Abstract
It is currently unknown whether any secular trends exist in the incidence and outcomes of hip fracture in kidney transplant recipients (KTR). We identified first-time KTR (1997–2010) who had >1 year of Medicare coverage and no recorded history of hip fracture. New hip fractures were identified from corresponding diagnosis and surgical procedure codes. Outcomes studied included time to hip fracture, type of surgery received and 30-day mortality. Of 69 740 KTR transplanted in 1997–2010, 597 experienced a hip fracture event during 155 341 person-years of follow-up for an incidence rate of 3.8 per 1000 person-years. While unadjusted hip fracture incidence did not change, strong confounding by case mix was present. Using year of transplantation as a continuous variable, the hazard ratio (HR) for hip fracture in 2010 compared with 1997, adjusted for demographic, dialysis, comorbid and most transplant-related factors, was 0.56 (95% confidence interval [CI]: 0.41–0.77). Adjusting for baseline immunosuppression modestly attenuated the HR (0.68; 95% CI: 0.47–0.99). The 30-day mortality was 2.2 (95% CI: 1.3–3.7) per 100 events. In summary, hip fractures remain an important complication after kidney transplantation. Since 1997, case-mix adjusted posttransplant hip fracture rates have declined substantially. Changes in immunosuppressive therapy appear to be partly responsible for these favorable findings.
Keywords: Bone disease, fracture, kidney transplant, mortality, outcomes
Introduction
Hip fracture is a major health concern in the general population, resulting in substantial mortality and morbidity (1). The risk of hip fracture is increased severalfold in patients with end-stage renal disease on dialysis compared to the general population (2).
Hip fracture is also an important complication following kidney transplantation. A 2002 study examined the risk of hip fracture in kidney transplant recipients (KTR) and estimated the fracture rate at 3.3 events per 1000 person-years, a 34% higher risk compared with patients receiving dialysis who were waitlisted for transplantation (3). In KTR, bone health is influenced by several factors including preexisting osteitis fibrosa cystica associated with secondary hyperparathyroidism and osteopenia associated with advanced age and/or vitamin D deficiency, as well as by posttransplant factors including use of immunosuppressive drugs and ongoing disturbances in the phosphate–calcium– parathyroid hormone–vitamin D axis (4,5).
Much of the epidemiological information on the rates and correlates of hip fracture in KTR comes from older studies, based on cohorts from the 1990s. However, significant changes have occurred since then, including longer time spent on dialysis (“vintage”) due to increased kidney transplant waiting times; newer approaches to immuno-suppression, including trends toward steroid-minimizing protocols; and differences in recipient and donor characteristics. Thus, it is imperative to update and carefully reexamine the epidemiology of hip fracture in KTR. We conducted the present study to determine national trends in the incidence, treatment and short-term outcomes of hip fractures after kidney transplantation.
Methods
Study population
From the US Renal Data System, we identified all adult (≥18 years) patients who received their first kidney transplant between January 1997 and December 2010. Patients were included in the cohort if they had uninterrupted Medicare Part A and B coverage in the year prior to transplant and had at least one valid claim filed to Medicare during that period. Patients were excluded if they had a history of hip fracture recorded in the year prior to transplant, had a history of prior solid organ transplants or had a simultaneous pancreas–kidney transplant.
Variable of interest
The variable of interest was year of transplant, 1997–2010. We initially examined year as a categorical variable (with year 1997 as the reference year); however, since there were no obvious departures from the linearity assumption, we subsequently considered year as a continuous variable for the main analyses.
Patient characteristics
We abstracted an array of recipient and donor characteristics from US Renal Data System files (Table 1). We categorized immunosuppression after Kirk et al (6): use of any induction therapy (thymoglobulin, basiliximab, daclizumab, alemtuzumab or OKT3); and discharge maintenance immuno-therapies based on (1) tacrolimus, (2) mycophenolate mofetil, (3) mammalian target of rapamycin inhibitor including sirolimus and everolimus or (4) steroid based.
Table 1.
