Abstract
Objective
Stressful events enhance risk for weight gain and adiposity. Ghrelin and leptin, two hormones that are implicated in appetite regulation, may link stressful events to weight gain; a number of rodent studies suggest that stressors increase ghrelin production. The present study investigated the links among daily stressors, ghrelin and leptin, and dietary intake in humans.
Method
Women (N = 50) completed three study appointments that were scheduled at least 2 weeks apart. At each visit, women arrived fasting and ate a standardized breakfast and lunch. Blood samples were collected 45 minutes after each meal. Women completed a self-report version of the Daily Inventory of Stressful Events (DISE) at each appointment. Two composites were created from the DISE data, reflecting the number of stressors that did and did not involve interpersonal tension.
Results
Women who experienced more stressors involving interpersonal tension had higher ghrelin and lower leptin levels than those who experienced fewer interpersonal stressors. Furthermore, women who experienced more interpersonal stressors had a diet that was higher in calories, fat, carbohydrates, protein, sugar, sodium, and fiber, and marginally higher in cholesterol, vegetables (but not fruits), vitamin A, and vitamin C. Stressors that did not involve interpersonal tension were unrelated to ghrelin and leptin levels or any of the dietary components examined.
Conclusions
These data suggest that ghrelin and leptin may link daily interpersonal stressors to weight gain and obesity.
Keywords: ghrelin, leptin, eating, obesity, stressful events, interpersonal relationships
Obesity contributes to a host of medical problems, including Type 2 diabetes, cardiovascular disease, and premature mortality, and is thus a major public health concern (Billington et al., 2000). Stressful events enhance risk for weight gain and adiposity (Korkeila et al., 1998; Solomon et al., 2011; Wardle et al., 2011). For example, a recent meta-analysis concluded that people who experienced more stressors gained more body fat over time than those who experienced fewer stressors (Wardle et al., 2011). However, the relationship between stressful events and weight gain is not without controversy; almost three quarters of the studies examined in the meta-analysis reported no relationship between stressful events and weight gain, whereas others demonstrated that people who experience more stressors eat less and lose weight (Torres and Nowson, 2007; Wardle et al., 2011). Accordingly, there may be contextual factors that influence the links among stressful events, eating behavior, and weight gain (see Torres and Nowson, 2007 for a related argument).
One possibility is that interpersonal stressors, such as marital disagreements, are qualitatively different from other types of stressors and thus have differential effects on appetite and eating behavior. The desire for social connection is a strong impetus behind human behavior (Leary and Cox, 2008; Maslow, 1968). The importance of the need to belong is not surprising given the significance of group living for humans’ survival throughout their evolutionary past; humans were most likely to thrive when they were part of a network of people who were invested in their welfare and who looked out for their well-being (Tooby and Cosmides, 1996). Over time, this ultimately led to a fundamental need to form close and caring bonds with other people (Baumeister and Leary, 1995). Because the need for social connection is central to human nature, the failure to fulfill this need should be detrimental to mental and physical health. Moreover, the disruption of social bonds may be a uniquely potent form of stress that affects a person’s appetite and eating behavior. For example, experiencing interpersonal stress should motivate people to attempt to restore their sense of social connection (Pickett and Gardner, 2005). Recent research demonstrated that eating comfort food caused people to spontaneously think about their relationships, and simply thinking about comfort food decreased loneliness (Troisi and Gabriel, 2011). Accordingly, feeling hungry and eating in response to interpersonal stressors may allow people to feel socially reconnected, suggesting a distinctive role for interpersonal stressors in eating behavior and obesity.
Ghrelin and leptin, two hormones that are implicated in appetite regulation, may link interpersonally stressful events to eating behavior and weight gain. Although a person’s eating behavior is multiply determined, ghrelin and leptin provide two internal eating-related signals. Ghrelin, an appetite-stimulating hormone, is one factor that promotes food consumption (Klok et al., 2007). For example, ghrelin reliably rises before a meal and declines after eating (Cummings et al., 2001), and pre-meal rises are related to increased feelings of hunger (Cummings et al., 2004). One innovative study demonstrated that people felt hungrier and consumed more food when they received a ghrelin injection compared with saline (Wren et al., 2001). Leptin, an appetite-suppressing hormone, rises following a meal and suppresses food intake in cooperation with other peptides (Klok et al., 2007). Furthermore, fat cells are a primary source of leptin, which is elevated among overweight people (Considine et al., 1996). Consistent with its satiety-related effects, leptin-deficient medical patients who received leptin treatment were less hungry and lost considerable weight (Licinio et al., 2004).
A number of rodent studies suggest that stressors increase ghrelin and reduce leptin production. For example, a tail pinch stressor elevated ghrelin gene expression in mice (Asakawa et al., 2001). Both chronic social defeat and continuous restraint stress increased plasma ghrelin production among mice (Chuang et al., 2011; Lutter et al., 2008; Ochi et al., 2008). Rats had higher plasma ghrelin levels after a water avoidance stressor compared with pre-stress levels (Kristenssson et al., 2006). Furthermore, mice that experienced chronic social defeat stress and rats that experienced chronic restraint stress had lower leptin levels compared with no-stress controls (Chuang et al., 2010; de Oliveira et al., 2014).
The rodent literature provides a useful framework for beginning to unpack the links among stressors, ghrelin, and leptin. However, humans are an ultra-social species who evolved specific cognitive skills for interpreting the social world (Herrmann et al., 2007; Pagel, 2012). Accordingly, research with human participants provides a way to more clearly delineate the importance of interpersonal versus other types of stressors. Accordingly, the goal of the current study was to fill this gap in the human literature by investigating the links among daily interpersonal and non-interpersonal stressors, ghrelin, and leptin.
The Current Study
We addressed the question of whether women who experienced more stressors involving interpersonal tension would have higher ghrelin and lower leptin levels than women who experienced fewer interpersonal stressors. Furthermore, if interpersonal stressors are related to more ghrelin and less leptin, people experiencing more interpersonal stressors should consume more food. Accordingly, we investigated whether stressors involving interpersonal tension were related to a woman’s typical diet. We also examined whether non-interpersonal stressors predicted ghrelin and leptin levels and dietary intake, allowing us to test whether the effects of stressful events were specific to interpersonal stressors. We focused on non-obese women, because obesity can alter the link between ghrelin and eating behavior (Buss et al., 2014), and obese people often develop cellular leptin resistance (Myers et al., 2010).
