Abstract
Objective
To determine whether pregnancies resulting in early preterm birth (< 30 weeks) were more likely than term pregnancies to have elevated mid-trimester tumor necrosis factor alpha (TNF-α) levels co-occurring with lipid patterns suggestive of hyperlipidemia.
Study Design
Patterns were examined using stored non-fasted serum samples from 108 California and 734 Iowa singleton pregnancies collected as part of statewide prenatal screening. The frequency of elevated mid-pregnancy serum TNF-α levels and lipid patterns suggestive of hyperlipidemia (e.g. elevated total cholesterol (TC), low-density-lipoproteins (LDLs), or triglycerides (TGs), decreased high-density lipoproteins (HDLs)) (considered independently and by co-occurrence) were compared in pregnancies resulting in early preterm birth to those resulting in term birth using logistic regression models.
Results
While no differences between preterm and term pregnancies were evident when TNF-α or target lipid abnormalities occurred in isolation, early preterm pregnancies were two to four times more likely than term pregnancies to have elevated TNF-α levels co-occurring with indicators of hyperlipidemia (37.5% versus 13.9% in the California sample (adjusted OR 4.0, 95% CI 1.1 – 16.3) and 26.3% versus 14.9% in the Iowa sample (adjusted OR 2.7, 95% CI 1.1 – 6.3)). Observed differences were not explicable to any maternal or infant characteristics.
Conclusion
Pregnancies resulting in early preterm birth were more likely than term pregnancies to have elevated mid-pregnancy TNF-α levels co-occurring with lipid patterns suggestive of hyperlipidemia. Patterns offer clues for further study of the signaling of early parturition in preterm birth.
INTRODUCTION
While a number of studies have reported a link between preterm birth and increased mid-pregnancy levels of tumor necrosis factor alpha (TNF-α)1-3 as well as preterm birth and mid-pregnancy lipid levels4-6 the combined influence of these factors on the risk of preterm birth has not been explored.
Given the established link between TNF-α and lipid release from adipocytes (TNF-α induced lipolysis)7-9 as well as the association between TNF-α and lipid metabolism10;11 we hypothesized that in some instances preterm birth risk could be associated with the co-occurrence of increased TNF-α levels and lipid levels. Most specifically when elevated TNF-α occurs in combination with hyperlipidemia (e.g. suggested by increased total cholesterol (TC), low-density-lipoproteins (LDLs), or triglycerides (TGs) or low high-density-lipoproteins (HDLs)). The importance of these combined influences on risk have been investigated in studies focused on gestational diabetes and preeclampsia12;13 as well as studies focused on obesity, cardiovascular disease, insulin sensitivity, and metabolic syndrome.11;14-17
Here we employ second trimester serum samples collected as part of routine second trimester screening for aneuploidies and neural tube defects (NTDs) to investigate potential interrelationships between TNF-α and lipid levels for their contribution to risk in pregnancies resulting in early preterm birth (< 30 weeks) compared to term pregnancies. We examine these associations in two nested case-control samples drawn from California and Iowa cohorts.
MATERIALS AND METHODS
The relationships between mid-pregnancy TNF-α, lipids patterns, and early preterm birth (< 30 weeks) were examined in two independent samples of pregnancies from California (n = 108) and Iowa (n = 734). Included were women who participated in routine prenatal screening for aneuploides and NTDs and delivered a live born singleton at or after 20 weeks completed gestation. Included from California were 72 case pregnancies resulting in early preterm birth and 36 singleton control pregnancies resulting in term birth (≥ 37 completed weeks gestation). Case and control pregnancies were drawn from a larger cohort of more than 1.4 million pregnancies who underwent routine prenatal screening in 2005 – 2008. Cohort details have been described elsewhere.18 In brief, all of the included cases and controls had detailed information available via prenatal screening and linked birth certificate records and all cases had detailed neonatal intensive care unit (NICU) data available through the California Perinatal Quality Care Collaborative (CPQCC) database which stores clinical data on over 90% of all neonates who receive neonatal intensive care in California.19 None of the cases or controls had any indication of chromosomal or structural defects, smoking, diabetes or amniotic fluid abnormalities present in prenatal screening, birth certificate, or for cases, NICU records. Early preterm cases were stratified by presence or absence of brochopulmonary dysplasia (BPD) (n = 36 per group, 72 total early preterm cases). The source study data focused on BPD as an outcome.18
Included from Iowa were 57 case pregnancies resulting in early preterm birth and 677 singleton control pregnancies resulting in term birth. Details regarding the cohort from which Iowa cases and controls were drawn have been described elsewhere.6 In brief, all included pregnancies belonged to a cohort of 12,057 women who underwent routine statewide prenatal screening in 2009 and 2010. No other exclusions were made from the Iowa sample.