Characteristics of patients with end-stage renal disease at the time of their first kidney transplant
| All years |
1997–2001 |
2002–2006 |
2007–2010 |
|||||
|---|---|---|---|---|---|---|---|---|
| Count | % | Count | % | Count | % | Count | % | |
| Total (row percent) | 69 740 | 100.00 | 19 970 | 28.63 | 26 686 | 38.26 | 23 084 | 33.10 |
| Patient demographics | ||||||||
| Age at transplant (years) | 51 (40–60) | 48 (37–58) | 51 (40–61) | 53 (42–62) | ||||
| Female | 27 219 | 39.03 | 8011 | 40.12 | 10 421 | 39.05 | 8787 | 38.07 |
| Race | ||||||||
| White | 39 711 | 56.94 | 12 066 | 60.42 | 15 160 | 56.81 | 12 485 | 54.09 |
| Black | 24 548 | 35.20 | 6647 | 33.28 | 9313 | 34.90 | 8588 | 37.20 |
| Other | 5462 | 7.83 | 1257 | 6.29 | 2207 | 8.27 | 1998 | 8.66 |
| Missing | 19 | 0.03 | 0 | 0.00 | 6 | 0.02 | 13 | 0.06 |
| Hispanic ethnicity | 11 627 | 16.67 | 3011 | 15.08 | 4587 | 17.19 | 4029 | 17.45 |
| Missing | 453 | 0.65 | 179 | 0.90 | 155 | 0.58 | 119 | 0.52 |
| BMI at transplant (kg/m2) | ||||||||
| <18.5 | 1435 | 2.06 | 512 | 2.56 | 541 | 2.03 | 382 | 1.65 |
| 18.5–24.9 | 20 514 | 29.41 | 6174 | 30.92 | 8063 | 30.21 | 6277 | 27.19 |
| 25.0–29.9 | 20 235 | 29.01 | 5121 | 25.64 | 7966 | 29.85 | 7148 | 30.97 |
| ≥30 | 17 770 | 25.48 | 3886 | 19.46 | 7000 | 26.23 | 6884 | 29.82 |
| Missing | 9786 | 14.03 | 4277 | 21.42 | 3116 | 11.68 | 2393 | 10.37 |
| Cause of end-stage renal disease | ||||||||
| Diabetes | 22 636 | 32.46 | 5994 | 30.02 | 8798 | 32.97 | 7844 | 33.98 |
| Hypertension | 17 124 | 24.55 | 4659 | 23.33 | 6476 | 24.27 | 5989 | 25.94 |
| Glomerulonephritis | 17 232 | 24.71 | 5414 | 27.11 | 6613 | 24.78 | 5205 | 22.55 |
| Other | 12 175 | 17.46 | 3510 | 17.58 | 4678 | 17.53 | 3987 | 17.27 |
| Missing | 573 | 0.82 | 393 | 1.97 | 121 | 0.45 | 59 | 0.26 |
| Dialysis vintage | ||||||||
| <2.5 | 18 824 | 26.99 | 6681 | 33.46 | 6923 | 25.94 | 5220 | 22.61 |
| 2.5–5 | 30 774 | 44.13 | 9723 | 48.69 | 11 465 | 42.96 | 9586 | 41.53 |
| >5 | 20 142 | 28.88 | 3566 | 17.86 | 8298 | 31.09 | 8278 | 35.86 |
| Most recent modality | ||||||||
| Hemodialysis | 58 755 | 84.25 | 16 168 | 80.96 | 22 724 | 85.15 | 19 863 | 86.05 |
| Peritoneal dialysis | 10 470 | 15.01 | 3561 | 17.83 | 3800 | 14.24 | 3109 | 13.47 |
| Missing | 515 | 0.74 | 241 | 1.21 | 162 | 0.61 | 112 | 0.49 |
| Nursing home stay | 1543 | 2.21 | 542 | 2.71 | 539 | 2.02 | 462 | 2.00 |
| Hospital days | 2 (0–8) | 3 (0–9) | 2 (0–8) | 2 (0–7) | ||||
| Nonnephrology clinic visits | 16 (8–27) | 14 (7–24) | 16 (8–27) | 17 (9–29) | ||||
| Comorbidities | ||||||||
| Diabetes | 27 234 | 39.05 | 6885 | 34.48 | 10 584 | 39.66 | 9765 | 42.30 |
| Cancer | 2148 | 3.08 | 477 | 2.39 | 808 | 3.03 | 863 | 3.74 |
| Coronary artery disease | 5962 | 8.55 | 1752 | 8.77 | 2380 | 8.92 | 1830 | 7.93 |
| Cerebrovascular disease | 4976 | 7.14 | 1178 | 5.90 | 1900 | 7.12 | 1898 | 8.22 |
| Cerebral hemorrhage | 333 | 0.48 | 97 | 0.49 | 125 | 0.47 | 111 | 0.48 |
| Alcohol | 406 | 0.58 | 122 | 0.61 | 157 | 0.59 | 127 | 0.55 |
| Tobacco | 2518 | 3.61 | 532 | 2.66 | 1031 | 3.86 | 955 | 4.14 |
| Peripheral vascular disease | 9076 | 13.01 | 2280 | 11.42 | 3490 | 13.08 | 3306 | 14.32 |
| Hypertension | 50 789 | 72.83 | 13 144 | 65.82 | 19 544 | 73.24 | 18 101 | 78.41 |
| Valve disease | 6917 | 9.92 | 1679 | 8.41 | 2704 | 10.13 | 2534 | 10.98 |
| Heart failure | 12 294 | 17.63 | 3290 | 16.47 | 4701 | 17.62 | 4303 | 18.64 |
| Chronic pulmonary disease | 6725 | 9.64 | 1548 | 7.75 | 2614 | 9.80 | 2563 | 11.10 |
| Atrial fibrillation | 3407 | 4.89 | 703 | 3.52 | 1336 | 5.01 | 1368 | 5.93 |
| Other arrhythmias | 3895 | 5.59 | 1112 | 5.57 | 1436 | 5.38 | 1347 | 5.84 |
