Abstract
Shear stress secondary to increased pulmonary blood flow (PBF) is elevated in some children born with congenital cardiac abnormalities. However, the majority of these patients do not develop pulmonary edema, despite high levels of permeability inducing factors. Previous studies have suggested that laminar fluid shear stress can enhance pulmonary vascular barrier integrity. However, little is known about the mechanisms by which this occurs. Using microarray analysis, we have previously shown that Sox18, a transcription factor involved in blood vessel development and endothelial barrier integrity, is up-regulated in an ovine model of congenital heart disease with increased PBF (shunt). By subjecting ovine pulmonary arterial endothelial cells (PAEC) to laminar flow (20 dyn/cm2), we identified an increase in trans-endothelial resistance (TER) across the PAEC monolayer that correlated with an increase in Sox18 expression. Further, the TER was also enhanced when Sox18 was over-expressed and attenuated when Sox18 expression was reduced, suggesting that Sox18 maintains the endothelial barrier integrity in response to shear stress. Further, we found that shear stress up-regulates the cellular tight junction protein, Claudin-5, in a Sox18 dependent manner, and Claudin-5 depletion abolished the Sox18 mediated increase in TER in response to shear stress. Finally, utilizing peripheral lung tissue of 4 week old shunt lambs with increased PBF, we found that both Sox18 and Claudin-5 mRNA and protein levels were elevated. In conclusion, these novel findings suggest that increased laminar flow protects endothelial barrier function via Sox18 dependent up-regulation of Claudin-5 expression.
Keywords: Sox18, Claudin-5, pulmonary barrier function, pulmonary artery endothelial cells, shear stress
INTRODUCTION
Elevated pulmonary blood flow (PBF) is a hallmark of congenital heart diseases (CHD), such as atrial septal defect (ASD), ventricular septal defect (VSD), and patent ductus arteriosus (PDA). We have previously developed an ovine model of CHD (shunt) with increased PBF by the in utero placement of a vascular graft between the aorta and the main pulmonary artery (Reddy et al., 1995). After spontaneous delivery, these lambs develop a significant left-to-right shunt, which exposes the pulmonary vasculature to increased blood flow and shear stress (Reddy et al., 1995). These shunted lambs display morphological and physiological features that mimic children born with VSD and, at 1 month of age, have dramatically elevated PBF, pulmonary artery pressure (Reddy et al., 1995), and increased levels of the endothelial barrier disruptive agonists: vascular endothelial growth factor (VEGF), thrombin, and transforming growth factor (TGF)-β1 (Mata-Greenwood et al., 2003a; Mata-Greenwood et al., 2003b). However, studies in humans and in animals with increased PBF have shown that, even though barrier disruptive agents are elevated (Lin et al., 2011; Tannenbaum et al., 1996; Zhou et al., 1998; Zhu et al., 2012), there is little lung edema (Alpan et al., 1989; Alpan et al., 1991; Sakuma et al., 2002; Waller et al., 1996). For example, although the patency of the ductus arteriosus has been associated with increased levels of TGF-β1 (Tannenbaum et al., 1996; Zhou et al., 1998), water accumulation and net protein transudation into the lung are not affected (Alpan et al., 1989; Alpan et al., 1991). Indeed, with the exception of acute perioperative pulmonary edema due to excess fluid administration, volutrauma, heart failure, or aspiration, patients with ASD (Gruner et al., 2012; Sanders et al., 1988; Singhi et al., 2010) or patients undergoing lung resection (Alvarez et al., 2003) do not in general present with increased lung permeability. In another study, postoperative edema could not be detected in patients after lobectomy (Waller et al., 1996).
Interestingly, in rodents, alveolar fluid clearance actually increased 14 and 28 days after pneumonectomy (Sakuma et al., 2002), despite increased levels of TGF-β1 (Zhu et al., 2012) and VEGF (Lin et al., 2011). Together these studies suggest that shear stress secondary to sustained increases in PBF might be acting as a barrier protective force in the lung. Indeed, previous studies have shown that fluid shear enhances pulmonary vascular barrier integrity (Shikata et al., 2005); however, the mechanisms by which shear stress regulates the pulmonary endothelial barrier are unclear.
Vascular cells are capable of sensing external mechanical forces and transforming the mechanical signal into a biological response. To date, there are several putative mechanoreceptors that allow endothelial cells to respond to shear stress (Barakat et al., 1999; Tzima et al., 2005). Through these receptors, shear stress can regulate various cellular functions, including the synthesis of nitric oxide and prostacyclin, cell shape, motility, and the expression and organization of cell junction proteins which link adjacent cells. Many of these functions appear to be regulated at the transcriptional level, and shear stress has been shown to regulate the endothelial transcriptome through the presence of specific cis-acting sequence elements, termed shear stress responsive elements (SSRE) (Chu and Peters, 2008; Dekker et al., 2002; Silberman et al., 2009; Tressel et al., 2007). Previously, using microarray analysis of 3-day old shunt lambs with increased PBF, we have identified the increased expression of the angiogenesis related transcription factor, Sox18, which may also regulate endothelial barrier function (Tian et al., 2011). Sox18 belongs to the Sex-Determining Region on the Y chromosome (SRY)-related High Mobility Group (HMG) box group-F family of transcription factors, which includes Sox7, Sox17, and Sox18 (Dunn et al., 1995; Kanai et al., 1996; Taniguchi et al., 1999). The HMG domain of Sox18 binds in the minor groove of the DNA helix and recognizes the consensus sequence 5’-AACAAAG-3’ (Hosking et al., 1995). The transactivation domain then facilitates the transcription of the associated gene. Sox18 is expressed in endothelial cells and is important for embryonic vasculogenesis, lymphangiogenesis (Francois et al., 2008), and adult angiogenesis. Sox18 genetic mutations underlying human hypotrichosis-lymphedema-telangiectasia (HLT) (Irrthum et al., 2003) and the ragged (Ra) mice (James et al., 2003; Pennisi et al., 2000b) are associated with respiratory distress, severe edema, and superficial hemorrhage (Hosking et al., 2001; Irrthum et al., 2003; James et al., 2003; Pennisi et al., 2000b), indicating an important role for Sox18 in endothelial development and likely, barrier function.
Physically, the endothelial barrier is maintained by the adherence of adjacent cells through the participation of two major junctional structures: zonula adherens and zonula occludens. The adherens junctions are formed by the transmembrane proteins of the cadherin family, which mediate intercellular adhesion by organizing multiple protein complexes. Vascular endothelial (VE)-cadherin is the most prominent cadherin present in endothelial cells independent of the vascular bed (Breviario et al., 1995). Tight junctions also mediate cell-cell adhesion and are able to regulate the paracellular diffusion of certain solutes. These junctions are formed by homophilic interactions between transmembrane proteins (Claudin, Occludin, Junctional Adhesion Molecule-A [JAM-A]) and corresponding intracellular proteins (zonula occludens-1, 2, 3, Protein 4.1), which mediate anchorage to actin microfilaments.
Claudin-5 is an important member of the endothelial tight junction proteins (Morita et al., 1999), which has been shown to have an important role in preventing the permeability of smaller molecules (Nitta et al., 2003). Although Sox18 has been shown to increase the expression of Claudin-5 (Fontijn et al., 2008), little is known about the regulation of Sox18 expression or its role in maintaining endothelial barrier integrity in the pulmonary vasculature in response to shear stress. Thus, the purpose of this study was to determine if Sox18 is up-regulated by shear and whether this is a potential mechanism by which shear stress regulates pulmonary barrier function. We found that in pulmonary arterial endothelial cells shear stress increased the expression of Sox18 which led to enhanced Claudin-5 levels and improved barrier function. In addition, the silencing of Claudin-5 abolished the Sox18 mediated increase in barrier function in response to shear stress.
MATERIAL AND METHODS
Materials
Polyclonal anti-Sox18 (rabbit) antibody, Monoclonal anti-β-actin (mouse) antibody (Clone: AC-15), Polyclonal anti-F2 (thrombin) (rabbit) antibody, α-amanitin, Recombinant human thrombin, and Recombinant human TGF-β1 were from Sigma-Aldrich (St. Louis, MO); Recombinant human VEGF165 and Chromatin immunoprecipitation assay kits were from EMD Biosciences (Philadelphia, PA); Polyclonal anti-Claudin-5 (rabbit) antibody, Polyclonal anti-ZO-1 (rabbit) antibody, Polyclonal anti-Claudin-1 (rabbit) antibody, Alexa Fluor 594 conjugated donkey anti-rabbit antibody, Alexa Fluor 488 conjugated donkey anti-rabbit antibody, Alexa Fluor 350 conjugated phalloidin, Texas Red-X conjugated phalloidin, and Monoclonal anti-ZO-1 (mouse) antibody (Clone: ZO1-1A12) Alexa Fluor 594 Conjugate were from Invitrogen Life Technologies (Grand Island, NY); Polyclonal anti-TGF-β1 (rabbit) antibody, Polyclonal anti-Sox7 (rabbit) antibody, Polyclonal anti-Claudin-3 (goat) antibody, Polyclonal anti-Claudin-12 (rabbit) antibody, Sox18 siRNA, and Claudin-5 siRNA were from Santa Cruz biotechnology (Santa Cruz, CA); pCMV6-Sox18 and pCMV6-Claudin-5 were from Origene Technologies (Rockville, MD); Polyclonal anti-VE-Cadherin (rabbit) antibody was from Cayman Chemical (Ann Arbor, MI); DeadEnd Fluorometric TUNEL kit was from Promega Corporation (Madison, WI); RNeasy kit, QuantiTect Reverse Transcription Kit, and QuantiTect SYBR Green PCR Master Mix were from Qiagen (Valencia, CA); Static and flow arrays were from Applied Biophysics (Albany, NY).
Ovine model of congenital heart disease with increased pulmonary blood flow
As we have previously described (Reddy et al., 1995), pregnant ewes (137–141 days of gestation, term = 145 days) were anesthetized using local anesthesia (2% lidocaine hydrochloride) and inhalational anesthesia (1–3% isoflorane). Under sterile conditions, an 8.0mm Gore-Tex vascular graft (~2mm long; W.L. Gore and Associates, Milpitas, CA, USA) was placed between the ascending aorta and the main pulmonary artery in the fetal lamb (shunt). Three weeks after spontaneous delivery, shunt and age matched control lambs were fasted for 24 h with free access to water. To facilitate intubation with a 6.5–7.0mm outer diameter cuffed endotracheal tube, the lambs were anesthetized with ketamine hydrochloride (0.3mg/kg/min), diazepam (0.002mg/kg/min), and fentanyl citrate (1.0µg/kg/h) and then mechanically ventilated with 21% oxygen using a Healthdyne pediatric time-cycled pressure-limited ventilator. A midsternotomy incision was then performed using strict aseptic technique. Patency of the vascular graft was confirmed by inspection and changes in oxygen saturation. Peripheral lung tissue was obtained using a side-biting vascular clamp from randomly selected lobes, and the incisions were cauterized. For each of four biopsies, approximately 300mg of peripheral lung were obtained, which were stored at −80°C until analyzed. All lambs were euthanized with a lethal injection of pentobarbital sodium at the end of each protocol in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All animal protocols and procedures were approved by the Committees on Animal Research at the University of California, San Francisco and at Georgia Regents University, Augusta.
Measurement of lung permeability
In the anesthetized animal, the chest cavity was opened. For wet to dry lung weight assessment, peripheral lung samples were taken from the caudal portion of the cranial lobe of the left lung. The lung tissues were briefly rinsed with 1x phosphate-buffered saline (PBS), immediately weighed (wet), and placed in fresh formalin overnight. The samples were then oven-baked at 70°C for 5 days before re-weighing (dry). Wet/Dry weight ratios were calculated.
Cell culture
Primary cultures of ovine pulmonary artery endothelial cells (PAEC) were isolated as described previously (Wedgwood et al., 2001). Cells were maintained in Dulbecco’s Modified Eagle Medium with 1gm glucose, L-glutamine, and sodium pyruvate, supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA) and 1% antibiotic-antimycotic solution (Thermo Fisher Scientific, Rockford, IL, USA) at 37°C in a humidifier with 5% CO2 and 95% air. Cells used were seeded at 50% confluence and utilized when 100% confluent. All experiments were conducted in cells between passages 5 and 15.
Over-expression of Sox18 and Claudin-5
PAEC were transfected with pCMV6-Sox18 plasmid or pCMV6-Claudin-5 plasmid using Effectene Transfection Reagent (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. An empty pCMV6 plasmid was used as a control. Over-expression was confirmed by immunoblot analysis.
Small interfering RNA-mediated knockdown
PAEC were transfected with 20 nM of Sox18 or Claudin-5 small interfering RNA (siRNA) (Santa Cruz biotechnology, Santa Cruz, CA, USA) with the use of HiPerFect transfection reagent (Qiagen), according to the manufacturer’s instructions. A scrambled siRNA was used as a negative control. Validation of the gene silencing effect on Sox18 and Claudin-5 in PAEC was confirmed by immunoblot analysis.
Shear stress
Laminar shear stress was applied using a cone-plate viscometer that accepts six-well culture plates, as described previously (Wedgwood et al., 2001). The cells were washed with 1× phosphate-buffered saline (PBS) and incubated in serum free medium overnight. PAEC were exposed to 20 dyn/cm2 of shear stress for the indicated amount of time. This method achieves laminar flow rates that represent physiological levels of laminar shear stress in human pulmonary arteries, which are approximately 20 dyn/cm2 (Tang et al., 2012).
Real-time RT-PCR analysis
Real-time RT-PCR was employed to verify the transcriptional regulation of Sox18 and Claudin-5 by shear stress, as described previously (Tian et al., 2011). Primers were designed by PrimerQuest (Integrated DNA Technologies, Coralville, IA, USA). Primer sequences were as follows: Sox18, (forward) 5’-gcaaagcaccaatgtagcaa-3’, (reverse) 5’-tttggaggaaaggtgaggtg-3’; Claudin-5, (forward) 5’- atttcagcttcccggtgaagtactcg-3’, (reverse) 5’- cgcctcagacgtagttcttcttgt-3’. Real time RT-PCR was carried out in two steps. First, total RNA was extracted from lung tissues or cell lysates with the RNeasy kit (Qiagen), and 1 µg total RNA was reverse-transcribed with the QuantiTect Reverse Transcription Kit (Qiagen) in a total volume of 20 µl. Quantitative real-time PCR was conducted on Mx4000 (Agilent Technologies, Santa Clara, CA, USA) with 2 µl of RT product, 12.5 µl of QuantiTect SYBR Green PCR Master Mix (Qiagen), and primers (400 nM) in a total volume of 25 µl. The following thermocycling conditions were used: 95°C for 10 min, followed by 95°C for 30 s, 55°C for 60 s, and 72°C 30 s for 45 cycles. Each set of primers displayed a single melting peak and PCR reaction efficiency between 90% and 110%. The threshold cycles (Ct) of a serially diluted control sample were plotted to generate a standard curve. Each sample was calculated by interpolating its Ct on the standard curve and then normalized to β-actin mRNA levels.