Information on maternal characteristics and related data
In both samples information on maternal characteristics, measurements on routine second trimester serum screening tests, and information on infant characteristics were abstracted from state prenatal screening program data (the California Prenatal Screening Program within the Genetic Disease Screening Program (GDSP) and the Iowa Prenatal Screening Program) and from state birth certificate records. Included from screening records was maternal race/ethnicity, weight at testing, age at testing and gestational weeks at time of second trimester screening. Other data extracted from birth certificates included parity, birth weight, and gestational age. For Iowa, information on body mass index (BMI), diabetes, congenital defects (chromosomal or other), and whether the preterm birth was spontaneous or medically indicated was also abstracted from birth certificates. In California, prenatal screening records and hospital discharge records (for cases and controls) and NICU records (for cases) were used along with birth certificates to make exclusions for smoking history, diabetes, structural or chromosomal defects, and to determine if the preterm birth was spontaneous or medically indicated NICU records were also used to identify cases with and without BPD and other indicators of case morbidity including intraventricular hemorrhage (IVH) and retinopathy of prematurity (ROP). In both California and Iowa samples, case pregnancies resulting in spontaneous preterm birth were considered to be those where vital statistic birth certificate records (California and Iowa) or hospital discharge records (California) indicated “premature labor” or premature rupture of membranes (PROM). Case pregnancies resulting in medically indicated premature birth were considered to be those without “premature labor” or PROM for whom there was a flag for “medical induction” or “assisted rupture of membranes” (AROM) or for whom there was a cesarean section given birth < 30 weeks and none of the aforementioned flags. Case pregnancies without any of these specific codes were considered as having an “unknown” preterm birth subtype.
Serum samples and biochemical testing
In both California and Iowa samples, nonfasting serum was drawn between 15 and 20 weeks of gestation. Serum was stored in 1 milliliter vacutubes at -80C until thawed for testing. TNF-α in California serum samples was measured by the Stanford Human Immune Monitoring Center (HIMC) as part of a Human 51-plex Luminex kit. Testing was done in accordance with manufacturer protocols. Details regarding the use of this kit by the HIMC have been described elsewhere.20 Median fluorescence intensity (MFI) values were reported for TNF-α using Masterplex software (Hitashi Corp.). Cytokines at the University of Iowa were measured using a Millipore high sensitivity kit (HSCYTO-60SPMX13) and run on a Biorad Bioplex 200 instrument. TNF- α data was analyzed using Biorad Bioplex Manager software. Lipids in both samples (total cholesterol (TC), low-density-lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides (TGs)) were measured at the State Hygienic Laboratory at the University of Iowa using a Roche Diagnostics c111 Cobas Analyzer (Basel, Switzerland).
Analyses
Serum TNF-α levels and lipid patterns suggestive of hyperlipidemia (including elevated TC, LDL, TGs, elevated ratios of TG:HDL, LDL:HDL, low HDL levels, or a low ratio of HDL:TC) 16;21-24 were compared in case and control pregnancies using logistic regression models. Elevated TNF-α and lipid patterns suggestive of hyperlipidemia were evaluated as independent predictors as well as combined predictors for risk of preterm birth. Elevated and decreased serum levels of analytes were defined by generating quartiles for California and Iowa samples separately based on the distribution of each analyte in control pregnancies. Biomarker level at or in the 4th quartile (4Q) was considered as high for TNF-α and as a potential indicator of hyperlipidemia for TC, LDLs, TGs, TG:HDL, LDL:HDL. Biomarker level at or in the 1st quartile (1Q) was considered as an potential indicator of hyperlipidemia for HDLs and HDL:TC. Models were adjusted for maternal weight and gestational week at serum draw to account for differences in blood volume and normal variations in lipid levels during pregnancy.25-27 Differences in maternal characteristics at time of testing for cases and controls and differences in maternal and infant characteristics based on having an elevated TNF- α and any at-risk lipid pattern among preterm birth cases were examined using chi-square and the Wilcoxon Rank-Sum test.
All analyses were done using Statistical Analysis Software (SAS) version 9.3 (Cary, NC). Methods and protocols for the study were approved by the Committee for the Protection of Human Subjects within the Health and Human Services Agency of the State of California, the Institutional Review Board of Stanford University, and the Institutional Review Board of the University of Iowa.
RESULTS
Early preterm cases and term controls in the California sample were primarily of White or Hispanic race/ethnicity (30.6 and 62.5% in cases and 27.8 and 52.8% in controls). All cases and controls in the Iowa sample were White. In California, early preterm cases weighed more at mid-pregnancy serum draw than term controls (161.9 compared to 140.6 pounds, z = -2.8, p < .01). In Iowa, early preterm cases were less likely than controls to have given birth previously (40.3 versus 58.6%, p < .05) (Table 1).
Table 1.
