Abstract
Objective
To determine whether patients with metastatic non-clear-cell renal cell carcinoma (RCC) benefit from cytoreductive nephrectomy (CN).
Patients and Methods
We used the Surveillance, Epidemiology, and End Results (SEER) programme to identify a population-based sample of 4914 patients diagnosed with metastatic RCC between 2000 and 2009.
Of the 4914 patients, 591 had non-clear-cell histology.
The median follow-up was 20 months.
The primary outcome measure was RCC-specific mortality.
Results
Approximately 64% of patients underwent CN.
Patients with non-clear-cell histology who underwent CN had lower RCC-specific and all-cause mortality than those who did not (P < 0.001 in both cases).
After adjustment for age, gender, race, marital status, year of diagnosis, geographical location and histology, the associations between CN and lower RCC-specific mortality (hazard ratio [HR] 0.62, 95% confidence interval [CI] 0.48–0.80, P < 0.001) and between CN and all-cause mortality (HR 0.45, 95% CI 0.37–0.55, P < 0.001) remained highly significant.
Among patients diagnosed between 2006 and 2009 (targeted therapy era), the results remained unchanged (HR 0.50, 95% CI 0.34–0.72, P < 0.001 and HR 0.43, 95% CI 0.31–0.59, P < 0.001, respectively).
An interaction model found lower all-cause mortality for all histologies after CN.
Conclusions
Patients from the SEER programme with metastatic non-clear-cell RCC, including those treated in the targeted therapy era, appear to derive a survival benefit from CN, an association which remained significant regardless of histological subtype.
This observation suggests that CN should remain standard in patients with advanced RCC who are deemed to be surgical candidates.
Keywords: clear cell, cytoreductive nephrectomy, mortality, non-clear-cell, renal cell carcinoma, survival
Introduction
It is estimated that 65 000 new cases of kidney cancer were diagnosed in 2012 in the United States, with 13 500 associated deaths [1]. Although the incidence of localized, asymptomatic renal cancers has increased in recent years, largely as a result of the increased use of cross-sectional abdominal imaging, about one third of patients still present with metastatic RCC [2]. An enhanced understanding of the underlying pathogenic mechanism in RCC formation has led to the development of targeted therapies against vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR), which have gained widespread use in recent years [3,4].
In patients with metastatic RCC, the role of cytoreductive nephrectomy (CN) is well established [5–7]; however, it is important to note that RCC is a heterogeneous disease with different histological and genetic subclassifications. While clear cell is the most common subtype, non-clear-cell variants account for ~15% of cases [2]. Notably, the two randomized trials that have shown the benefit of CN in patients with metastatic RCC were conducted in the pre-targeted therapy era and the results were not stratified by histological subtype [5,6]. Retrospective studies investigating CN outcomes in patients with metastatic RCC of non-clear-cell histology have generally been small and underpowered [8,9]. Consequently, the question of whether CN confers a survival benefit among patients with non-clear-cell histology remains unresolved. In addition, the issue of whether a benefit from CN exists among patients treated in the targeted therapy era also remains unclear, despite some positive evidence from retrospective studies and subgroup analysis of large clinical trials [4,8,10].
We used the Surveillance, Epidemiology, and End Results (SEER) database [11] to determine whether CN was associated with improved RCC-specific mortality and overall survival in patients with non-clear-cell, metastatic RCC and also examined this potential association in patients treated in the targeted therapy era.