| Dementia | 567 | 0.81 | 148 | 0.74 | 234 | 0.88 | 185 | 0.80 |
| Rheumatologic disease | 3128 | 4.49 | 843 | 4.22 | 1170 | 4.38 | 1115 | 4.83 |
| Liver disease | 6694 | 9.60 | 2547 | 12.75 | 2124 | 7.96 | 2023 | 8.76 |
| Hyperparathyroidism | 6840 | 9.81 | 2540 | 12.72 | 2692 | 10.09 | 1608 | 6.97 |
| Patient blood type | ||||||||
| A | 23 154 | 33.20 | 6892 | 34.51 | 8839 | 33.12 | 7423 | 32.16 |
| AB | 2697 | 3.87 | 732 | 3.67 | 1006 | 3.77 | 959 | 4.15 |
| B | 10 119 | 14.51 | 2812 | 14.08 | 3857 | 14.45 | 3450 | 14.95 |
| O | 33 667 | 48.28 | 9468 | 47.41 | 12 951 | 48.53 | 11 248 | 48.73 |
| Missing | 103 | 0.15 | 66 | 0.33 | 33 | 0.12 | 4 | 0.02 |
| Panel reactive antibody titer | ||||||||
| 0–10% | 45 192 | 64.80 | 13 154 | 65.87 | 17 628 | 66.06 | 14 410 | 62.42 |
| 11–80% | 12 797 | 18.35 | 3401 | 17.03 | 4543 | 17.02 | 4853 | 21.02 |
| ≥80 | 4139 | 5.93 | 977 | 4.89 | 1571 | 5.89 | 1591 | 6.89 |
| Missing | 7612 | 10.91 | 2438 | 12.21 | 2944 | 11.03 | 2230 | 9.66 |
| Transplant characteristics | ||||||||
| Donor age | 39 (25–51) | 38 (23–49) | 40 (24–50) | 41 (26–52) | ||||
| Missing | 4590 | 6.58 | 2359 | 11.81 | 1873 | 7.02 | 358 | 1.55 |
| Female donor | 30 342 | 43.51 | 8694 | 43.54 | 11 725 | 43.94 | 9923 | 42.99 |
| Missing | 142 | 0.20 | 108 | 0.54 | 30 | 0.11 | 4 | 0.02 |
| Hispanic donor | 10 598 | 15.20 | 2648 | 13.26 | 4248 | 15.92 | 3702 | 16.04 |
| Missing | 150 | 0.22 | 115 | 0.58 | 31 | 0.12 | 4 | 0.02 |
| Donor type | ||||||||
| Living | 11 844 | 16.98 | 3360 | 16.83 | 4805 | 18.01 | 3679 | 15.94 |
| Standard deceased | 40 280 | 57.76 | 11 717 | 58.67 | 15 335 | 57.46 | 13 228 | 57.30 |
| Expanded criteria | 9131 | 13.09 | 2288 | 11.46 | 3374 | 12.64 | 3469 | 15.03 |
| Donation after cardiac death | 4079 | 5.85 | 301 | 1.51 | 1361 | 5.10 | 2417 | 10.47 |
| Missing | 4406 | 6.32 | 2304 | 11.54 | 1811 | 6.79 | 291 | 1.26 |
| Number of HLA mismatches | ||||||||
| 0 | 5904 | 8.47 | 1940 | 9.71 | 2449 | 9.18 | 1515 | 6.56 |
| 1–3 | 18 292 | 26.23 | 7110 | 35.60 | 6365 | 23.85 | 4817 | 20.87 |
| 4-6 | 42 163 | 60.46 | 10 214 | 51.15 | 16 499 | 61.83 | 15 450 | 66.93 |
| Missing | 3381 | 4.85 | 706 | 3.54 | 1373 | 5.15 | 1302 | 5.64 |
| Cold ischemia time (h) | ||||||||
| <12 | 18 767 | 26.91 | 4608 | 23.07 | 6856 | 25.69 | 7303 | 31.64 |
| 12–24 | 27 068 | 38.81 | 7842 | 39.27 | 9938 | 37.24 | 9288 | 40.24 |
| >24 | 13 225 | 18.96 | 4610 | 23.08 | 4736 | 17.75 | 3879 | 16.80 |
| Missing | 10 680 | 15.31 | 2910 | 14.57 | 5156 | 19.32 | 2614 | 11.32 |
| Immunosuppression | ||||||||
| Induction | ||||||||
| Thymoglobulin | 24 162 | 34.65 | 3358 | 16.82 | 9934 | 37.23 | 10 870 | 47.09 |
| Alemtuzumab | 4187 | 6.00 | 0 | 0.00 | 1480 | 5.55 | 2707 | 11.73 |
| OKT3 | 2116 | 3.03 | 1879 | 9.41 | 164 | 0.61 | 73 | 0.32 |
| Basiliximab | 12 265 | 17.59 | 3088 | 15.46 | 5275 | 19.77 | 3902 | 16.90 |
| Daclizumab | 7450 | 10.68 | 2545 | 12.74 | 3090 | 11.58 | 1815 | 7.86 |
| Maintenance | ||||||||
| Tacrolimus | 44 677 | 64.06 | 7372 | 36.92 | 17 981 | 67.38 | 19 324 | 83.71 |
| Cyclosporine | 18 427 | 26.42 | 11 581 | 57.99 | 5309 | 19.89 | 1537 | 6.66 |
| Mammalian target of rapamycin—inhibitors | 5714 | 8.19 | 1821 | 9.12 | 3089 | 11.58 | 804 | 3.48 |
| Mycophenolate mofetil | 57 026 | 81.77 | 15 268 | 76.45 | 21 438 | 80.33 | 20 320 | 88.03 |
| Azathioprine | 2704 | 3.88 | 2324 | 11.64 | 274 | 1.03 | 106 | 0.46 |
| Steroids | 63 598 | 91.19 | 19 370 | 97.00 | 23 878 | 89.48 | 20 350 | 88.16 |
| Missing | 3351 | 4.80 | 361 | 1.81 | 1567 | 5.87 | 1423 | 6.16 |
All values are presented as percent or median (interquartile range).