Maternal characteristics in early preterm pregnancies compared to term pregnancies.
| California |
Iowa |
|||
|---|---|---|---|---|
| Cases < 30 Weeks |
Controls ≥ 37 Weeks |
Cases < 30 Weeks |
Controls ≥ 37 Weeks |
|
| % 100.0 (n = 72) | % 100.0 (n =36) | % 100.0 (n = 57) | % 100.0 (n = 677) | |
| Race/Ethnicity | ||||
| White | 30.6 | 27.8 | 100.0 | 100.0 |
| Hispanic | 62.5 | 52.8 | 0 | 0 |
| Black | 1.4 | 8.3 | 0 | 0 |
| Asian | 4.2 | 8.3 | 0 | 0 |
| Other | 1.4 | 2.8 | 0 | 0 |
| Maternal Age (Years) | ||||
| < 18 Years | 1.4 | 2.8 | 0 | 1.6 |
| 18 – 34 | 62.2 | 86.1 | 89.5 | 86.6 |
| ≥ 35 | 27.8 | 11.1 | 10.5 | 11.8 |
| Parity | ||||
| 1 | 41.7 | 38.9 | 59.7 | 41.4 |
| ≥ 2 | 58.3 | 61.1 | 40.3 | 58.6a |
| Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
|
|---|---|---|---|---|
| Weight at Test (pds) | 161.9 (42.2)a | 140.6 (32.4) | 177.4 (48.9) | 171.4 (41.7) |
| Week at Test (15-20) | 16.8 (1.3) | 16.9 (1.4) | 16.7 (1.3) | 16.8 (1.3) |
SD, Standard Deviation; pds, pounds.
p = < .01
When evaluated without consideration of co-occurring indicators of hyperlipidemia, California cases, but not Iowa cases, were nearly three times more likely than term controls to have elevated TNF-α levels (odds ratio (OR) = 2.8. 95% confidence interval (CI) 1.1 to 7.0). When lipids were evaluated without consideration of cooccurrence with TNF-α levels, in both samples case pregnancies were more than twice as likely as controls to have elevated TGs (OR 3.4, 95% CI 1.4 to 8.4 in California and OR 2.1, 95% CI 1.2 to 3.7 in Iowa) or unusually low HDL:TC (OR 3.2, 95% CI 1.3 to 8.3 in California and OR 2.0, 95% CI 1.1 to 3.5 in Iowa). These risks for lipids were attenuated among cases and controls with TNF-α levels in the 4Q. Risks associated with lipids remained and were often magnified when cases and controls were examined in combination with TNF-α levels in the 4Q (Table 2).
Table 2.
Mid-pregnancy tumor necrosis factor-alpha (TNF-α) and lipids (by quartile) in early preterm pregnancies compared to term pregnancies: Independent biomarker models and by co-occurrence.
| Sample | California |
Iowa |
||
|---|---|---|---|---|
| Cases < 30 Weeks |
Controls ≥ 37 Weeks |
Cases < 30 Weeks |
Controls ≥ 37 Weeks |
|
| n = (%) OR (95% CI)a 72 (100.0) |
n = (%) Referent 36 (100.0) |
n = (%) OR (95% CI)a 57 (100.0) |
n = (%) Referent 677 (100.0) |
|
| Independent Models | ||||
| TNF-α 4Qb | 33 (45.8) 2.8 (1.1 – 7.0) |
9 (25.0) | 18 (31.6) 1.3 (0.7 – 2.3) |
173 (25.6) |
| TC 4Qb | 18 (25.0) 0.9 (0.3 – 2.4) |
9 (25.0) | 19 (33.3) 1.5 (0.8 – 2.7) |
171 (25.3) |
| HDL 1Qb | 33 (45.8) 2.4 (1.0 – 5.9) |
9 (25.0) | 25 (43.9) 2.2 (1.3 – 3.8) |
175 (25.9) |
| LDL 4Qb | 26 (36.1) 1.6 (0.6 – 4.2) |
9 (25.0) | 16 (28.1) 1.2 (0.6 – 2.1) |
171 (25.3) |
| TGs 4Qb | 38 (52.8) 3.4 (1.4 – 8.4) |