Western Blot analysis
Cells or peripheral lung tissue were lysed in Triton X-100 lysis buffer (containing protease- and phosphatase- inhibitors), as we have previously described (Aggarwal et al., 2013). Homogenates were then centrifuged at 20,000 g at 4°C for 20min, the tissue supernatant was collected, and protein concentration was quantified by the BCA Protein Assay (Thermo Fisher Scientific). Tissue and cell extracts (50 µg) were resolved using 4–20% Tris-SDS-Hepes PAGE, electrophoretically transferred to Immuno-Blot PVDF membrane (Bio-Rad Laboratories, Hercules, CA, USA), and then blocked with 5% nonfat dry milk in Tris-buffered saline. The membranes were probed with antibodies against Sox18 (1:250 dilution), Sox7 (1:500 dilution), Claudin-5 (1:500 dilution), Claudin-1 (1:500 dilution), Claudin-3 (1:500 dilution), Claudin-12 (1:500 dilution), zonula occludens-1 (ZO-1) (1:250 dilution), vascular endothelial growth factor (VEGF) (1:500), transforming growth factor beta 1 (TGF-β1) (1:500), and thrombin (1:500). Protein expression was normalized by re-probing with anti β-actin (1:1000). T24 cell lysate (Santa Cruz biotechnology) was used as a positive control for Sox7, Claudin-1, Claudin-3, and Claudin-12, according to the manufacturer’s instructions. Reactive bands were visualized using chemiluminescence (Thermo Fisher Scientific) on a Kodak 440CF image station. Band intensity was quantified using Kodak 1D image processing software.
Measurements of transendothelial monolayer resistance
The transmonolayer electrical resistance of PAEC grown on gold electrodes was measured with the electrical cell impedance sensor technique, as previously described (Catravas et al., 2010) using the electrical cell-substrate impedance sensing (ECIS) system (Applied Biophysics, Albany, NY, USA). After 24 h of transfection with either pCMV6-Sox18, Sox18 siRNA, or Claudin-5 siRNA, PAEC were trypsinized, counted, and seeded at equal densities (5 × 105 cells/ml) into eight-well ECIS plates (400 µl/well). The ECIS plates were placed into the cell culture incubator for 48 h to monitor the changes in electrical resistance. As the cells became confluent and the electrical resistance achieved a steady state. For additional experiments, PAEC were transfected with pCMV6 or pCMV6-Sox18 for 24 h, plated onto ECIS arrays as described, and then, after 48 h post-transfection, the PAEC were exposed to VEGF (500ng/ml), thrombin (100nM), or TGF-β1 (10ng/µl) for the indicated amount of time. The resistance data were normalized to the initial voltage and plotted as normalized resistance.
Effects of laminar shear stress on electrical resistance of PAEC monolayers
The effects of laminar shear stress on the permeability of PAEC monolayers were assessed by the ECIS technique, as previously described (DePaola et al., 2001; Shikata et al., 2005). This apparatus consists of a flow array, two media reservoirs, and a pump that propels the medium through the flow array via 1.6 mm gas-permeable silicone tubing. The ECIS flow array consists of eight 250 µm diameter electrodes located at the base of a flow channel measuring 50 mm long, 5 mm in width, and 0.4 mm in height. The medium is pumped from the outlet reservoir into the inlet reservoir, enters the flow chamber over the PAEC seeded onto the electrodes, and exits via the outlet reservoir. The shear stress is determined using the equation τ = (6Uµ)/H, where τ is the shear stress in dyn/cm2, U is the flow velocity in cm/s, µ is the viscosity of the culture medium (0.009 P), and H is the height of the chamber (0.04 cm). The flow rate (ml/min) of a Heidolph pump 5201 was set to deliver the desired flow velocity (flow rate [ml/min] divided by 60 and divided again by the cross sectional area of the channel in cm2 [0.02 cm2]) necessary to achieve 5 or 20 dyn/cm2. After 24 h of transfection with either pCMV6-Sox18, Sox18 siRNA, or Claudin-5 siRNA, PAEC were trypsinized, counted, and seeded at equal densities (5 × 105 cells/ml) into the flow ECIS array (300 µl/array). For rescue experiments, cells were transfected with either scrambled siRNA or Claudin-5 siRNA for 24 h. Then, the cells were transfected with either pCMV6 or pCMV6-Claudin-5. Twenty-four hours later, the cells were seeded into the flow ECIS array for analysis, as described above. The ECIS arrays were placed into the cell culture incubator for 24 h to equilibrate under no-flow conditions. Prior to the onset of flow, thirty minutes of resistance data were collected to establish the baseline values. All monolayers were exposed to laminar shear stress of 5 or 20 dyn/cm2, and data were collected continuously during the exposure to flow. The resistance data were normalized to the initial voltage and plotted as normalized resistance.
Cell Imaging
For immunofluorescence experiments, PAEC monolayers were seeded on collagen covered coverslips and transfected for 48 h with either pCMV6-Sox18, Sox18 siRNA, or Claudin-5 siRNA. The cells were washed with 1xPBS and incubated in serum free medium overnight prior to the experiment. The cells were then subjected or not to 20 dyn/cm2 of shear stress for 3 h, briefly washed with 1× PBS containing Ca2+ and Mg2+, and subsequently fixed in a PBS solution containing 4% paraformaldehyde (15 min), as previously described (Aggarwal et al., 2013). After permeabilization using 0.25% Triton X-100 (30 min) and blocking in 5% BSA (1 h), the cells were stained with either a zonula occludens-1 (ZO-1) specific antibody conjugated with Alexa Fluor 594 (1:100) or a VE-cadherin specific antibody (1:100) followed by incubation with Alexa Fluor 594 or Alexa Fluor 488 conjugated donkey anti-rabbit antibodies (1:200). Stress fibers were stained with either Alexa Fluor 350 conjugated phalloidin (1:200) or Texas Red-X conjugated phalloidin (1:200). The coverslips were mounted in anti-fade media, viewed at 40×, and photographed with a Zeiss Axio Observer video imaging system using Zeiss Axiovision software. Inter- and intra- cellular gap analysis was performed using ImageJ software. Gaps within each image were outlined, and the total gap area (µm2) was calculated and used for statistical analysis.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed using PAEC (~1.0×106) cultured in 10cm plates, serum starved overnight and then exposed to shear stress (20 dyn/cm2, 3h) using the ChIP Assay Kit (EMD Biosciences, Philadelphia, PA, USA), as previously described (Kumar et al., 2008). DNA-protein interactions were cross-linked with 1% formaldehyde for 10 min at 37°C. The cells were harvested, and the lysates were sonicated to 200–1000bp fragments. The supernatants were incubated overnight at 4°C with either an anti-Sox18 antibody (7µg/sample) or rabbit IgG (as a negative control). After the DNA was precipitated with ethanol, it was re-suspended in 20µL of dH2O, and the DNA concentration was determined. Equal concentrations of DNA (65ng) were used as PCR template. The Sox18 binding site in the human Claudin-5 promoter is located at chromosome 22 position 19513030–19513036 (171–177 bp upstream of the transcriptional start site). The primer pairs used to amplify the Sox18 binding site in the Claudin-5 promoter were (forward) 5’-aggagagacaaagggacacg-3’ and (reverse) 5’-acaccagtggacctttcgag-3’, resulting in a fragment of 218bp. A positive control (input) was obtained from sonicated lysates that underwent reverse cross-link and phenol/chloroform extraction.
Cell counting
PAEC were grown in 10cm plates to 75% confluence, transfected with pCMV6-Sox18 or Sox18 siRNA, as previously described, and incubated at 37°C for 24 h. The cells were trypsinized and seeded onto 6-well plates at densities of 5.5 × 105 and 5.0 × 105 cells per well, respectively. The cells were then counted after 24 h and 48 h. To evaluate cellular proliferation under shear conditions, the cells were allowed to adhere to the 6-well plates for 24 h, subjected to 6 h and 12 h of shear stress (20 dyn/cm2), trypsinized, and then counted with a hemocytometer (Cascade Biologicals, Portland, OR, USA).
TUNEL Assay
PAEC monolayers were seeded onto coverslips and transfected for 48 h with either pCMV6, pCMV6-Sox18, scrambled siRNA, or Sox18 siRNA. The cells were washed with PBS then incubated in serum free medium overnight prior to the experiment. The cells were then exposed or not to shear stress (20 dyn/cm2, 3 h). Following treatment, the cells were fixed in PBS solution containing 4% paraformaldehyde (15 min). The cells were analyzed for the presence of apoptotic nuclei using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI), according to the manufacturer’s instructions. Images were captured using fluorescent microscopy using the 20X objective lens. The TUNEL and DAPI stained nuclei were quantified using ImageJ software. The results are presented as a percentage of TUNEL positive/total nuclei in the field, as previously described (Sun et al., 2014).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 4.01 (GraphPad Software, San Diego, CA, USA). The mean ± SEM was calculated in all experiments, and statistical significance determined either by the unpaired t-test (for 2 groups), oneway ANOVA (for ≥ 3 groups) with Newman-Kuels post-hoc testing, or two-way ANOVA with Bonferroni post-hoc testing. A value of p < 0.05 was considered significant.
RESULTS
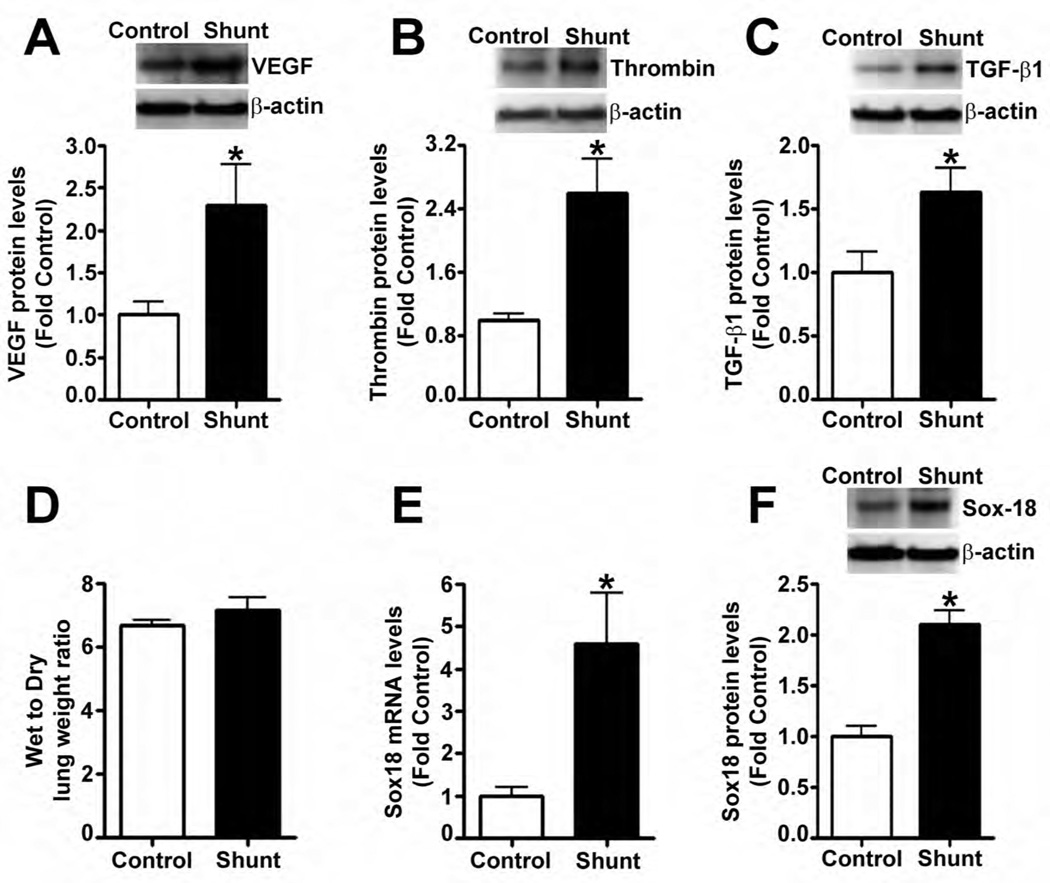
Previous studies have shown that fluid shear stress enhances pulmonary vascular barrier integrity (Birukov et al., 2002; Seebach et al., 2000; Shikata et al., 2005). However, little is known about the mechanisms by which shear regulates endothelial barrier function. We have developed an animal model of increased pulmonary shear stress by placing an aorta-pulmonary artery vascular graft in the late gestational fetal lamb (shunt) (Reddy et al., 1995). At 4-weeks of age, these shunt lambs had elevated levels of endothelial barrier disruptive agents, VEGF (Fig. 1 A), thrombin (Fig. 1 B), and TGF-β1 (Fig. 1 C); however, the lung leak in these lambs was not different than age matched control lambs (Fig. 1 D). From previously published microarray data, we have identified the angiogenesis-related transcription factor, Sox18, as being up-regulated in 3-day old shunt lambs (Tian et al., 2011). Utilizing quantitative real-time RT-PCR (qRT-PCR), we confirmed the up-regulation of Sox18 at the mRNA (Fig. 1 E) and protein (Fig. 1 F) level in peripheral lung tissue of 4-week old shunt lambs.
Fig. 1. Lung barrier function is not disrupted in lambs with increased pulmonary blood flow.
Protein extracts prepared from the peripheral lung tissue of 4-week old shunt and age matched control lambs were subjected to immunoblot analysis using a specific antiserum raised against vascular endothelial growth factor (VEGF), thrombin, and transforming growth factor (TGF)-β1. Densitometric analysis indicated that VEGF (A), thrombin (B), and TGF-β1 (C) protein levels were significantly increased in shunt compared to control lambs. The accumulation of water in lung tissue was used to determine barrier function in vivo. The wet to dry lung weight ratio of shunt lambs was not different than age matched control lambs (7.1 ± 1.4 in 12 shunts vs. 6.7 ± 0.5 in 11 control lambs; p = 0.3) (D). Total RNA was isolated from the peripheral lung tissues of 4-week old shunt or control lambs. Messenger RNA levels for Sox18 were elevated in shunt lambs compared to age matched control lambs, as determined by SYBR Green real-time RT-PCR analysis (E). The protein levels of Sox18 were significantly increased in peripheral lung tissue of shunt lambs (F). Values are mean ± SEM, n=4–12. *p<0.05 vs. sham operated control.