In order to examine our hypotheses, we conducted secondary analyses using data from a study about yoga practice and inflammation (Kiecolt-Glaser et al., 2010). Women were assessed on three separate days; we utilized 2 blood samples from each visit, both of which were collected 45 minutes after participants ate a standardized meal.
Method
Participants
Due to the nature of the parent study, we recruited women who had some form of hatha yoga experience through online ads and notices posted in yoga studios. Women were categorized as either novice (n = 25) or expert (n = 25) yoga practitioners; women with an intermediate level of expertise were excluded (Kiecolt-Glaser et al., 2010). Interested participants were ineligible if they had health problems with obvious immunological or endocrinological consequences (e.g., cancer, recent surgeries, diabetes, etc.) or were taking related medications. We also excluded women who currently smoked, used statins, beta blockers, or psychoactive drugs, consumed excessive amounts of alcohol, had a convulsive disorder, or had a BMI ≥ 30.
The final sample (N = 50) was primarily White (n = 44) and their average age was 41.32 (SD = 10.33, range = 30–65). Over half of the sample was married or were in a domestic partnership (n = 29) and all participants had at least some college education. Novice and expert yoga practitioners did not differ in terms of race, age, marital status, and education level. Furthermore, all participants completed all study visits. Additional samples characteristics are listed in Table 1. The project was approved by the Ohio State University Institutional Review Board and all participants provided written informed consent before participating.
Table 1.
Characteristics of the 50 women included in the study analyses of ghrelin, leptin and interpersonal stressors
| Characteristic | Category/Description | Number(%) | Mean(SD) |
|---|---|---|---|
| Race | White | 44(88) | --- |
| Black | 3(6) | --- | |
| Other | 3(4) | --- | |
|
| |||
| Education | High school or below | 0(0) | --- |
| Some college/College graduate | 26(52) | --- | |
| Graduate/Professional training | 24(48) | --- | |
|
| |||
| Marital Status | Single | 13(26) | --- |
| Married/Domestic partner | 29(58) | --- | |
| Separated/divorced/widowed | 8(16) | --- | |
|
| |||
| BMI (categorical) | Normal weight (< 25 kg/m2) | 34(68) | --- |
| Overweight (≥25 kg/m2) | 16(32) | --- | |
|
| |||
| Sleep quality (PSQI – categorical) | Poor sleep quality (< 5) | 13(26.0) | --- |
| Good sleep quality (≥ 5) | 37(74.0) | --- | |
|
| |||
| # interpersonal stressors | 0 reported across all visits | 14(29.2) | --- |
| At least 1 reported at 1 visit | 18(37.5) | --- | |
| At least 1 reported at 2 visits | 10(20.8) | --- | |
| At least 1 reported at 3 visits | 6(12.5) | --- | |
|
| |||
| # non-interpersonal stressors | 0 reported across all visits | 13(27.1) | --- |
| At least 1 reported at 1 visit | 16(33.3) | --- | |
| At least 1 reported at 2 visits | 11(22.9) | --- | |
| At least 1 reported at 3 visits | 8(16.7) | --- | |
|
| |||
| BMI (continuous) | N/A | --- | 23.21(2.77) |
|
| |||
| Sleep quality (PSQI – continuous) | N/A | --- | 4.24(2.18) |
|
| |||
| Age | N/A | --- | 41.32(10.33) |
|
| |||
| Ghrelin levels at T1 (pg/ml) | Averaged across all 3 visits | --- | 1053.06(332.71) |
|
| |||
| Ghrelin levels at T2 (pg/ml) | Averaged across all 3 visits | --- | 1161.89(378.35) |
|
| |||
| Leptin levels at T1 (ng/ml) | Averaged across all 3 visits | --- | 9.25(5.37) |
|
| |||
| Leptin levels at T2 (ng/ml) | Averaged across all 3 visits | --- | 8.99(5.20) |
Note: N = 50, except for number of stressors (N = 48). T1 refers to the blood draw after breakfast whereas T2 is after lunch. Both ghrelin and leptin levels represent untransformed values.
Study Procedure
In this randomized cross-over study, women completed three study appointments that were scheduled at least 2 weeks apart. Each visit corresponded to a different condition that was relevant to the parent study: yoga, movement control, and video control. The order of the three conditions was randomly assigned. Every visit followed the same timeline, differing only in the condition randomized for that visit.
All participants arrived at the Clinical Research Center (CRC), a hospital research unit, at 7:30 a.m. after fasting since the previous evening. At 7:45 a.m., women ate a standardized breakfast. They were allowed to choose between one of two breakfast options that were designed to provide equivalent carbohydrates, protein, fats, and calories across individuals and study visits, and women were asked to eat the entire meal. The meals were designed and supervised by a PhD level bionutritionist who was part of the CRC full-time staff.
After breakfast, each participant had a heparin well placed in one arm for subsequent blood draws. Women rested in a hospital bed for a 20-minute relaxation period and then provided a blood sample 45 minutes after the start of breakfast. At 10:15 a.m., participants completed their assigned movement condition for that session, which took 75 minutes. At 12:20 p.m., women ate a standardized lunch. Similar to the breakfast, they were allowed to choose between different lunch options that were designed be equivalent across participants and study visits, and women were asked to eat the entire meal. The second blood sample was drawn at 1:10 p.m., 50 minutes after the start of lunch.
Movement Conditions
The three conditions used in the parent study were not the focus of current investigation and are thus only briefly summarized (see Kiecolt-Glaser et al., 2010 for details). Iyengar yoga, the form of hatha yoga used in this study, emphasizes safe and comfortable postures based on each person’s body type and needs. A restorative session was selected rather than a vigorous sequence because the poses could be performed by both novice and experienced practitioners.
The first control condition consisted of walking on a treadmill at 0.5 miles per hour. This walking speed best approximated women’s heart rate during the restorative yoga session. The second control condition was a video sequence about physics experiments for a high school classroom, as well as segments from two lectures on polymers and quantum mechanics.
