Abstract
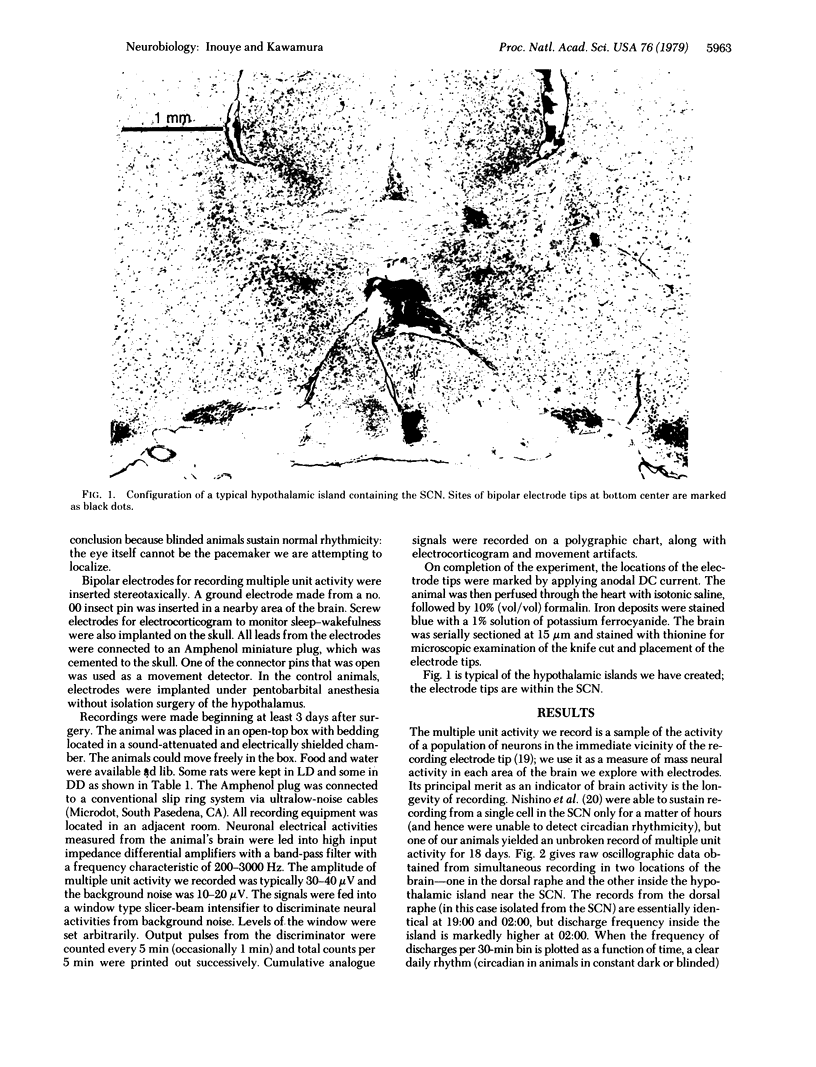
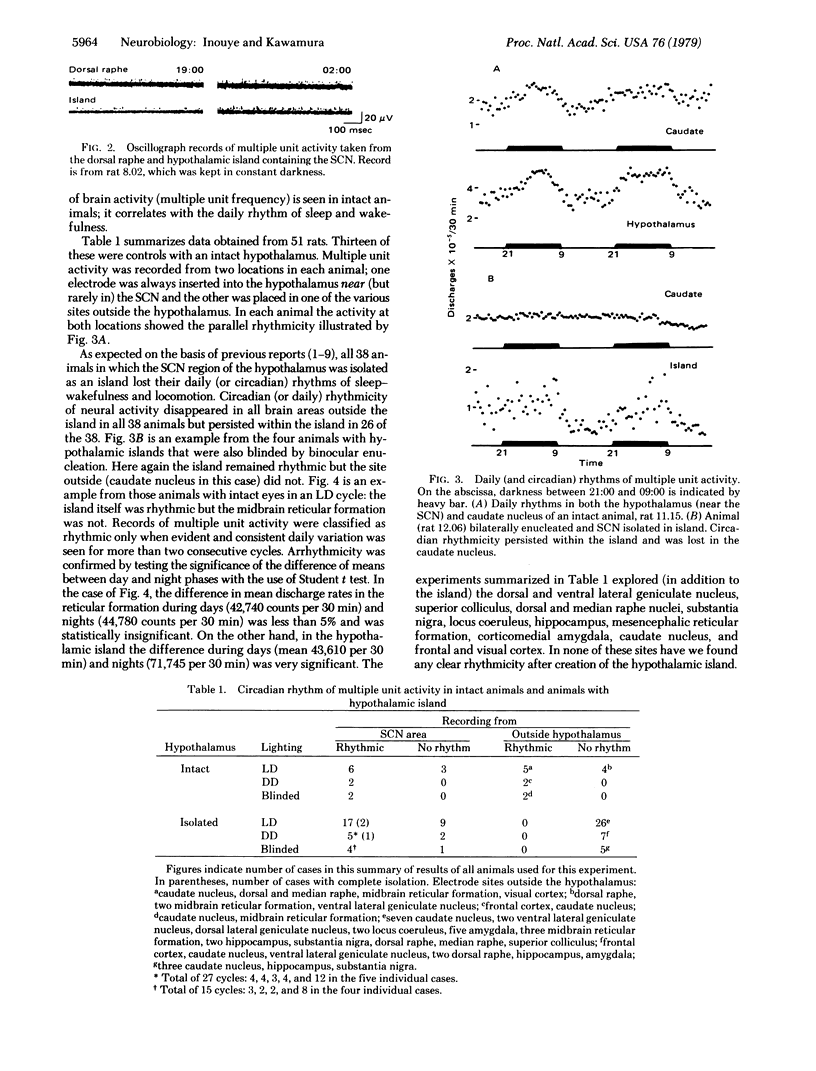
The experimental work described tested the prosposition that the suprachiasmatic nucleus of the hypothalamus is an autonomous circadian pacemaker. Simultaneous recording from two extracellular electrodes indicated neural (multiple unit) activity at two sites in the brain, one of which is in or near the suprachiasmatic nucleus and the other in one of many other brain locations. Both sites in intact rats displayed clear circadian rhythmicity of spontaneous neural activity. In experimental animals, a Halasz knife was used to create an island of hypothalamic tissue that contained the suprachiasmatic nuclei. In such animals that were also blinded by bilateral ocular enucleation, circadian rhythmicity was lost at all brain locations recorded outside the island, but it persisted within the island that contained the suprachiasmatic nuclei. The rhythmicity of the island is thus not dependent on afferent inputs from elsewhere in the brain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREWS R. V., FOLK G. E., Jr CIRCADIAN METABOLIC PATTERNS IN CULTURED HAMSTER ADRENAL GLANDS. Comp Biochem Physiol. 1964 Apr;11:393–409. doi: 10.1016/0010-406x(64)90006-4. [DOI] [PubMed] [Google Scholar]
- Binkley S. A., Riebman J. B., Reilly K. B. The pineal gland: a biological clock in vitro. Science. 1978 Dec 15;202(4373):1198–1120. doi: 10.1126/science.214852. [DOI] [PubMed] [Google Scholar]
- Daan S., Damassa D., Pittendrigh C. S., Smith E. R. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus). Proc Natl Acad Sci U S A. 1975 Sep;72(9):3744–3747. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T. Circadian rhythm of serotonin N-acetyltransferase activity in organ culture of chicken pineal gland. Science. 1979 Mar 23;203(4386):1245–1247. doi: 10.1126/science.424750. [DOI] [PubMed] [Google Scholar]
