Abstract
Background
Bayley-III scales are currently used to evaluate outcomes of term infants following hypothermia therapy, while all prior reported outcomes in this population have used Bayley-II.
Objective
To determine the incidence of abnormal neurodevelopmental outcomes using Bayley III and the predictive value of MRI in infants who received systemic hypothermia.
Methods
We conducted a prospective cohort study of inborn infants who underwent hypothermia for moderate/severe neonatal encephalopathy from 10/2005–11/2011.
Results
80 newborns underwent hypothermia (incidence of 1/1000). Of the survivors, 89% had Bayley-III performed around 24 months of age. An abnormal outcome using Bayley-III <85 occurred in 50%, while Bayley III <70 occurred in 13%. MRI predicted Bayley III < 85 with sensitivity of 73%, specificity of 84%, PPV of 84%, NPV of 74%.
Conclusions
A Bayley-III 85 cut off identifies a disability rate of 50%, and MRI was predictive of abnormal outcomes. Findings can be useful for counseling of families and planning of future studies using Bayley III.
Keywords: Neonatal encephalopathy, hypothermia, magnetic resonance imaging, Bayley-scores, neurodevelopmental outcomes
Perinatal hypoxic-ischemic encephalopathy (HIE) remains a frequent cause of cerebral palsy, mental retardation, learning disability, and epilepsy. (1) Hypothermia therapy for moderate to severe HIE has reduced significantly death or disability at 18 to 24 months of age.(2–7) Bayley-Scales of Infant and Toddler Development, 2nd edition (Bayley-II) and brain magnetic resonance imaging (MRI), have mostly been utilized for assessing, predicting, and counseling about neurodevelopmental outcomes in these infants.(8–10) The need for standardized assessment of outcomes among survivors of HIE who underwent hypothermia as part of clinical care has been cited as a critical need area by the National Institute of Child Health and Human Development (NICHD). (11)
Published clinical neuro-protection trials have used the Bayley-II score of <70 as part of the criteria for moderate and/or severe disability. (2–7)In 2006, the Bayley-II was restructured in a new standardized third edition (Bayley-III) to provide distinct scores for cognitive, expressive and receptive language, fine and gross motor function, as well as updated normative data for the general population(12). Neonatal follow-up programs including ours have recently incorporated the new Bayley-III as the standard for developmental assessments.
The objectives of this study were to 1) assess the neurodevelopmental outcomes of cooled infants using the new Bayley-III scales, and 2) determine the incidence of each of moderate (70–84) vs. severe (<70) developmental delays and the MRI predictive values of Bayley-III outcomes in a 6 year prospective inborn cohort delivered at Parkland Memorial Hospital (PMH), Dallas, TX.
METHODS
This prospective cohort study included all inborn infants of 36 weeks’ gestation and birth weight 1800 grams with moderate to severe HIE who underwent systemic hypothermia therapy from 10/2005 to 11/2011 in the neonatal intensive care unit (NICU) at PMH, a large public tertiary care center with approximately 14,000 births annually. The study was initiated in October 2005 when systemic hypothermia was implemented as standard care for neonatal HIE and the follow-up in 2007 corresponded to the starting time of Bayley-III implementation for assessment of outcomes. The study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and waiver of consent was obtained.
Patients
Criteria for systemic hypothermia therapy were perinatal acidemia and moderate or severe HIE on a standardized neurological examination. These criteria were identical to those used in the NICHD Neonatal Research Network study of whole body hypothermia(4). Perinatal acidemia was determined by sampling of umbilical cord arterial blood that was obtained routinely on all deliveries from double clamped sections of umbilical cord. The neurological examination consisted of a modified Sarnat staging (13) done by neonatal faculty who were certified by a gold standard examiner to perform such evaluation in a standardized manner. Specifically, the physical examination assessed 1) level of consciousness, 2) spontaneous activity, 3) posture, 4) tone, 5) primitive reflexes, and 6) autonomic nervous system evaluation of pupils and spontaneous respirations. Infants diagnosed with moderate or severe encephalopathy (NE) received hypothermia therapy. Hypothermia was maintained for 72 hours and was achieved by placing the newborn on a cooling blanket (Blanketrol II, Cincinnati Sub-Zero) and maintaining the esophageal temperature at 33.5°C by the blanket servomechanism.