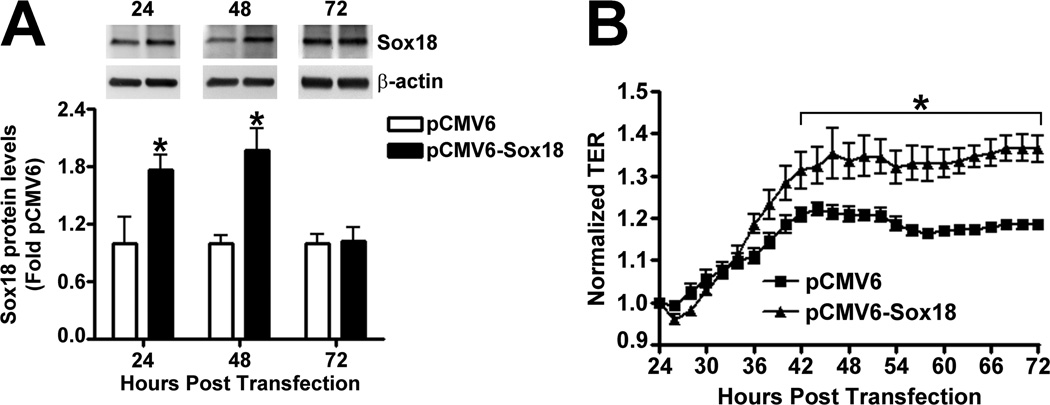
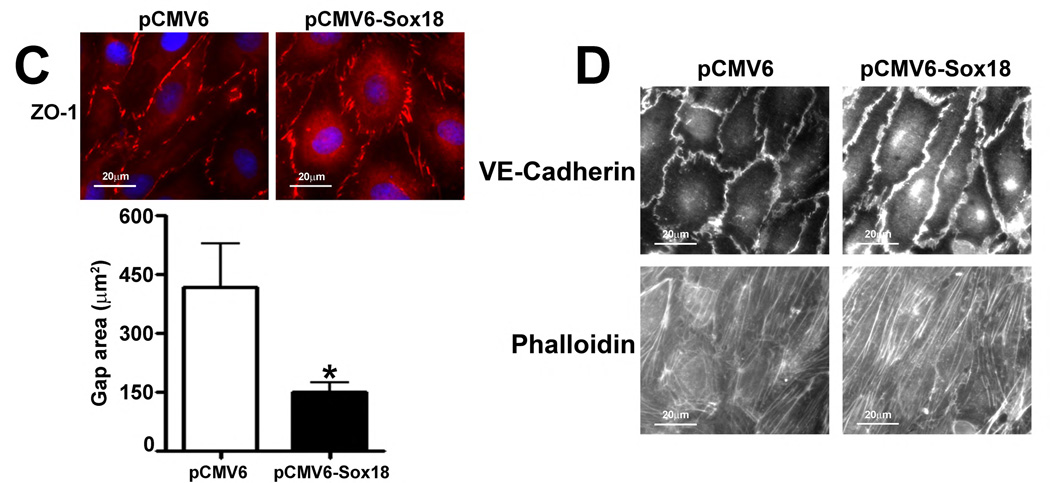
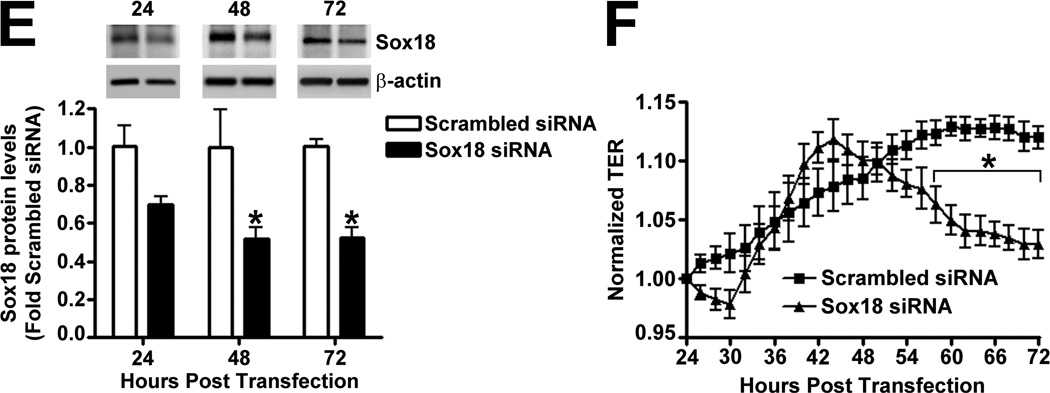
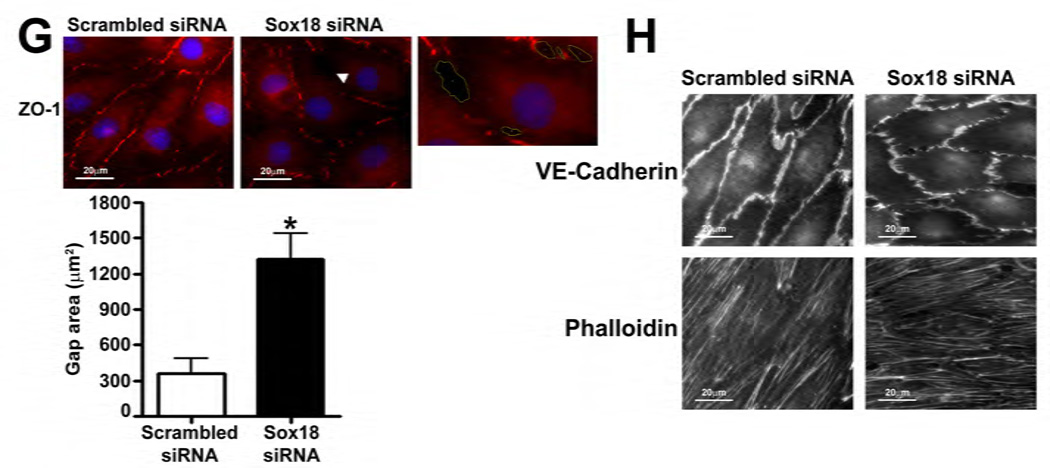
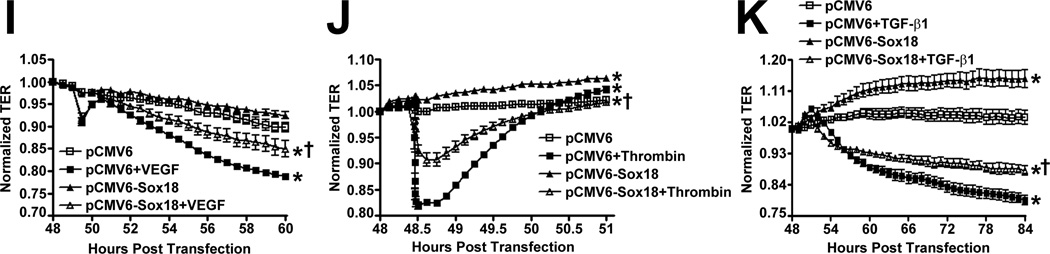
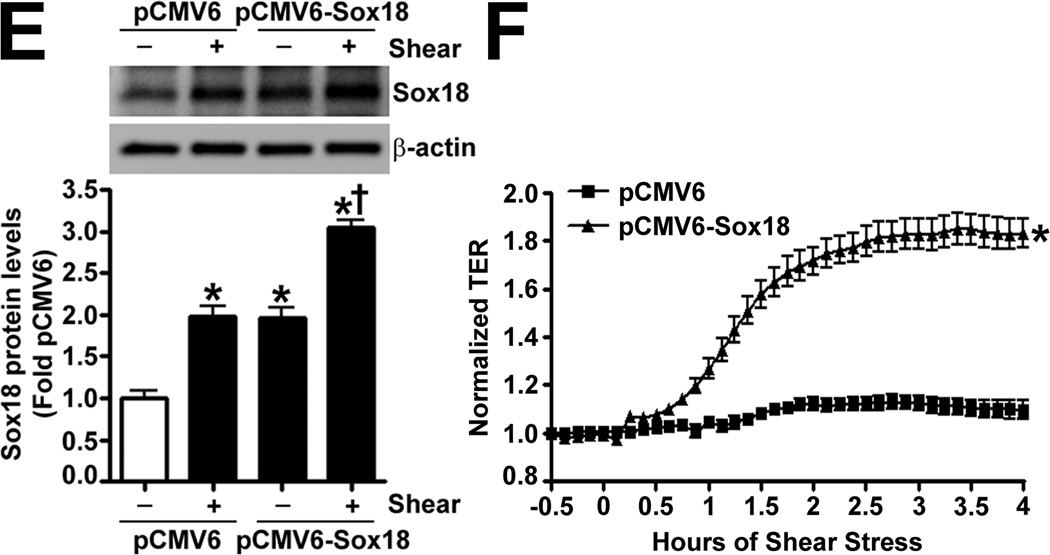
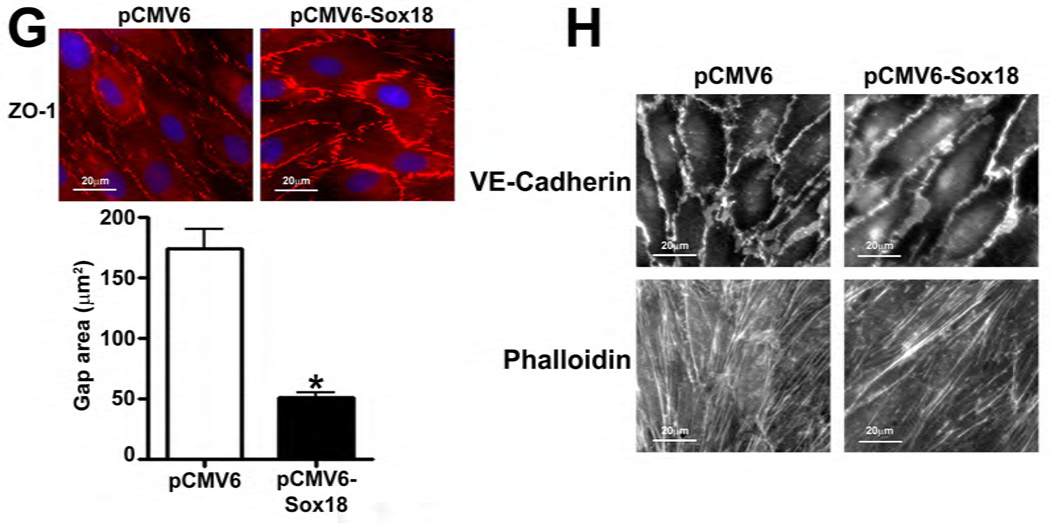
To determine if Sox18 regulates endothelial barrier function, we over-expressed the transcription factor in PAEC (Fig. 2 A) and found that the transendothelial electrical resistance (TER) of confluent endothelial monolayers was significantly enhanced after 48 h of transfection (Fig. 2 B). Similarly, intercellular gaps were reduced in PAEC monolayers expressing Sox18, as indicated by ZO-1 immunostaining of tight junctions (Fig. 2 C). Sox18 over-expression also produced an increase in VE-cadherin staining, although no changes in stress fiber formation were observed (Fig. 2 D). Conversely, the silencing of Sox18 expression in PAEC (Fig. 2) resulted in decreased TER (Fig. 2) resulted in decreased TER (Fig. 2 E) resulted in decreased TER (Fig. 2 F), an increase in intercellular gap formation (Fig. 2 G), and disrupted VE-cadherin staining, and again no changes in stress fiber formation were observed (Fig. 2 H). In addition, Sox18 over-expression in PAEC attenuated the VEGF (Fig. 2 I), thrombin (Fig. 2 J), and TGF-β1 (Fig. 2 K) induced drop in TER. These results suggested that Sox18 plays a significant role in maintaining endothelial barrier function.
Fig. 2. Sox18 protects endothelial barrier function in pulmonary artery endothelial cells.
PAEC were transfected with either pCMV6 or pCMV6-Sox18 for 24–72 h. Immunoblot analysis indicated an increase in Sox18 protein levels after 24 h and 48 h of transfection (A). PAEC monolayers grown on gold microelectrodes (B) or glass coverslips (C & D) were transfected with either pCMV6 or pCMV6-Sox18. After 42 h post transfection, the normalized trans-endothelial resistance (TER) was significantly increased in pCMV6-Sox18 transfected PAEC (B). In addition, immunofluorescent staining of tight junctions was performed using an Alexa Fluor 594 conjugated Zonula occludens-1 (ZO-1) antibody; the nuclei were stained with DAPI. After 48 h, the over-expression of pCMV6-Sox18 decreased inter-cellular gap formation (C). Immunofluorescent analysis of adherens junctions indicated increased VE-cadherin staining but no changes in stress fiber formation (D). In contrast, the siRNA mediated knockdown of Sox18 decreased Sox18 protein levels after 48 and 72 h (E) and the TER after 58 h of transfection (F). Additionally, the immunofluorescent staining of inter-cellular tight junctions demonstrated that, after 48 h, the Sox18 depleted monolayers displayed enhanced gap formation (G). Similarly, these cells displayed a loss of junctional VE-cadherin staining but no alterations in stress fiber formation (H). Further, when the PAEC transfected with pCMV6 were exposed to VEGF (500ng/ml) (I), thrombin (100nM) (J), or TGF-β1 (10ng/µl) (K) for the indicated amount of time, there was a gradual decrease in TER. However, the transfection of cells with pCMV6-Sox18 attenuated the decline in TER induced by VEGF (I), thrombin (J), and TGF-β1 (K). Values are mean ± SEM, n=3–6. *p<0.05 vs. pCMV6 (AC, I–K) or Scrambled siRNA (E–G); † P<0.05 vs. pCMV6+VEGF (I), pCMV6+Thrombin (J), pCMV6+TGF-β1 (K).