We ascertained comorbidities in the 365 days prior to transplant from one inpatient or two outpatient claims (on different days) using appropriate International Classification of Diseases, 9th Revision (ICD-9) diagnosis codes (see Table S1 and Supplemental Methods) from institutional claims and physician supplier files.
Outcome
The main outcome of interest was time from kidney transplantation to first hip fracture after transplant. We ascertained hip fracture from the presence of any 820.×× or 821.×× ICD-9 diagnosis code in hospital billing claims with an accompanying procedure code (Table S2) within 7 days prior to or after the hip fracture ICD-9 code. Patients were censored at end of study (December 31, 2010), loss of Medicare A and B coverage, at 3 years posttransplant (when most patients under 65 years of age lose their Medicare coverage), or at death.
We further examined the type of treatment received within 7 days of coded hip fracture. We ascertained treatment type from ICD-9 procedure codes as listed in Table S2, divided into four main categories: partial hip replacement, total hip replacement, internal fixation and other, which included reduction, repair not otherwise specified and no treatment identified. A third outcome of interest was 30-day mortality (“case fatality”) following hip fracture.
Statistical analysis
We summarized baseline characteristics by groups defined by year of transplant (1997–2001, 2002–2006 and 2007–2010). We computed unadjusted hip fracture incidence rates, defined as the number of events over total person-time observed, by year of transplant as well as by time (years) since transplant. We also calculated the 3-year cumulative incidence of fracture stratified by age (below 60 years vs. 60 years or older) and sex.
We applied multivariable Cox proportional hazards models to calculate the adjusted cause-specific hazard ratio (HR) for each year (categorical vs. the referent of 1997). We tested the linearity of year using contrasts and calculated the HR for a 1-year difference in the year of transplant (continuous). Using the linearity assumption of year of transplant, we also computed the HR (and 95% confidence interval [CI]) for each year's transplant cohort to those who received their allograft in 1997. Censoring events and their respective proportions are listed in Table S3. We generated results using three models: Model 1, adjusted for demographic variables; Model 2, additionally adjusted for BMI, comorbidities, healthcare utilization and dialysis history; Model 3, additionally adjusted for most transplant variables; and Model 4, additionally adjusted for use of induction and categories of baseline immunosuppression. No violations of the proportionality assumption were detected. Changes in absolute risk over time were calculated using the method proposed by Austin (7). Since hip fracture incidence was not constant over time, we focused on the first year following transplantation in which hip fracture incidence was highest. We conducted companion analyses considering death as a competing risk. We report counts and percentages of each treatment type received after hip fracture and modeled changes in treatment over time. We also report the number of 30-day case-fatalities following hip fracture. Detailed methods and results of these analyses are provided in Tables S4 and S5. This study was approved by an institutional review board at Stanford University. All analyses were conducted using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC) and R (The R Project for Statistical Computing, Vienna, Austria).
Missing data
About 42% of patients had at least one variable missing. The percentage of data missing ranged from less than 1% for recipient race to 18% for BMI. We assumed a missing at random mechanism and used standard multiple imputation techniques through a multivariable normal model to obtain 14 imputed data sets. To each imputed data set, we fit the Cox proportional hazard model and the results were combined using the rules described by Little and Rubin (8). All variables in Model 4 were included in the imputation model. To augment the imputation of BMI, we also included information on BMI at time of transplant waitlisting or, if missing, BMI from the Medical Evidence Report, and time between BMI reporting and transplantation. For technical details and results, see Supplemental Methods and Table S6.
Results
Figure 1 describes how we assembled the cohort. Baseline characteristics of the 69 740 transplant recipients studied, stratified by era of transplantation, are shown in Table 1. In brief, more recently transplanted recipients were older, and had higher BMI, longer dialysis vintage and generally higher rates of previously diagnosed comorbidities; more kidneys were derived from donation after cardiac death and expanded criteria donors. In more recent years, there were higher number of HLA mismatches, lower cold ischemia times and differential use of immunosuppressive therapy.
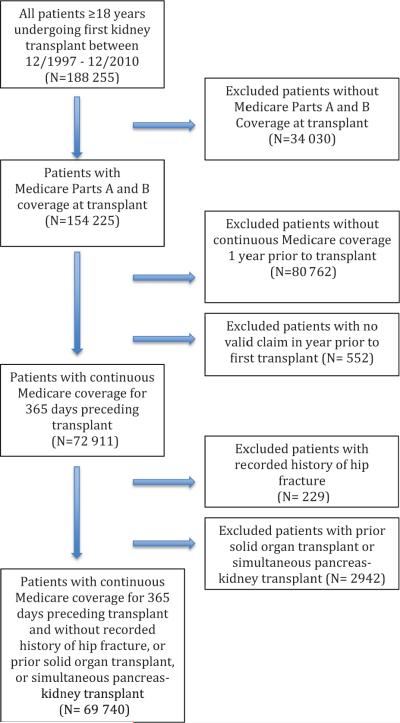
Figure 1.
Flow diagram of cohort derivation.