- Halász B., Pupp L. Hormone secretion of the anterior pituitary gland after physical interruption of all nervous pathways to the hypophysiotrophic area. Endocrinology. 1965 Sep;77(3):553–562. doi: 10.1210/endo-77-3-553. [DOI] [PubMed] [Google Scholar]
- Ibuka N., Inouye S. I., Kawamura H. Analysis of sleep-wakefulness rhythms in male rats after suprachiasmatic nucleus lesions and ocular enucleation. Brain Res. 1977 Feb 11;122(1):33–47. doi: 10.1016/0006-8993(77)90660-6. [DOI] [PubMed] [Google Scholar]
- Ibuka N., Kawamura H. Loss of circadian rhythm in sleep-wakefulness cycle in the rat by suprachiasmatic nucleus lesions. Brain Res. 1975 Oct 10;96(1):76–81. doi: 10.1016/0006-8993(75)90574-0. [DOI] [PubMed] [Google Scholar]
- Jacklet J. W. Circadian rhythm of optic nerve impulses recorded in darkness from isolated eye of Aplysia. Science. 1969 May 2;164(3879):562–563. doi: 10.1126/science.164.3879.562. [DOI] [PubMed] [Google Scholar]
- Kasal C. A., Menaker M., Perez-Polo J. R. Circadian clock in culture: N-acetyltransferase activity of chick pineal glands oscillates in vitro. Science. 1979 Feb 16;203(4381):656–658. doi: 10.1126/science.569904. [DOI] [PubMed] [Google Scholar]
- Moberg G. P., Clark C. R. Effect of adrenalectomy and dexamethasone treatment on circadian running in the rat. Pharmacol Biochem Behav. 1976 May;4(5):617–619. doi: 10.1016/0091-3057(76)90207-0. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Eichler V. B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972 Jul 13;42(1):201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Klein D. C. Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res. 1974 May 10;71(1):17–33. doi: 10.1016/0006-8993(74)90188-7. [DOI] [PubMed] [Google Scholar]