9 (25.0) | 24 (42.1) 2.1 (1.2 – 3.7) |
172 (25.4) |
| HDL:TC 1Qb | 40 (55.6) 3.2 (1.3 – 8.3) |
9 (25.0) | 24 (42.1) 2.0 (1.1 – 3.5) |
179 (26.4) |
| TGs:HDL 4Qb | 43 (59.7) 4.2 (1.7 – 10.7) |
9 (25.0) | 21 (36.8) 1.7 (1.0 – 3.1) |
170 (25.1) |
| LDL:HDL 4Qb | 34 (47.2) 2.3 (0.9 – 6.1) |
9 (25.0) | 25 (43.9) 2.3 (1.3 – 4.0) |
172 (25.4) |
| Lipids where TNF-α < 4Q | ||||
| TC 4Q | 7 (9.7) 0.5 (0.1 – 1.5) |
6 (16.7) | 14 (24.6) 1.4 (0.7 – 2.6) |
131 (19.4) |
| HDL 1Q | 8 (22.2) 1.3 (0.5 – 3.5) |
21 (29.2) | 13 (22.8) 1.3 (0.7 – 2.5) |
124 (18.3) |
| LDL 4Q | 8 (22.2) 0.6 (0.2 – 1.8) |
13 (18.1) | 12 (21.1) 1.1 (0.6 – 2.2) |
132 (19.5) |
| TGs 4Q | 19 (26.4) 1.7 (0.6 – 4.7) |
6 (16.7) | 15 (26.3) 1.5 (0.8 – 2.8) |
130 (19.2) |
| HDL:TC 1Q | 23 (31.9) 1.2 (0.4 – 3.2) |
8 (22.2) | 14 (24.6) 1.3 (0.7 – 2.5) |
134 (19.8) |
| Lipids where TNF-α < 4Q | ||||
| TGs:HDL 4Q | 21 (29.2) 1.4 (0.5 – 3.6) |
8 (22.2) | 13 (22.8) 1.3 (0.7 – 2.4) |
126 (18.6) |
| LDL:HDL 4Q | 17 (23.6) 0.8 (0.3 – 2.3) |
8 (22.2) | 14 (24.6) 1.4 (0.7 – 2.6) |
132 (19.5) |
| Lipids where TNF-α 4Q | ||||
| TC 4Q | 11 (15.3) 2.0 (0.5 – 8.4) |
3 (8.3) | 5 (8.8) 1.5 (0.6 – 3.9) |
40 (5.9) |
| HDL 1Q | 12 (16.7)c | 1 (2.8) | 12 (21.1) 3.1 (1.5 – 6.3) |
52 (7.7) |
| LDL 4Q | 13 (18.1)c | 1 (2.8) | 4 (7.0) 1.2 (0.4 – 3.5) |
39 (5.8) |
| TGs 4Q | 19 (26.4) 4.4 (1.2 – 16.8) |
3 (8.3) | 9 (15.8) 2.7 (1.2 – 6.0) |
43 (6.4) |
| HDL:TC 1Q | 17 (23.6)c | 1 (2.8) | 10 (17.5) 2.8 (1.3 – 6.1) |
46 (6.8) |
| TGs:HDL 4Q | 22 (30.6)c | 1 (2.8) | 8 (14.0) 2.3 (1.0 – 5.1) |
45 (6.7) |
| LDL:HDL 4Q | 17 (23.6)c | 1 (2.8) | 11 (19.3) 3.7 (1.8 – 7.7) |
40 (5.9) |
TNF-α, tumor necrosis factor-alpha; OR, Odds Ratio; 95% CI, 95% confidence interval; 1Q, 1st quartile; 4Q, 4th quartile; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TGs, triglycerides
Adjusted for gestational week at serum draw and maternal weight.
Wherein = 1st and 4th quartile cut points were derived from the term controls.
Not calculated due to frequency in referent sample ≤ 1.
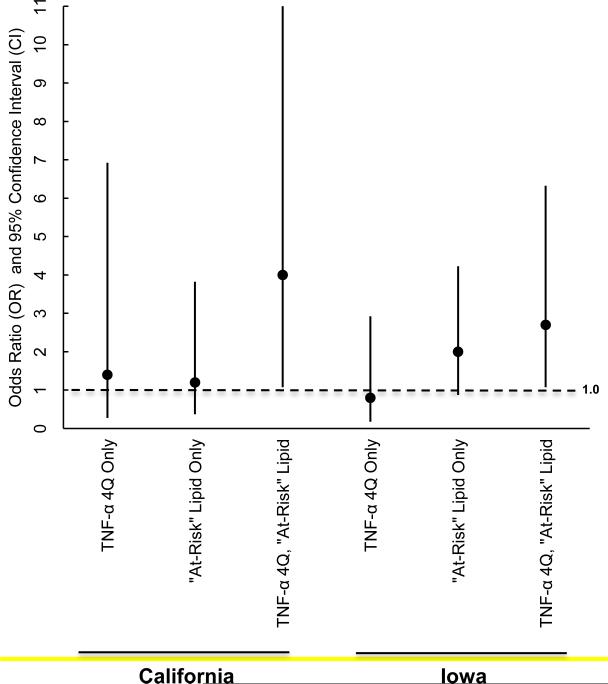
When the frequency of co-occurring elevated TNF-α and any lipid patterns suggestive of hyperlipdemia were compared for cases and controls, 37.5% of early preterm cases in California and 26.3% of early preterm cases in Iowa (compared to 13.9% of term controls in California and 14.9% in Iowa) were found to have a TNF-α level in the 4Q and one or more at-risk lipid pattern suggestive of hyperlipidemia resulting in ORs of 4.0 (95% CI 1.1 to 16.3 ) and 2.7 ( 95% CI 1.1 to 6.3) respectively after adjustment for gestational week and maternal weight at serum draw). No significant between group differences were noted when elevated TNF-α or at-risk lipid patterns occurred in isolation (Table 3) (Figure 1).
Table 3.
