Abstract
One of the hallmarks of Alzheimer disease is the pathological aggregation of tau protein into paired helical filaments (PHFs) and neurofibrillary tangles. Here we describe the in vitro assembly of recombinant tau protein and constructs derived from it into PHFs. Though whole tau assembled poorly, constructs containing three internal repeats (corresponding to the fetal tau isoform) formed PHFs reproducibly. This ability depended on intermolecular disulfide bridges formed by the single Cys-322. Blocking the SH group, mutating Cys for Ala, or keeping tau in a reducing environment all inhibited assembly. With constructs derived from four-repeat tau (having the additional repeat no. 2 and a second Cys-291), PHF assembly was blocked because Cys-291 and Cys-322 interact within the molecule. PHF assembly was enabled again by mutating Cys-291 for Ala. The synthetic PHFs bound the dye thioflavin S used in Alzheimer disease diagnostics. The data imply that the redox potential in the neuron is crucial for PHF assembly, independently or in addition to pathological phosphorylation reactions.
Full text
PDF

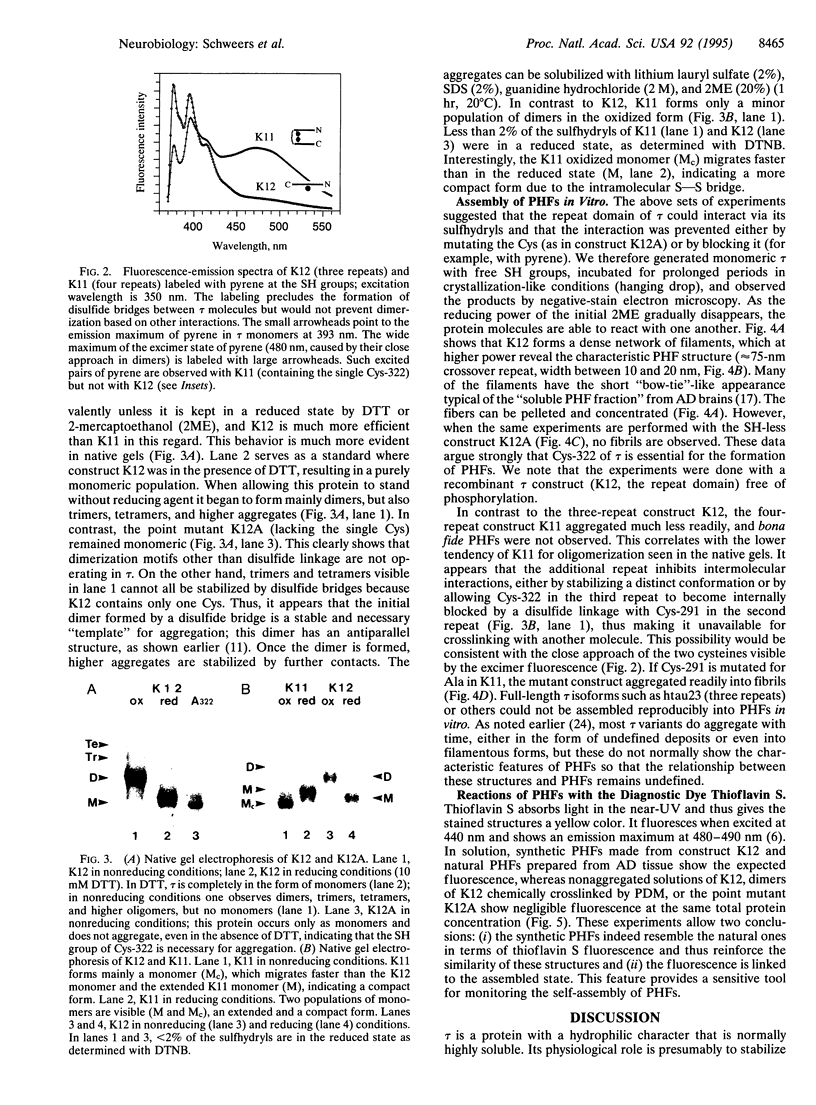
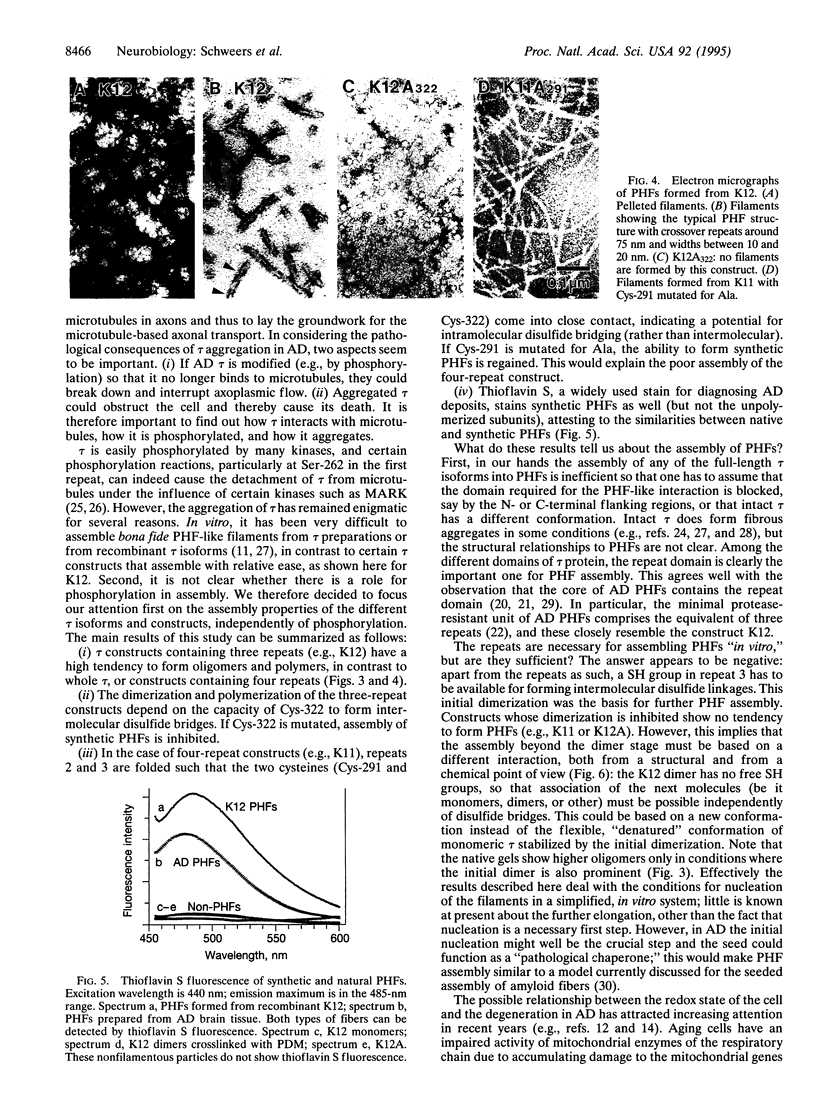

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H. Expression and processing of pathologic proteins in Alzheimer's disease. Hippocampus. 1993;3(Spec No):227–237. [PubMed] [Google Scholar]
- Behl C., Davis J. B., Lesley R., Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994 Jun 17;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Biernat J., Gustke N., Drewes G., Mandelkow E. M., Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993 Jul;11(1):153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- Biernat J., Mandelkow E. M., Schröter C., Lichtenberg-Kraag B., Steiner B., Berling B., Meyer H., Mercken M., Vandermeeren A., Goedert M. The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J. 1992 Apr;11(4):1593–1597. doi: 10.1002/j.1460-2075.1992.tb05204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R. A., Olesen O. F., Smith M. J., Jakes R., Goedert M. Assembly of Alzheimer-like filaments from full-length tau protein. FEBS Lett. 1994 Jan 10;337(2):135–138. doi: 10.1016/0014-5793(94)80260-2. [DOI] [PubMed] [Google Scholar]
- Drewes G., Trinczek B., Illenberger S., Biernat J., Schmitt-Ulms G., Meyer H. E., Mandelkow E. M., Mandelkow E. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem. 1995 Mar 31;270(13):7679–7688. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Eanes E. D., Page D. L. The relation of the properties of Congo red-stained amyloid fibrils to the -conformation. J Histochem Cytochem. 1972 Oct;20(10):821–826. doi: 10.1177/20.10.821. