Abstract
Chronotype is a construct reflecting individual differences in diurnal preference. Although chronotype has been extensively studied in school-age children, adolescents, and adults, data on young children are scarce. This study describes chronotype and its relationship to the timing of the circadian clock and sleep in 48 healthy children ages 30–36 months (33.4±2.1 months; 24 males). Parents completed the Children’s Chronotype Questionnaire (CCTQ) ~2 weeks before the start of the study. The CCTQ provides three measures of chronotype: midsleep time on free days, a multi-item morningness/eveningness score, and a single item chronotype score. After 5 days of sleeping on their habitual schedule (assessed with actigraphy and sleep diaries), children participated in an in-home salivary dim light melatonin onset assessment. Average midsleep time on free days was 1:47±0:35, and the average morningness/eveningness score was 26.8±4.3. Most toddlers (58.4%) were rated as “definitely a morning type” or “rather morning than evening type,” while none (0%) was rated as “definitely evening type.” More morning-types (midsleep time on free days and morningness/eveningness score, respectively) had earlier melatonin onset times (r=0.45, r=0.26), earlier habitual bedtimes (r=0.78, r=0.54), sleep onset times (r=0.80, r=0.52), sleep midpoint times (r=0.90, r=0.53), and wake times (r=0.74, r=0.34). Parental ratings using the single item chronotype score were associated with melatonin onset (r=0.33) and habitual bedtimes (r=0.27), sleep onset times (r=0.33), and sleep midpoint times (r=0.27). Morningness may best characterize circadian preference in early childhood. Associations between chronotype and circadian physiology and sleep timing suggest adequate validity for the CCTQ in this age group. These findings have important implications for understanding the marked variability in sleep timing during the early years of life.
Keywords: Chronotype, DLMO, Circadian Phase, Sleep, Children, Circadian Phase, Morningness/Eveningness
INTRODUCTION
Chronotype is a construct reflecting individual differences in diurnal preference. In adolescents and adults, evening chronotype is associated with negative social and health consequences (Gelbmann et al. 2012; Roenneberg et al. 2012). Results from an established literature also indicate an inverted U-shaped distribution of chronotype across age, with a shift towards eveningness from school-age to adolescence and a subsequent shift back to morningness from adulthood into older age (Carskadon et al. 1993; Roenneberg et al. 2004; Werner et al. 2009; Wolfson et al. 2003). Published data on chronotype in early childhood are scarce; however, existing studies suggest young children show a relatively strong preference for morningness (Nakade et al. 2012; Wickersham 2006).
Chronotype is assessed with questionnaires asking individuals about their “feeling-best rhythms” (Carskadon et al. 1993; Horne and Ostberg 1976; Wolfson et al. 2003) or sleep timing on “free” days (Roenneberg, 2003). Similar constructs were adapted in the Children’s Chronotype Questionnaire (CCTQ) (Werner et al. 2009), a parent-report scale for use with 4- to 11-year-olds. The CCTQ provides three measures of chronotype: midsleep time on free days (MSF), a multi-item morningness/eveningness (ME) score, and a single item chronotype (CT) score. Although chronotype in adults is related to salivary dim light melatonin onset (DLMO) time (Duffy et al. 2001; Laberge et al. 2000; Wright et al. 2013), the association between toddlers’ chronotype and circadian physiology remains an unexplored area of research.
Results from numerous studies suggest chronotype, in addition to genetics, light exposure, and accumulated sleep debt, interact to influence variability in sleep behavior (Katzenberg et al. 1998; Lazar et al. 2012). Indeed, adults self-reporting a preference for morningness are more likely to have earlier bedtimes, sleep midpoints, and wake times (Duffy et al. 2001; Laberge et al. 2000; Wright et al. 2013). Chronotype may also play an important role in determining sleep duration and perceived sleep need, although results are mixed (Roepke and Duffy 2010). Examining chronotype-sleep links are of particular importance in early childhood because it is a stage marked by substantial inter-individual differences in the timing and duration of sleep (Iglowstein et al. 2003). Sleep problems are also prevalent during this developmental stage, affecting about 25% of young children (Owens 2008).