Co-occurrence of mid-pregnancy tumor necrosis factor-alpha (TNF-α) and any at-risk lipid pattern in early preterm pregnancies compared to term pregnancies.
| Sample | California |
Iowa |
||
|---|---|---|---|---|
| Cases |
Controls |
Cases |
Controls |
|
| n = (%) OR (95% CI)a 72 (100.0) |
n = (%) 36 (100.0) |
n = (%) OR (95% CI)a 57 (100.0) |
n = (%) Referent 677 (100.0) |
|
| No TNF-α 4Qb, No “At-risk”c Lipid | 10 (13.9) | 9 (25.0) Referent |
10 (17.5) | 206 (30.4) Referent |
| TNF-α 4Q Only | 6 (8.3) 1.4 (0.3 – 6.9) |
4 (11.1) | 3 (5.3) 0.8 (0.2 – 2.9) |
72 (10.6) |
| “At-risk”c Lipid Only | 29 (40.3) 1.2 (0.4 – 3.8) |
18 (50.0) | 29 (50.9) 2.0 (0.9 – 4.2) |
298 (44.0) |
| TNF-α 4Q and “At-risk”c Lipid | 27 (37.5) 4.0 (1.1 – 16.3) |
5 (13.9) | 15 (26.3) 2.7 (1.1 – 6.3) |
101 (14.9) |
TNF-α, tumor necrosis factor-alpha; OR, Odds Ratio; 95% CI, 95% confidence interval; 4Q, 4th quartile
Adjusted for gestational week at serum draw and maternal weight.
Wherein 1st and 4th quartile cut points were derived from the term controls.
Total cholesterol (TC), low-density lipoprotein (LDL), triglycerides (TGs), TG: high-density lipoprotein (HDL), or LDL:HDL in the 4Q and/or HDL or HDL:TC ratio in the 1st quartile (1Q).
Figure 1.
Co-occurrence of mid-trimester tumor necrosis factor-alpha (TNF-α) in the 4th quartile (4Q) and lipid riska in pregnancies resulting in early preterm birthb.
aTotal cholesterol (TC), low-density lipoprotein (LDL), triglycerides (TGs), TG: highdensity (HDL), or LDL:HDL in the 4Q and/or HDL or HDL:TC ratio in the 1st quartile (1Q).
bCompared to pregnancies ending in term birth, adjusted for gestational week and maternal weight at serum draw.
Maternal and infant characteristics were similar for case pregnancies with one or more lipid pattern suggestive of hyperlipidemia and a TNF-α in the 4Q versus case pregnancies without such co-occurrence. Although the pattern did not reach statistical significance, in both California and Iowa, cases with co-occurring lipid patterns suggestive of hyperlipidemia and a TNF-α in the 4Q were about twice as likely to have a BMI > 30 than were cases without such co-occurrence (18.5 versus 8.9% in California and 40.0 versus 21.4% in the Iowa (Table 4).
Table 4.
Maternal and infant characteristics in early preterm pregnancies with and without a mid-pregnancy tumor necrosis factor-alpha (TNF-α) level in the 4th quartile (4Q) co-occurring with an “at-risk”a lipid pattern.
| Sample | Preterm (< 30 Weeks), California |
Preterm (< 30 Weeks), Iowa |
||
|---|---|---|---|---|
| TNF-α 4Q, “At-risk”a Lipid Pattern |
TNF-α 4Q, “At-risk”a Lipid Pattern |
|||
| No |
Yes |
No |
Yes |
|
| % 100 (n = 45) | % 100.0 (n = 27) | % 100.0 (n = 42) | % 100.0 (n = 15) | |
| Maternal | ||||
| Prepregnancy BMI | ||||
| > 30 | 8.9 | 18.5 | 21.4 | 40.0 |
| ≤ 30 | 91.1 | 81.5 | 78.6 | 60.0 |
| < 18 | b | b | 12.4 | 0 |
| 18-25 | b | b | 52.4 | 33.3 |
| >25-30 | b | b | 23.8 | 26.7 |
| Hypertension – Any | 17.8 | 14.8 | 33.3 | 20.0 |
| Diabetes | ||||
| Prepregnancy | c | c | 2.4 | 0 |
| Gestational | c | c | 4.8 | 0 |
| Previous PTB | 2.2 | 11.1 | 9.5 | 6.7 |
| Type of PTB | ||||
| Spontaneous | 73.3 | 77.8 | 54.8 | 73.3 |
| PROM | 20.0 | 18.5 | 31.0 | 40.0 |
| Medically Indicated | 17.8 | 14.8 | 45.2 | 26.7 |
| Unknown | 8.9 | 7.4 | -- | -- |
| Infant | Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
|---|---|---|---|---|
| Weeks Gestation | 27.7 (1.7) | 27.3 (1.6) | 25.9 (2.6) | 26.5 (2.6) |
| Birth Weight (grams) | 1016.8 (292.3) | 961.5 (278.5) | 808 (320.8) | 943.2 (442.9) |
TNF-α, tumor necrosis factor-alpha; 4Q, 4th quartile; BMI, Body Mass Index; AFP, alpha-fetoprotein; MoM, multiple of the median; hCG, human chorionic gonadotrophin; uE3, unconjugated estriol; INH, inhibin; BPD, brochopulmonary dysplasia, ROP, retinopathy of prematurity; IVH, intraventricular hemmorhage
Total cholesterol (TC), low-density lipoprotein (LDL), triglycerides (TGs), TG: high-density (HDL), or LDL:HDL in the 4Q and/or HDL or HDL:TC ratio in the 1st quartile (1Q).
Not available.
Excluded from initial sample.