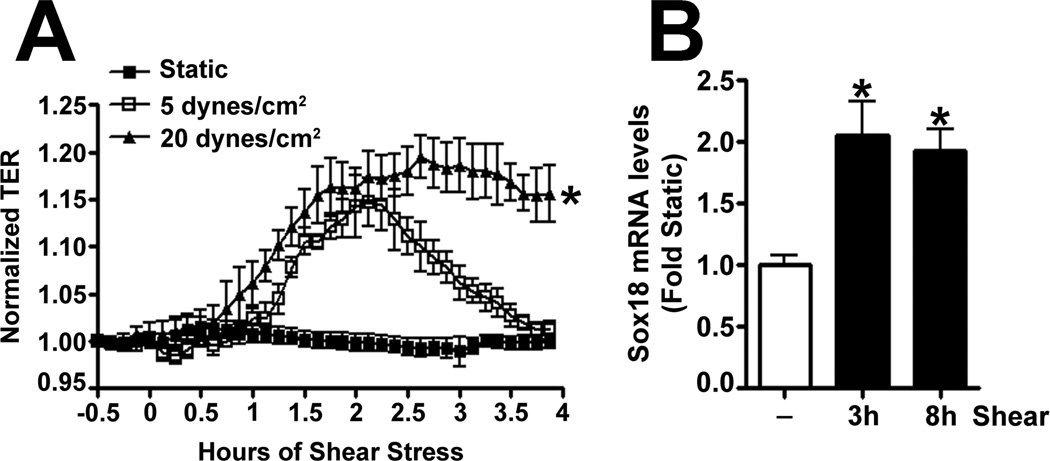
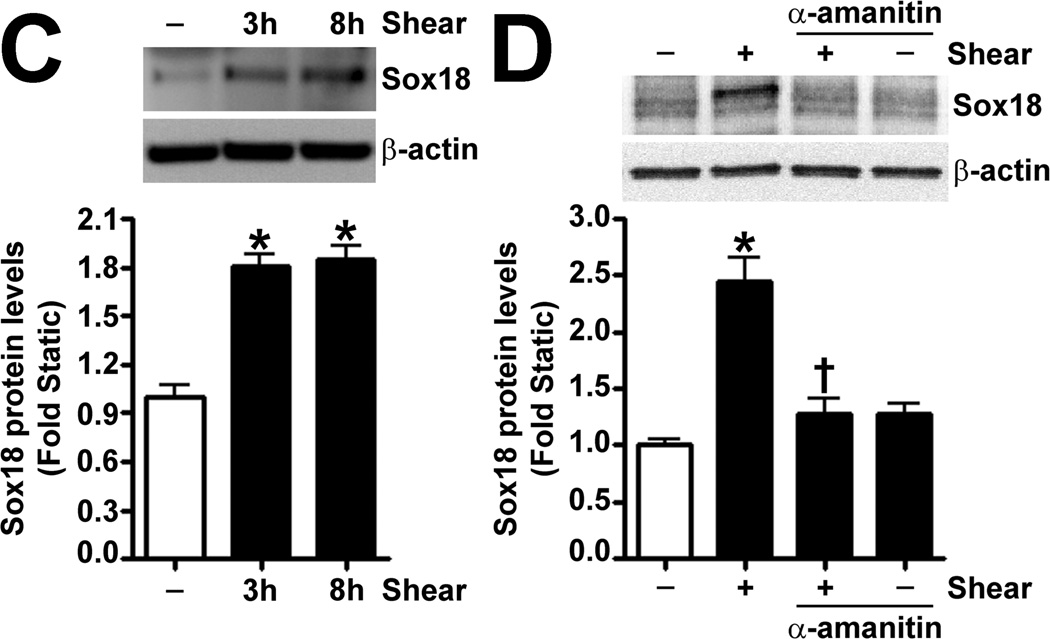
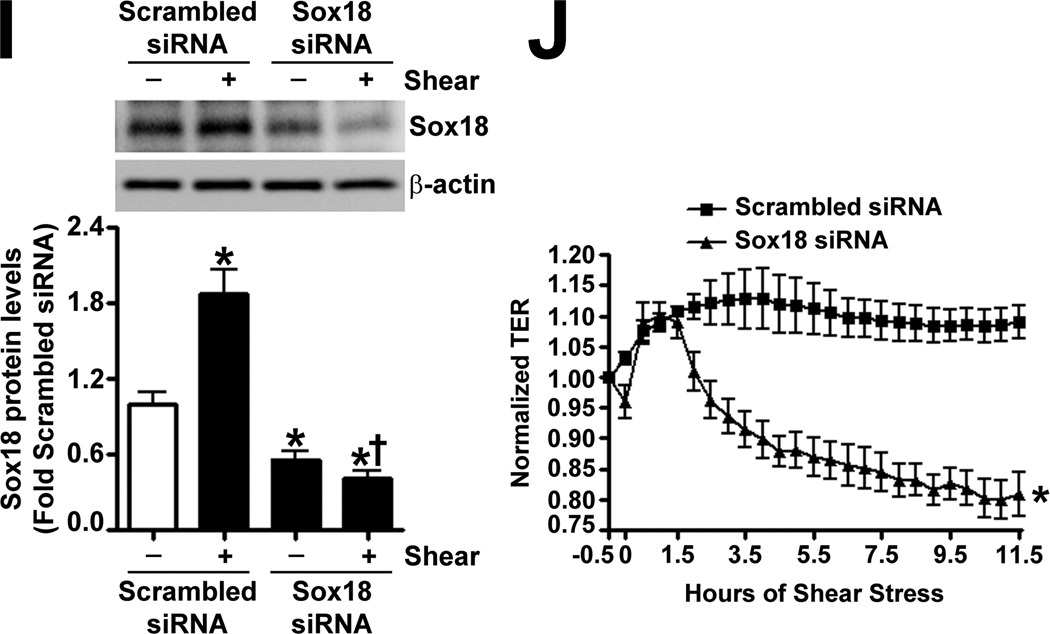
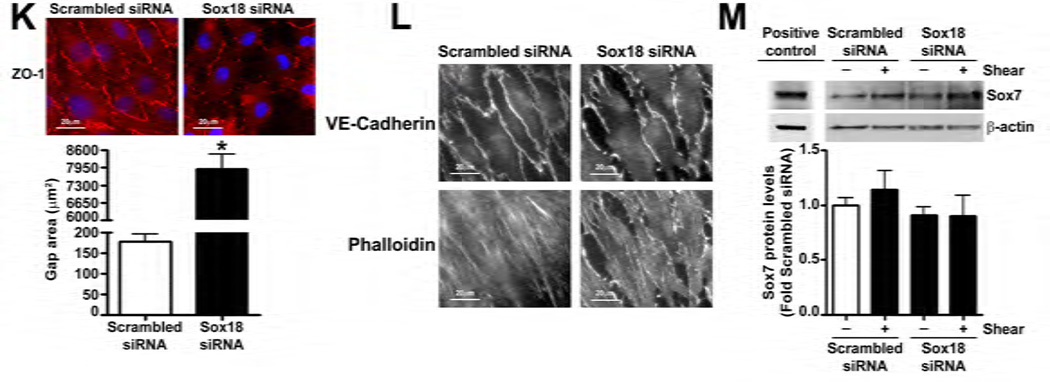
To determine if shear stress regulates Sox18 levels, we subjected PAEC to 5- and 20-dyn/cm2 of shear and found a sustained increase in TER at 20 dyn/cm2 (Fig. 3 A), which corresponded to an increase in Sox18 mRNA (Fig. 3 B) and protein levels (Fig. 3 C). Further, 24 h pre-treatment with an RNA polymerase inhibitor, α -amanitin (10 µg/mL), prevented the increase in Sox18 protein levels upon shear (Fig. 3 D), supporting the conclusion that shear stress increases Sox18 expression by increasing its transcription. In addition, the over-expression of Sox18 in PAEC subjected to shear stress (Fig. 3 E) enhanced TER (Fig. 3 F), reduced intercellular gaps (Fig. 3 G), and increased VE-cadherin staining (Fig. 3 H) compared to pCMV6 transfected cells (Fig. 3 H). Again, no changes in stress fibers were observed (Fig. 3 H). Conversely, the siRNA-mediated knockdown of Sox18 (Fig. 3 I) abrogated the shear induced increase in TER (Fig. 3 J), increased transendothelial gap formation (Fig. 3 K), and reduced VE-cadherin staining (Fig. 3 L). Stress fibers did not appear to be influenced by Sox18 depletion (Fig. 3 L). In addition, there were no changes to Sox7 protein levels upon Sox18 knockdown in the presence of shear (Fig. 3 M). These findings suggest that shear augments Sox18 levels, which in turn enhances the shear dependent tightening of endothelial barrier.
Fig. 3. Shear stress induced endothelial barrier protection is mediated by Sox18 in pulmonary artery endothelial cells.
Under 5 and 20 dyn/cm2 of shear stress, the trans-endothelial resistance (TER) across PAEC monolayers increased sharply with a maximal increase in resistance at 3 h; however, the resistance declined back to static control levels in the presence of 5 dyn/cm2, while the TER remained above the static control in the presence 20 dyn/cm2 of shear stress (A). Further, in comparison to static conditions, PAEC subjected to 20 dyn/cm2 of shear stress for 3 and 8 h had significantly higher Sox18 mRNA expression, as determined by SYBR Green real-time RT-PCR analysis (B) and protein levels (C), as indicated by immunoblot analysis. The increase in Sox18 protein levels after 3 h of shear was abrogated by 24 h pre-treatment with the transcriptional inhibitor, α-amanitin (D). Sox18 protein levels were further increased in PAEC transfected with pCMV6-Sox18 for 48 h and then exposed to 20 dyn/cm2 of shear stress for 3 h (E). The transfection of PAEC with pCMV6-Sox18 prior to exposure to 20 dyn/cm2 of shear stress resulted in a significant increase in the normalized TER compared to pCMV6 transfected cells (F). In addition, immunofluorescent staining with Alexa Fluor 594 conjugated Zonula occludens-1 (ZO-1) antibody and nuclear staining with DAPI indicated reduced gap formation in the cells transfected with Sox18 for 48 h and then subjected to 20 dyn/cm2 of shear stress for 3 h (G). Similarly, immunofluorescent analysis of adherens junctions showed enhanced VE-cadherin staining, although stress fiber formation was unchanged (H). In contrast, the shear (3h, 20 dyn/cm2) mediated increase in Sox18 protein levels was mitigated in the presence of Sox18 siRNA (I). PAEC transfected with an Sox18 siRNA exhibited a decrease in normalized TER (J), enlarged inter-cellular gaps (K), and diminished VE-cadherin fluorescent signal (L) upon exposure to 20 dyn/cm2 of shear stress compared to scrambled siRNA transfected cells. Sox7 protein levels were not altered by Sox18 depletion in the presence or absence of shear (M). Values are mean ± SEM, n=3–8. *p<0.05 vs. Static (A-D), pCMV6 (E-G), Scrambled siRNA (I-K, M); † P<0.05 vs. pCMV6+Shear (E) or Scrambled siRNA+Shear (I).
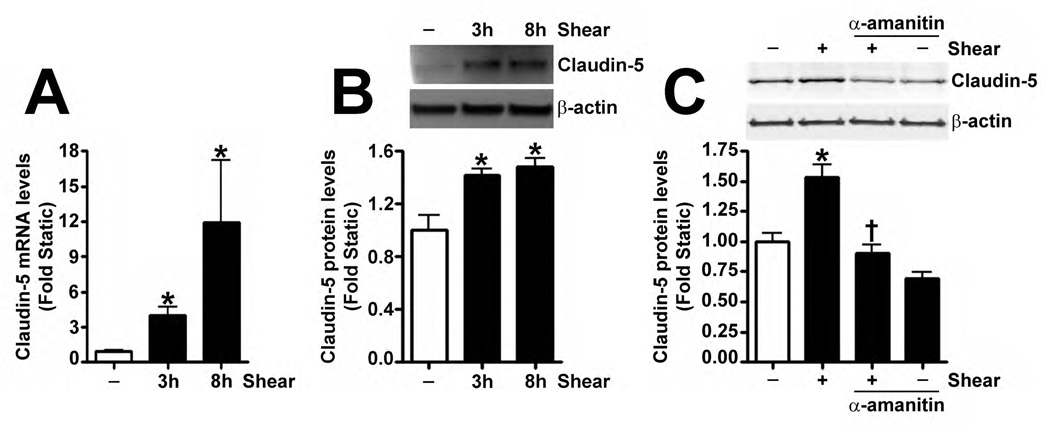
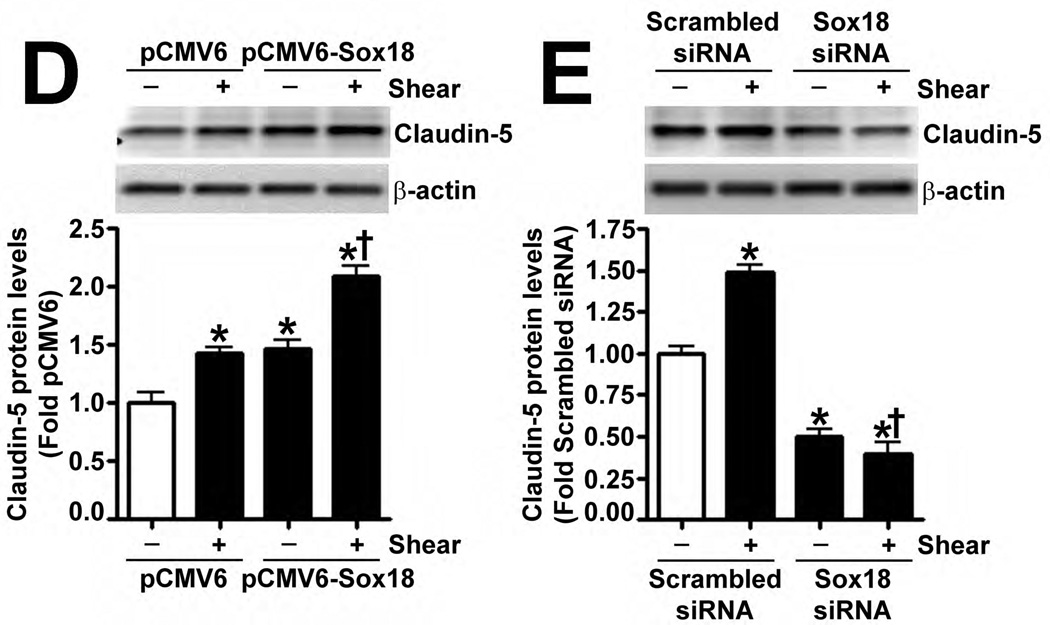
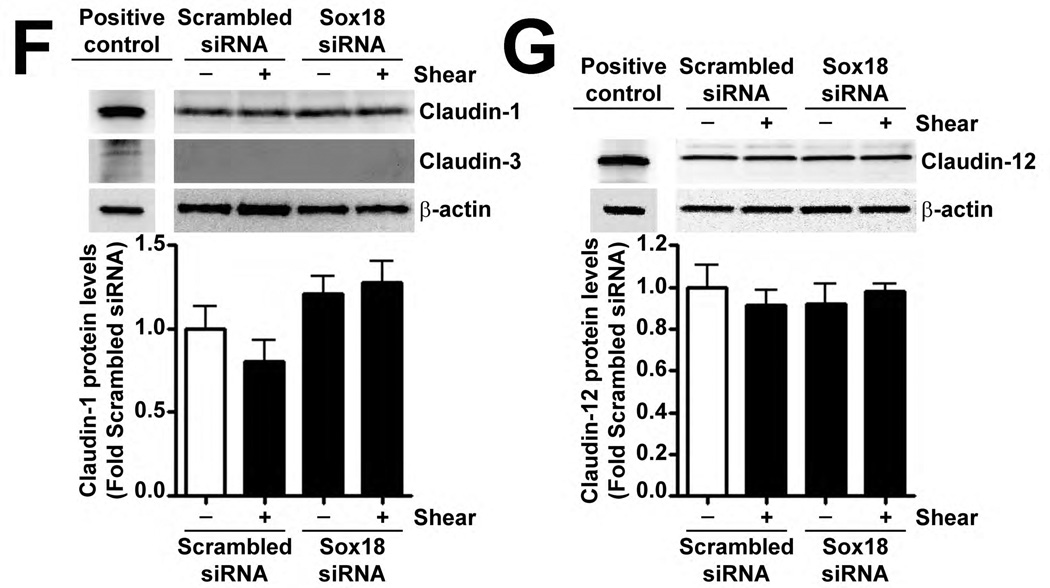
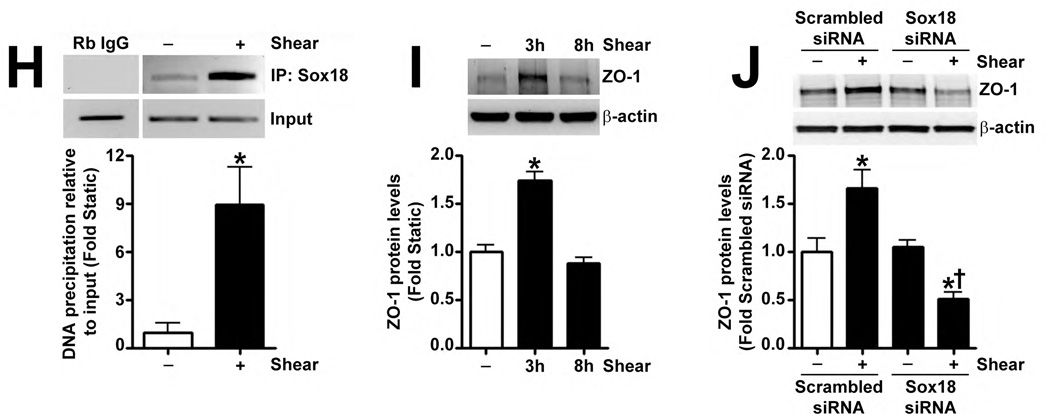
Recently, Sox18 has been shown to up-regulate the tight junction protein, Claudin-5, in HUVECs (Fontijn et al., 2008). Thus, we investigated whether the shear mediated up-regulation of Sox18 affected Claudin-5 levels in PAEC. The exposure of these cells to 20 dyn/cm2 of shear for 3 h and 8 h resulted in an increase in Claudin-5 mRNA (Fig. 4 A) and protein (Fig. 4 B) levels. Further, 24 h pre-treatment with an RNA polymerase inhibitor, α-amanitin (10 µg/mL), prevented the increase in Claudin-5 protein levels upon shear (Fig. 4 C), supporting the conclusion that shear stress increases Claudin-5 expression by increasing its transcription. The over-expression of Sox18 in these cells further enhanced Claudin-5 protein levels in the presence of shear (Fig. 4 D), while the knockdown of Sox18 attenuated the shear mediated increase in Claudin-5 protein levels (Fig. 4 E). However, Sox18 depletion did not alter Claudin-1 (Fig. 4 F) or Claudin-12 (Fig. 4 G) protein levels in the presence of shear. Claudin-3 was not detected (Fig. 4 F). Additionally, using ChIP analysis, we found that shear significantly enhanced the binding of Sox18 to the endogenous Claudin-5 promoter (Fig. 4 H). Further, we found that shear stress transiently increased the levels of the Zonula occludens-1 (ZO-1) protein (Fig. 4 I), while decreasing Sox-18 levels with a specific siRNA, attenuated the ability of shear stress to increase ZO-1 expression (Fig. 4 J).