Patients and Methods
Patient Population and Study Design
We used the SEER database to identify 5505 patients diagnosed between 2000 and 2009 with metastatic RCC (including 611 patients with non-clear-cell histology) for whom data relating to the presence or absence of CN were available. Sponsored by the National Cancer Institute, the SEER programme captures ~97% of incident cancers and covers ~26% of the US population [11]. Registries report information on age, date of diagnosis, demographics, tumour characteristics, extent of disease, surgical treatment, radiation therapy, overall survival and RCC-specific survival. All available registries were used in our study. Our cohort included patients with the following histologies: papillary, RCC (chromophobe type), and medullary/collecting duct carcinoma (patients with clear-cell histology were included as a reference group, as discussed below). Patients with sarcomatoid histology were excluded from our study. The following surgeries were deemed to qualify as CN: complete/total/simple nephrectomy, radical nephrectomy, partial/subtotal nephrectomy, any nephrectomy in combination with resection of other organs, and nephrectomy not otherwise specified; these procedures were performed at the time of or after the diagnosis of metastatic cancer. No patient in this study underwent nephrectomy before the development of metastatic disease. Of patients undergoing CN, 3.9% underwent a partial or subtotal nephrectomy and the remaining 96.1% underwent a complete, total, simple or radical nephrectomy. Notably, 61.0% of patients undergoing CN underwent surgical evaluation of the regional lymph nodes. Patients were excluded from the analysis if they had missing information regarding age at diagnosis, race or marital status, leaving 4914 patients (of whom 591 patients had non-clear-cell histology) eligible for analysis for the endpoint of overall survival. Of these, 2994 patients (61%) underwent CN and 1920 patients (39%) did not.
Statistical Analysis
Baseline patient characteristics were compared using the t-test for normally distributed continuous variables, a Wilcoxon test for non-normally distributed continuous variables, and the chi-squared test for categorical variables. Death from RCC, the primary endpoint of the study, was shown in patients who did and did not undergo CN using cumulative incidence curves, which were subsequently compared using Gray’s test [12]. Fine and Gray’s competing risks regression analysis [13] was used to assess the impact of CN on RCC-specific mortality after adjustment for the following covariates: age, gender, marital status, race, histology, year of diagnosis, geographical location and use of CN. A competing risks analysis was performed for the endpoint of RCC-specific mortality, given that deaths from other causes preclude patients from dying from RCC, and such patients should be distinguished from those who remain alive but are censored [14]. For the analysis of overall survival (the secondary endpoint) between patients who did and did not undergo CN, Kaplan–Meier curves were generated and were compared using the log-rank test. A multivariable Cox proportional hazards regression analysis was performed using the same covariates as those in the competing risks analysis. To determine the effect of CN in the targeted therapy era, the analysis was repeated in the subset of patients diagnosed between 2006 and 2009. In addition, to examine the relative effect of CN on each histology, an interaction model was generated between histology and use of CN (using patients with clear-cell histology as a reference). The year of diagnosis was categorized as 2000–2005 (before the targeted therapy era), or 2006–2009 (targeted therapy era). For the purposes of the competing risks analysis, 668 patients with an unknown cause of death were excluded, leaving 4246 patients eligible for analysis (including 487 patients with non-clear-cell histology). Of these 4246 patients, 2632 patients (62%) underwent CN and 1614 (38%) did not. The median follow-up in surviving patients was 20 months. All P values were two-sided and a threshold of 0.05 was considered to indicate statistical significance. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). Competing risks regression was performed using R version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria). The study was approved by the institutional review board at our institution; a waiver for informed consent was obtained.
Results
Patient Characteristics
Demographic and clinical characteristics, as stratified by the use of CN vs non-use of CN, are shown in Table 1. Patients who underwent CN were younger than those who did not and were more likely to be male and married. In addition, significant differences in race, histology and geographical location were seen between the two cohorts but no differences in year of diagnosis were noted. In comparing patients who had clear-cell histology with those with non-clear-cell histology, significant differences in unidimensional tumour size were noted: median (inter-quartile range) 8.5 (6.0–11.0) and 7.8 (5.0–12.0) cm, respectively (P = 0.03). Of patients undergoing CN who had lymph node evaluation, 45.3% were found to have positive nodes.
Table 1.