COMMENT
To our knowledge, this is the first study to examine whether risk for early preterm birth is related to the co-occurrence of elevated mid-pregnancy TNF-α and lipid patterns indicative of hyperlipidemia. We found that when lipid patterns suggestive of hyperlipidemia were considered in concert with elevated TNF-α levels, elevated risks emerged that were consistent across California and Iowa samples. In both samples, preterm births were more likely than term births to have co-occurring indicators of hyperlipidemia and TNF-α in the 4Q (37.5% of early preterm cases versus 13.9% of term controls in California (adjusted OR = 4.0, 95% CI 1.1 to 16.3 ) and 26.3% of early preterm cases versus 14.9% of term controls in Iowa (adjusted OR = 2.7, 95% CI 1.1 to 6.3).
While no studies that have focused on early preterm birth have examined the cooccurrence reported here, several studies have examined the relationship between TNF-α and preterm birth2;3;28-31 or lipid patterns suggestive of hyperlipidemia and preterm birth.4;6;32-35 While a number of studies have observed a positive association between preterm birth and elevated TNF-α2;3;28 or preterm birth and indicators of hyperlipidemia,4;6;35 other studies have observed no such association.29-31;33;34 In the current study, we observed evidence for such inconsistency as well. For example, whereas in California samples an elevated TNF-α and an elevated TGs:HDL ratio was associated with an increased risk for early preterm birth before consideration of cooccurrence, no such pattern was observed in Iowa samples. These findings and the observed agreement across the California and Iowa samples when co-occurrence was considered suggests that discrepancies across studies may be related to failure to consider the co-occurrence in other studies.
No specific studies have evaluated the relationship between early preterm birth, TNF-α, and indicators of hyperlipidemia, studies focused on other outcomes in pregnant and non-pregnant samples have observed a similar pattern of co-occurrence between hyperlipidemia and elevated TNF-α and therefore, may provide some insight with respect to the present observations. Of particular note are two recent studies focused on late-onset gestational diabetes and preeclampsia that observed an association between the outcomes of interest and the co-occurrence of hyperlipidemia and elevated TNF-α and also observed associations with other markers that suggested pathways of influence that may be relevant to the current study.
Recently, Lopez-Tinoco and colleagues12 found that in addition to being more likely to have elevated TNF-α levels and indicators of hyperlipidemia (including elevated TGs and low HDLs), pregnancies with late-onset gestational diabetes were more likely than control pregnancies to have increased leptin and decreased adiponectin levels. These investigators also found that patterns of analytes were associated with obesity. These findings may be particularly relevant to the present study given that this constellation of risks (obesity, diabetes, hyperlipidemia, increased TNF-α, increased leptin, and decreased adiponectin) have been associated more broadly with adiposity and insulin resistance in non-pregnant populations 11;14-17;36-38 and with the release of lipids into the bloodstream via lipoloysis.37;39 As such, it may be that the patterns observed in the present study and the pathways involved are related to adipokine abnormalities more broadly and to presence of both increased adiposity and insulin resistance. Such a possible link with these broader pathogeneses may explain why nearly twice as many pregnancies in both samples resulting in preterm birth < 30 weeks and exhibited hyperlipidemia and had increased TNF-α levels were obese compared to pregnancies resulting in preterm birth < 30 weeks without this marker pattern (18.5 versus 8.9% in California and 40.0 versus 21.4% in Iowa). Although the observed differences were not statistically different, further consideration of the potential role of overweight along with co-occurring factors, like insulin resistance, seems warranted.
A recent mouse knock-out study may also be relevant to the present study and suggest pathways of influence given their observation of a relationship between hyperlipidemia, increased TNF-α, and indicators of apoptosis in a preeclampsia model.13 These investigators found that when hyperlipidemia was triggered by knock-out of apoE (a ligand involved in the uptake and clearance of atherogenic lipoproteins) it was accompanied by increased placental TNF-α as well as higher levels of placental markers associated with apoptosis including caspase-3 and Bcl-2-associated X protein (Bax). Such findings may be important to the present analyses in that they suggest that the increased frequency of hyperlipidemia and elevated TNF-α levels observed in early preterm cases might have genetic underpinnings related to apoE, other ligands, and lipoproteins and they also point to placental dysfunction and apoptosis a potential mechanism by which early preterm birth risks may have been realized in our populations of study.
While strengths of the present study include its novel focus and the use of two population-based case-control datasets, there are limitations present in both datasets as well as analytical limitations that could impact the generalization of findings. Specifically, because the dataset in California was drawn from a previous study focused on BPD,18 it was enriched with BPD among cases (36 of 72 with). Observed results, however, were not found to be associated with BPD. As such, , future studies will benefit from the use of larger case groups which in turn, will allow for the evaluation of patterns by cut points across all markers that are more typical of abnormality of value. In future analyses a larger case group will also allow for more detailed consideration of adiposity indicators as well as measurement of other factors that may have close links to the observed results including insulin resistance and other markers of apoptosis (e.g. free fatty acids).