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann R. P., Erickson A. C., Johnson G. V. Tau self-association: stabilization with a chemical cross-linker and modulation by phosphorylation and oxidation state. J Neurochem. 1995 Mar;64(3):1209–1215. doi: 10.1046/j.1471-4159.1995.64031209.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Morishima-Kawashima M., Takio K., Suzuki M., Titani K., Ihara Y. Protein sequence and mass spectrometric analyses of tau in the Alzheimer's disease brain. J Biol Chem. 1992 Aug 25;267(24):17047–17054. [PubMed] [Google Scholar]
- Hyman B. T., Van Hoesen G. W., Beyreuther K., Masters C. L. A4 amyloid protein immunoreactivity is present in Alzheimer's disease neurofibrillary tangles. Neurosci Lett. 1989 Jul 3;101(3):352–355. doi: 10.1016/0304-3940(89)90559-4. [DOI] [PubMed] [Google Scholar]
- Inouye H., Fraser P. E., Kirschner D. A. Structure of beta-crystallite assemblies formed by Alzheimer beta-amyloid protein analogues: analysis by x-ray diffraction. Biophys J. 1993 Feb;64(2):502–519. doi: 10.1016/S0006-3495(93)81393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Kirschner D. A., Abraham C., Selkoe D. J. X-ray diffraction from intraneuronal paired helical filaments and extraneuronal amyloid fibers in Alzheimer disease indicates cross-beta conformation. Proc Natl Acad Sci U S A. 1986 Jan;83(2):503–507. doi: 10.1073/pnas.83.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo J., Honda T., Mori H., Hamada Y., Miura R., Ogawara M., Ihara Y. The carboxyl third of tau is tightly bound to paired helical filaments. Neuron. 1988 Nov;1(9):827–834. doi: 10.1016/0896-6273(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114(1):33–38. [PubMed] [Google Scholar]
- Ksiezak-Reding H., Yen S. H. Structural stability of paired helical filaments requires microtubule-binding domains of tau: a model for self-association. Neuron. 1991 May;6(5):717–728. doi: 10.1016/0896-6273(91)90169-z. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993 Mar;2(3):404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg-Kraag B., Mandelkow E. M. Isoforms of tau protein from mammalian brain and avian erythrocytes: structure, self-assembly, and elasticity. J Struct Biol. 1990 Oct-Dec;105(1-3):46–53. doi: 10.1016/1047-8477(90)90097-v. [DOI] [PubMed] [Google Scholar]
- Litersky J. M., Johnson G. V. Phosphorylation by cAMP-dependent protein kinase inhibits the degradation of tau by calpain. J Biol Chem. 1992 Jan 25;267(3):1563–1568. [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E. Tau as a marker for Alzheimer's disease. Trends Biochem Sci. 1993 Dec;18(12):480–483. doi: 10.1016/0968-0004(93)90011-b. [DOI] [PubMed] [Google Scholar]