Baseline demographic and clinical characteristics.
| Characteristic | No CN group, N = 1998 | CN group, N = 3057 | P |
|---|---|---|---|
| Mean (SD) age, years, | 66 (12) | 60 (11) | <0.001 |
| Gender, n (%) | 0.02 | ||
| Male | 1319 (66) | 2117 (69) | |
| Female | 679 (34) | 940 (31) | |
| Race, n (%) | 0.002 | ||
| White | 1410 (71) | 2244 (73) | |
| Black | 201 (10) | 231 (8) | |
| Hispanic | 270 (14) | 391 (13) | |
| Asian | 88 (4) | 165 (5) | |
| Native American | 27 (1) | 23 (1) | |
| Unknown | 2 (0) | 3 (0) | |
| Marital status, n (%) | <0.001 | ||
| Unmarried | 805 (40) | 923 (30) | |
| Married | 1117 (56) | 2073 (68) | |
| Unknown | 76 (4) | 61 (2) | |
| Histology, n (%) | 0.01 | ||
| Clear-cell | 1775 (89) | 2669 (87) | |
| Papillary | 162 (8) | 236 (8) | |
| RCC, chromophobe type | 29 (1) | 71 (2) | |
| Collecting duct/medullary | 32 (2) | 81 (3) | |
| Year of diagnosis, n (%) | 0.11 | ||
| 2000–2005 | 984 (49) | 1575 (52) | |
| 2006–2009 | 1014 (51) | 1482 (48) | |
| Geographic location, n (%) | <0.001 | ||
| West | 1037 (52) | 1780 (58) | |
| Midwest | 309 (15) | 384 (13) | |
| Southeast | 380 (19) | 533 (17) | |
| Northeast | 272 (14) | 360 (12) |
Percentages may not add up to 100 because values have been rounded.
Primary Endpoint: Cancer-Specific Survival
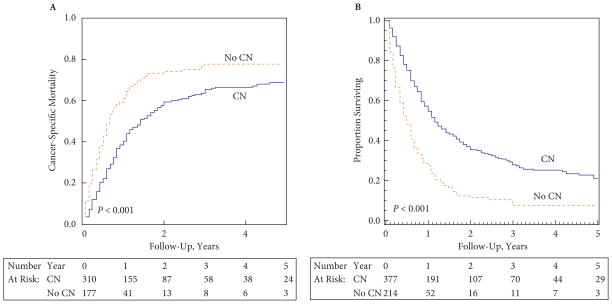
Cumulative incidence estimates of RCC-specific mortality in patients with non-clear-cell histology, as stratified by use of CN vs non-use of CN, are shown in Fig. 1A. Patients undergoing CN had a lower likelihood of RCC-specific mortality (Gray’s test P < 0.001). The 2-year estimates of RCC-specific mortality in patients with non-clear-cell histology who did and did not undergo CN were 59.2% (95% CI 53.1–64.8%) and 74.2% (95% CI 66.4–80.4%), respectively, P < 0.001. Respective estimates in the clear-cell population were 48.7% (95% CI 46.5–50.9%) and 74.3% (95% CI 71.9–76.7%), P < 0.001. Among patients who did vs those who did not undergo CN, the 2-year estimates of non-RCC mortality in the non-clear-cell and clear-cell cohorts were 5.6% (95% CI 3.4–8.8%) vs 14.3% (95% CI 9.5–20.0%) and 5.8% (95% CI 4.8–6.8%) vs 11.8% (95% CI 10.2–13.7%), respectively (P = 0.006 in the non-clear-cell and P < 0.001 in the clear-cell cohort, respectively). Among patients with non-clear-cell histology, after adjustment for age at diagnosis, gender, race, marital status, year of diagnosis, geographical location and histology, Fine and Gray’s regression analysis showed that patients who underwent CN had lower estimates of RCC-specific mortality (hazard ratio [HR] 0.62, 95% CI 0.48–0.80, P < 0.001). In the targeted therapy era (2006–2009), the association between CN and RCC-specific mortality in patients with non-clear-cell histology remained significant (HR 0.50, 95% CI 0.34–0.72, P < 0.001).
Fig. 1.
(A) Cumulative incidences of RCC-specific mortality and (B) Kaplan–Meier estimates of overall survival in patients with non-clear-cell RCC, as stratified by the use of CN. Dashed line: no CN; solid line: CN.