Acknowledgments
Statement of Financial Support: Supported by NIH/NHLBI grants (RC2 HL101748, RO1 HD-57192, and R01 HD-52953), the March of Dimes Prematurity Center at Stanford University School of Medicine, Bill and Melinda Gates Millennium grants (OPP52256 and RSDP 5K12 HD-00849-23), and March of Dimes grants (6-FY11-261 and FY10-180).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None of the authors have a conflict of interest.
The results HAVE NOT been presented at any scientific meetings. An abstract related to these findings has been accepted for poster presentation at the 2014 Annual Meeting of the Society if Maternal and Fetal Medicine (SMFM)
REFERENCES
- 1.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166(5):1576–1587. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 2.Pearce BD, Grove J, Bonney EA, Bliwise N, Dudley DJ, Schendel DE, et al. Interrelationship of cytokines, hypothalamic-pituitary-adrenal axis hormones, and psychosocial variables in the prediction of preterm birth. Gynecol Obstet Invest. 2010;70(1):40–46. doi: 10.1159/000284949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D'Anna K, Argys L, et al. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun. 2012;26(4):650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer MS, Kahn SR, Rozen R, Evans R, Platt RW, Chen MF, et al. Vasculopathic and thrombophilic risk factors for spontaneous preterm birth. Int J Epidemiol. 2009;38(3):715–723. doi: 10.1093/ije/dyp167. [DOI] [PubMed] [Google Scholar]
- 5.Mudd LM, Holzman CB, Catov JM, Senagore PK, Evans RW. Maternal lipids at mid-pregnancy and the risk of preterm delivery. Acta Obstet Gynecol Scand. 2012;91(6):726–735. doi: 10.1111/j.1600-0412.2012.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alleman BW, Smith AR, Byers HM, Bedell B, Ryckman KK, Murray JC, et al. A proposed method to predict preterm birth using clinical data, standard maternal serum screening, and cholesterol. Am J Obstet Gynecol. 2013;208(6):472–11. doi: 10.1016/j.ajog.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green A, Dobias SB, Walters DJ, Brasier AR. Tumor necrosis factor increases the rate of lipolysis in primary cultures of adipocytes without altering levels of hormone-sensitive lipase. Endocrinology. 1994;134(6):2581–2588. doi: 10.1210/endo.134.6.8194485. [DOI] [PubMed] [Google Scholar]
- 8.Plomgaard P, Fischer CP, Ibfelt T, Pedersen BK, van HG. Tumor necrosis factor- alpha modulates human in vivo lipolysis. J Clin Endocrinol Metab. 2008;93(2):543–549. doi: 10.1210/jc.2007-1761. [DOI] [PubMed] [Google Scholar]
- 9.Yang X, Zhang X, Heckmann BL, Lu X, Liu J. Relative contribution of adipose triglyceride lipase and hormone-sensitive lipase to tumor necrosis factor-alpha (TNF-alpha)-induced lipolysis in adipocytes. J Biol Chem. 2011;286(47):40477–40485. doi: 10.1074/jbc.M111.257923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan H, Hacohen N, Golub TR, Van PL, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51(5):1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Xun K, Chen L, Wang Y. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct. 2009;27(7):407–416. doi: 10.1002/cbf.1596. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Tinoco C, Roca M, Fernandez-Deudero A, Garcia-Valero A, Bugatto F, Aguilar-Diosdado M, et al. Cytokine profile, metabolic syndrome and cardiovascular disease risk in women with late-onset gestational diabetes mellitus. Cytokine. 2012;58(1):14–19. doi: 10.1016/j.cyto.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Mao L, Zhou Q, Zhou S, Wilbur RR, Li X. Roles of apolipoprotein E (ApoE) and inducible nitric oxide synthase (iNOS) in inflammation and apoptosis in preeclampsia pathogenesis and progression. PLoS One. 2013;8(3):e58168. doi: 10.1371/journal.pone.0058168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohmura E, Hosaka D, Yazawa M, Tsuchida A, Tokunaga M, Ishida H, et al. Association of free fatty acids (FFA) and tumor necrosis factor-alpha (TNF-alpha) and insulin-resistant metabolic disorder. Horm Metab Res. 2007;39(3):212–217. doi: 10.1055/s-2007-970421. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116(3):219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin DA, McMurray RG, Hackney AC, Harrell JS. Relationship between cardiovascular risk factors and adipokines in adolescents. Horm Res Paediatr. 2011;76(2):123–129. doi: 10.1159/000327852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae YJ, Kim SH, Chung JH, Song SW, Kim KS, Kim MK, et al. Evaluation of adiposity-related biomarkers as metabolic syndrome indicators. Clin Nutr Res. 2013;2(2):91–99. doi: 10.7762/cnr.2013.2.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelliffe-Pawlowski LL, Shaw GM, Currier RJ, Stevenson DK, Baer RJ, O'Brodovich HM, et al. Association of early-preterm birth with abnormal levels of routinely collected first- and second-trimester biomarkers. Am J Obstet Gynecol. 2013 doi: 10.1016/j.ajog.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould JB. The role of regional collaboratives: the California Perinatal Quality Care Collaborative model. Clin Perinatol. 2010;37(1):71–86. doi: 10.1016/j.clp.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Stringer EA, Baker KS, Carroll IR, Montoya JG, Chu L, Maecker HT, et al. Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: evidence of inflammatory pathology. J Transl Med. 2013;11:93. doi: 10.1186/1479-5876-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256(20):2835–2838. [PubMed] [Google Scholar]