Questionnaires
Towards the beginning of each visit, women completed the Daily Inventory of Stressful Events (DISE; Almeida et al., 2002), a commonly used measure of daily stress with good concurrent validity. Participants completed a self-report version of the DISE, indicating whether certain types of daily stressors had occurred the prior day (Mroczek et al., 2013). Accordingly, the DISE had the potential to tap into both acute and chronic stressors. For example, participants were asked “In the last 24 hours, did anything happen in your workplace or volunteer setting that most people would consider stressful?” and “In the last 24 hours, did anything happen at home that most people would consider stressful?” In both scenarios, a participant could report a stressor that was an ongoing chronic stressor (e.g., one of many arguments over the past few months with a spouse), or one that only happened that day, and thus was relatively more acute in nature. Following prior research, participants were also asked whether the stressor involved an argument or disagreement with another person (Almeida et al., 2002; Mroczek et al., 2013). We created two composites from the DISE, reflecting the total number of stressors that did and did not involve interpersonal tension on the day before each visit (Almeida et al., 2002); both composites were based on stressors that participants self-identified as involving interpersonal tension or not.
To assess women’s typical diet, we administered the Food Frequency Questionnaire (FFQ) at an initial screening visit. Taken from the National Cancer Institute’s Health Habits and History Questionnaire, the FFQ assessed participant’s typical intake of select foods and nutrients (Patterson et al., 1999). The FFQ is widely used among different populations and shows convergent validity with other dietary measures (Subar et al., 2001). Women reported the type, frequency, and quantity of foods and beverages they consumed in the past 90 days. Software from the National Cancer Institute allowed us to calculate dietary intake of key food groups (Kristal et al., 1999).
During their screening visit, women also completed the Pittsburgh Sleep Quality Index, assessing sleep quality over the past month via a combination of subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction (PSQI; Buysse et al., 1989). The PSQI has good internal consistency and can distinguish between people with and without sleep disturbances, indicating acceptable discriminant validity (Buysse et al., 1989; Carpenter and Andrykowski, 1998). At each visit, participants were asked how many hours they slept the night before and how rested they felt when they woke up that morning. The sleep measures provided a way to assess the links among interpersonal stressors, ghrelin, and leptin independent of sleep, which can influence both hormones (Knutson et al., 2007; Taheri et al., 2004).
Endocrine Assays
All blood samples for a participant were collected via a catheter, frozen after collection, and analyzed within the same assay run. Determinations for leptin and total ghrelin were made using their respective RIA kits per kit instructions (Millipore Corporation, St. Charles, MO 63304). For leptin, the intra-assay coefficient of variation (CV) was 4.2% and inter-assay CV was 4.5%; sensitivity was 0.5 ng/ml as previously reported (Kiecolt-Glaser et al., 2012). For total ghrelin, the intra-assay CV was 6.4% and inter-assay CV was 16.3%; sensitivity was .09 ng/ml.
Data Analytic Strategy
The hormone data were moderately skewed. Accordingly, each measure was square root transformed prior to analyses. Linear mixed models were used to account for the correlations within a participant, both within and across conditions. A random subject-specific effect captured the within-subject correlations across conditions (yoga vs. movement vs. video) and also across sample times (after breakfast vs. after lunch). All analyses were conducted in SPSS version 19.0 (IBM, New York).
We investigated whether stressors involving interpersonal tension predicted ghrelin and leptin levels. Specifically, we conducted an analysis with the number of interpersonal stressors at each visit as the predictor and either ghrelin or leptin levels as the outcome. First, we investigated a model with no covariates. Next, we added a series of control variables (described below) and the fixed effects for the 3-way interpersonal stressor by condition by sample time interaction, and all corresponding 2-way interactions. This allowed us to test whether condition or sample time modified the effects of interpersonal stressors. Due to the complexity of the analytic model and our limited sample size, higher order interactions that were not significant or marginally significant were dropped (p values > 0.10). Remaining significant interactions were decomposed using simple slopes tests. We followed the same data analytic strategy using the number of non-interpersonal stressors at each visit as the predictor.
We conducted two sets of ancillary analyses. For both sets of analyses, we examined whether stressors involving interpersonal tension predicted participants’ dietary intake. Our diet measure assessed women’s food intake over a 90 day period prior to the study. Accordingly, we created an interpersonal stressor composite by averaging the number of interpersonal stressors each woman reported across visits. This composite provided a window into a participant’s typical day by serving as an index of how many stressors she would experience on an average day.
For the first set of ancillary analyses, we conducted a set of linear regressions examining whether the average number of interpersonal stressors across visits predicted women’s typical diet in terms of quantity of foods consumed. Specifically, we examined calories, three macronutrients (fat, carbohydrates, and protein), and several other dietary components (cholesterol, sugar, sodium, fiber, fruits, vegetables, vitamin A, and vitamin C). We followed the same data analytic strategy for non-interpersonal stressors.
In the second set of auxiliary analyses, we conducted a set of linear regressions testing whether the average number of interpersonal stressors across visits predicted the percentage of calories due to fat, carbohydrates, and protein. We followed the same data analytic strategy for non-interpersonal stressors. These accompanying analysis allowed us to investigate whether interpersonal stressors were linked to the overall quantity of macronutrients women were consuming (which would be supported by ancillary analysis #1) versus the type of macronutrients people were consuming (which would be supported by ancillary analysis #2).
Potential confounds were selected based on their theoretical and empirical relationships to stressful events, ghrelin, and leptin (Haqq et al., 2003; Kiecolt-Glaser et al., 2012; Knutson et al., 2007; Shiiya et al., 2002; Taheri et al., 2004). Every model adjusted for age, body mass index (BMI: kg/m2), sleep, and prior yoga experience (novice vs. expert). We had multiple sleep measures available. In the primary analyses, feeling rested in the morning (or the lack thereof) was more strongly related to ghrelin and leptin levels than hours of sleep the night before, perhaps because people vary in their usual waking time and the amount of sleep they typically need (Van Dongen et al., 2005). Accordingly, the feeling rested item was used as a covariate in our primary analyses. In the secondary analyses investigating women’s diet over a 3-month period, we used the PSQI, measuring longer-term sleep quality.
