Drug Deliv Rev. 2000;41:303–13. doi: 10.1016/S0169-409X(00)00048-X. [DOI] [PubMed] [Google Scholar]
- 4.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–35. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 5.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci. 2009;1165:113–20. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–8. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Citi S. The Cytoplasmic Plaque Proteins of the Tight Junction. Tight Junctions: CRC Press, 2001. [Google Scholar]
- 8.Melkamu T, Squillace D, Kita H, O’Grady SM. Regulation of TLR2 expression and function in human airway epithelial cells. J Membr Biol. 2009;229:101–13. doi: 10.1007/s00232-009-9175-3. [DOI] [PubMed] [Google Scholar]
- 9.Soong G, Reddy B, Sokol S, Adamo R, Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest. 2004;113:1482–9. doi: 10.1172/JCI200420773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lennartz DJLaMR. Protein Kinase C and Toll-Like Receptor Signaling. Enzyme Research 2011; 2011:7 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–8. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol. 2001;152:1183–96. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puchelle E, Zahm J-M, Tournier J-M, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:726–33. doi: 10.1513/pats.200605-126SF. [DOI] [PubMed] [Google Scholar]
- 14.Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166–78. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- 15.Borchard G. Understanding the Mechanisms of Local Pulmonary Drug Absorption and Metabolism: An in Vitro Model of the Airway Epithelium. In: Gradoń L, Marijnissen J, eds. Optimization of Aerosol Drug Delivery: Springer Netherlands, 2003:295-307. [Google Scholar]
- 16.Borchard G, Cassará ML, Roemelé PEH, Florea BI, Junginger HE. Transport and local metabolism of budesonide and fluticasone propionate in a human bronchial epithelial cell line (Calu-3) J Pharm Sci. 2002;91:1561–7. doi: 10.1002/jps.10151. [DOI] [PubMed] [Google Scholar]
- 17.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–38. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Morr M, Takeuchi O, Akira S, Simon MM, Mühlradt PF. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur J Immunol. 2002;32:3337–47. doi: 10.1002/1521-4141(2002012)32:12<3337::AID-IMMU3337>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–64. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 20.Nakanishi H, Brewer KA, Exton JH. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993;268:13–6. [PubMed] [Google Scholar]
- 21.Rezaee F, Meednu N, Emo JA, Saatian B, Chapman TJ, Naydenov NG, De Benedetto A, Beck LA, Ivanov AI, Georas SN. Polyinosinic:polycytidylic acid induces protein kinase D-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. J Allergy Clin Immunol. 2011;128:1216–, e11. doi: 10.1016/j.jaci.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuki T, Yoshida H, Akazawa Y, Komiya A, Sugiyama Y, Inoue S. Activation of TLR2 enhances tight junction barrier in epidermal keratinocytes. J Immunol. 2011;187:3230–7. doi: 10.4049/jimmunol.1100058. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton KO, Topp E, Makagiansar I, Siahaan T, Yazdanian M, Audus KL. Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J Pharmacol Exp Ther. 2001;298:1199–205. [PubMed] [Google Scholar]
- 24.Voss S, Ulmer AJ, Jung G, Wiesmüller K-H, Brock R. The activity of lipopeptide TLR2 agonists critically depends on the presence of solubilizers. Eur J Immunol. 2007;37:3489–98. doi: 10.1002/eji.200737537. [DOI] [PubMed] [Google Scholar]
- 25.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paschoud S, Bongiovanni M, Pache J-C, Citi S. Claudin-1 and claudin-5 expression patterns differentiate lung squamous cell carcinomas from adenocarcinomas. Mod Pathol. 2007;20:947–54. doi: 10.1038/modpathol.3800835. [DOI] [PubMed] [Google Scholar]
- 27.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–40. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–54. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 29.Fanning AS, Van Itallie CM, Anderson JM. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell. 2012;23:577–90. doi: 10.1091/mbc.E11-09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guillemot L, Hammar E, Kaister C, Ritz J, Caille D, Jond L, Bauer C, Meda P, Citi S. Disruption of the cingulin gene does not prevent tight junction formation but alters gene expression. J Cell Sci. 2004;117:5245–56. doi: 10.1242/jcs.01399. [DOI] [PubMed] [Google Scholar]
- 32.Guillemot L, Schneider Y, Brun P, Castagliuolo I, Pizzuti D, Martines D, Jond L, Bongiovanni M, Citi S. Cingulin is dispensable for epithelial barrier function and tight junction structure, and plays a role in the control of claudin-2 expression and response to duodenal mucosa injury. J Cell Sci. 2012;125:5005–14. doi: 10.1242/jcs.101261. [DOI] [PubMed] [Google Scholar]
- 33.Teusch N, Lombardo E, Eddleston J, Knaus UG. The low molecular weight GTPase RhoA and atypical protein kinase Czeta are required for TLR2-mediated gene transcription. J Immunol. 2004;173:507–14. doi: 10.4049/jimmunol.173.1.507. [DOI] [PubMed] [Google Scholar]
- 34.Sparrow MP, Omari TI, Mitchell HW. The epithelial barrier and airway responsiveness. Can J Physiol Pharmacol. 1995;73:180–90. doi: 10.1139/y95-027. [DOI] [PubMed] [Google Scholar]
- 35.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120:1233–44, quiz 1245-6. doi: 10.1016/j.jaci.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 36.Stewart CE, Torr EE, Mohd Jamili NH, Bosquillon C, Sayers I. Evaluation of differentiated human bronchial epithelial cell culture systems for asthma research. J Allergy (Cairo) 2012;2012:943982. doi: 10.1155/2012/943982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrhardt C, Kneuer C, Bies C, Lehr CM, Kim KJ, Bakowsky U. Salbutamol is actively absorbed across human bronchial epithelial cell layers. Pulm Pharmacol Ther. 2005;18:165–70. doi: 10.1016/j.pupt.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Cardellini P, Davanzo G, Citi S. Tight junctions in early amphibian development: detection of junctional cingulin from the 2-cell stage and its localization at the boundary of distinct membrane domains in dividing blastomeres in low calcium. Dev Dyn. 1996;207:104–13. doi: 10.1002/(SICI)1097-0177(199609)207:1<104::AID-AJA10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]