Fig. 4. Shear stress up-regulates the cellular tight junction proteins, Claudin-5 and Zonula occludens-1, in a Sox18 dependent manner in pulmonary artery endothelial cells.
PAEC were subjected to static conditions or 20 dyn/cm2 of shear stress for 3 and 8 h. Messenger RNA levels for Claudin-5 were significantly higher in PAEC exposed to shear compared to static culture conditions, as determined by SYBR Green real-time RT-PCR analysis (A). Similarly, immunoblot analysis indicated that Claudin-5 protein levels were significantly higher in PAEC exposed to 3h and 8h of 20 dyn/cm2 of shear stress (B). The increase in Claudin-5 protein levels after 3 h of shear was abrogated by 24 h pre-treatment with the transcriptional inhibitor, α-amanitin (C). PAEC transfected with pCMV6-Sox18 or Sox18 siRNA for 48 h were exposed to 3 h of 20 dyn/cm2 of shear stress. Immunoblot analysis showed that the over-expression of Sox18 potentiates (D), while the knockdown of Sox18 attenuates (E), the shear mediated increase in Claudin-5 levels. Claudin-1 (F) and Claudin-12 (G) protein levels were not altered by Sox18 depletion in the presence or absence of shear. Claudin-3 was not detected (F). Chromatin immunoprecipitation indicated that shear stress induces a significant increase in the binding of Sox18 to the endogenous Claudin-5 promoter (H). Immunoblot analysis also indicated that Zonula occludens-1 (ZO-1) protein levels were significantly increased in PAEC exposed to 3h of 20 dyn/cm2 of shear stress compared to static controls (I). In addition, PAEC were transfected with Sox18 siRNA for 48 h and exposed to 3 h of 20 dyn/cm2 of shear stress. Immunoblot analysis showed that the silencing of Sox18 attenuates the shear mediated increase in ZO-1 protein levels (J). Values are mean ± SEM, n=4–6. *p<0.05 vs. Static (A-C, H, I), pCMV6 (D), Scrambled siRNA (E-G, J); † P<0.05 vs. pCMV6+Shear (D) or Scrambled siRNA+Shear (E, J).
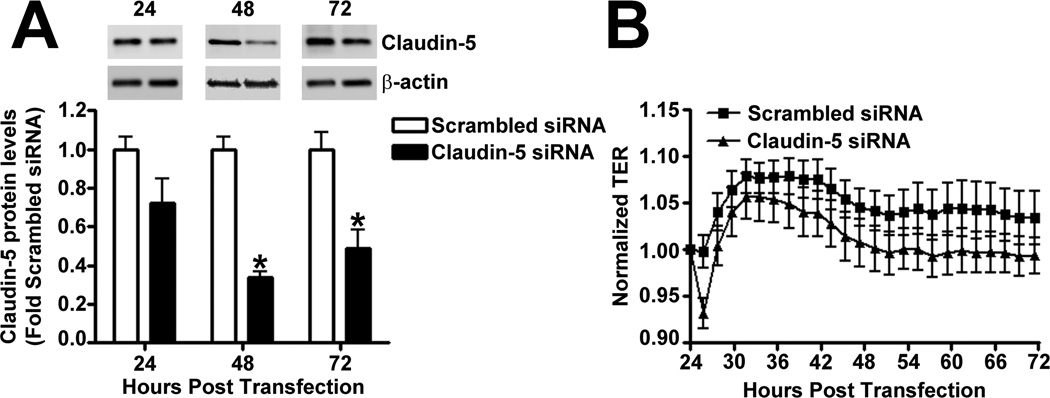
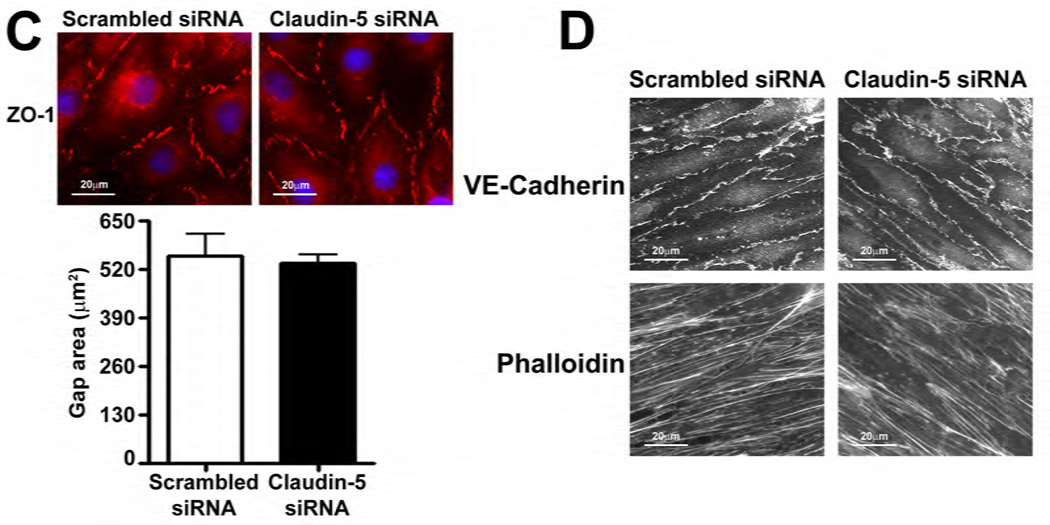
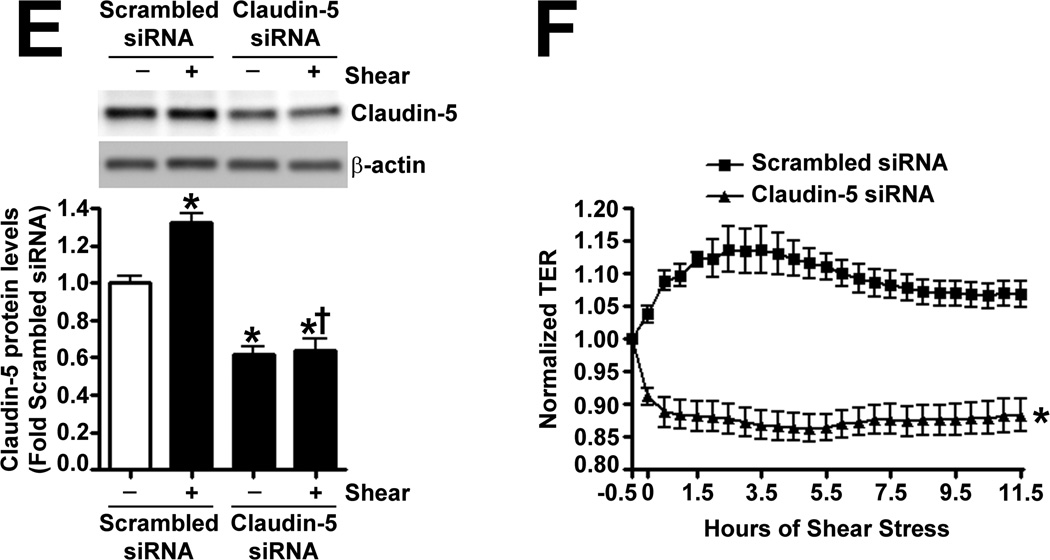
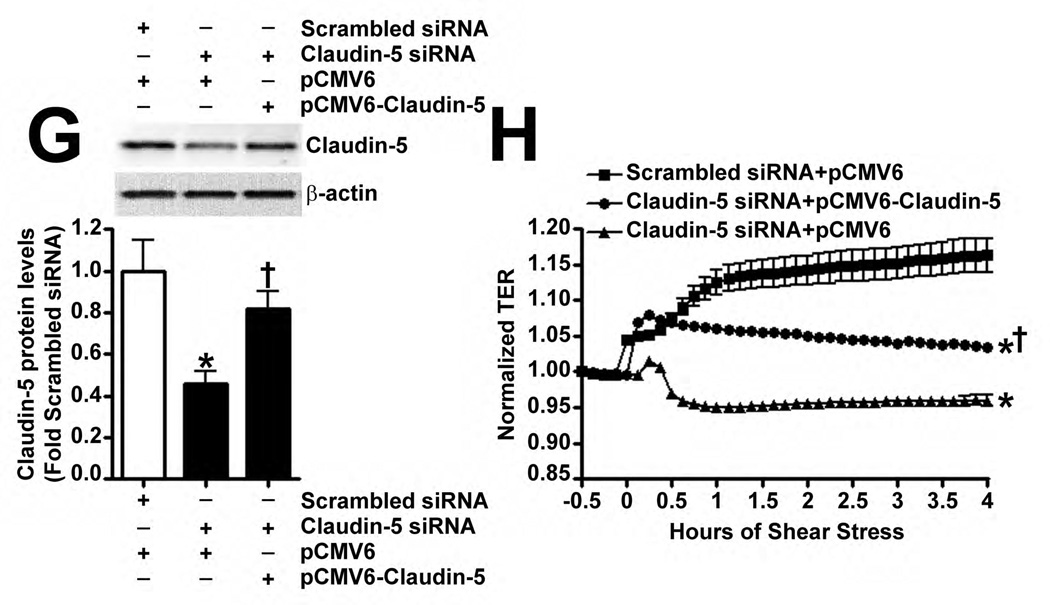
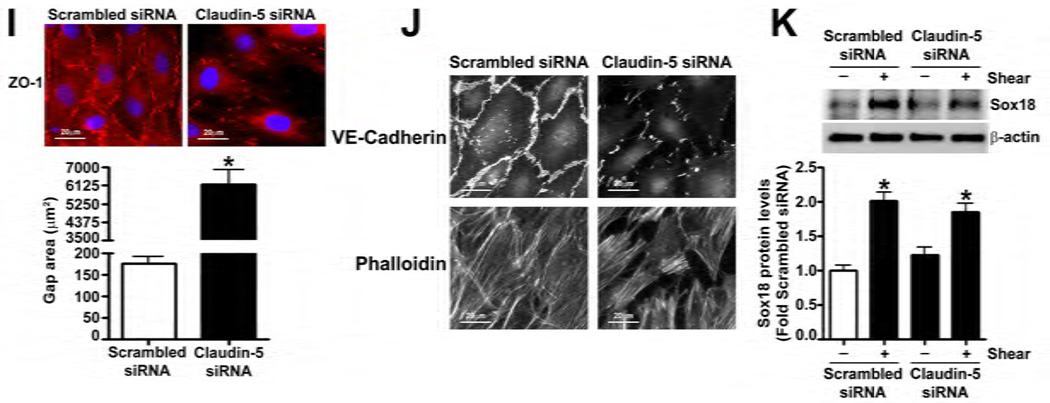
The effect of Claudin-5 on the endothelial barrier was established by silencing Claudin-5 in PAEC using siRNA (Fig. 5 A). Under static conditions, Claudin-5 depletion did not significantly alter normalized TER (Fig. 5 B), intercellular gap formation (Fig. 5 C), VE-cadherin staining (Fig. 5 D), or stress fiber formation (Fig. 5 D). When Claudin-5 protein levels were decreased, using an siRNA approach (Fig. 5 E), and the cells exposed to shear stress, there was a significant drop in TER (Fig. 5 F). However, when Claudin-5 expression was restored, by transfecting the cells with a Claudin-5 mammalian expression plasmid, (Fig. 5 G), barrier function increased with shear (Fig. 5 H). The knockdown of Claudin-5 under shear also increased intercellular gap formation (Fig. 5 I) and disrupted VE-cadherin staining (Fig. 5 J) but did not alter stress fiber formation (Fig. 5 J), even though Sox18 levels remained elevated with shear (Fig. 5 K). This suggests that the Sox18 dependent barrier enhancement under shear stress is mediated by Claudin-5.