In sum, the primary analyses focused on relationships between the number of stressors the day before each visit (as a continuous predictor) and ghrelin or leptin levels at each visit. The ancillary analyses examined the link between average number of stressors reported across all three visits (as a continuous predictor) and women’s typical dietary intake. Furthermore, the ghrelin, leptin, and dietary analyses all included the same covariates, although the sleep measure differed based on the timeframe of the corresponding dependent measure. Degrees of freedom vary slightly from analysis to analysis because of small amounts of missing data.
Results
Preliminary Analyses
Number of stressors within each visit
Within the video condition visit, 59.6% of women reported 0 stressors involving interpersonal tension, 31.9% reported 1 interpersonal stressor, 6.4% reported 2 interpersonal stressors, and 2.1% reported 3 interpersonal stressors. The corresponding breakdowns within the movement control condition were 59.6%, 23.4%, 17.0%, and 0%. Within the yoga condition, the frequencies were 62.5%, 27.1%, 10.4%, and 0%.
Within the video condition, 51.1% of women reported 0 non-interpersonal stressors, 42.6% reported 1 non-interpersonal stressor, 6.4% reported 2 non-interpersonal stressors, and 0% reported 3 non-interpersonal stressors. The corresponding breakdowns within the movement control condition were 61.7%, 23.4%, 10.6%, and 4.3%. Within the yoga condition, the frequencies were 56.3%, 31.3%, and 12.5%.
Number of stressors averaged across visits
Approximately 12.5% of women reported one or more stressors involving interpersonal tension at all 3 assessments, 20.8% during at least 2 assessments, 37.5% at only one assessment, and 29.2% did not report any interpersonal stressors. The corresponding breakdowns for non-interpersonal stressors were 16.7%, 22.9%, 33.3%, and 27.1% respectively. All reported coefficients are unstandardized.
Primary Analyses
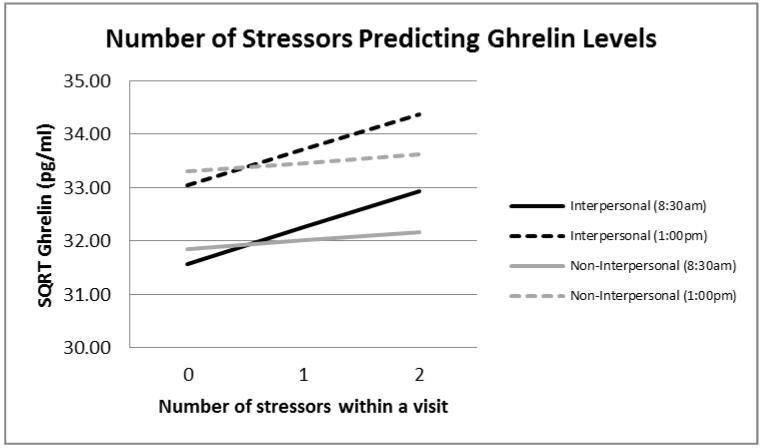
First, we examined an unadjusted model with the number of interpersonal stressors at each visit as the predictor and ghrelin levels as the outcome. Women who experienced more stressors involving interpersonal tension had higher ghrelin levels than those who experienced fewer interpersonal stressors, b = .68, F(1,204)= 3.35, p = .068, although this effect was only marginally significant. Next, we added our a priori selected covariates and also tested whether the interpersonal stressor effect was moderated by condition or time of day. Ghrelin levels broken down by type of stressor, number of stressors at each visit, and time of day are graphed in Figure 1. As predicted, women who experienced more stressors involving interpersonal tension had higher ghrelin levels than those who experienced fewer interpersonal stressors, b = .67, F(1,201)= 3.97, p = .048. Women also had higher ghrelin levels later in the day compared with earlier, b = 1.45, F(1,192)= 11.26, p = .001. However, none of the interactions with sample time or condition were significant, indicating that the strength of the relationship between interpersonal stressors and ghrelin was the same across conditions (i.e., visits) and at both sampling time points, all p values > .366. Non-interpersonal stressors were unrelated to ghrelin levels, except there was a significant non-interpersonal stressor by time by condition interaction predicting ghrelin levels, F(1,109)= 3.85, p = .024. This interaction was driven by higher ghrelin levels later in the day compared to earlier for everyone except women experiencing more non-interpersonal stressors in the video condition. This interaction was not replicated with the leptin data and is thus not discussed further.
Figure 1.
Number of stressors predicting ghrelin values.
Note. N = 47. Estimated marginal means were calculated from two mixed models, one predicting ghrelin levels (SQRT) from number of interpersonal stressors at each visit, controlling for BMI, age, sleep, and yoga experience, and the other with non-interpersonal stressors as the key predictor.
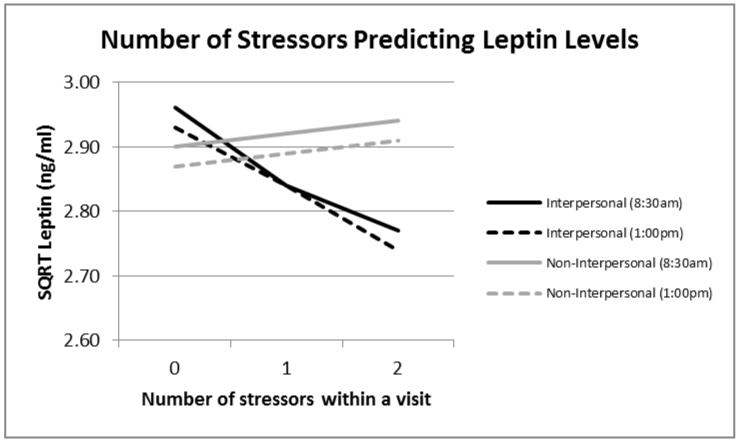
Next, we examined an unadjusted model with the number of interpersonal stressors at each visit as the predictor and leptin levels as the outcome. Women who experienced more stressors involving interpersonal tension had lower leptin than those who experienced fewer interpersonal stressors, b = −.12, F(1,191)= 3.90, p = .050. Next, we added our a priori selected covariates and also tested whether the interpersonal stressor effect was moderated by condition or time of day. Leptin levels broken down by type of stressor, number of stressors at each visit, and time of day are graphed in Figure 1. As expected, women who experienced more stressors involving interpersonal tension had lower leptin than those who experienced fewer interpersonal stressors, b = −.10, F(1,184)= 4.20, p = .042. Time of day was unrelated to leptin levels, b = −.03, F(1,180)= 0.22, p = .641. Furthermore, none of the interactions with condition or sampling time were significant, indicating that the strength of the relationship between interpersonal stressors and leptin was the same across conditions and at both sampling time points, all p values > .105. Non-interpersonal stressors were unrelated to leptin levels in either the unadjusted or adjusted models, p values > .434.