In this study, parents of 30- to 36-month olds (n=48) completed the CCTQ ~2 weeks before starting the study. During the 6-day protocol, children slept on their habitual schedule verified with actigraphy and a daily sleep diary. Children then participated in a 6-h salivary DLMO assessment performed in family’s homes. We used these data to (a) describe parent-reported chronotype in toddlers, (b) test whether chronotype is positively correlated with children’s circadian physiology (DLMO), (c) assess the concordance between reported chronotype and objective actigraphic measures of sleep timing, and (d) explore associations between circadian preference and 24-h sleep duration.
MATERIALS AND METHODS
Participants
Participants were 48 healthy 30- to 36-month-old children (33.4±2.1 months; 24 females; 83% Caucasian, 17% mixed-race) from two cohorts: Providence, RI (n=19) and Boulder, CO (n=29). The majority of children (n=43) took regular daytime naps (3.7±1.6 days/week; nappers). Five children slept only at night (non-nappers). Families were recruited at community events and via flyers and laboratory website advertisements. Parents completed a telephone screening and then questionnaires to evaluate study criteria. Children were included if they were 30–36 months of age at the time of assessment and were excluded if they (a) participated in co-sleeping (i.e., sharing a bed all or part of the night with another individual >2 times/month); (b) had a bedtime/wake time sleep schedule that differed by >2h between weekdays and weekends; (c) traveled beyond 2 time zones within 3 months of the study; (d) regularly used medications affecting sleep, alertness, or the circadian system; (e) had reported/diagnosed sleep problems; (f) had developmental disabilities, neurologic/metabolic disorders, chronic medical conditions, lead poisoning, or head injury involving loss of consciousness; (g) had a conceptual age <38 weeks or >42 weeks; (h) were low birth weight (<5.5 lbs); or (i) had a family history (first degree) of diagnosed narcolepsy, psychosis, or bipolar disorder.
All parents signed an informed consent approved by the IRB. Families were compensated with $50 cash, and children received a $50 United States savings bond after completing the study.
Protocol
About 2 weeks before the start of the study, parents completed the CCTQ. During the first 5 study days, children slept on their habitual schedule, and researchers made several in-home visits to train children in providing saliva samples. The DLMO assessment occurred on study day 6. During the entire protocol, caffeine or medications affecting sleep and/or circadian rhythms were proscribed. Parents provided daily reports of their child’s sleep patterns and protocol compliance via telephone or email. Children were studied neither during summer months (June to August) nor during the 1-week following daylight saving time changes.
Measures
Children’s Chronotype Questionnaire [CCTQ; (Werner et al. 2009)]
The CCTQ is a 27-item, parent report questionnaire that provides three measures of chronotype in children: (1) midsleep time on free days (MSF); (2) a multi-item morningness/eveningness (ME) score; (3) and a single item chronotype (CT) score. The MSF requires parents to report on several sleep parameters (i.e., bedtime, lights-off time, sleep latency, wake time, get out of bed time, and time to be fully alert). “Scheduled” days are those when the child’s sleep patterns are influenced by their own or their family’s structured activities (e.g., school, day care, work, athletics, church). “Free” days are those when the child’s sleep patterns are not influenced by individual or family activities; thus, representing a time when the child would more likely to sleep at his/her circadian preference. MSF was computed as (sleep onset time + sleep period)/ 2 on free days. The ME score is calculated using a series of 10 questions asking about the child’s “feeling-best” rhythm for various activities. Based upon findings from 4- to 11-year-old Swiss children, scores range from 10 (extreme morningness) to 49 (extreme eveningness), with scores ≤23 corresponding to morning types, 24–32 to intermediate types, and ≥33 to evening types (Werner et al. 2009). The CT score was a single item measure in which parents reported their child’s chronotype on a 5-point scale (1 = “definitely a morning type,” 2 = “rather a morning type,” 3 = “neither a morning type nor an evening type,” 4 = “rather an evening type than a morning type,” or 5 = “definitely an evening type”). The validity (concordance with actigraphic sleep parameters) and test-retest reliability of the CCTQ with 4- to 11-year-olds have been established as adequate for research instruments (Werner et al. 2009).