Secondary Endpoint: Overall Survival
Kaplan–Meier estimates of overall survival in patients with non-clear-cell histology who did and did not undergo CN are shown in Fig. 1B. Patients who underwent CN had greater estimates of overall survival than those who did not (median survival 14 vs 6 months, respectively, log-rank P < 0.001). The 2-year overall survival estimates in patients with non-clear-cell histology who did and did not undergo CN were 35.6% (95% CI 30.4–40.7%) and 11.6% (95% CI 7.3–17.0%), respectively. On multivariable Cox proportional hazards regression analysis among patients with non-clear-cell histology after adjustment for age at diagnosis, gender, race, marital status, year of diagnosis, geographical location and histology, the use of CN was associated with a significant improvement in overall survival (HR 0.45, 95% CI 0.37–0.55, P < 0.001). Similarly, when the data were limited to patients treated in the targeted therapy era, the association between CN and overall survival in patients with non-clear-cell histology remained highly significant (HR 0.43, 95% CI 0.31–0.59, P < 0.001, respectively).
Impact of CN on Survival in Specific Non-Clear-Cell Histologies
RCC-specific mortality
A multivariable Fine and Gray’s regression model incorporating the interaction terms histology and use of CN is shown in Table 2. In patients with all histologies, the use of of CN was associated with lower estimates of RCC-specific mortality, although in medullary/collecting duct carcinoma the P value for this association did not reach significance (P = 0.05). Relative to patients with clear-cell histology (reference group), there was no significant difference in the effect of CN in patients with chromophobe or collecting duct/medullary histology, suggesting that patients with either of these types of non-clear-cell RCC derive similar benefit to that derived by those with clear-cell histology. Patients with papillary histology still had lower RCC-specific mortality when treated with CN, but the magnitude of this benefit was not as large as that seen in patients with clear-cell histology (HR 0.71 vs 0.48, P interaction = 0.01).
Table 2.
Fine and Gray’s multivariable competing risks regression for RCC-specific mortality, with the interaction terms histology and use of CN.
| Variable | HR (95% CI) | P | ||
|---|---|---|---|---|
| Age, per year increase | 0.998 (0.995–1.002) | 0.34 | ||
| Gender | ||||
| Male | Reference | – | ||
| Female | 1.15 (1.06–1.25) | <0.001 | ||
| Race | ||||
| Caucasian | Reference | – | ||
| African-American | 0.94 (0.81–1.09) | 0.37 | ||
| Hispanic | 0.93 (0.82–1.05) | 0.25 | ||
| Asian-American | 0.77 (0.64–0.92) | 0.004 | ||
| Native American | 1.04 (0.72–1.50) | 0.83 | ||
| Marital status | ||||
| Unmarried | Reference | |||
| Married | 0.97 (0.89–1.05) | 0.41 | ||
| Year of diagnosis | ||||
| 2000–2005 | Reference | – | ||
| 2006–2009 | 0.82 (0.76–0.89) | <0.001 | ||
| Geographical location | ||||
| West | Reference | – | ||
| Midwest | 1.01 (0.99–1.13) | 0.85 | ||
| Southeast | 0.96 (0.86–1.07) | 0.49 | ||
| Northeast | 0.91 (0.80–1.02) | 0.10 | ||
|
| ||||
| CN | Relative effect of CN on RCC- specific mortality* | P-interaction | ||
|
| ||||
| Among patients with clear-cell histology | 0.48 (0.44–0.53) | <0.001 | Ref | – |
| Among patients with chromophobe histology | 0.32 (0.16–0.65) | 0.002 | 0.66 (0.32–1.34) | 0.25 |
| Among patients with collecting duct/medullary histology | 0.52 (0.27–1.01) | 0.05 | 1.08 (0.55–2.10) | 0.83 |
| Among patients with papillary histology† | 0.71 (0.53–0.96) | 0.02 | 1.47 (1.09–2.00) | 0.01 |
Values below represent effect of CN on RCC-specific mortality among individual non-clear-cell histologies relative to the reference of clear cell histology.