Ancillary Analyses
First, we examined women’s diet in terms of overall quantities of food consumed. Women who experienced more stressors involving interpersonal tension across all three visits had a typical diet that was higher in calories, fat, carbohydrates, protein, sugar, sodium, and fiber compared with those who experienced fewer interpersonal stressors (see Table 1). Their diet also included more cholesterol, vegetables (but not fruits), vitamin A, and vitamin C, although these effects were only marginally significant. Non-interpersonal stressors were unrelated to any of the dietary components examined (see Table 2).
Table 2.
Fully adjusted regression analyses: average number of stressors across visits involving interpersonal tension predicting dietary outcomes.
| Predictor: Average number of stressors involving interpersonal tension | ||||
|---|---|---|---|---|
|
| ||||
| Outcome | Unstandardized beta coefficient (b) |
Standard Error | t | p |
| Calories (kcal) | 652.45 | 170.20 | 3.83 | <.001 |
| Fat (g) | 20.39 | 7.38 | 2.76 | .009 |
| Carbohydrates (g) | 77.21 | 23.97 | 3.22 | .003 |
| Protein (g) | 35.32 | 8.13 | 4.35 | <.001 |
| Cholesterol (mg) | 89.19 | 47.69 | 1.873 | .069 |
| Sugars (g) | 32.32 | 12.32 | 2.62 | .012 |
| Sodium (mg) | 1287.70 | 332.57 | 3.87 | <.001 |
| Fiber (g) | 8.48 | 2.85 | 2.98 | .005 |
| Fruit (#/day) | 0.20 | 0.51 | 0.38 | .705 |
| Vegetables (#/day) | 0.87 | 0.51 | 1.69 | .099 |
| Vitamin A (IU) | 5117.15 | 3088.64 | 1.66 | .105 |
| Vitamin C (mg) | 38.11 | 22.18 | 1.72 | .093 |
Note: N = 46. All analyses controlled for age, BMI, sleep quality, and yoga experience
Next, we investigated the percentage of calories in a woman’s diet due to three macronutrients: fat, carbohydrates, and protein. Both interpersonal stressors and non-interpersonal were unrelated to percentage of calories due to all three macronutrients, p values > .112.
Discussion
The current study demonstrated that women who experienced more stressors involving interpersonal tension had higher ghrelin and lower leptin levels than those who experienced fewer interpersonal stressors. Furthermore, women who experienced more interpersonal stressors had a typical diet that was significantly higher in calories, fat, carbohydrates, protein, sugar, sodium, and fiber, and and marginally higher in cholesterol, vegetables (but not fruits), vitamin A, and vitamin C. However, the percentage of calories in a woman’s diet due to fat, carbohydrates, and protein was unrelated to interpersonally stressful events. Furthermore, stressors that did not involve interpersonal tension were unrelated to ghrelin and leptin levels or any of the dietary components examined.
A number of rodent studies have demonstrated that stressors increase ghrelin production (Asakawa et al., 2001; Chuang et al., 2011; Kristenssson et al., 2006; Lutter et al., 2008; Ochi et al., 2008). Although these studies provide a useful foundation to build upon, humans are an ultra-social species who evolved specific cognitive skills for interpreting the social world (Herrmann et al., 2007; Pagel, 2012). Accordingly, the present results fill an important gap in the human literature by investigating the relationships between daily stressors and both ghrelin and leptin. Furthermore, the current research distinguished between interpersonal and non-interpersonal stressors, demonstrating that only stressors involving interpersonal tension were related to ghrelin and leptin levels and dietary intake. These data suggest that interpersonal stressors are qualitatively different than other types of stressors in terms of their effect on two appetite-relevant hormones and a person’s typical diet.
The present data demonstrated that women who experienced more interpersonal stressors had a typical diet that was higher in all three macronutrients (i.e., fat, carbohydrates, and protein) and a variety of micronutrients. However, the percentage of calories in women’s diets due to fat, carbohydrates, and protein was similar between women experiencing more or fewer interpersonal stressors. Accordingly, interpersonally stressed women were eating more macronutrients without altering the proportion of their diet that came from these macronutrients. Taken together, the hormone and dietary data add to the burgeoning literature about ghrelin, leptin, and eating behavior. For example, a growing body of research suggests that ghrelin increases consumption of hedonically pleasant food, such as food high in sugar and fat, among non-obese rodents and humans (Buss et al., 2014; Disse et al., 2010; Perello et al., 2010). The current study raises the possibility that interpersonally stressful events may also be a determining factor that shapes the links among ghrelin, leptin, and eating behavior. Accordingly, an excellent target for future research is examining the effects of interpersonal stressors on ghrelin, leptin, and actual food consumption.
Women in the current study had higher ghrelin levels later in their visit compared with earlier. However, leptin did not change based on time of day. Prior work examining diurnal changes in ghrelin and leptin has revealed inconsistent patterns (Birketvedt et al., 2012; Bodosi et al., 2004; Sánchez et al., 2004). This variation may be due to differences in macronutrient consumption and sample timing across studies; ghrelin levels largely depend on macronutrient consumption (Koliaki et al., 2010), which may obfuscate any diurnal patterns. Taken together, prior research and the present data begin to paint a picture about timing and macronutrient-related factors that influence ghrelin and leptin levels.
Obesity contributes to a host of medical problems and is thus a major public health concern (Billington et al., 2000). The current data suggest that ghrelin and leptin may link interpersonally stressful events to overeating and weight gain. However, eating behavior is multiply determined. Accordingly, other physiological and psychological mechanisms may work independently or in tandem with changes in ghrelin and leptin levels to influence eating behavior. For example, sleep deprivation is linked to increased food consumption (Knutson et al., 2007). In addition, environmental factors, such as food package size and lighting conditions, alter dietary intake (Wansink, 2004). The stringent exclusion criteria used in this study and the use of standardized meals helped rule out potential confounding factors that might explain the links among interpersonal stressors ghrelin, and leptin. In addition, the current results were independent of participants’ age, BMI, sleep, and yoga experience. Consequently, interpersonal stressors were related to ghrelin and leptin levels and dietary intake independent of participants’ demographic characteristics, health, and health behaviors.
