Abstract
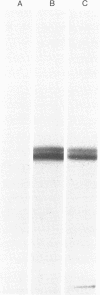
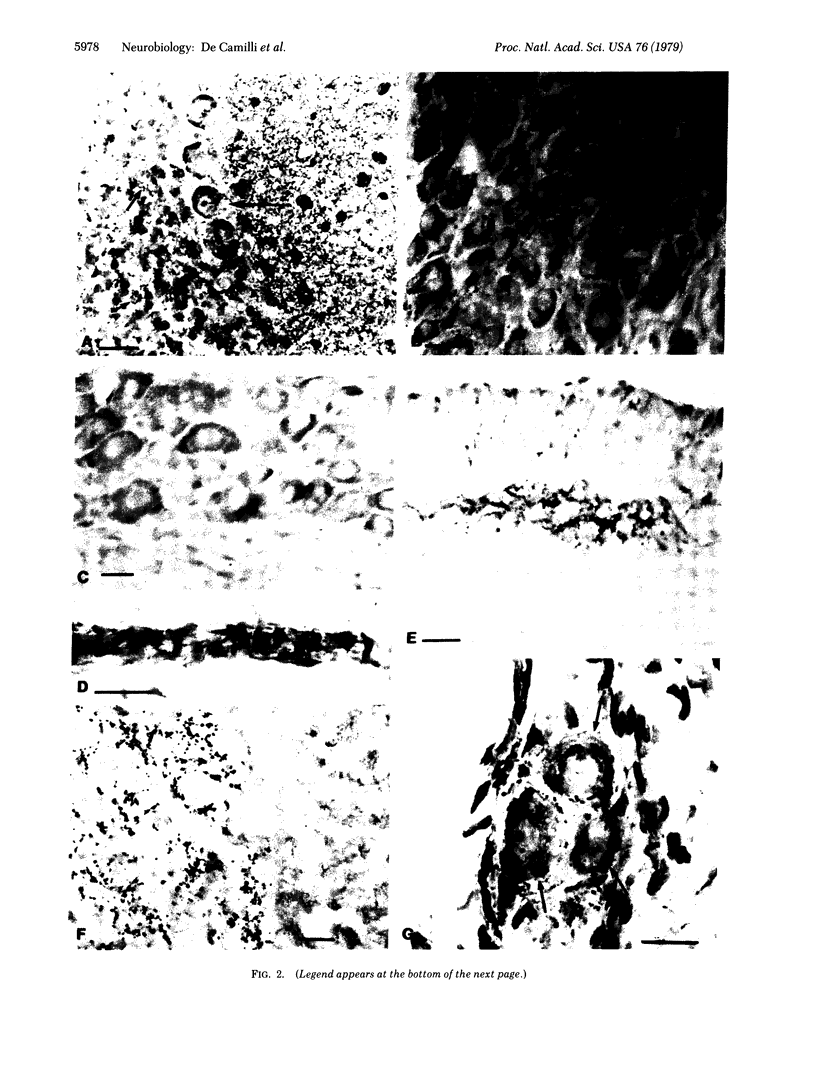
Protein I, a naturally occurring substrate for cyclic AMP-dependent and calcium-dependent protein kinases, previously has been found only in mammalian brain, where it has been demonstrated to be located in neurons. Various tissues and organs outside the brain have now been examined for the possible occurrence of protein I, by using both an immunohistochemical approach and a chemical procedure involving radioimmunolabeling of polyacrylamide gels. Protein I has been found in the inner plexiform layer of the retina, in the posterior pituitary, and in the autonomic nervous system. In tissue composed predominantly of cells other than nerve cells, immunoreactivity was present only where innervation was present. Protein I appeared to be localized in some, but not all, nerve terminals and synapses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair W. S., Jurivich D., Goodenough U. W. Localization of cellular antigens in sodium dodecyl sulfate-polyacrylamide gels. J Cell Biol. 1978 Oct;79(1):281–285. doi: 10.1083/jcb.79.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A. Monoamine-containing fibres in the pituitary neuro-intermediate lobe of the pig and rat. Z Zellforsch Mikrosk Anat. 1968;89(4):573–589. doi: 10.1007/BF00336180. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Ueda T., Battenberg E., Greengard P. Immunocytochemical localization, in synapses, of protein I, an endogenous substrate for protein kinases in mammalian brain. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5982–5986. doi: 10.1073/pnas.76.11.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom F., Battenberg E., Rossier J., Ling N., Guillemin R. Neurons containing beta-endorphin in rat brain exist separately from those containing enkephalin: immunocytochemical studies. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1591–1595. doi: 10.1073/pnas.75.3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ehinger B. Synaptic organization of the dopaminergic neurons in the rabbit retina. J Comp Neurol. 1978 Jul 15;180(2):203–220. doi: 10.1002/cne.901800202. [DOI] [PubMed] [Google Scholar]
- Fried G., Lagercrantz H., Hökfelt T. Improved isolation of small noradrenergic vesicles from rat seminal ducts following castration. A density gradient centrifugation and morphological study. Neuroscience. 1978;3(12):1271–1291. doi: 10.1016/0306-4522(78)90147-1. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legg P. G. Fluorescence studies on neural structures and endocrine cells in the pancreas of the cat. Z Zellforsch Mikrosk Anat. 1968;88(4):487–495. doi: 10.1007/BF00571795. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Norberg K. A., Olson L. Adrenergic innervation of the salivary glands in the rat. Z Zellforsch Mikrosk Anat. 1965 Oct 12;68(2):183–189. doi: 10.1007/BF00342427. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Dreyfus C. F., Gershon M. D., Hökfelt T., Elde R. P., Nilsson G., Said S., Goldstein M. VIP-, enkephalin-, substance P- and somatostatin-like immunoreactivity in neurons intrinsic to the intestine: immunohistochemical evidence from organotypic tissue cultures. Brain Res. 1978 Oct 27;155(2):239–248. doi: 10.1016/0006-8993(78)91020-x. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Lundberg J. M., Hökfelt T., Terenius L., Brandt J., Elde R. P., Goldstein M. Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience. 1978;3(12):1169–1186. doi: 10.1016/0306-4522(78)90137-9. [DOI] [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]
- ZELANDER T., EKHOLM R., EDLUND Y. The ultrastructural organization of the rat exocrine pancreas. III. Intralobular vessels and nerves. J Ultrastruct Res. 1962 Aug;7:84–101. doi: 10.1016/s0022-5320(62)80029-x. [DOI] [PubMed] [Google Scholar]