- Montejo de Garcini E., Carrascosa J. L., Nieto A., Avila J. Collagenous structures present in brain contain epitopes shared by collagen and microtubule-associated protein tau. J Struct Biol. 1990 Mar;103(1):34–39. doi: 10.1016/1047-8477(90)90083-o. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Titani K., Ihara Y. Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments. Neuron. 1993 Jun;10(6):1151–1160. doi: 10.1016/0896-6273(93)90063-w. [DOI] [PubMed] [Google Scholar]
- Novak M., Kabat J., Wischik C. M. Molecular characterization of the minimal protease resistant tau unit of the Alzheimer's disease paired helical filament. EMBO J. 1993 Jan;12(1):365–370. doi: 10.1002/j.1460-2075.1993.tb05665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweers O., Schönbrunn-Hanebeck E., Marx A., Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J Biol Chem. 1994 Sep 30;269(39):24290–24297. [PubMed] [Google Scholar]
- Shearman M. S., Ragan C. I., Iversen L. L. Inhibition of PC12 cell redox activity is a specific, early indicator of the mechanism of beta-amyloid-mediated cell death. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1470–1474. doi: 10.1073/pnas.91.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga M. K., Hagen T. M., Ames B. N. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Carney J. M., Starke-Reed P. E., Oliver C. N., Stadtman E. R., Floyd R. A., Markesbery W. R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J., Weisgraber K. H., Goedert M., Saunders A. M., Huang D., Corder E. H., Dong L. M., Jakes R., Alberts M. J., Gilbert J. R. Hypothesis: microtubule instability and paired helical filament formation in the Alzheimer disease brain are related to apolipoprotein E genotype. Exp Neurol. 1994 Feb;125(2):163–174. doi: 10.1006/exnr.1994.1019. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Lee V. M. Paired helical filament tau in Alzheimer's disease. The kinase connection. Am J Pathol. 1994 Mar;144(3):449–453. [PMC free article] [PubMed] [Google Scholar]
- Wille H., Drewes G., Biernat J., Mandelkow E. M., Mandelkow E. Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol. 1992 Aug;118(3):573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski H. M., Wen G. Y., Kim K. S. Comparison of four staining methods on the detection of neuritic plaques. Acta Neuropathol. 1989;78(1):22–27. doi: 10.1007/BF00687398. [DOI] [PubMed] [Google Scholar]
- Wolvetang E. J., Johnson K. L., Krauer K., Ralph S. J., Linnane A. W. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994 Feb 14;339(1-2):40–44. doi: 10.1016/0014-5793(94)80380-3. [DOI] [PubMed] [Google Scholar]