Brain MRI
MRI was performed within 2 weeks of age to depict the pattern and extent of brain injury. Images were acquired after feeding and with swaddling (“bundle and feed”). Sequences included sagittal, axial T1, and axial T2 imaging. The MR studies were interpreted using the NICHD brain injury pattern scale by two experienced pediatric neuroradiologists who were blinded to clinical outcomes(14).
Neurodevelopmental follow-up
The primary outcome was predefined as a composite of death or moderate to severe delays at 24 months of age. Neurodevelopmental testing was performed at Children’s Medical Center, Dallas using the Bayley-III at 12, 24, and 36 months of age. The assessments were administered by a trained pediatric developmental specialist with 11 years of experience, in conjunction with a neurodevelopmental pediatrician with 25 years of experience. Both were blinded to the patients’ MR findings and neonatal course and were assisted by a certified translator-interpreter for non-English speaking subjects(15, 16).
For subjects who underwent serial neurodevelopmental assessments, the BSID-III closest to 24 months was used for this study. A moderate delay was defined by BSID-III 1–2 standard deviations below the norm, i.e. lowest composite score of 70–84 in any of 3 domains: cognitive, language, and motor. Severe delay was defined as any BSID-III composite score > 2 standard deviations below the norm, i.e. < 70 on any of the three tested domains or a complete inability to assign a score due to severe mental deficiency or cerebral palsy (CP). (16)
Statistical analysis
Statistical analysis was performed using Sigma Plot 11.0 (SPPSS, Chicago, IL) with results reported in a tabular format as the mean ± SD, median (25–75%) or number (percentage). One infant who were not testable in a specific domain due to severe CP were analyzed and represented in the severe delay category. Nonparametric analyses and Fisher exact test for categorical variables was used when indicated. The predictive values of MRI in relation to development of the abnormal composite outcome of death or developmental delays were calculated using sensitivity, specificity, positive and negative predictive values.
RESULTS
During the six year study period, 331 (0.004%) of 86,371 deliveries had perinatal acidosis detected from sampling of the umbilical cord arterial blood. Of these 331 newborns, 90 (27%) had signs of moderate to severe HIE on neurological examination and received systemic hypothermia therapy at a median age of 5 ± 1 hours. Hypothermia was maintained for 72 hours with the lowest and highest mean esophageal temperatures being 32.5 ± 0.6°C and 33.9 ± 0.5°C, respectively. None of the infants had a temperature above 37°C following cessation of cooling.
Maternal and infant characteristics of this inborn, largely Hispanic cohort are summarized in Table 1. Moderate encephalopathy was present in 80 (89%) newborns, while severe encephalopathy occurred in 10 (11%). Intrapartum complications were frequent, with meconium exposure in 44%, maternal chorioamnionitis in 29%, and placental abruption in 9% of deliveries.
Table 1.
Characteristics in Infants who underwent Systemic Hypothermia Therapy.