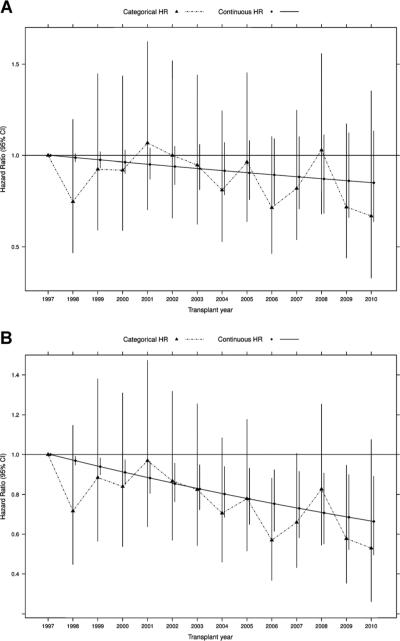
The 69 740 KTR were followed for a total of 155 341 person-years (mean: 2.2 years; median: 3.0 years), during which 597 hip fracture events were recorded, for an unadjusted incidence rate of 3.8 (95% CI: 3.5–4.1) events per 1000 person-years. The event rate was higher in the first year after transplant when compared to the second or third years of follow-up (4.8, 3.3 and 3.2 per 1000 person-years, respectively). The hip fracture incidence after kidney transplantation appeared unchanged over the 14 years in unadjusted analysis. In categorical analysis, the HR for patients transplanted in 2010 was 0.67 (95% CI: 0.33–1.35) compared with patients transplanted in 1997. Given the patterns of the HRs across categorical years, it appeared reasonable to use a linear functional form for trend to maximize power (Figure 2): the unadjusted HR for each more recent year was 0.99 (95% CI: 0.97–1.01). These estimates were confounded, however, by the observed trends in the characteristics of transplant recipients.
Figure 2. Comparisons of estimated categorical and linear trends in the incidence of hip fracture in recipients of a first kidney transplant.
(A) Unadjusted. (B) Adjusted for age, sex, race and Hispanic ethnicity (Model 1). Exact numbers for hazard ratios and 95% confidence intervals are available in Tables S3 and S4.
Adjustment for demographic characteristics (Model 1) alone yielded a HR for continuous year of 0.97 (95% CI: 0.95–0.99), which translated into an estimated HR of 0.66 (95% CI: 0.50–0.89) for the comparison between 1997 and 2010 (Figures 2 and 3). Additional adjustment for comorbid conditions and dialysis-related factors (Model 2) further magnified the reduction in adjusted hip fracture risk, whereas adding most transplant characteristics (Model 3) did not alter the strength of the association (Figure 3). However, additional adjustment for baseline immunosuppression (Model 4) attenuated the reduction in risk. Estimates of sub-distribution and cause-specific HRs were almost identical, arguing against any meaningful informative censoring for death by transplant year (Tables S4 and S5). On an absolute scale, and calculated for the first year in which hip fracture rate was highest, Model 3 corresponds to 0.29 fewer hip fractures per 1000 patient-years for each more recent year of transplantation; Model 4 attenuates this reduction to 0.17 fewer hip fractures per 1000 patient-years. The 3-year cumulative incidence of fracture stratified by age and sex showed that the incidence of hip fracture was similar for both sexes, but higher for age group ≥60 years (Figure 4).
Figure 3. Temporal trends in the incidence of hip fracture in recipients of a first kidney transplant.
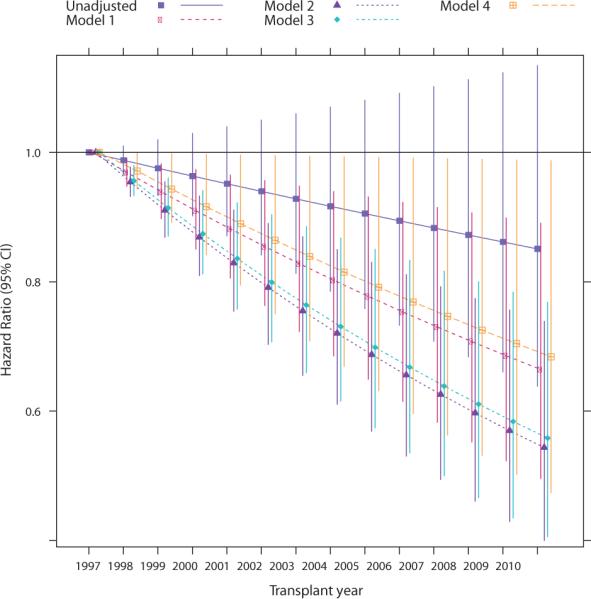
Note: Model 1 is adjusted for demographic variables (age, sex, race, Hispanic ethnicity); Model 2 is additionally adjusted for all comorbidities, dialysis-related and healthcare utilization variables in Table 1 and for BMI; Model 3 is additionally adjusted for all transplant variables in Table 1; Model 4 is additionally adjusted for induction and baseline immunosuppression. p-Values for linear year in Models 1–3 were <0.001. Exact numbers for hazard ratios and 95% confidence intervals are available in Table S4.
Figure 4.
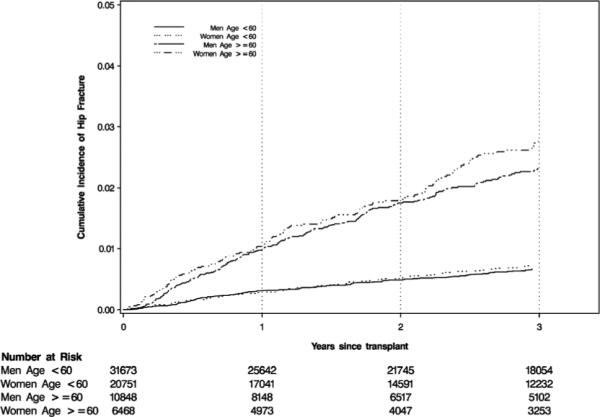
Three-year cumulative hip fracture incidence in recipients of a first kidney transplant, stratified by age and sex.