- Morin L. P., Fitzgerald K. M., Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977 Apr 15;196(4287):305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Mouret J., Coindet J., Debilly G., Chouvet G. Suprachiasmatic nuclei lesions in the rat: alterations in sleep circadian rhythms. Electroencephalogr Clin Neurophysiol. 1978 Sep;45(3):402–408. doi: 10.1016/0013-4694(78)90191-8. [DOI] [PubMed] [Google Scholar]
- Nishino H., Kiyomi K., Brooks C. M. The role of suprachiasmatic nuclei of the hypothalamus in the production of circadian rhythm. Brain Res. 1976 Aug 6;112(1):45–59. doi: 10.1016/0006-8993(76)90333-4. [DOI] [PubMed] [Google Scholar]
- Schwartz W. J., Gainer H. Suprachiasmatic nucleus: use of 14C-labeled deoxyglucose uptake as a functional marker. Science. 1977 Sep 9;197(4308):1089–1091. doi: 10.1126/science.887940. [DOI] [PubMed] [Google Scholar]
- Stephan F. K., Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson M. H., Watson-Whitmyre M. Nucleus suprachiasmaticus: the biological clock in the hamster? Science. 1976 Jan 16;191(4223):197–199. doi: 10.1126/science.942799. [DOI] [PubMed] [Google Scholar]
- Terkel J., Johnson J. H., Whitmoyer D. I., Sawyer C. H. Effect of adrenalectomy on a diurnal (circadian) rhythm in hypothalamic multiple unit activitiy in the female rat. Neuroendocrinology. 1974;14(2):103–113. doi: 10.1159/000122250. [DOI] [PubMed] [Google Scholar]
- Van den Pol A. N., Powley T. A fine-grained anatomical analysis of the role of the rat suprachiasmatic nucleus in circadian rhythms of feeding and drinking. Brain Res. 1979 Jan 12;160(2):307–326. doi: 10.1016/0006-8993(79)90427-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman N. H., Menaker M. The pineal gland: a pacemaker within the circadian system of the house sparrow. Proc Natl Acad Sci U S A. 1979 Feb;76(2):999–1003. doi: 10.1073/pnas.76.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]