| a) Maternal characteristics | N= 90 |
|---|---|
|
| |
| Ethnicity (Hispanic; black; white) | 77 (83%); 13 (14%); 3 (3%) |
|
| |
| Rupture of membranes (hr, mean ± SD) | 13 ± 20 |
|
| |
| Urgent Cesarean delivery | 48 (53%) |
|
| |
| Hypertension | 14 (16%) |
|
| |
| Diabetes mellitus | 8 (9%) |
|
| |
| Eclampsia | 2 (2%) |
|
| |
| Intrapartum complications | |
|
| |
| Meconium | 40 (44%) |
|
| |
| Umbilical cord prolapse | 2 (2%) |
|
| |
| Umbilical cord avulsion | 2 (2%) |
|
| |
| Placental abruption | 8 (9%) |
|
| |
| Shoulder dystocia | 2 (2%) |
|
| |
| Maternal chorioamnionitis | 26 (29%) |
|
| |
| b) Infant characteristics | |
|
| |
| Birth weight (g) | 3430 ± 584 |
|
| |
| Gestational age (wk) | 39 ± 2 |
|
| |
| Gender: Male | 55 (61%) |
|
| |
| Apgar 1 minute: | 2 (0–7) |
|
| |
| Apgar 5 minute: | 4 (0–9) |
|
| |
| Intubation | 65 (72%) |
|
| |
| Umbilical cord gas pH | 6.97 ± 0.17 |
| base deficit | 19± 2 |
|
| |
| Degree of encephalopathy at< 6 hrs | |
| Moderate | 80 (88%) |
| Severe | 10 (12%) |
Morbidities in the NICU included pulmonary hypertension with receipt of nitric oxide therapy in 13 (14%) and subcutaneous fat necrosis in 2 (2%) neonates. Ten (11%) infants in total died; all occurred in the first week of age following withdrawal of care. Of the 80 survivors, 70 (88%) have attained target follow-up age needed for Bayley testing (Figure 1).
FIGURE 1.
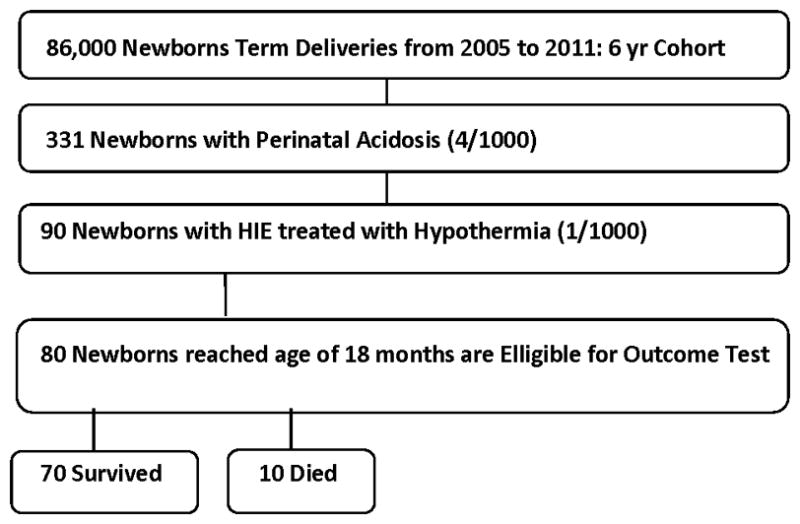
Flow Chart of Patient Population.
Bayley-III at 24 months (Table 2)
Table 2.
Results of Bayley-III at 18–24 Months of Age
| Number of Infants | N=62 |
|---|---|
| 1. Cognitive Score: Average | 85(70–90) |
| ≥ 85 | 33 (53%) |
| 70 – 84 | 21 (34%) |
| < 70 | 8 (13%) |
| 2. Language Score: Average | 83 (74–91) |
| ≥ 85 | 35 (56%) |
| 70 – 84 | 20 (32%) |
| < 70 | 7 (11%) |
| 3. Motor Score: Average | 85 (73–97) |
| ≥ 85 | 42 (68%) |
| 70 – 84 | 13 (21%) |
| < 70 | 7 (10%) |
| 4. Overall Worst Bayley-III category (in any language, motor or cognitive scores) | |
| ≥ 85 | 31 (50%) |
| 70 – 84 | 23 (37%) |
| < 70 | 8 (13%) |
Average for each score represented in bold as Median &Inter-quartile (25–75) range.