Fig. 5. Shear stress enhances the endothelial barrier through the Sox18 mediated increase in Claudin-5 in pulmonary artery endothelial cells.
PAEC were transfected with either Scrambled siRNA or Claudin-5 siRNA for 24–72 h. Immunoblot analysis indicated a decrease in Claudin-5 protein levels after 48 h and 72 h of transfection (A) Under static conditions, the knockdown of Claudin-5 did not alter endothelial barrier function, as determined by the normalized transendothelial resistance (TER) (B) and by the formation of intercellular gaps (C) indicated by the immunostaining of the tight junction protein, Zonula occludens-1 (ZO-1), using an Alexa Fluor 594 conjugated ZO-1 antibody. No changes in VE-cadherin or stress fiber formation were observed (D). Under shear conditions (20 dyn/cm2, 3h), the silencing of Claudin-5 expression with Claudin-5 siRNA (E) resulted in an immediate decrease in TER compared to scrambled siRNA transfected cells (F). When Claudin-5 was over-expressed in the Claudin-5 depleted cells (G), the increase in barrier function with shear was partially restored (H). Claudin-5 depletion in the presence of shear stress (20 dyn/cm2, 3h) increased intercellular gap formation (I) and disrupted the pattern of VE-cadherin staining (J) but did not alter stress fiber formation (J). Further, immunoblot analysis showed that the exposure of PAEC to Claudin-5 siRNA did not attenuate the shear mediated increase in Sox18 protein levels (K). Values are mean ± SEM, n=3–7. *p<0.05 vs. Scrambled siRNA, Scrambled siRNA+pCMV6 (G-H); † P<0.05 vs. Scrambled siRNA+Shear, Claudin-5 siRNA+pCMV6 (G-H).
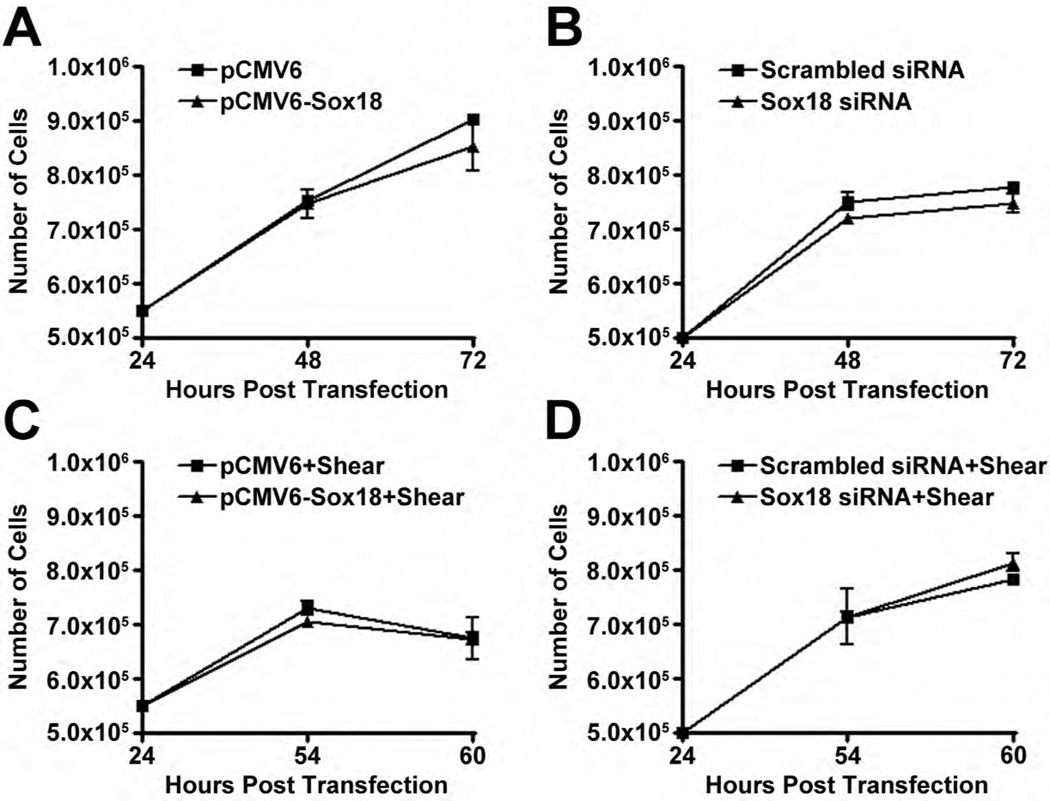
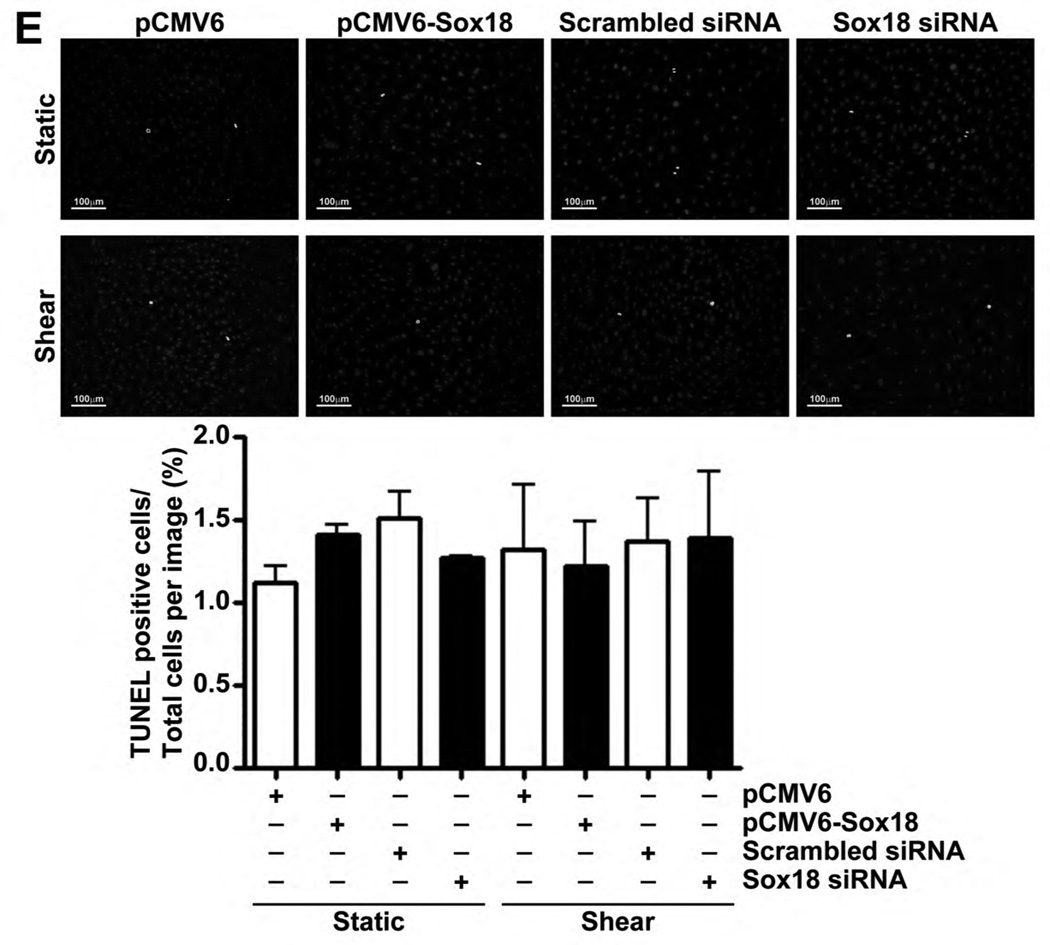
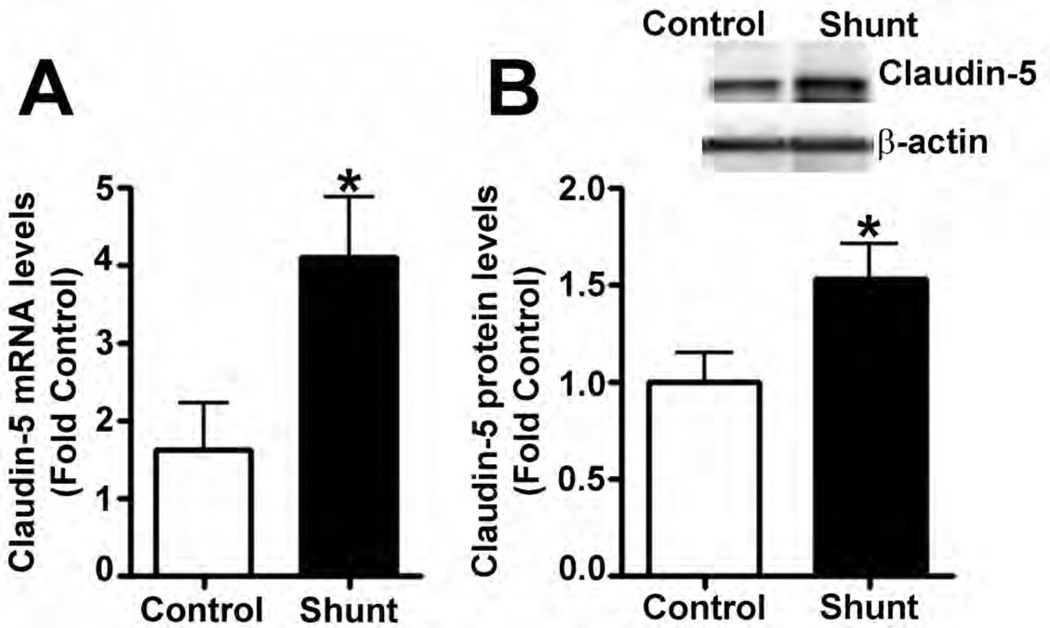
Our data also demonstrated that Sox18 over-expression or depletion did not influence cell numbers (Fig. 6 A–D) or apoptotic cell death (Fig. 6 E) under either static or shear conditions, indicating that Sox18 does not appear to influence barrier function by modulating cellular growth in PAEC. Finally, in our in vivo studies, we found that Claudin-5 mRNA (Fig. 7 A) and protein levels (Fig. 7 B) are elevated in the lungs of shunt, compared to age matched control, lambs. These results suggest that the Sox18 dependent increase in Claudin-5 may play a significant role in protecting the pulmonary endothelium under conditions of high PBF.
Fig. 6. The modulation of Sox18 expression does not alter the proliferation of pulmonary artery endothelial cells.
PAEC transfected with either pCMV6-Sox18 or Sox18 siRNA for 24 h were trypsinized, seeded onto a 6-well plate at a density of 5.5 × 105 or 5.0 × 105 cells per well, respectively, and grown for an additional 48 h at 37°C in an incubator. After 48 h and 72 h post transfection, the cellular proliferation was evaluated by counting the cell number with a hemocytometer. The PAEC expressing pCMV6-Sox18 (A) or Sox18 siRNA (B) showed no difference in their cell count compared to their respective controls. Further, PAEC transfected with either pCMV6-Sox18 or Sox18 siRNA for 24 h were trypsinized, seeded onto a 6-well plate at a density of 5.5 × 105 or 5.0 × 105 cells per well, respectively, and grown for an additional 24 h at 37°C in an incubator. The cells were then exposed to 6 h or 12 h of shear (20 dyn/cm2), and the cellular proliferation was evaluated by counting the cell number with a hemocytometer. The PAEC expressing pCMV6-Sox18 (C) or Sox18 siRNA (D) showed no difference in their cell counts compared to their respective controls. To evaluate apoptotic cell death, PAEC were transfected for 48 h with either pCMV6, pCMV6-Sox18, scrambled siRNA, or Sox18 siRNA. The cells were then subjected or not to shear (20 dyn/cm2, 3 h). No differences in cellular apoptosis were observed with either Sox18 over-expression or Sox18 silencing in the presence or absence of shear (E). Values are mean ± SEM, n=3.
Fig. 7. Claudin-5 expression is increased in shunt lambs.
Total RNA was isolated from peripheral lung tissues of 4-week old shunt or sham-operated control lambs. Messenger RNA levels for Claudin-5 were significantly increased in shunt lambs compared to age matched control lambs, as determined by SYBR Green real-time RT-PCR analysis (A). In addition, immunoblot analysis demonstrated that the protein levels of Claudin-5 were significantly elevated in the peripheral lung tissues of shunt lambs as compared to sham-operated control lambs (B). Values are mean ± SEM, n=5, *p<0.05 vs. sham-operated control.
DISCUSSION
Pulmonary endothelial cells can adapt to shear stress by modulating signaling events to protect against endothelial injury. In models of elevated PBF, there is little evidence of lung edema prior to the onset of cardiac failure (Alpan et al., 1989; Alpan et al., 1991; Sakuma et al., 2002), despite the presence of barrier disruptive agonists, such as VEGF, TGF-β1, and thrombin, suggesting that elevated pulmonary shear stress enhances the alveolar-capillary barrier. In our present study, we have identified a new mechanism by which endothelial cells adapt to laminar shear stress by up-regulating Sox18 to prevent the disruption of the alveolar-capillary barrier. Interestingly, in the absence of Sox18 up-regulation, laminar shear stress becomes a barrier disruptive force in pulmonary arterial endothelial cells. The endothelial barrier enhancing effects of shear described here are consistent with previous reports indicating barrier protection in the pulmonary circulation. However, there is still controversy regarding the effect of shear stress on endothelial barrier function. This may be due to the fact that endothelial cells are heterogeneous and possess highly specialized functions depending on their location in the body. Thus, the effects can be diverse with shear stress being shown to be barrier protective or barrier disruptive, depending on the flow pattern, the duration of exposure to shear, and the tissue bed from which the endothelial cells are derived. For example, while low levels (Conklin et al., 2002; Himburg et al., 2004; Yoshida et al., 1995) of oscillatory shear stress (Miao et al., 2005) are associated with barrier disruption and increased LDL permeability at vessel branch points and curvatures, higher levels (Himburg et al., 2004; Yoshida et al., 1995) of laminar shear stress (Seebach et al., 2000) are associated with barrier protection, decreased LDL permeability, and athero-protection. Similarly, acute shear stress, for 1 hour, has been described to increase permeability, while chronic exposure results in a strengthening of the endothelial barrier (Warboys et al., 2010). Lastly, endothelial cells lining skeletal muscle (Shibata and Kamiya, 1992) and fenestrated endothelial cells, such as glomerular capillary endothelial cells (Bevan et al., 2011) and liver sinusoidal endothelial cells (Braet et al., 2004), appear to be more permeable under conditions of increased shear stress, while endothelial cells derived from the central nervous system (Cucullo et al., 2011; Walsh et al., 2011) or lung (Shikata et al., 2005) are less permeable. Previous studies have attempted to elucidate the signaling pathways by which laminar shear stress increases barrier function. In the pulmonary circulation, an increase in laminar flow has been shown to reduce cell motility (Dieterich et al., 2000) and enhance the endothelial barrier by activating the small GTPase, Rac1, phosphorylating focal adhesion kinase at tyrosine 576, and by promoting the peripheral accumulation of the actin-binding protein, cortactin, enhancing the cortical actin cytoskeleton (Birukov et al., 2002; Seebach et al., 2000; Shikata et al., 2005). In brain microvascular endothelial cells, shear stress has been shown to stimulate Tiam1/Rac1 signaling and p-Tyr occludin dephosphorylation through VE-cadherin signaling (Walsh et al., 2011). Our data support the diversity of shear effects on the endothelial barrier by showing that 20 dyn/cm2 of laminar shear stress increased the barrier function of PAEC, while barrier function at 5 dyn/cm2 of laminar shear stress stabilized at, or slightly below, static control levels.