We excluded interested participants with a BMI ≥ 30 kg/m2, and thus none of the women in our sample were obese (Centers for Disease Control and Prevention, 2014). Obesity is related to cellular leptin resistance, although it remains unclear whether obesity causes this resistance or vice versa (Myers et al., 2010). One interesting extension of the present research would be testing whether the relationships among interpersonal stressors, ghrelin, and leptin generalize to obese individuals. In fact, a recent study demonstrated that ghrelin was linked to caloric intake and hedonic eating among overweight but not obese people (Buss et al., 2014), suggesting potential differential effects of interpersonal stressors among obese people. Along similar lines, prior research indicates that the links among acute stressors, ghrelin, and food consumption may be altered by eating-related problems, such as binge eating disorder or emotional eating (Raspopow et al., 2014, 2010; Rouach et al., 2007), suggesting another possible extension of the current findings.
One critical avenue for additional research is exploring mechanisms that link interpersonal stressors to elevated ghrelin and leptin. One rodent study demonstrated that a β3-adrenergic antagonist attenuated the effects of chronic social defeat on leptin production (Chuang et al., 2010). In addition, direct stimulation of the sympathetic nervous system in rats elevated ghrelin levels (Mundinger, 2006). Accordingly, one promising mechanism is sympathetic nervous system activation. However, the research examining the links among stressors, ghrelin, and leptin is in its infancy, particularly among humans.
Another intriguing avenue for future research is understanding why stressors involving interpersonal tension, but not other stressors, were related to ghrelin and leptin. One possibility is that feeling hungry in response to interpersonal stress is socially adaptive. The need for social connection is fundamental to human nature. Consequently, experiencing interpersonal stressors should motivate people to try and connect with others in order to restore their sense of belonging. Furthermore, eating and social connection are intricately linked; eating was a highly social activity throughout human evolution (Wrangham, 2010), and people still often eat around other people. In addition, recent research demonstrated that eating comfort food caused people to spontaneously think about their relationships, and simply thinking about comfort food decreased loneliness (Troisi and Gabriel, 2011). Consequently, people may feel hungrier when they experience interpersonal stressors because they have either implicitly or explicitly learned that eating either helps them feel socially connected and/or provides them with an opportunity for social connection.
The current sample consisted of women who were primarily white, one limitation of the present results. The study hypotheses were also designed and tested after data collection for the study was complete. Accordingly, researchers should design additional studies to a priori test the relationships among interpersonal stressors, ghrelin and leptin, and dietary intake in more diverse samples. In the present study, it is possible that some participants did not completely finish their standardized meals, another limitation. However, none of the women were obese, they were allowed to choose from multiple standardized meal options, and they were instructed to eat the entire meal, making this possibility less likely. Nonetheless, given the influence of calorie consumption on ghrelin levels (le Roux et al., 2005), an important direction for additional research is to examine actual food consumption and whether this differs from self-reported dietary intake. Although all participants were given standardized meals, no pre-meal blood samples were obtained. Accordingly, the results evident in this study may be due to specific meal responses, more chronic hormone dysregulation, or a combination of the two. In addition, the inventory of stressful events utilized in this study captures stressful events that occurred within the prior 24 hours. Accordingly, these events could reflect both daily stressors that represent a chronic source of distress and those that are acute one-time occurrences. Understanding the relationships among acute versus chronic stressors, ghrelin, and leptin before and after a meal is an important direction for future research. Another key question is to determine whether more recent stressors have a stronger effect on appetite-regulation than those that are less recent.
The ghrelin assay used in this study was based on total levels, and thus it is unclear how active ghrelin may be related to interpersonal stressors. However, previous research demonstrated that overweight women with higher total ghrelin levels consumed more calories, particularly hedonically pleasant food, than those with lower levels, supporting the importance of total ghrelin in eating behavior (Buss et al., 2014).
In sum, women who experienced more stressors involving interpersonal tension had higher ghrelin and lower leptin levels and reported consuming more food than those who experienced fewer interpersonal stressors. These data suggest that ghrelin and leptin, two hormones that are implicated in appetite regulation, may link daily interpersonal stressors to weight gain and obesity.
Highlights.
More interpersonal stressors were related to higher ghrelin and lower leptin
Interpersonal stressors were also linked to a diet higher in calories
Non-interpersonal stressors were unrelated to ghrelin and leptin levels and diet
Figure 2.
Number of stressors and leptin values.
Note. N = 47. Estimated marginal means were calculated from two mixed models, one predicting leptin levels (SQRT) from number of interpersonal stressors at each visit, controlling for BMI, age, sleep, and yoga experience, and the other with non-interpersonal stressors as the key predictor.
Table 3.
Fully adjusted regression analyses: average number of stressors across visits not involving interpersonal tension predicting dietary outcomes.
| Predictor: Average number of stressors not involving interpersonal tension | ||||
|---|---|---|---|---|
|
| ||||
| Outcome | Unstandardized beta coefficient (b) |
Standard Error | t | p |
| Calories (kcal) | 14.94 | 193.20 | 0.08 | .939 |
| Fat (g) | 0.87 | 7.82 | 0.11 | .912 |
| Carbohydrates (g) | −2.31 | 26.11 | −0.09 | .930 |
| Protein (g) | 1.54 | 9.57 | 0.16 | .873 |
| Cholesterol (mg) | 30.32 | 48.04 | 0.63 | .532 |
| Sugars (g) | 10.28 | 12.85 | 0.80 | .428 |
| Sodium (mg) | −194.34 | 377.32 | −0.52 | .609 |
| Fiber (g) | −3.48 | 3.00 | −1.16 | .254 |
| Fruit (#/day) | −0.17 | 0.50 | −0.35 | .731 |
| Vegetables (#/day) | −0.24 | 0.51 | −0.46 | .648 |
| Vitamin A (IU) | −1877.00 | 3085.44 | −0.61 | .546 |
| Vitamin C (mg) | −24.24 | 21.98 | −1.10 | .277 |
Note: N = 46. All analyses controlled for age, BMI, sleep, and yoga experience
Acknowledgments
Funding Sources
Work on this project was supported in part by NIH grants R21 AT002971, K05 CA172296, R21 CA158868, P30 CA016058, and UL1RR025755, as well as American Cancer Society Postdoctoral Fellowship Grant 121911-PF-12-040-01-CPPB and a Pelotonia Postdoctoral Fellowship from the Ohio State University Comprehensive Cancer Center.