Categories scored as normal (>85); moderate-mild delays (70–84); severe delays (<70)
Of the 70 survivors, 62 (89%) of children had neurodevelopmental outcomes testing at a mean ± SD age of 20 ± 2.9 months. Thirty-one (50%) children had normal Bayley-III scores of >85 in all domains (cognitive, language, motor). Twenty-three (37%) children had scores of 70–84 on any one of the three domains, while an additional 8 (13%) had scores of <70 indicative of severe delays. A primary outcome of combined death/developmental delays occurred in 41 (51%) where 10 died and 31 had abnormal Bayley-III in the complete cohort of 80 patients. Infants assessed by clinical examination to have severe NE as compared to moderate NE had significantly more (p=0.04) developmental delays, which occurred in 25 (46%) in moderate vs. in severe NE 6 (86%).
Outcome predictions with Brain MRI
All 62 surviving infants who had Bayley-III testing had a brain MRI performed at a median age of 8 days (range, 4–14 days). Additionally, 5 of the 10 infants who died had an MRI performed prior to their death and were included. Table 3 describes the NICHD MRI scoring classification and associated outcomes at 18–24 months in 67 infants. MRI was normal in 35 (52%) infants, of which 74% had normal outcomes. All infants with MRI scores of ≥2 indicative of basal ganglia injury or watershed infarction with cerebral devastation had concomitant cognitive and motor delays with a specificity of 100% CI (90%–100%) and a PPV of 100% CI (68%–100%).
Table 3.
Brain MRI and Death or Bayley IIII Developmental Delays at 24 Months.
| MRI (N=67) |
Normal/No Delays (Bayley-III >85) |
Moderate Delays (Bayley-III 70–85) |
Death or Severe Delays (Bayley-III <70) |
|---|---|---|---|
| Score 0 (n=35) | 26 (74%) | 7(20%) | 2 (6%) |
| Score 1a (n=5) | 1(20%) | 4(80%) | 0 (0%) |
| Score 1b(n=12) | 4(33%) | 7(58%) | 1(8%) |
| Score 2a (n=4) | 0 | 2(50%) | 2(50%) |
| Score 2b (n=7) | 0 | 2(28%) | 5(72%) |
| Score 3 (n=4) | 0 | 1(25%) | 3(75%) |
| Score | Interpretation |
|---|---|
| 0 | Normal |
| 1A | Minimal cerebral lesions only with no involvement of basal ganglia (BG) or thalamus (T) or anterior limb of the internal capsule or posterior limb and no infarction |
| 1B | More extensive cerebral lesions (>2) without BG or watershed infarction |
| 2A | Any BG, anterior or posterior limb of the internal capsule involvement |
| 2B | BG, anterior or posterior limb of the internal capsule involvement, watershed and cerebral lesions |
| 3 | Cerebral hemispheric devastation |
5 of the infants who died had MRI available were included in the severe delays category.
A low injury score on MRI such as 1a and 1b was also associated with delays in 12/17 (70%) of the infants. Such delays were mostly moderate in nature and included in all patients a score of 70–85 in both cognitive and language domains, except for one infant who had severe delays <70 in all three domains.
The predictive values of any MRI abnormalities (score ≥1) for having any disability using a Bayley-III cutoff <85 at 18–24 months demonstrated a sensitivity with 95 % confidence intervals (CI ) of 73% (63%–83%), specificity of 84%(70%–90%), positive predictive value (PPV) of 84%(71%–93%), and negative (NPV) of 74%(62%–82%). The calculated positive likelihood ratio was 5; CI (2, 12).
DISCUSSION
In this prospective cohort inborn population study, the incidence of moderate-to-severe HIE treated with hypothermia therapy was 1/1000 and 50% of these children had abnormal Bayley-III outcomes using 85 as a cut off value, while only 13% of cooled survivors had abnormal outcomes when a cut off value of 70 was used.