Interaction analyses example: results for patients with papillary histology can be interpreted as follows. Patients with papillary features have lower RCC-specific mortality when treated with CN (HR 0.71, 95% CI 0.53–0.96, P = 0.02). This association was not as great as in patients with clear-cell histology, as the HR for patients with papillary histology treated with CN is of significantly less magnitude than that seen in patients with clear-cell histology who were treated with CN. The HR presented in the second column from the right (HR = 1.47) can be thought of as the quotient of the HR for the effect of CN on patients with papillary (0.71) and clear-cell (0.48) histologies, respectively, with associated 95% CI and P value (HR = 1.47, 95% CI 1.09–2.00, P = 0.01).
All-cause mortality
On multivariable Cox proportional hazards modelling, incorporating the interaction terms histology and the use of CN (Table 3), patients with all histologies had lower estimates of all-cause mortality after undergoing CN. Relative to patients with clear-cell histology, there was no significant difference in the effect of CN in patients with collecting duct/medullary histology, although patients with chromophobe histology did appear to derive more benefit from CN than did those with clear-cell histology (HR 0.17 vs 0.40, P interaction = 0.002). Patients with papillary histology were found to have an overall survival benefit when treated with CN, although, again, the magnitude of this benefit was not as large as in patients with clear-cell histology (HR 0.53 vs 0.40, P interaction = 0.02).
Table 3.
Cox multivariable proportional hazards regression for all-cause mortality, with the interaction terms histology and use of CN.
| Variable | HR (95% CI) | P | ||
|---|---|---|---|---|
| Age, per year increase | 1.005 (1.002–1.008) | 0.002 | ||
| Gender | ||||
| Male | Reference | – | ||
| Female | 1.06 (0.99–1.14) | 0.11 | ||
| Race | ||||
| Caucasian | Reference | – | ||
| African American | 1.09 (0.96–1.23) | 0.19 | ||
| Hispanic | 1.03 (0.92–1.14) | 0.62 | ||
| Asian American | 0.78 (0.66–0.92) | 0.003 | ||
| Native American | 0.80 (0.57–1.11) | 0.18 | ||
| Marital status | ||||
| Unmarried | Reference | – | ||
| Married | 0.87 (0.81–0.93) | <0.001 | ||
| Year of diagnosis | ||||
| 2000–2005 | Reference | – | ||
| 2006–2009 | 0.89 (0.83–0.96) | 0.002 | ||
| Geographical location | ||||
| West | Reference | – | ||
| Midwest | 0.97 (0.88–1.08) | 0.58 | ||
| Southeast | 0.98 (0.90–1.08) | 0.74 | ||
| Northeast | 0.88 (0.79–0.98) | 0.02 | ||
|
| ||||
| CN | Relative effect of CN on all-cause mortality* | P-interaction | ||
|
| ||||
| Among patients with clear cell histology | 0.40 (0.37–0.43) | <0.001 | Ref | – |
| Among patients with chromophobe histology | 0.17 (0.10–0.29) | <0.001 | 0.44 (0.26–0.73) | 0.002 |
| Among patients with collecting duct/medullary histology | 0.44 (0.28–0.68) | <0.001 | 1.09 (0.70–1.70) | 0.69 |
| Among patients with papillary histology | 0.53 (0.42–0.67) | <0.001 | 1.33 (1.04–1.70) | 0.02 |
Values below represent effect of CN on all-cause mortality among individual non-clear cell histologies relative to the reference of clear cell histology.
CI, Confidence interval; HR, Hazard Ratio; RCC, Renal Cell Carcinoma; Ref, Reference.
Discussion
In this study using the SEER database, we found that CN was associated with lower RCC-specific mortality and better overall survival in patients with metastatic, non-clear-cell RCC. Given that patients in all the histological subtype groups benefitted from CN, our findings suggest that histology alone should not preclude a patient from undergoing CN; rather the decision for an individual patient to undergo this intervention should depend on factors such as surgical candidacy, comorbid conditions and performance status. In addition, our study suggests that biopsy before CN may not be of significant value in patients whose histology is unknown if CN is planned. One exception could be patients with central renal pelvic tumours suspicious for TCC.