Our data indicate that laminar shear stress enhances barrier function in pulmonary artery endothelial cells through the transcription factor, Sox18. Sox18 is one member of a multigene family containing at least 20 different members that are organized into subfamilies A–H based on sequence similarities in the DNA-binding HMG domain (Wegner, 2010). Interestingly, Sox18 genetic mutations in Ra mice and HLT syndrome in humans are both associated with severe edema (Irrthum et al., 2003; James et al., 2003; Pennisi et al., 2000b). In both cases truncated forms of Sox18 are produced which compete with other members of the Sox F subfamily (Sox7 and Sox17) to act in a dominant negative manner, thereby precluding any protective mechanism offered by genomic redundancy within the Sox F subfamily (Hosking et al., 2001). In addition, while the genetic deletion of the Sox18 HMG domain in mice with a mixed genetic background resulted in no obvious cardiovascular defects (Pennisi et al., 2000a), the same mutation on a C57BL/6 genetic background caused extensive generalized edema and death by 14.5dpc, suggesting Sox7 and 17 can compensate for Sox18 only under certain conditions (Hosking et al., 2009). However, in our studies, the knockdown of Sox18 resulted in the disruption of the endothelial barrier under both static and shear stress conditions without changes in Sox7 expression, suggesting that the other members of Sox F subfamily cannot offset the loss of Sox18, at least in relation to endothelial barrier function.
Although our data clearly demonstrate that laminar shear stress increases Sox18 expression, the mechanism by which this mechanical force translates into a molecular signal is unresolved. As mentioned earlier, several mechanoreceptors on the endothelial cells, including flow sensitive ion channels (K+, Cl-, Na+, Ca2+) (Barakat et al., 1999) and a complex of PECAM-1, VE-cadherin, and VEGFR2 (Tzima et al., 2005), respond to shear stress, allowing for the downstream activation of transcription factors. These transcription factors, once activated, bind to SSRE’s within the promoters of shear responsive genes. For example, the transcriptional changes in response to acute shear stress are mainly elicited by the activator protein 1 (AP-1) and nuclear factor κB (NF-κB) (Lan et al., 1994). In addition, it has been previously shown that shear stress activates NF-κB through the integrin-p38 MAPK signaling cascade (Orr et al., 2005). These transcription factors are generally known to induce pro-inflammatory and pro-coagulant genes, such as monocyte chemotactic protein-1 (MCP-1), E-selectin, intercellular adhesion molecule 1 (ICAM-1), and tissue factor (TF) (Resnick and Gimbrone, 1995). Indeed a preliminary analysis of the Sox18 promoter using MatInspector revealed the presence of several NF-κB binding sites (Cartharius et al., 2005). In addition, long term shear stress exposure has also been shown to confer antiinflammatory and anti-coagulant properties to cultured endothelial cells (Gimbrone et al., 2000; Zhang and Friedman, 2011) and this appears to be mediated by Krüppel-like factor 2 (KLF2) and nuclear factor erythroid 2-like 2 (Nrf2) (Dai et al., 2007; Dekker et al., 2005). Nrf2 is responsible for the shear-mediated transcription of antioxidant genes (Chen et al., 2003). Putative cis elements for KLF2 and Nrf2 are also present in the Sox18 promoter (Cartharius et al., 2005). Therefore, it is possible that Sox18 may be regulated by these classical mechanosensory transcription factors. In support of this possibility, both KLF2 (Lin et al., 2010) and Nrf2 (Jin et al., 2009) are reported to be barrier protective in the lung. However, further studies will be required to determine which of these elements is actually responsible for shear stress induced Sox18 expression.
Shear stress mediated increases in peripheral actin cytoskeletal proteins (Thi et al., 2004), ZO-1 (Walsh et al., 2011), occludin (Colgan et al., 2007), and VE-cadherin (Walsh et al., 2011) has also been documented. In addition, shear has been shown to increase the mRNA expression of occludin, ZO-1 and −2, VE-cadherin, catenin α2 and β1, actin α2, and Claudin-3 and −5 in brain microvascular endothelial cells (Colgan et al., 2007; Cucullo et al., 2011; Walsh et al., 2011) and VE-cadherin, ZO-1, and Claudin-5 in mouse embryonic stem cell-derived endothelial cells (Nikmanesh et al., 2012). Accordingly, in our study, we found that laminar shear increased Claudin-5 mRNA expression in PAEC; although, we saw no changes in the protein levels of Claudin-1 or Claudin-12 in the presence of shear. In agreement with these previous studies, we also found that Claudin-5 and ZO-1 protein levels were increased by shear stress. However, the increase in ZO-1 protein levels was transient, while the expression of Claudin-5 was sustained. These findings suggest that Claudin-5 may play a more important role in maintaining barrier protection under sustained shear stress than ZO-1. Recent studies have suggested that Claudin-5 expression may be regulated by Sox18 (Fontijn et al., 2008), and our results demonstrate that the up-regulation of Claudin-5 by laminar shear stress is dependent upon the transcription factor, Sox18. Similarly, in lambs with increased PBF, the protein levels of Sox18 and Claudin-5 were elevated, and the barrier function in these lambs was not attenuated, despite increases in barrier disruptive agonists, suggesting Sox18 and Claudin-5 are important for the maintenance of barrier function in vivo. Interestingly, Claudin-5 knockdown under static conditions did not alter permeability, even though Sox18 knockdown did increase permeability for reasons that are unclear. However, previous studies have shown that Claudin-5 knockdown can produce different effects on permeability depending on the type of endothelial cell (Fontijn et al., 2006; Kluger et al., 2013). In addition, Sox18 over-expression provided barrier protection against several well-known permeability inducing agents: VEGF, TGF-β1, and thrombin, which also suggests that Sox18 may influence signaling pathways, besides Claudin 5, related to barrier function. Indeed, several gene targets of Sox18 have now been characterized including VCAM-1, µ-opioid receptor, ephrin B2, semaphorin 3G, ROBO4 (Samant et al., 2011), and matrix metalloprotease 7 (Hoeth et al., 2012), which may have additional effects on permeability. Claudin-5 is important for paracellular permeability and the maintenance of cell polarity (Krause et al., 2008; Piehl et al., 2010) through homo- or hetero- philic interactions with adjacent cells (Piontek et al., 2008). Although Claudin-5 is largely considered to be an endothelial specific tight junction member (Morita et al., 1999), it is also expressed in other cell types (Turksen and Troy, 2004). Claudin-5 up-regulation has been shown to enhance the blood-brain barrier (Honda et al., 2006), reduce paracellular cation permeability (Wen et al., 2004), and improve endothelial barrier function (Kluger et al., 2013). Furthermore, Claudin-5 knockout mice die within 1 day after birth with a compromised blood-brain barrier (Nitta et al., 2003). In addition to its regulation by Sox18, the expression of Claudin-5 has been shown to be up-regulated by ETS-related gene (ERG) (Morrow et al., 2009; Yuan et al., 2012) and down-regulated by VEGF (Argaw et al., 2009), TNF-a/NF-κB (Aslam et al., 2012), and FoxO1/β-catenin (Taddei et al., 2008). However, to our knowledge, this is the first report describing a transcriptional mechanism by which laminar shear stress positively regulates Claudin-5 expression and endothelial barrier function. In addition, our results also suggest that Sox18 may enhance barrier function through other cell-cell junction effectors besides Claudin-5. In support of this, in PAEC where Sox18 expression was silenced, ZO-1 protein levels did not increase when the cells were exposed to shear stress. Although, it is unclear whether this is a direct affect of Sox18 on the ZO-1 promoter or an indirect effect due to the loss of Claudin-5, leading to the degradation of the ZO-1 protein. In addition, our results also demonstrated that the over-expression of Sox18 enhanced VE-cadherin staining, while Sox18 depletion disrupted VE-cadherin fluorescent signal. However, it is again unclear whether this is due to changes in protein expression or the peripheral translocation of the junctional protein. Further studies will be required to test these possibilities.
In conclusion, our study has identified a novel mechanism by which shear stress enhances pulmonary endothelial barrier function through the Sox18 mediated increase in Claudin-5 expression. This important homeostatic mechanism for maintaining pulmonary endothelial barrier integrity, if disrupted, could possibly lead to pulmonary edema under conditions of increased PBF prior to the onset of cardiac failure. Although, during cardiac failure, it is possible that these compensatory barrier protective mechanisms are either suppressed or overwhelmed by the increased hydrostatic forces within the vasculature resulting in massive pulmonary edema.
ACKNOWLEDGMENTS
This research was supported in part by the grants: P01HL0101902 (to SMB), HL60190 (to SMB), HL67841 (to SMB), HL084739 (to SMB), R21HD057406 (to SMB), HL61284 (to JRF), K12HD047349 (to SAD), and T32HD049303 (to SAD) all from the National Institutes of Health, by a grant from the Foundation Leducq to (SMB and JRF), a predoctoral fellowship from the Southeast Affiliates of the American Heart Association: 12PRE12060224 (to CMG), and a Cardiovascular Discovery Institute Seed Award (to SK). The authors have no conflicts of interest to declare.
REFERENCES
- Aggarwal S, Gross CM, Kumar S, Dimitropolou C, Sharma S, Gorshkov BA, Sridhar S, Lu Q, Bogatcheva NV, Jezierska-Drutel AJ, Lucas R, Verin AD, Catravas JD, Black SM. DDAH II Over-Expression Attenuates Lipopolysaccharide Mediated Lung Leak in Acute Lung Injury. Am J Respir Cell Mol Biol doi: 10.1165/rcmb.2013-0193OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpan G, Mauray F, Clyman RI. Effect of patent ductus arteriosus on water accumulation and protein permeability in the lungs of mechanically ventilated premature lambs. Pediatr Res. 1989;26(6):570–575. doi: 10.1203/00006450-198912000-00011. [DOI] [PubMed] [Google Scholar]
- Alpan G, Scheerer R, Bland R, Clyman R. Patent ductus arteriosus increases lung fluid filtration in preterm lambs. Pediatr Res. 1991;30(6):616–621. doi: 10.1203/00006450-199112000-00026. [DOI] [PubMed] [Google Scholar]
- Alvarez JM, Panda RK, Newman MA, Slinger P, Deslauriers J, Ferguson M. Postpneumonectomy pulmonary edema. J Cardiothorac Vasc Anesth. 2003;17(3):388–395. doi: 10.1016/s1053-0770(03)00071-5. [DOI] [PubMed] [Google Scholar]
- Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106(6):1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Ahmad N, Srivastava R, Hemmer B. TNF-alpha induced NFkappaB signaling and p65 (RelA) overexpression repress Cldn5 promoter in mouse brain endothelial cells. Cytokine. 2012;57(2):269–275. doi: 10.1016/j.cyto.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Barakat AI, Leaver EV, Pappone PA, Davies PF. A flow-activated chloride-selective membrane current in vascular endothelial cells. Circ Res. 1999;85(9):820–828. doi: 10.1161/01.res.85.9.820. [DOI] [PubMed] [Google Scholar]
- Bevan HS, Slater SC, Clarke H, Cahill PA, Mathieson PW, Welsh GI, Satchell SC. Acute laminar shear stress reversibly increases human glomerular endothelial cell permeability via activation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2011;301(4):F733–F742. doi: 10.1152/ajprenal.00458.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol. 2002;26(4):453–464. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- Braet F, Shleper M, Paizi M, Brodsky S, Kopeiko N, Resnick N, Spira G. Liver sinusoidal endothelial cell modulation upon resection and shear stress in vitro. Comp Hepatol. 2004;3(1):7. doi: 10.1186/1476-5926-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breviario F, Caveda L, Corada M, Martin-Padura I, Navarro P, Golay J, Introna M, Gulino D, Lampugnani MG, Dejana E. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler Thromb Vasc Biol. 1995;15(8):1229–1239. doi: 10.1161/01.atv.15.8.1229. [DOI] [PubMed] [Google Scholar]
- Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21(13):2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- Catravas JD, Snead C, Dimitropoulou C, Chang AS, Lucas R, Verin AD, Black SM. Harvesting, identification and barrier function of human lung microvascular endothelial cells. Vascul Pharmacol. 2010;52(5–6):175–181. doi: 10.1016/j.vph.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278(2):703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- Chu TJ, Peters DG. Serial analysis of the vascular endothelial transcriptome under static and shear stress conditions. Physiol Genomics. 2008;34(2):185–192. doi: 10.1152/physiolgenomics.90201.2008. [DOI] [PubMed] [Google Scholar]
- Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, Cummins PM. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol. 2007;292(6):H3190–H3197. doi: 10.1152/ajpheart.01177.2006. [DOI] [PubMed] [Google Scholar]
- Conklin BS, Zhong DS, Zhao W, Lin PH, Chen C. Shear stress regulates occludin and VEGF expression in porcine arterial endothelial cells. J Surg Res. 2002;102(1):13–21. doi: 10.1006/jsre.2001.6295. [DOI] [PubMed] [Google Scholar]
- Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011;12:40. doi: 10.1186/1471-2202-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101(7):723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100(5):1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167(2):609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaola N, Phelps JE, Florez L, Keese CR, Minnear FL, Giaever I, Vincent P. Electrical impedance of cultured endothelium under fluid flow. Ann Biomed Eng. 2001;29(8):648–656. doi: 10.1114/1.1385811. [DOI] [PubMed] [Google Scholar]
- Dieterich P, Odenthal-Schnittler M, Mrowietz C, Kramer M, Sasse L, Oberleithner H, Schnittler HJ. Quantitative morphodynamics of endothelial cells within confluent cultures in response to fluid shear stress. Biophys J. 2000;79(3):1285–1297. doi: 10.1016/S0006-3495(00)76382-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TL, Mynett-Johnson L, Wright EM, Hosking BM, Koopman PA, Muscat GE. Sequence and expression of Sox-18 encoding a new HMG-box transcription factor. Gene. 1995;161(2):223–225. doi: 10.1016/0378-1119(95)00280-j. [DOI] [PubMed] [Google Scholar]