Footnotes
Contributors
Lisa M. Jaremka: substantial contributions to the analysis and interpretation of data, primary person responsible for writing and revising the article, final approval of the version to be published
Martha A. Belury: helped design the study, helped revise the article for important intellectual content, final approval of the version to be published
Rebecca R. Andridge: helped design the study, substantial contributions to the analysis and interpretation of data, helped revise the article for important intellectual content, final approval of the version to be published
William B. Malarkey: helped design the study, helped revise the article for important intellectual content, final approval of the version to be published
Ronald Glaser: helped design the study, helped revise the article for important intellectual content, final approval of the version to be published
Lisa Christian: helped design the study, helped revise the article for important intellectual content, final approval of the version to be published
Charles F. Emery: helped design the study, helped revise the article for important intellectual content, final approval of the version to be published
Janice K. Kiecolt-Glaser: primary person responsible for designing the study, substantial contributions to the analysis and interpretation of data, helped revise the article for important intellectual content, final approval of the version to be published
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida DM, Wethington E, Kessler RC. The daily inventory of stressful events: An interview-based approach for measuring daily stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. doi:10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, Katsuura G, Makino S, Fujino MA, Kasuga M. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74:143–147. doi: 10.1159/000054680. doi:10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. doi:10.1037/0033-2909.117.3.497. [PubMed] [Google Scholar]
- Billington CJ, Epstein LH, Goodwin NJ, Hill JO, Pi-Sunyer FX, Rolls BJ, Stern J, Wadden TA, Weinsier RL, Wilson GT, Wing RR, Yanovski SZ, Hubbard VS, Hoofnagle JH, Everhart J, Harrison B. Overweight, obesity, and health risk. Arch. Intern. Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- Birketvedt GS, Geliebter A, Kristiansen I, Firgenschau Y, Goll R, Florholmen JR. Diurnal secretion of ghrelin, growth hormone, insulin binding proteins, and prolactin in normal weight and overweight subjects with and without the night eating syndrome. Appetite. 2012;59:688–692. doi: 10.1016/j.appet.2012.07.015. doi:10.1016/j.appet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Bodosi B, Gardi J, Hajdu I, Szentirmai E,F, Obal J, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: Effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. doi:10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- Buss J, Havel PJ, Epel E, Lin J, Blackburn E, Daubenmier J. Associations of ghrelin with eating behaviors, stress, metabolic factors, and telomere length among overweight and obese women: Preliminary evidence of attenuated ghrelin effects in obesity? Appetite. 2014;76:84–94. doi: 10.1016/j.appet.2014.01.011. doi:10.1016/j.appet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the pittsburgh sleep quality index. Journal of Psychosomatic Research. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. doi:10.1016/S0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [accessed 4.2.14];Overweight and Obesity [WWW Document] 2014 URL http://www.cdc.gov/obesity/adult/defining.html.
- Chuang J-C, Krishnan V, Yu HG, Mason B, Cui H, Wilkinson MB, Zigman JM, Elmquist JK, Nestler EJ, Lutter M. A β3-adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biological Psychiatry, Synaptic Development in Mood Disorders. 2010;67:1075–1082. doi: 10.1016/j.biopsych.2009.12.003. doi:10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J-C, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121:2684–2692. doi: 10.1172/JCI57660. doi:10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. doi:10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. American Journal of Physiology - Endocrinology and Metabolism. 2004;287:E297–E304. doi: 10.1152/ajpendo.00582.2003. doi:10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. doi:10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- De Oliveira C, Scarabelot VL, Souza A. de, Oliveira C.M. de, Medeiros LF, Macedo I.C. de, Marques Filho PR, Cioato SG, Caumo W, Torres ILS. Obesity and chronic stress are able to desynchronize the temporal pattern of serum levels of leptin and triglycerides. Peptides. 2014;51:46–53. doi: 10.1016/j.peptides.2013.10.024. doi:10.1016/j.peptides.2013.10.024. [DOI] [PubMed] [Google Scholar]
- Disse E, Bussier A-L, Veyrat-Durebex C, Deblon N, Pfluger PT, Tschöp MH, Laville M, Rohner-Jeanrenaud F. Peripheral ghrelin enhances sweet taste food consumption and preference, regardless of its caloric content. Physiol. Behav. 2010;101:277–281. doi: 10.1016/j.physbeh.2010.05.017. doi:10.1016/j.physbeh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Haqq AM, Farooqi IS, O’Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL, LaFranchi SH, Purnell JQ. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi Syndrome. JCEM. 2003;88:174–178. doi: 10.1210/jc.2002-021052. doi:10.1210/jc.2002-021052. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Call J, Hernàndez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science. 2007;317:1360–1366. doi: 10.1126/science.1146282. doi:10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Christian L, Preston H, Houts CR, Malarkey WB, Emery CF, Glaser R. Stress, inflammation, and yoga practice. Psychosom Med. 2010;72:113–121. doi: 10.1097/PSY.0b013e3181cb9377. doi:10.1097/PSY.0b013e3181cb9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Christian LM, Andridge R, Hwang BS, Malarkey WB, Belury MA, Emery CF, Glaser R. Adiponectin, leptin, and yoga practice. Physiology & Behavior. 2012;107:809–813. doi: 10.1016/j.physbeh.2012.01.016. doi:10.1016/j.physbeh.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obesity Reviews. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. doi:10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Medicine Reviews. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. doi:10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliaki C, Kokkinos A, Tentolouris N, Katsilambros N. The effect of ingested macronutrients on postprandial ghrelin response: A critical review of existing literature data. International Journal of Peptides. 20102010:e710852. doi: 10.1155/2010/710852. doi:10.1155/2010/710852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkeila M, Kaprio J, Rissanen A, Koskenvuo M, Sorensen TIA. Predictors of major weight gain in adult Finns: Stress, life satisfaction and personality traits. Int. J. Obes. 1998;22:949–957. doi: 10.1038/sj.ijo.0800694. doi:10.1038/sj.ijo.0800694. [DOI] [PubMed] [Google Scholar]