The present study indicates that CN is associated with reductions in RCC-specific and all-cause mortality of ~38% and 55% among patients with non-clear-cell histology. The HR for overall survival among patients with non-clear-cell histology in this study (HR 0.45) was similar to that found in a randomized trial evaluating the benefit of CN in patients of all histologies (HR 0.54) [6]. Yet, despite the proven benefit of CN [5,6], our study indicates that only 64% of patients undergo this potentially life-extending operation, suggesting that CN remains underused. The use of CN does, however, appear to be increasing with time; a SEER-based study which included patients diagnosed between 1989 and 2004 found that upfront CN was performed in only 31% of patients [15]. It is important to note, however, that the SEER database only recorded cancer-directed treatments within the first 4 and 12 months of diagnosis through 1998 and after 1998, respectively [16].
The findings of the present study are relatively novel, as published manuscripts that specifically address the role of CN in patients with non-clear-cell RCC are lacking (Table 4). Notably, however, two recently published abstracts have examined this issue. In a study by Williams et al. [17] in a population-based cohort of patients with metastatic RCC, CN was associated with better RCC-specific survival in patients with both clear-cell and non-clear-cell histologies, although specific HRs for these associations and a quantitative comparison of the differential effect of CN on each histology was not available in that study. Conversely, in an abstract published by Kenney et al. [18] in which 90 patients with non-clear-cell histologies from a single institution were examined, CN was not found to be associated with improved overall survival (P = 0.62). Notably, neither study has been published in manuscript form.
Table 4.
Summary of previous studies on CN and non-clear-cell RCC.
| First author | Year published | N | Histology | Population/Study type | Comparison | Main findings/features |
|---|---|---|---|---|---|---|
| Flanigan [5,7] | 2001 | 241 | CC/NCC | RCT | CN + IFN vs IFN alone | Addition of CN to IFN improved OS (median OS: 8 vs 11 months, P = 0.05) No analysis of histology |
| Mickisch [6] | 2001 | 85 | CC | RCT | CN + IFN vs IFN alone | Addition of CN to IFN improved OS (HR 0.54, 95% CI 0.31–0.94) No analysis of histology |
| Kassouf [9] | 2007 | 606 | CC/NCC | Single institution | CC vs NCC (all had CN) | On MVA, disease-specific survival worse in patients with NCC vs CC (10 vs 20 months, respectively, P < 0.001) No assessment of CN vs no CN possible |
| Shuch [26] | 2009 | 417 | CC/NCC | Single institution | Sarcomatoid vs non-sarcomatoid (all had CN) | Median survival in sarcomatoid vs non-sarcomatoid groups 4.9 vs 17.7 months (P < 0.001; HR (on MVA) 1.88 (P < 0.001) No assessment of CN vs no CN possible |
| Zini [27] | 2009 | 5 372 | CC/NCC | Population-based | CN vs no CN | Patients not undergoing CN had increased risk of all-cause mortality (HR 2.5, P < 0.001) No analysis by histology |
| Choueiri [8] | 2011 | 314 | CC/NCC | Multicentre | CN vs no CN (all had anti-VEGF therapy) | On MVA, CN associated with improved OS (HR 0.68, P = 0.04) |
| You [28] | 2011 | 78 | CC | Single institution | CN vs no CN (all received targeted therapy) | No significant improvement in OS (median OS 22 vs 14 months, respectively, P = 0.13) Patients with sarcomatoid features had poorer OS on MVA (HR 2.7, P = 0.02) |
| Aben [29] | 2011 | 328 | CC/NCC | Population-based | CN vs no CN | On MVA using a propensity score, CN associated with improved overall survival (HR 0.52, 95% CI 0.37–0.73) No analysis by histology |
| Williams [17] | 2012 (Abstract) | 27 415 | CC/NCC | Population-based | CN vs no CN | Improved OS in clear-cell and NCC histology with CN (no quantitative comparison of effect of CN on different histologies) |
| Kenney [18] | 2012 (Abstract) | 90 | NCC | Single institution | CN vs no CN | CN not associated with improved OS (P = 0.62) |
CC, clear-cell; IFN, interferon; MVA, multivariable analysis; NCC, non-clear-cell; OS, overall survival; RCT, randomized controlled trial.