Sleep Diary
Parents completed a daily 26-item sleep diary throughout the study. Diary questions asked about bedtime, lights-out time, sleep onset latency, wake time, get out of bed time, nap start time, nap end time, and intervals the actigraphy was not worn by the child. Diary data were used to ensure compliance with the study protocol and to facilitate scoring of actigraphy data (Acebo et al. 2005).
Actigraphy
Actigraphy is non-invasive tool for objectively assessing sleep patterns in field settings. Children wore model AW64 (Minimitter; Bend, OR)] or AW Spectrum (Philips/Respironics; Pittsburg, PA) actigraphs on their non-dominant wrist throughout the duration of the study to obtain uninterrupted recordings of sleep/wakefulness states via measurements of motor activity. Actiware-Sleep V5.59 software was used to estimate 1-min epochs of sleep or wakefulness from activity levels produced in the surrounding ±2-min interval. This algorithm was applied to portions of the record identified as sleep through a combination of diary reports and event markers pressed at “lights-out” and “lights-on.” In comparison to videosomnography in young children, this algorithm shows high overall epoch-by-epoch agreement (94%) and is excellent in detecting sleep (sensitivity=97%); however, it overestimates wake during the sleep period (specificity=24%) (Sitnick et al. 2008). Standard scoring rules were applied to each sleep episode – sleep start was the first of 3 epochs of sleep after lights-out, and sleep end was the last of 5 epochs of sleep before lights-on (Acebo et al. 2005). Sleep episodes were excluded when the (a) actigraph was off for all/part of the sleep period, (b) concurrent diary report was not available, or (c) the episode included external motion (e.g., sleeping in a car or stroller). Actigraphic estimates of sleep parameters aggregated across study days 1–5 included the following: lights-out time (bedtime), sleep start (onset) time, sleep midpoint, sleep end time (wake time), and 24-h sleep duration (number of minutes between sleep start time to sleep end time for naps and nighttime sleep). Actigraphy data were not available due to non-compliance (n=1) and technical failure (n=3); in these cases, we used daily diary reports to compute sleep variables. Of the 44 children with actigraphy data, variables were aggregated across 5 days/nights in 85% of children, 4 days/nights in 12% of children, and 3 days/nights in 3% of children.
Salivary Dim Light Melatonin (DLMO) Assessment. DLMO was assessed with an in-home protocol on the final day of the study. Participants entered dim light conditions (0.01 – 10.90 lux at angle of gaze) at least 1-h before the first saliva sample, where they remained throughout the data collection interval. Children provided saliva samples (~2 mL) every 30-min for 6-h ending 1-h past their average bedtime (12 samples total) from parental reports during study days 1–5. With help from a researcher, children rinsed their mouths with water >15 min before each saliva sample. Children remained in a sitting posture for 5-min before and during each saliva sample. Saliva samples were collected by having children chew on a braided dental cotton roll (Henry Schein Inc., Denver, Pennsylvania, USA) for 1–2 min. Lux levels were obtained with each saliva sample using a light meter (Extech Instruments, Nashua, NH, USA) held approximately 5 cm adjacent to the child’s eye and directed in the angle of gaze. Samples were immediately centrifuged and refrigerated on-site and then frozen (−20°C) within 12 h. Assays were performed at the Bradley Hospital Sleep and Chronobiology Laboratory (Providence, RI, USA) or SolidPhase Inc. (Portland, Maine, USA) using radioimmunoassay (ALPCO Diagnostics, Salem, New Hampshire, USA), minimum detection 0.2 pg/mL. Circadian DLMO phase was defined as the clock time evening salivary melatonin concentrations increased and remained above a 4pg/mL threshold using linear interpolation between successive samples citation. This is a well-accepted standard with children and adolescents (Carskadon et al. 1997) and was derived from reports that salivary melatonin concentrations are about 40% of plasma levels in healthy young adults (10pg/mL is the most common DLMO threshold for plasma melatonin) (Deacon and Arendt 1994).