The observed rate of severe delays using Bayley III in this study is considerably less than the published literature using Bayley II.(2–7) For instance, The NICHD Neonatal Research Network’s randomized trial of hypothermia, which used a similar cooling protocol has reported twice the rate in the current study with a MDI or PDI <70 in 27%. (4)
A recent cohort from UK also reported the same phenomena of fewer children classified with severe delay using the Bayley-III than the Bayley-II (17) in infants with neonatal encephalopathy who were cooled using different criteria from TOBY trial. Our patient population is different from other published studies in that it is comprised of 83% Hispanic population, in contrast with the NICHD trial, which had more equal distribution of white, black and other races. We also have a higher proportion of infants with moderate encephalopathy and fewer infants with severe encephalopathy in this cohort.
The fact remains that the possibility of underestimating disabilities when using the Bayley-III does not appear specific to a particular population or group since it has also been reported in outcome studies of other populations such as preterm infants (18–20) and children with cardiac lesions.(21)
The differences observed are therefore more likely related to the inherent structure of the Bayley-III examination which was developed in 2006 to allow for separate scores of cognitive, language, motor domains, receptive and expressive communication. The Bayley Technical Manual reports approximately 7 point lower difference when comparing Bayley-II vs. III for each of the Mental Developmental Index (MDI) vs. the Composite Cognitive Score and the Psychomotor Developmental Index (PDI) vs. the Motor Composite Score, respectively(12). The inherent differences between the two versions preclude direct comparisons. Previously published trials for neonatal encephalopathy (2–7) have used the Bayley-II MDI scores of <70, which incorporates both cognitive and language items, while Bayley-III Cognitive Composite only includes the former; and have used PDI<70, which comprises both gross and fine motor items as does the Bayley-III Motor Composite. When we analyzed the data using a Bayley III cutoff of <85 in any one of the three domains, we found a 50% rate of death/disability in the overall cohort, which are closer rates to the published literature used in prognosis and counseling of families.
MRI is a recognized surrogate marker of outcome in neonatal HIE(10) and the basal ganglia/thalamic and watershed injury patterns represent the hallmark of HIE injury.(22, 23) Hypothermia has reduced the incidence and severity of brain injury while preserving the overall predictive value of MRI as a marker of death or disability.(24) However, correlations with Bayley-III scores have not been published to date. We used the NICHD MRI classification to assess the predictive values of MRI for abnormal Bayley-III outcomes and found high MRI brain injury scores; e.g. 2a, 2b and 3 to be 100% predictive of abnormal outcomes. Findings are consistent with Shankaran et al who reported a two-fold increase in odds of death or disability with increased in the MRI severity. (14) An unexpected finding in the current study was that even low scores, i.e. 1a and b showing evidence of focal white matter injury, were associated with cognitive deficits at 24 months of age in about two thirds of patients.
The current study findings, if replicated, would raise clinical concerns that these subtle MRI lesions that could escape detection especially by radiologists not skilled in the interpretation of these studies, may be associated with worse prognosis. It is also possible that conventional imaging may not be sensitive to injury, and that more advanced imaging and diffusion studies are needed to detect subtle white matter injury and improve our abilities to predict higher mental functions such as language and cognition.
Major strengths of the study are: a) the long standing follow up clinic with the ability to provide serial Bayley testing, as well as over 85% follow up rates at 24 months of age; b) a large patient population; and adherence to strict cooling protocols based on screening neurological examination performed by a certified neonatologist.
One of the main study limitations is that it did not control for maternal education, socioeconomic status, and the home environment after NICU discharge. Since our study population is predominantly Hispanic, and inborn, the study findings of high incidence of white matter injury in association with abnormal developmental outcomes will need to be replicated in other settings. Whether above findings are related to Bayley III underestimating or Bayley II overestimating disability cannot be extrapolated from this study. The study is focused by design on Bayley-III, and did not include CP, deafness or blindness and should only be interpreted accordingly. The optimal cut-off values that best differentiate between mild, moderate, and severe disability either in terms of current functioning or prediction of later function are still not established. Regardless of the ranges used, it should be noted that each level represents a spectrum of impairment, or conversely, functional ability.