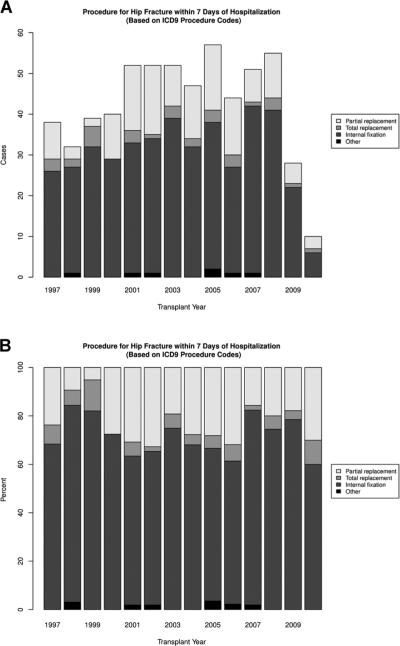
The majority of hip fractures were treated with internal fixation (70.5%), whereas 23.1% underwent partial hip replacement surgery, and 5.1% received a total hip replacement. The remaining 1.1% of patients underwent reduction, or their repair was not specified. We did not observe any significant trends in these treatment patterns over time (adjusted p = 0.74; Figure 5). Following the 597 hip fractures studied, 13 patients died within 30 days of the hip fracture event for a case fatality rate of 2.2 (95% CI: 1.3–3.7) per 100 events.
Figure 5. Trends in the surgical treatment received for hip fracture.
(A) Number of hip fracture treatments, by category and year. (B) Proportion of hip fracture treatments, by category and year. Note: Other includes reduction and repair not otherwise specified. Tests for trend of the use of internal fixation versus other treatment were not significant in unadjusted or any of the adjusted models.
Discussion
Our study demonstrates that the demographic-adjusted incidence of hip fracture in US KTR has declined significantly since the late 1990s. When additionally adjusted for changes in case mix, the incidence of hip fracture was 45% lower in patients transplanted in 2010 than in 1997. However, due to low incidence of the event, this large reduction in relative hazard translates into a rather small decline in absolute hip fracture rates, by approximately 0.3 fewer events per 1000 person-years for each more recent year of transplantation. The trend toward declining adjusted risk of hip fracture in more recent years was attenuated with further adjustment for early posttrans-plant immunosuppressive therapy, suggesting that changes in the immunosuppressive regimen were partly responsible for the reduction in the risk of hip fracture. We observed no temporal trends in the treatment of hip fracture. The 30-day mortality following hip fracture in KTR was relatively low, lower than that of the general population at 2.2 per 100 events. This may be due to a relatively younger study population (median age 51 years) compared to studies of hip fracture in general populations, which are usually conducted in much older populations.
Bone loss and fractures are well-known complications of solid organ transplantation. Bone loss of about 3.9% in the femoral neck has been shown to occur as early as 3 months after transplantation (9). Most fractures in KTR are appendicular rather than axial (10–12). However, hip fractures have been commonly reported (13). Compared to the general population, KTR are at a much higher risk for fractures (10,12). Using the National Health Interview Survey, Ramsey-Goldman et al (10) reported a 34-fold higher fracture risk for women aged 45–64 with a kidney transplant compared to the general population. Ball et al (3) found that the incidence rate of hip fractures was 3.3 per 1000 person-years in patients transplanted between 1990 and 1999, which was 34% higher in adjusted analysis compared with patients receiving dialysis who were on the kidney transplant waitlist during that time. Their reported incidence rate is comparable to ours of 3.8 per 1000 person-years. Furthermore, hip fracture risk is highest in the immediate posttransplant period and has been estimated to decline by 1% for each additional month from the transplant procedure (3). We found that the hip fracture incidence was almost 5 events per 1000 person-years in the first posttransplant year, but closer to 3 events per 1000 person-years in the 2 subsequent years.
There are several possible explanations for the decline in the adjusted risk of hip fracture in KTR. Immunosuppressive regimens changed considerably during the era studied (14). When adjusted for the baseline immunosuppression, the decline in risk was attenuated, suggesting that changes in immunosuppressive therapy may be partly responsible for the reduction. Tacrolimus largely replaced cyclosporine by the early 2000s and some data suggest that, relative to cyclosporine, tacrolimus may preserve bone mineral density (15). Corticosteroids are a well-established risk factor for posttransplant fractures, and the adoption of steroid-sparing and steroid-minimizing regimens could have contributed to the diminished adjusted risk (13,16).
There are several other possible explanations for the decline in the demographic-adjusted rates of hip fracture. The use of other (nonimmunosuppressive) medications may also have favorably influenced the reduction. A decline in incidence of hip fracture in the general population has been partly attributed to increased bisphosphonate use (17). While routine use is not recommended in patients with significantly impaired kidney function, including patients with chronic kidney disease stages 4 and 5 and patients on dialysis (18), bisphosphonates have been shown to attenuate bone loss in KTR (19). Activated vitamin D derivatives and the calcimimetic cinacalcet have also yielded improvements in bone mineral density (20,21). However, studies in KTR have not shown a significant decrease in hip or other fracture risk with bisphosphonates, 25-hydroxy vitamin D or other agents (22). Finally, changes in lifestyle, including enhanced physical activity (23), reduced smoking and alcohol use (24,25) could have improved bone health in KTR. In addition, acute rejection rates (and the corresponding requirement for pulsed steroids) fell considerably between 1997 and the mid-2000s (18,26).