Actigraphic estimates of sleep and DLMO times in a subset of this sample have been previously published (LeBourgeois et al. 2013a).
Data Processing and Analysis
Chronotype data were collected and managed using the Research Electronic Data Capture (REDCap) tool hosted at University of Colorado. REDCap is a secure, web-based application designed to support data collection for research studies (Harris et al. 2009).
Statistical analyses were performed with PASW Statistics Package 21.0 (IBM Corp. Armonk, NY, USA). Summary measures are presented as frequency distributions, M±SD, median, or range. Pearson correlations were used to assess associations between continuous variables, and spearman correlations were used to assess associations between ordinal variables. One-tailed tests were used to examine the following hypotheses: (a) chronotype is positively associated with DLMO and actigraphic sleep timing measures and (b) chronotype measures are positively correlated. Two-tailed tests were used to explore correlations between chronotype and sleep duration and age. Sex and napping status differences in chronotype were examined with two-tailed independent t-tests. Finally, a paired t-test was used to test differences between midsleep time on free and scheduled days.
RESULTS
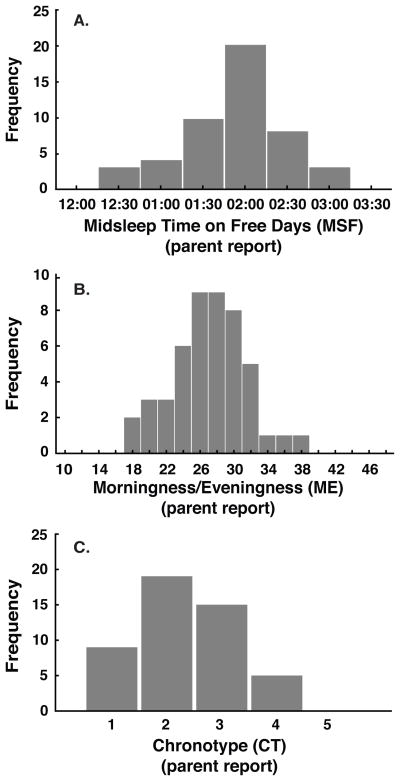
Table 1 reports descriptive statistics for the three CCTQ chronotype measures (MSF, ME, CT), DLMO, and actigraphic sleep measures. Frequency histograms of MSF, ME, and CT are illustrated in Fig 1. Using the single-item chronotype (CT) score, 18.8% of children were rated by parents as “definitely a morning type,” 39.6% as “rather a morning than evening type,” 31.3% as “neither a morning nor an evening type,” 10.4% as “rather an evening type,” and 0% as “definitely an evening type” (Fig 1C). We observed significant inter-correlations between the three chronotype measures: MSF with ME (r=0.60, p<.001); MSF with CT (r=0.27, p<.05); and ME with CT (r=0.50, p<.001). Midsleep time on days when children’s sleep was influenced by structured activities (scheduled days) was 01:53 ± 0:34, which differed from midsleep time on free days 01:47 ± 0:35, t(47) = 2.9, p < 0.05, d = 0.20.
Table 1.
Descriptive statistics for chronotype, circadian, and actigraphic sleep measures (n=48).
| All Subjects | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| MSF | 01:47 | 0:35 | 00:15 | 02:57 |
| ME | 26.8 | 4.3 | 18 | 37 |
| CT | 2.0 (Median) | 1 | 4 | |
| DLMO | 19:26 | 0:51 | 17:35 | 21:07 |
| Bedtime | 20:12 | 0:38 | 19:03 | 22:00 |
| Sleep Start Time | 20:38 | 0:45 | 19:12 | 22:31 |
| Midsleep Time | 01:41 | 0:38 | 00:00 | 02:55 |
| Wake Time | 06:46 | 0:43 | 04:41 | 08:16 |
| 24-h Sleep Duration (h) | 11.7 | 0.7 | 10.3 | 13.04 |
Abbreviations: dim light melatonin onset (DLMO); midsleep on free days (MSF); morningness/ eveningness (ME) score; chronotype (CT) score
Figure 1.