Conclusions
Study findings are relevant to counseling families in the era of Bayley III and suggest using a cut off value of 85 for early interventions referral and planning of future neuroprotective trials. The high incidence of isolated white matter injury in this study and its association with cognitive deficits at 24 months of age merits further evaluations in larger groups of patients.
Acknowledgments
Funding: Dr. Chalak is supported by Grant K23HD069521-01A11 and Gerber Foundation grant.
Abbreviations
- NE
Neonatal encephalopathy
- MRI
Magnetic resonance imaging
- BG
Basal ganglia
- WS
Watershed infarct
- PVWMI
Periventricular white matter injury
- Bayley
Bayley-Scales of Infant and Toddler Development
Footnotes
Financial disclosure: Nothing to declare
Conflict of interest: Nothing to declare
Author Contributions:
- Lina Chalak, MD drafted the manuscript, contributed to study conception and design, performed statistical analysis and data interpretation, and finalized the manuscript as submitted after feedback from the other authors.
- Tara DuPont, MD contributed to the conception and design, data acquisition and initial analyses, and presented the preliminary findings of the first 50 patients at the meeting of the American Academy of Pediatrics in October, 2011 (poster).
- Pablo J. Sánchez, MD contributed to conception and study design, analysis and interpretation of data, critical review and revision of the manuscript, and approved the submitted version.
- Ashley Lucke, MD provided the follow-up data, revised excel sheets, and approved the final submitted version.
- Roy J. Heyne, MD performed the follow-up assessments and data acquisition, critically analyzed and interpreted the data, critically reviewed and revised the manuscript, and approved the final submitted version.
- Micheal Morriss, MD acquired the MRI data and reviewed the MRIs and interpreted the results critically, scored the MRI, critically reviewed the manuscript, and approved the final submitted version
- Nancy Rollins, MD contributed to study conception and design, acquired the MRI data and reviewed the MRIs and interpreted the results critically, scored the MRI, critically reviewed the manuscript, and approved the final submitted version.
References
- 1.Levene ML, Kornberg J, Williams TH. The incidence and severity of post-asphyxial encephalopathy in full-term infants. Early Hum Dev. 1985 May;11(1):21–6. doi: 10.1016/0378-3782(85)90115-x. Epub 1985/05/01.eng. [DOI] [PubMed] [Google Scholar]
- 2.Zhou WH, Cheng GQ, Shao XM, Liu XZ, Shan RB, Zhuang DY, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010 Sep;157(3):367–72. 72 e1–3. doi: 10.1016/j.jpeds.2010.03.030. Epub 2010/05/22.eng. [DOI] [PubMed] [Google Scholar]
- 3.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010 Oct;126(4):e771–8. doi: 10.1542/peds.2009-2441. Epub 2010/09/22.eng. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005 Oct 13;353(15):1574–84. doi: 10.1056/NEJMcps050929. Epub 2005/10/14.eng. [DOI] [PubMed] [Google Scholar]
- 5.Azzopardi D, Brocklehurst P, Edwards D, Halliday H, Levene M, Thoresen M, et al. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 2008;8:17. doi: 10.1186/1471-2431-8-17. Epub 2008/05/02.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011 Aug;165(8):692–700. doi: 10.1001/archpediatrics.2011.43. Epub 2011/04/06.eng. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005 Feb 19–25;365(9460):663–70. doi: 10.1016/S0140-6736(05)17946-X. Epub 2005/02/22.eng. [DOI] [PubMed] [Google Scholar]