We found that the magnitude of the RCC-specific and overall survival benefit associated with CN may be smaller in patients with papillary histology. Although other investigators have found that patients with papillary histology and metastatic disease may have poor survival [19], papillary histology is generally not thought of as an adverse prognostic factor (although patients with type II papillary carcinoma may have more aggressive disease than those with type I) [20]. The benefit that CN provides this population has not been adequately evaluated in previous studies. Our finding that patients with papillary histology may not benefit from CN to the same degree as those with clear-cell histology should not deter patients with papillary histology from pursuing CN, given that an RCC-specific and all-cause mortality benefit still exists in this population.
Targeted therapies with VEGF and mTOR inhibitors have been mostly studied in clear-cell RCC, where they have been shown to be effective [3,4,21]; however, when administered to patients with non-clear-cell histology, the effects appear to be of lesser magnitude [22]. Given the relatively limited efficacy of targeted therapies in patients with non-clear-cell histology, CN may play an important role in decreasing tumour burden in such patients, and our results substantiate the use of CN in this population. Consequently, we conclude that CN should remain a standard option for patients with non-clear-cell histology managed in the targeted therapy era [8].
Several limitations of our study deserve mention; most relate to the limitations of SEER data. First, data relating to the burden of metastatic disease are not available in the SEER database, nor are laboratory values associated with prognosis in patients with metastatic RCC [23]. Second, the SEER database does not record data relating to performance status or comorbidity, factors known to affect outcomes in patients with cancer and influence the decision for a surgical procedure [24]; therefore, it is possible that selection bias, whereby healthier patients opted for CN, may be in part responsible for the main findings of our study. We were able to mitigate the influence of this potential bias partially by using a competing risks analysis for the main endpoint of our study, RCC-specific survival. Third, data relating to systemic therapy are largely not available in the SEER database. Given the rapid adoption of targeted therapies after the publication of randomized studies showing a benefit from targeted agents in early 2006 [3,4,25], we accounted for the impact of targeted therapy by including the year of diagnosis (2000–2005 vs 2006–2009) as a covariate in our multivariable model, and by performing a separate analysis of patients treated between 2006 and 2009. In addition, given that patients with non-clear-cell RCC who underwent CN were healthier than those who did not (as evidenced by the lower non-RCC mortality in the former population), it is possible that patients treated with CN were more likely to receive systemic therapy, regardless of the year of diagnosis; however, it is unlikely such a difference could have accounted for the benefit of CN with regard to RCC-specific mortality, given that both RCC-specific mortality and non-RCC specific mortality were also lower in the CN arm among patients with clear-cell histology, a population for whom CN has been definitively shown via randomized trials to confer a survival advantage [5,6]. Our results suggest that the same could be true in patients with non-clear-cell histology; i.e. patients undergoing CN may be healthier and treated more aggressively than those who do not undergo CN, but CN itself does appear to exert an RCC-specific survival benefit as well. Finally, a detailed review of the histology by expert genitourinary pathologists or a unified central pathology review was not possible because of the limitations of the database.
In conclusion, our findings suggest that patients from the SEER programme with metastatic non-clear-cell RCC appear to derive a survival benefit from CN. Additional studies on this subject, including verification of these findings in an independent dataset with baseline information regarding performance status and disease burden, are warranted.