Frequency distributions of 48 young children assessed with the Children’s Chronotype Questionnaire (CCTQ; lower values = more morningness, higher values = more eveningness): midsleep time on free days in 30-min bins (MSF; 1A); morningness/eveningness score (ME; 1B); and chronotype score (CT; 1C). For the CT score, 1 = “definitely a morning type,” 2 = “rather a morning type,” 3 = “neither a morning type nor an evening type,” 4 = “rather an evening type than a morning type,” or 5 = “definitely an evening type.”
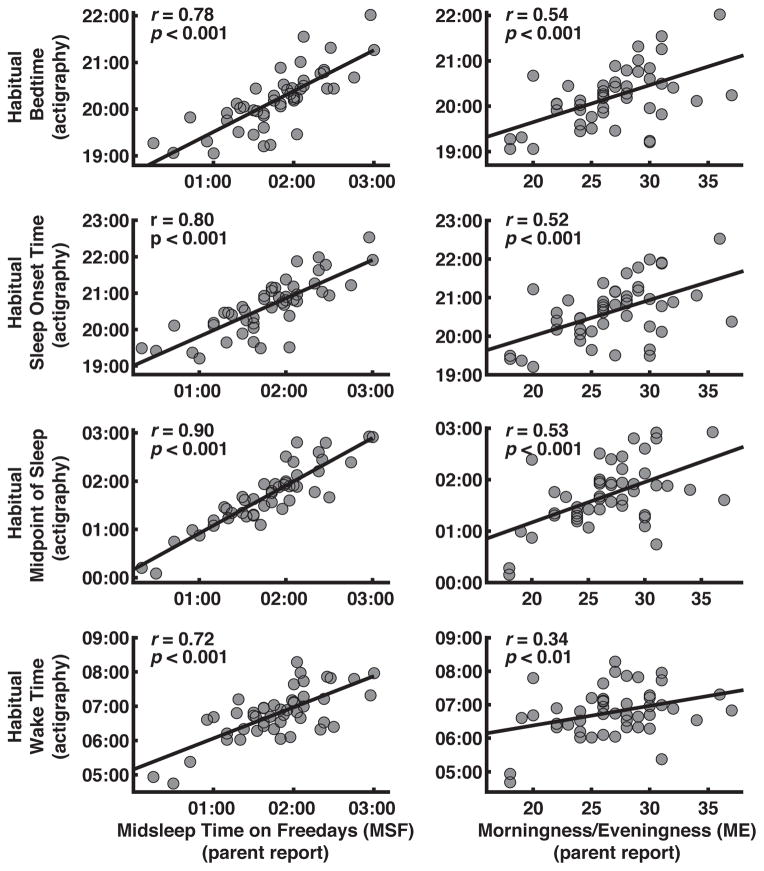
Correlation coefficients quantifying associations between the three chronotype variables and DLMO and sleep measures are shown in Table 2. Toddlers with earlier DLMOs were more likely to have earlier MSFs and a stronger preference for morningness as reported by parents (Fig 2). Children with an earlier parental-reported circadian preference as assessed with MSF or the ME scale, had earlier objectively measured habitual bedtimes, sleep midpoint times, and wake times (Fig 3). Chronotype as rated with the single-item CT score was positively associated with DLMO, bedtime, sleep onset time, and sleep midpoint time. We found no correlations between any of the chronotype measures and 24-h sleep duration. Chronotype did not differ by sex or napping status and was not associated with age.
Table 2.