- 8.Cotten CM, Shankaran S. Hypothermia for hypoxic-ischemic encephalopathy. Expert Rev Obstet Gynecol. 2010 Mar 1;5(2):227–39. doi: 10.1586/eog.10.7. Epub 2010/07/14.Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barks JDE. Technical Aspects of Starting a Neonatal Cooling Program. Clinics in Perinatology. 2008 Dec;35(4):765–75. doi: 10.1016/j.clp.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998 Jan;19(1):143–9. Epub 1998/02/12.eng. [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, et al. Hypothermia and Other Treatment Options for Neonatal Encephalopathy: An Executive Summary of the Eunice Kennedy Shriver NICHD Workshop. J Pediatr. 2011 Aug 27; doi: 10.1016/j.jpeds.2011.08.004. Epub 2011/08/31.Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.2006. BsoidNSTHA. Bayley scales of infant and toddler development. Harcourt Assessment. (3) 2006 [Google Scholar]
- 13.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976 Oct;33(10):696–705. doi: 10.1001/archneur.1976.00500100030012. Epub 1976/10/01.eng. [DOI] [PubMed] [Google Scholar]
- 14.Shankaran S, Barnes PD, Hintz SR, Laptook AR, Zaterka-Baxter KM, McDonald SA, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child. 2012 Jul 17; doi: 10.1136/archdischild-2011-301524. Epub 2012/07/19.Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997 Apr;39(4):214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. Epub 1997/04/01.eng. [DOI] [PubMed] [Google Scholar]
- 16.Palisano RJ, Hanna SE, Rosenbaum PL, Russell DJ, Walter SD, Wood EP, et al. Validation of a model of gross motor function for children with cerebral palsy. Phys Ther. 2000 Oct;80(10):974–85. Epub 2000/09/26.eng. [PubMed] [Google Scholar]
- 17.Jary S, Whitelaw A, Walloe L, Thoresen M. Comparison of Bayley-2 and Bayley-3 scores at 18 months in term infants following neonatal encephalopathy and therapeutic hypothermia. Dev Med Child Neurol. 2013 Aug 9; doi: 10.1111/dmcn.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. 2012 Aug;161(2):222–8. e3. doi: 10.1016/j.jpeds.2012.01.057. Epub 2012/03/17.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore T, Johnson S, Haider S, Hennessy E, Marlow N. Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. J Pediatr. 2012 Apr;160(4):553–8. doi: 10.1016/j.jpeds.2011.09.047. Epub 2011/11/04.eng. [DOI] [PubMed] [Google Scholar]
- 20.Lowe JR, Erickson SJ, Schrader R, Duncan AF. Comparison of the Bayley II Mental Developmental Index and the Bayley III Cognitive Scale: are we measuring the same thing? Acta Paediatr. 2012 Feb;101(2):e55–8. doi: 10.1111/j.1651-2227.2011.02517.x. Epub 2011/11/08.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acton BV, Biggs WS, Creighton DE, Penner KA, Switzer HN, Thomas JH, et al. Overestimating neurodevelopment using the Bayley-III after early complex cardiac surgery. Pediatrics. 2011 Oct;128(4):e794–800. doi: 10.1542/peds.2011-0331. Epub 2011/09/29.eng. [DOI] [PubMed] [Google Scholar]
- 22.Rutherford MA, Counsell SJ, Cowan FM, Azzopardi D, Edwards D, Renowden S, et al. Magnetic resonance imaging patterns of brain injury following hypothermia in neonates with hypoxic ischemic encephalopathy. PEDIATRIC RESEARCH. 2004 Apr;55(4):583a-a. [Google Scholar]
- 23.Rutherford M, Srinivasan L, Dyet L, Ward P, Allsop J, Counsell S, et al. Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol. 2006 Jul;36(7):582–92. doi: 10.1007/s00247-006-0164-8. Epub 2006/06/14.eng. [DOI] [PubMed] [Google Scholar]
- 24.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010 Jan;9(1):39–45. doi: 10.1016/S1474-4422(09)70295-9. Epub 2009/11/10.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]