The conventional treatment of hip fracture is surgical repair. About 71.5% of the fractured patients in our study underwent internal fixation, while approximately 23% underwent total or partial hip replacement. Formal statistical testing did not identify any trends in the relative use of these treatments. However, the choice of treatment does provide information about the type of hip fracture sustained; partial or complete hip replacement is mainly used for intracapsular femoral neck fractures and internal fixation for peritrochanteric fractures. For femoral neck fractures and peritrochanteric fractures in the general Medicare population the adjusted percent of each treatment was internal fixation 26.9% and 94.8%, partial hip replacement 77.8% and 8.2%, and total hip replacement 2.7% and 0.5%, respectively (27). The predominance of internal fixation in our population suggests that the majority of fractures were peritrochanteric and contrasts with data from the elderly population where the incidence of peritrochanteric and intracapsular fractures is roughly equal (28).
The 30-day mortality after sustaining a hip fracture was 2.2 per 100 events. This rate is considerably lower than in the general population. Brauer et al (17) examined trends in 30-day mortality after hip fracture in older persons from the general population, and found that the adjusted 30-day mortality decreased from 5.9% to 5.2% in women and from 11.9% to 9.3% in men between 1985 and 2005. KTR constitute a relatively young and carefully screened population with disproportionately poor bone health, which may explain their comparatively high hip fracture incidence but low case fatality rate.
Our study does have certain limitations. There may be under-ascertainment of hip fractures by inclusion of only hospitalized patients, as we based our outcome definition on administrative claims data. However, nearly all patients with hip fractures require hospitalization and numerous studies have validated a claims-based approach for identification of hip fracture (29–31) with a sensitivity of 97% and a positive predictive value of 98%. We looked for procedural claims within 7 days of the coded hip fracture event and may have missed a small minority of patients who received surgical treatment at a later date. To our knowledge, there are no validation studies for the procedure codes for hip fracture treatment. However, given the cost of these procedures, it is unlikely that procedure codes would be ignored or omitted. We have no reason to suspect any temporal trend in ascertainment during the study period. Conversely, over-ascertainment of hip fractures is theoretically possible, but unlikely as we included only fracture events that were accompanied by claims for corresponding surgical treatments, confirming that hip fractures were present. Our study population represents a broad sample of US transplant recipients. However, by limiting our study to patients with ≥1 year of fee-for-service Medicare coverage, we excluded a considerable number of patients, mostly patients younger than 65 years who received preemptive transplantation, had private insurance or were on dialysis less than 1 year prior to transplantation, all of whom constitute a younger and relatively healthier segment of the KTR population. As with all observational studies, residual confounding from un-measured or incorrectly ascertained data cannot be excluded. However, most confounding occurred by reliably coded demographic characteristics and additional adjustment for clinical and transplant characteristics barely changed the inference from demographic-adjusted analyses. Patients younger than 65 years lose federal Medicare benefits 3 years after successful transplantation; therefore, in order to avoid censoring bias, we limited follow-up to 3 years posttransplant. However, most of the literature suggests that fracture risk is highest in the first few years following transplant (3,13). Finally, it is possible that steroid-sparing regimens that are not yet evident from data at discharge and whose use has certainly increased over the years would have further attenuated or even annulled any multivariate association of transplant year with hip fracture incidence. However, since we conducted multiple imputations for missing data and information on postbaseline immunosuppressant drug regimens was missing in large proportions of patients at 1 and 2 years after transplant, imputation models for time-dependent information did not converge.
In summary, we observed relatively high and steady rates of hip fracture in KTR since 1997, which correspond to statistically significant and clinically meaningful declines in age-adjusted, and age-, vintage- and comorbidity-adjusted risks. Additional well-designed comparative effectiveness studies are warranted to identify key drivers of hip fracture incidence and interventions to further reduce the risk of hip fracture and associated complications.
Supplementary Material
Acknowledgments
This study was funded by an unrestricted research fellowship grant from Sanofi to Dr. Sukumaran Nair. An Institutional Review Board at Stanford University approved this study. Data were provided through a data use agreement between the National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK) and Dr. Winkelmayer. An NIDDK officer reviewed this manuscript for privacy and approved of its publication.