- 22.Temelkova-Kurktschiev T, Hanefeld M. The lipid triad in type 2 diabetes - prevalence and relevance of hypertriglyceridaemia/low high-density lipoprotein syndrome in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2004;112(2):75–79. doi: 10.1055/s-2004-815753. [DOI] [PubMed] [Google Scholar]
- 23.Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34(7):583–588. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 24.Scott NJ, Cameron VA, Raudsepp S, Lewis LK, Simpson ER, Richards AM, et al. Generation and characterization of a mouse model of the metabolic syndrome: apolipoprotein E and aromatase double knockout mice. Am J Physiol Endocrinol Metab. 2012;302(5):E576–E584. doi: 10.1152/ajpendo.00222.2011. [DOI] [PubMed] [Google Scholar]
- 25.Brizzi P, Tonolo G, Esposito F, Puddu L, Dessole S, Maioli M, et al. Lipoprotein metabolism during normal pregnancy. Am J Obstet Gynecol. 1999;181(2):430–434. doi: 10.1016/s0002-9378(99)70574-0. [DOI] [PubMed] [Google Scholar]
- 26.Mankuta D, Elami-Suzin M, Elhayani A, Vinker S. Lipid profile in consecutive pregnancies. Lipids Health Dis. 2010;9:58. doi: 10.1186/1476-511X-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resi V, Basu S, Haghiac M, Presley L, Minium J, Kaufman B, et al. Molecular inflammation and adipose tissue matrix remodeling precede physiological adaptations to pregnancy. Am J Physiol Endocrinol Metab. 2012;303(7):E832–E840. doi: 10.1152/ajpendo.00002.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitoratos N, Papadias K, Makrakis E, Christodoulakos G, Panoulis K, Creatsas G. Association between serum tumor necrosis factor-alpha and corticotropin-releasing hormone levels in women with preterm labor. J Obstet Gynaecol Res. 2006;32(5):497–501. doi: 10.1111/j.1447-0756.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 29.Curry AE, Vogel I, Drews C, Schendel D, Skogstrand K, Flanders WD, et al. Mid-pregnancy maternal plasma levels of interleukin 2, 6, and 12, tumor necrosis factor-alpha, interferon-gamma, and granulocyte-macrophage colony-stimulating factor and spontaneous preterm delivery. Acta Obstet Gynecol Scand. 2007;86(9):1103–1110. doi: 10.1080/00016340701515423. [DOI] [PubMed] [Google Scholar]
- 30.Kramer MS, Kahn SR, Platt RW, Genest J, Chen MF, Goulet L, et al. Mid trimester maternal plasma cytokines and CRP as predictors of spontaneous preterm birth. Cytokine. 2010;49(1):10–14. doi: 10.1016/j.cyto.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Tsiartas P, Holst RM, Wennerholm UB, Hagberg H, Hougaard DM, Skogstrand K, et al. Prediction of spontaneous preterm delivery in women with threatened preterm labour: a prospective cohort study of multiple proteins in maternal serum. BJOG. 2012;119(7):866–873. doi: 10.1111/j.1471-0528.2012.03328.x. [DOI] [PubMed] [Google Scholar]
- 32.Catov JM, Bodnar LM, Kip KE, Hubel C, Ness RB, Harger G, et al. Early pregnancy lipid concentrations and spontaneous preterm birth. Am J Obstet Gynecol. 2007;197(6):610–617. doi: 10.1016/j.ajog.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Edison RJ, Berg K, Remaley A, Kelley R, Rotimi C, Stevenson RE, et al. Adverse birth outcome among mothers with low serum cholesterol. Pediatrics. 2007;120(4):723–733. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 34.Gunes T, Koklu E, Ozturk MA. Maternal and cord serum lipid profiles of preterm infants with respiratory distress syndrome. J Perinatol. 2007;27(7):415–421. doi: 10.1038/sj.jp.7211775. [DOI] [PubMed] [Google Scholar]
- 35.Magnussen EB, Vatten LJ, Myklestad K, Salvesen KA, Romundstad PR. Cardiovascular risk factors prior to conception and the length of pregnancy: population-based cohort study. Am J Obstet Gynecol. 2011;204(6):526–528. doi: 10.1016/j.ajog.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Harris RB. Direct and indirect effects of leptin on adipocyte metabolism. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohler H, Jr., Mokshagundam S, Winters SJ. Adipose tissue and reproduction in women. Fertil Steril. 2010;94(3):795–825. doi: 10.1016/j.fertnstert.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 38.Amitani M, Asakawa A, Amitani H, Inui A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. 2013;7:51. doi: 10.3389/fnins.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14(5):447–455. doi: 10.1016/s1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]