- Fontijn RD, Rohlena J, van Marle J, Pannekoek H, Horrevoets AJ. Limited contribution of claudin-5-dependent tight junction strands to endothelial barrier function. Eur J Cell Biol. 2006;85(11):1131–1144. doi: 10.1016/j.ejcb.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Fontijn RD, Volger OL, Fledderus JO, Reijerkerk A, de Vries HE, Horrevoets AJ. SOX-18 controls endothelial-specific claudin-5 gene expression and barrier function. Am J Physiol Heart Circ Physiol. 2008;294(2):H891–900. doi: 10.1152/ajpheart.01248.2007. [DOI] [PubMed] [Google Scholar]
- Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456(7222):643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- Gimbrone MA, Jr., Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–240. [DOI] [PubMed] [Google Scholar]
- Gruner C, Akkaya E, Kretschmar O, Roffi M, Corti R, Jenni R, Eberli FR. Pharmacologic preconditioning therapy prior to atrial septal defect closure in patients at high risk for acute pulmonary edema. J Interv Cardiol. 2012;25(5):505–512. doi: 10.1111/j.1540-8183.2012.00747.x. [DOI] [PubMed] [Google Scholar]
- Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, Friedman MH. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol. 2004;286(5):H1916–H1922. doi: 10.1152/ajpheart.00897.2003. [DOI] [PubMed] [Google Scholar]
- Hoeth M, Niederleithner H, Hofer-Warbinek R, Bilban M, Mayer H, Resch U, Lemberger C, Wagner O, Hofer E, Petzelbauer P, de Martin R. The transcription factor SOX18 regulates the expression of matrix metalloproteinase 7 and guidance molecules in human endothelial cells. Plos One. 2012;7(1):e30982. doi: 10.1371/journal.pone.0030982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Nakagawa S, Hayashi K, Kitagawa N, Tsutsumi K, Nagata I, Niwa M. Adrenomedullin improves the blood-brain barrier function through the expression of claudin-5. Cell Mol Neurobiol. 2006;26(2):109–118. doi: 10.1007/s10571-006-9028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking B, Francois M, Wilhelm D, Orsenigo F, Caprini A, Svingen T, Tutt D, Davidson T, Browne C, Dejana E, Koopman P. Sox7 and Sox17 are strain-specific modifiers of the lymphangiogenic defects caused by Sox18 dysfunction in mice. Development. 2009;136(14):2385–2391. doi: 10.1242/dev.034827. [DOI] [PubMed] [Google Scholar]
- Hosking BM, Muscat GE, Koopman PA, Dowhan DH, Dunn TL. Trans-activation and DNA-binding properties of the transcription factor, Sox-18. Nucleic Acids Res. 1995;23(14):2626–2628. doi: 10.1093/nar/23.14.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking BM, Wang SC, Chen SL, Penning S, Koopman P, Muscat GE. SOX18 directly interacts with MEF2C in endothelial cells. Biochem Biophys Res Commun. 2001;287(2):493–500. doi: 10.1006/bbrc.2001.5589. [DOI] [PubMed] [Google Scholar]
- Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, Fryns JP, Van Steensel MA, Vikkula M. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet. 2003;72(6):1470–1478. doi: 10.1086/375614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James K, Hosking B, Gardner J, Muscat GE, Koopman P. Sox18 mutations in the ragged mouse alleles ragged-like and opossum. Genesis. 2003;36(1):1–6. doi: 10.1002/gene.10190. [DOI] [PubMed] [Google Scholar]
- Jin W, Wang H, Ji Y, Zhu L, Yan W, Qiao L, Yin H. Genetic ablation of Nrf2 enhances susceptibility to acute lung injury after traumatic brain injury in mice. Exp Biol Med (Maywood) 2009;234(2):181–189. doi: 10.3181/0807-RM-232. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Kanai-Azuma M, Noce T, Saido TC, Shiroishi T, Hayashi Y, Yazaki K. Identification of two Sox17 messenger RNA isoforms, with and without the high mobility group box region, and their differential expression in mouse spermatogenesis. J Cell Biol. 1996;133(3):667–681. doi: 10.1083/jcb.133.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MS, Clark PR, Tellides G, Gerke V, Pober JS. Claudin-5 controls intercellular barriers of human dermal microvascular but not human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(3):489–500. doi: 10.1161/ATVBAHA.112.300893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778(3):631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Kumar S, Sun X, Wedgwood S, Black SM. Hydrogen peroxide decreases endothelial nitric oxide synthase promoter activity through the inhibition of AP-1 activity. Am J Physiol Lung Cell Mol Physiol. 2008;295(2):L370–L377. doi: 10.1152/ajplung.90205.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Q, Mercurius KO, Davies PF. Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun. 1994;201(2):950–956. doi: 10.1006/bbrc.1994.1794. [DOI] [PubMed] [Google Scholar]
- Lin M, Chamoto K, Gibney BC, Lee GS, Collings-Simpson D, Houdek J, Konerding MA, Tsuda A, Mentzer SJ. Angiogenesis gene expression in murine endothelial cells during post-pneumonectomy lung growth. Respir Res. 2011;12:98. doi: 10.1186/1465-9921-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, Atkins GB, Nayak L, Cui Y, Finigan JH, Jain MK. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30(10):1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Greenwood E, Meyrick B, Soifer SJ, Fineman JR, Black SM. Expression of VEGF and its receptors Flt-1 and Flk-1/KDR is altered in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2003a;285(1):L222–L231. doi: 10.1152/ajplung.00388.2002. [DOI] [PubMed] [Google Scholar]
- Mata-Greenwood E, Meyrick B, Steinhorn RH, Fineman JR, Black SM. Alterations in TGF-beta1 expression in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2003b;285(1):L209–L221. doi: 10.1152/ajplung.00171.2002. [DOI] [PubMed] [Google Scholar]
- Miao H, Hu YL, Shiu YT, Yuan S, Zhao Y, Kaunas R, Wang Y, Jin G, Usami S, Chien S. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J Vasc Res. 2005;42(1):77–89. doi: 10.1159/000083094. [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147(1):185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow CM, Tyagi G, Simon L, Carnes K, Murphy KM, Cooke PS, Hofmann MC, Hess RA. Claudin 5 expression in mouse seminiferous epithelium is dependent upon the transcription factor ets variant 5 and contributes to blood-testis barrier function. Biol Reprod. 2009;81(5):871–879. doi: 10.1095/biolreprod.109.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikmanesh M, Shi ZD, Tarbell JM. Heparan sulfate proteoglycan mediates shear stress-induced endothelial gene expression in mouse embryonic stem cell-derived endothelial cells. Biotechnol Bioeng. 2012;109(2):583–594. doi: 10.1002/bit.23302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol. 2005;169(1):191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi D, Bowles J, Nagy A, Muscat G, Koopman P. Mice null for sox18 are viable and display a mild coat defect. Mol Cell Biol. 2000a;20(24):9331–9336. doi: 10.1128/mcb.20.24.9331-9336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi D, Gardner J, Chambers D, Hosking B, Peters J, Muscat G, Abbott C, Koopman P. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat Genet. 2000b;24(4):434–437. doi: 10.1038/74301. [DOI] [PubMed] [Google Scholar]
- Piehl C, Piontek J, Cording J, Wolburg H, Blasig IE. Participation of the second extracellular loop of claudin-5 in paracellular tightening against ions, small and large molecules. Cell Mol Life Sci. 2010;67(12):2131–2140. doi: 10.1007/s00018-010-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 2008;22(1):146–158. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, Fineman JR. In utero placement of aortopulmonary shunts. A model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation. 1995;92(3):606–613. doi: 10.1161/01.cir.92.3.606. [DOI] [PubMed] [Google Scholar]
- Resnick N, Gimbrone MA., Jr Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 1995;9(10):874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- Sakuma T, Sagawa M, Hida M, Nambu Y, Osanai K, Toga H, Takahashi K, Ohya N, Matthay MA. Time-dependent effect of pneumonectomy on alveolar epithelial fluid clearance in rat lungs. J Thorac Cardiovasc Surg. 2002;124(4):668–674. doi: 10.1067/mtc.2002.122549. [DOI] [PubMed] [Google Scholar]
- Samant GV, Schupp MO, Francois M, Moleri S, Kothinti RK, Chun CZ, Sinha I, Sellars S, Leigh N, Pramanik K, Horswill MA, Remadevi I, Li K, Wilkinson GA, Tabatabai NM, Beltrame M, Koopman P, Ramchandran R. Sox factors transcriptionally regulate ROBO4 gene expression in developing vasculature in zebrafish. J Biol Chem. 2011;286(35):30740–30747. doi: 10.1074/jbc.M111.220665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C, Bittner V, Nath PH, Breatnach ES, Soto BS. Atrial septal defect in older adults: atypical radiographic appearances. Radiology. 1988;167(1):123–127. doi: 10.1148/radiology.167.1.2964675. [DOI] [PubMed] [Google Scholar]
- Seebach J, Dieterich P, Luo F, Schillers H, Vestweber D, Oberleithner H, Galla HJ, Schnittler HJ. Endothelial barrier function under laminar fluid shear stress. Lab Invest. 2000;80(12):1819–1831. doi: 10.1038/labinvest.3780193. [DOI] [PubMed] [Google Scholar]
- Shibata M, Kamiya A. Blood flow dependence of local capillary permeability of Cr-EDTA in the rabbit skeletal muscle. Jpn J Physiol. 1992;42(4):631–639. doi: 10.2170/jjphysiol.42.631. [DOI] [PubMed] [Google Scholar]
- Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res. 2005;304(1):40–49. doi: 10.1016/j.yexcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Silberman M, Barac YD, Yahav H, Wolfovitz E, Einav S, Resnick N, Binah O. Shear stress-induced transcriptional regulation via hybrid promoters as a potential tool for promoting angiogenesis. Angiogenesis. 2009;12(3):231–242. doi: 10.1007/s10456-009-9143-7. [DOI] [PubMed] [Google Scholar]
- Singhi AK, Mahesh K, Kumar RK. Pulmonary edema following transcatheter closure of atrial septal defect. Ann Pediatr Cardiol. 2010;3(1):90–91. doi: 10.4103/0974-2069.64359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Kumar S, Sharma S, Aggarwal S, Lu Q, Gross C, Rafikova O, Lee SG, Dasarathy S, Hou Y, Meadows ML, Han W, Su Y, Fineman JR, Black SM. Endothelin-1 Induces a Glycolytic Switch in Pulmonary Arterial Endothelial Cells via the Mitochondrial Translocation of Endothelial NO Synthase. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2013-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10(8):923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- Tang BT, Pickard SS, Chan FP, Tsao PS, Taylor CA, Feinstein JA. Wall shear stress is decreased in the pulmonary arteries of patients with pulmonary arterial hypertension: An image-based, computational fluid dynamics study. Pulm Circ. 2012;2(4):470–476. doi: 10.4103/2045-8932.105035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, Hiraoka Y, Ogawa M, Sakai Y, Kido S, Aiso S. Isolation and characterization of a mouse SRY-related cDNA, mSox7. Biochim Biophys Acta. 1999;1445(2):225–231. doi: 10.1016/s0167-4781(99)00047-0. [DOI] [PubMed] [Google Scholar]
- Tannenbaum JE, Waleh NS, Mauray F, Gold L, Perkett EA, Clyman RI. Transforming growth factor-beta protein and messenger RNA expression is increased in the closing ductus arteriosus. Pediatr Res. 1996;39(3):427–434. doi: 10.1203/00006450-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci U S A. 2004;101(47):16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Fratz S, Hou Y, Lu Q, Gorlach A, Hess J, Schreiber C, Datar SA, Oishi P, Nechtman J, Podolsky R, She JX, Fineman JR, Black SM. Delineating the angiogenic gene expression profile before pulmonary vascular remodeling in a lamb model of congenital heart disease. Physiol Genomics. 2011;43(2):87–98. doi: 10.1152/physiolgenomics.00135.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tressel SL, Huang RP, Tomsen N, Jo H. Laminar shear inhibits tubule formation and migration of endothelial cells by an angiopoietin-2 dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27(10):2150–2156. doi: 10.1161/ATVBAHA.107.150920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksen K, Troy TC. Barriers built on claudins. J Cell Sci. 2004;117(Pt 12):2435–2447. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Waller DA, Keavey P, Woodfine L, Dark JH. Pulmonary endothelial permeability changes after major lung resection. Ann Thorac Surg. 1996;61(5):1435–1440. doi: 10.1016/0003-4975(96)00103-8. [DOI] [PubMed] [Google Scholar]
- Walsh TG, Murphy RP, Fitzpatrick P, Rochfort KD, Guinan AF, Murphy A, Cummins PM. Stabilization of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signaling leading to modulation of pTyr-occludin levels. J Cell Physiol. 2011;226(11):3053–3063. doi: 10.1002/jcp.22655. [DOI] [PubMed] [Google Scholar]
- Warboys CM, Eric Berson R, Mann GE, Pearson JD, Weinberg PD. Acute and chronic exposure to shear stress have opposite effects on endothelial permeability to macromolecules. Am J Physiol Heart Circ Physiol. 2010;298(6):H1850–1856. doi: 10.1152/ajpheart.00114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedgwood S, Bekker JM, Black SM. Shear stress regulation of endothelial NOS in fetal pulmonary arterial endothelial cells involves PKC. Am J Physiol Lung Cell Mol Physiol. 2001;281(2):L490–498. doi: 10.1152/ajplung.2001.281.2.L490. [DOI] [PubMed] [Google Scholar]
- Wegner M. All purpose Sox: The many roles of Sox proteins in gene expression. Int J Biochem Cell Biol. 2010;42(3):381–390. doi: 10.1016/j.biocel.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24(19):8408–8417. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Okano M, Wang S, Kobayashi M, Kawasumi M, Hagiwara H, Mitsumata M. Hemodynamic-force-induced difference of interendothelial junctional complexes. Ann N Y Acad Sci. 1995;748:104–120. doi: 10.1111/j.1749-6632.1994.tb17311.x. discussion 120–101. [DOI] [PubMed] [Google Scholar]
- Yuan L, Le Bras A, Sacharidou A, Itagaki K, Zhan Y, Kondo M, Carman CV, Davis GE, Aird WC, Oettgen P. ETS-related gene (ERG) controls endothelial cell permeability via transcriptional regulation of the claudin 5 (CLDN5) gene. J Biol Chem. 2012;287(9):6582–6591. doi: 10.1074/jbc.M111.300236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Friedman MH. The Adaptive Response of Vascular Endothelial Cells to an Acute Increase in Shear Stress Magnitude. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Coulber C, Rabinovitch M. Tissue-specific and developmental regulation of transforming growth factor-beta1 expression in fetal lamb ductus arteriosus endothelial cells. Pediatr Res. 1998;44(6):865–872. doi: 10.1203/00006450-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Zhu R, He L, Xu J, Zhang Y, Hu Y. [Changes of TGF-beta1 and CTGF in rats with increased blood flow-induced pulmonary artery hypertension] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37(10):1013–1020. doi: 10.3969/j.issn.1672-7347.2012.10.008. [DOI] [PubMed] [Google Scholar]