Abbreviations
- CI
confidence interval
- HR
hazard ratio
- ICD
International Classification of Diseases
- KTR
kidney transplant recipient(s)
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51:364–370. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- 2.Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58:396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 3.Ball AM, Gillen DL, Sherrard D, et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA. 2002;288:3014–3018. doi: 10.1001/jama.288.23.3014. [DOI] [PubMed] [Google Scholar]
- 4.Weisinger JR, Carlini RG, Rojas E, Bellorin-Font E. Bone disease after renal transplantation. Clin J Am Soc Nephrol. 2006;1:1300–1313. doi: 10.2215/CJN.01510506. [DOI] [PubMed] [Google Scholar]
- 5.Alshayeb HM, Josephson MA, Sprague SM. CKD-mineral and bone disorder management in kidney transplant recipients. Am J Kidney Dis. 2013;61:310–325. doi: 10.1053/j.ajkd.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Kirk AD, Cherikh WS, Ring M, et al. Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am J Transplant. 2007;7:2619–2625. doi: 10.1111/j.1600-6143.2007.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin PC. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J Clin Epidemiol. 2010;63:46–55. doi: 10.1016/j.jclinepi.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. J. Wiley; Hoboken, NJ: 2002. [Google Scholar]
- 9.Almond MK, Kwan JT, Evans K, Cunningham J. Loss of regional bone mineral density in the first 12 months following renal transplantation. Nephron. 1994;66:52–57. doi: 10.1159/000187765. [DOI] [PubMed] [Google Scholar]
- 10.Ramsey-Goldman R, Dunn JE, Dunlop DD, et al. Increased risk of fracture in patients receiving solid organ transplants. J Bone Miner Res. 1999;14:456–463. doi: 10.1359/jbmr.1999.14.3.456. [DOI] [PubMed] [Google Scholar]
- 11.Nisbeth U, Lindh E, Ljunghall S, Backman U, Fellstrom B. Increased fracture rate in diabetes mellitus and females after renal transplantation. Transplantation. 1999;67:1218–1222. doi: 10.1097/00007890-199905150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Vautour LM, Melton LJ III, Clarke BL, Achenbach SJ, Oberg AL, McCarthy JT. Long-term fracture risk following renal transplantation: A population-based study. Osteoporos Int. 2004;15:160–167. doi: 10.1007/s00198-003-1532-y. [DOI] [PubMed] [Google Scholar]
- 13.Nikkel LE, Hollenbeak CS, Fox EJ, Uemura T, Ghahramani N. Risk of fractures after renal transplantation in the United States. Transplantation. 2009;87:1846–1851. doi: 10.1097/TP.0b013e3181a6bbda. [DOI] [PubMed] [Google Scholar]
- 14.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2011 Annual Data Report: Kidney. Am J Transplant 2013. 13(Suppl 1):11–46. doi: 10.1111/ajt.12019. [DOI] [PubMed] [Google Scholar]
- 15.Goffin E, Devogelaer JP, Lalaoui A, et al. Tacrolimus and low-dose steroid immunosuppression preserves bone mass after renal transplantation. Transplant Int. 2002;15:73–80. doi: 10.1007/s00147-001-0377-6. [DOI] [PubMed] [Google Scholar]
- 16.Nikkel LE, Mohan S, Zhang A, et al. Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am J Transplant. 2012;12:649–659. doi: 10.1111/j.1600-6143.2011.03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573–1579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moe SM, Drueke TB, Block GA, et al. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 19.Coco M, Glicklich D, Faugere MC, et al. Prevention of bone loss in renal transplant recipients: A prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol. 2003;14:2669–2676. doi: 10.1097/01.asn.0000087092.53894.80. [DOI] [PubMed] [Google Scholar]
- 20.Cho ME, Duan Z, Chamberlain CE, Reynolds JC, Ring MS, Mannon RB. Cinacalcet improves bone density in post-kidney transplant hyperparathyroidism. Transplant Proc. 2010;42:3554–3558. doi: 10.1016/j.transproceed.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Sevaux RGL, Hoitsma AJ, Corstens FHM, Wetzels JFM. Treatment with vitamin D and calcium reduces bone loss after renal transplantation: A randomized study. J Am Soc Nephrol. 2002;13:1608–1614. doi: 10.1097/01.asn.0000016082.70875.36. [DOI] [PubMed] [Google Scholar]
- 22.Palmer SC, McGregor DO, Strippoli GF. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst Rev. 2007:CD005015. doi: 10.1002/14651858.CD005015.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Spindler A, Paz S, Berman A, et al. Muscular strength and bone mineral density in haemodialysis patients. Nephrol Dial Transplant. 1997;12:128–132. doi: 10.1093/ndt/12.1.128. [DOI] [PubMed] [Google Scholar]
- 24.Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 25.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: Recognition of a major effect. BMJ. 1997;315:841–846. doi: 10.1136/bmj.315.7112.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Shaughnessy EA, Dahl DC, Smith CL, Kasiske BL. Risk factors for fractures in kidney transplantation. Transplantation. 2002;74:362–366. doi: 10.1097/00007890-200208150-00012. [DOI] [PubMed] [Google Scholar]
- 27.Fanuele JC, Lurie JD, Zhou W, Koval KJ, Weinstein JN. Variation in hip fracture treatment: Are black and white patients treated equally? Am J Orthop (Belle Mead NJ) 2009;38:E13–E17. [PubMed] [Google Scholar]
- 28.Wildner M, Bergmann KE. Re: “Heterogeneity of hip fracture: Age, race, sex, and geographic patterns of femoral neck and trochanteric fractures among the US elderly”. Am J Epidemiol. 1997;146:887–889. doi: 10.1093/oxfordjournals.aje.a009212. [DOI] [PubMed] [Google Scholar]
- 29.Virnig B, Durham SB, Folsom AR, Cerhan J. Linking the Iowa Women's Health Study cohort to Medicare data: Linkage results and application to hip fracture. American J Epidemiol. 2010;172:327–333. doi: 10.1093/aje/kwq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron JA, Lu-Yao G, Barrett J, McLerran D, Fisher ES. Internal validation of Medicare claims data. Epidemiology. 1994;5:541–544. [PubMed] [Google Scholar]
- 31.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.