- Kristal AR, Patterson RE, Shattuck A, Vizenor NC. Nutrient databases for food frequency questionnaires; Presented at the National Nutrient Databank Conference; 1999. [Google Scholar]
- Kristenssson E, Sundqvist M, Astin M, Kjerling M, Mattsson H, Dornonville de la Cour C, Håkanson R, Lindström E. Acute psychological stress raises plasma ghrelin in the rat. Regulatory Peptides. 2006;134:114–117. doi: 10.1016/j.regpep.2006.02.003. doi:10.1016/j.regpep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J. Clin. Endocrinol. Metab. 2005;90:1068–1071. doi: 10.1210/jc.2004-1216. doi:10.1210/jc.2004-1216. [DOI] [PubMed] [Google Scholar]
- Leary MR, Cox CB. Belongingness motivation: A mainspring of social action. In: Gardner WL, Shah JY, editors. Handbook of Motivation Science. Guilford Press; New York, NY: 2008. pp. 27–40. [Google Scholar]
- Licinio J, Caglayan S, Ozata M, Yildiz BO, Miranda P.B. de, O’Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong M-L. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. PNAS. 2004;101:4531–4536. doi: 10.1073/pnas.0308767101. doi:10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. doi:10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow AH. Toward a Psychology of Being. 2nd ed. D. Van Nostrand; Oxford, England: 1968. [Google Scholar]
- Mroczek DK, Stawski RS, Turiano NA, Chan W, Almeida DM, Neupert SD, Spiro A. Emotional reactivity and mortality: Longitudinal findings from the VA Normative Aging Study. J Gerontol B Psychol Sci Soc Sci. 2013:gbt107. doi: 10.1093/geronb/gbt107. doi:10.1093/geronb/gbt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundinger TO. Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology. 2006;147:2893–2901. doi: 10.1210/en.2005-1182. doi:10.1210/en.2005-1182. [DOI] [PubMed] [Google Scholar]
- Myers MG, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol. Metab. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. doi:10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi M, Tominaga K, Tanaka F, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Oshitani N, Higuchi K, Arakawa T. Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sciences. 2008;82:862–868. doi: 10.1016/j.lfs.2008.01.020. doi:10.1016/j.lfs.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Pagel M. Evolution: Adapted to culture. Nature. 2012;482:297–299. doi: 10.1038/482297a. doi:10.1038/482297a. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Annals of Epidemiology. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. doi:10.1016/S1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- Perello M, Sakata I, Birnbaum S, Chuang J-C, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol. Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. doi:10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CL, Gardner WL. The social monitoring system: Enhanced sensitivity to social cues as an adaptive response to social exclusion. In: Williams KD, Forgas J, von Hippel W, editors. The Social Outcast: Ostracism, Social Exclusion, Rejection, and Bullying. Psychology Press; New York: 2005. pp. 213–225. [Google Scholar]
- Raspopow K, Abizaid A, Matheson K, Anisman H. Psychosocial stressor effects on cortisol and ghrelin in emotional and non-emotional eaters: Influence of anger and shame. Hormones and Behavior. 2010;58:677–684. doi: 10.1016/j.yhbeh.2010.06.003. doi:10.1016/j.yhbeh.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Raspopow K, Abizaid A, Matheson K, Anisman H. Anticipation of a psychosocial stressor differentially influences ghrelin, cortisol and food intake among emotional and non-emotional eaters. Appetite. 2014;74:35–43. doi: 10.1016/j.appet.2013.11.018. doi:10.1016/j.appet.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Rouach V, Bloch M, Rosenberg N, Gilad S, Limor R, Stern N, Greenman Y. The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology. 2007;32:693–702. doi: 10.1016/j.psyneuen.2007.04.010. doi:10.1016/j.psyneuen.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Sánchez J, Oliver P, Picó C, Palou A. Diurnal rhythms of leptin and ghrelin in the systemic circulation and in the gastric mucosa are related to food intake in rats. Pflugers Arch - Eur J Physiol. 2004;448:500–506. doi: 10.1007/s00424-004-1283-4. doi:10.1007/s00424-004-1283-4. [DOI] [PubMed] [Google Scholar]
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S-I, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. JCEM. 2002;87:240–244. doi: 10.1210/jcem.87.1.8129. doi:10.1210/jc.87.1.240. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Jankord R, Flak JN, Herman JP. Chronic stress, energy balance and adiposity in female rats. Physiol. Behav. 2011;102:84–90. doi: 10.1016/j.physbeh.2010.09.024. doi:10.1016/j.physbeh.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America’s Table Study. Am. J. Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. doi:10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. doi:10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooby J, Cosmides L. Friendship and the banker’s paradox: Other pathways to the evolution of adaptations for altruism. In: Runciman WG, Smith JM, Dunbar RIM, editors. Evolution of Social Behaviour Patterns in Primates and Man. Oxford University Press; New York, NY: 1996. pp. 119–143. [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. doi:10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Troisi JD, Gabriel S. Chicken soup really is good for the soul: “Comfort food” fulfills the need to belong. Psychological Science. 2011;22:747–753. doi: 10.1177/0956797611407931. doi:10.1177/0956797611407931. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28:479–496. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- Wansink B. Environmental factors that increase the food intake and consumption volume of unknowing consumers. Annu. Rev. Nutr. 2004;24:455–479. doi: 10.1146/annurev.nutr.24.012003.132140. doi:10.1146/annurev.nutr.24.012003.132140. [DOI] [PubMed] [Google Scholar]
- Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A. Stress and adiposity: A meta-analysis of longitudinal studies. Obesity. 2011;19:771–778. doi: 10.1038/oby.2010.241. doi:10.1038/oby.2010.241. [DOI] [PubMed] [Google Scholar]
- Wrangham R. Catching fire: How cooking made us human. Basic Books; 2010. [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. JCEM. 2001;86:5992–5992. doi: 10.1210/jcem.86.12.8111. doi:10.1210/jc.86.12.5992. [DOI] [PubMed] [Google Scholar]