Abbreviations
- CN
cytoreductive nephrectomy
- HR
hazard ratio
- SEER
Surveillance, Epidemiology, and End Results
- VEGF
vascular endothelial growth factor
- mTOR
mammalian target of rapamycin
Footnotes
Conflict of Interest
There was no external financial support for this study. T.C. is a Consultant and/or on an Advisory Board for Pfizer Inc., Bayer AG, GlaxoSmithKline and Novartis. P.N. declares research funding from Varian Consulting, Ferring Inc., Astellas Pharma and Medivation. No other conflicts of interest declared.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–32. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 5.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 6.Mickisch GHJ, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–70. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 7.Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071–6. doi: 10.1097/01.ju.0000110610.61545.ae. [DOI] [PubMed] [Google Scholar]
- 8.Choueiri TK, Xie W, Kollmannsberger C, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011;185:60–6. doi: 10.1016/j.juro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Kassouf W, Sanchez-Ortiz R, Tamboli P, et al. Cytoreductive nephrectomy for metastatic renal cell carcinoma with nonclear cell histology. J Urol. 2007;178:1896–900. doi: 10.1016/j.juro.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Polcari AJ, Gorbonos A, Milner JE, Flanigan RC. The role of cytoreductive nephrectomy in the era of molecular targeted therapy. Int J Urol. 2009;16:227–33. doi: 10.1111/j.1442-2042.2008.02245.x. [DOI] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results (SEER) Program. Research Data (1973–2009) National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; ( www.seer.cancer.gov) released April 2012, based on the November 2011 submission. [Google Scholar]
- 12.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the sub distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 14.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–7. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 15.Jeldres C, Baillargeon-Gagne S, Liberman D, et al. A population-based analysis of the rate of cytoreductive nephrectomy for metastatic renal cell carcinoma in the United States. Urology. 2009;74:837–41. doi: 10.1016/j.urology.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Iezzoni LI, Ngo LH, Li D, Roetzheim RG, Drews RE, McCarthy EP. Early stage breast cancer treatments for younger Medicare beneficiaries with different disabilities. Health Serv Res. 2008;43 (5 Pt 1):1752–67. doi: 10.1111/j.1475-6773.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams S, Hakami AA, Ghavamian R, et al. Outcomes with nephrectomy for metastatic kidney cancer in the era of targeted therapy. J Urol. 2012;187 (4S):e724. [Google Scholar]
- 18.Kenney P, Chapin B, Culp S, et al. Does cytoreductive nephrectomy improve survival in non-clear cell renal cell carcinoma. J Urol. 2012;187 (4S):e727. [Google Scholar]
- 19.Motzer RJ, Bacik J, Mariani T, Russo P, Mazumdar M, Reuter V. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20:2376–81. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 20.Waldert M, Haitel A, Marberger M, et al. Comparison of type I and II papillary renal cell carcinoma (RCC) and clear cell RCC. BJU Int. 2008;102:1381–4. doi: 10.1111/j.1464-410X.2008.07999.x. [DOI] [PubMed] [Google Scholar]
- 21.Schutz FA, Je Y, Richards CJ, Choueiri TK. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J Clin Oncol. 2012;30:871–7. doi: 10.1200/JCO.2011.37.1195. [DOI] [PubMed] [Google Scholar]
- 22.Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26:127–31. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 23.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Filson CP, Redman BG, Dunn RL, Miller DC. Initial patterns of care with oral targeted therapies for patients with renal cell carcinoma. Urology. 2011;77:825–30. e1. doi: 10.1016/j.urology.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shuch B, Said J, La Rochelle JC, et al. Cytoreductive nephrectomy for kidney cancer with sarcomatoid histology–is up-front resection indicated and, if not, is it avoidable? J Urol. 2009;182:2164–71. doi: 10.1016/j.juro.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zini L, Capitanio U, Perrotte P, et al. Population-based assessment of survival after cytoreductive nephrectomy versus no surgery in patients with metastatic renal cell carcinoma. Urology. 2009;73:342–6. doi: 10.1016/j.urology.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 28.You D, Jeong IG, Ahn JH, et al. The value of cytoreductive nephrectomy for metastatic renal cell carcinoma in the era of targeted therapy. J Urol. 2011;185:54–9. doi: 10.1016/j.juro.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Aben KK, Heskamp S, Janssen-Heijnen ML, et al. Better survival in patients with metastasised kidney cancer after nephrectomy: a population-based study in the Netherlands. Eur J Cancer. 2011;47:2023–32. doi: 10.1016/j.ejca.2011.03.002. [DOI] [PubMed] [Google Scholar]