Correlation coefficients quantifying associations between the three Children’s Chronotype Questionnaire (CCTQ) parent-report measures and dim light melatonin onset time (DLMO) and actigraphic sleep measures (*p<0.05, **p<0.001).
| CCTQ Chronotype Measures | |||
|---|---|---|---|
| MSF | ME | CT | |
| DLMO | 0.45** | 0.26* | 0.32* |
| Bedtime | 0.78** | 0.54** | 0.27* |
| Sleep Onset Time | 0.80** | 0.52** | 0.33* |
| Midsleep Time | 0.90** | 0.53** | 0.27* |
| Wake Time | 0.72** | 0.34* | 0.11 |
| 24-h Sleep Duration (h) | −0.03 | −0.10 | −0.19 |
Abbreviations: dim light melatonin onset (DLMO); midsleep on free days (MSF); morningness/ eveningness (ME) score; chronotype (CT) score
Figure 2.
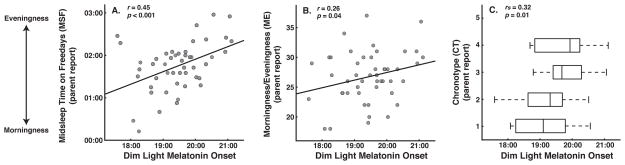
Scatterplots showing associations between dim light melatonin onset (DLMO) and parental-reported midsleep time on free days (MSF; 2A) and morningingness/eveningness scores (ME; 2B). Boxplots showing association between DLMO and the parental-reported single-item chronotype score (CT; 2C). Upper and lower borders of boxplots represent upper and lower quartiles, tails represent min and max scores, and the vertical midline represents the median. Lower MSF, ME, and CT values = more morningness, higher values = more eveningness.
Figure 3.
Scatterplots showing associations between parent-reported chronotype measures [midsleep time on free days (MSF); morningness/eveningness (ME) score; chronotype score (CT)] and actigraphic sleep measures. Lower MSF, ME, and CT values = more morningness, higher values = more eveningness.
DISCUSSION
In this study, we collected parental reports of chronotype in a sample of 30- to 36-month-old children with the Children’s Chronotype Questionnaire (CCTQ). We also obtained a reliable marker of circadian phase, the dim light melatonin onset (DLMO), and objective actigraphic estimates of sleep timing and duration. With these data, several important findings emerged. First, toddlers exhibit substantial inter-individual differences in chronotype, with a relative preference for morningness. Second, DLMO time was related to chronotype, such that toddlers with later circadian phases were more likely to be evening-types (“owls”). Third, toddler’s reportedly exhibiting a stronger morning preference had earlier bedtimes, sleep onset times, sleep midpoints, and wake times as measured with actigraphy. Findings are discussed in the context of understanding chronotype in the early years as it pertains to maturational changes in sleep and circadian rhythms, as well as to health and developmental outcomes.
Our results add to the ongoing discussion about changes in chronotype across the first two decades of life (Carskadon et al. 1993; Harada et al. 2007; Roenneberg et al. 2004; Wickersham 2006). Using the CCTQ, Werner and colleagues found MSF was 02:26 in a cohort of 4- to 11-year-old Swiss children (Werner et al. 2009), and in a large-scale study by Roenneberg and colleagues, MSF was roughly 02:55 in 10-year-olds, 04:30 in 15-year-olds, and 04:55 in 20 year-olds (Roenneberg et al. 2004). Although the current analysis was not based upon a community sample, we found 30–36 month-olds had an average MSF of 01:47. Similarly, the average ME score of our sample suggests an overall preference towards morningness in young children as compared to the 4- to 11-year-olds studied by Werner and colleagues (Werner et al. 2009). This trend was further supported by the percentage of parents responding to CT categories. None of our toddlers were rated as “definitely an evening type,” whereas 21% of Swiss school-age children were reported as such (Werner et al. 2009). Our findings also build upon those from several cross-sectional studies describing circadian preference in early childhood, all of which used parent-report, multi-item morningness/ eveningness questionnaires. For example, data from a large sample of Japanese children attending daycare/school showed a phase delay in chronotype from infancy to mid-adolescence. Additionally, an age-related change in the chronotype of preschool children was found by Wichersham (Wickersham 2006): 90% of 2-year-olds versus ~58% of 6-year-olds were reportedly morning types, with only 3% of the sample rated by their parent as having a preference for eveningness. Taken together, these findings provide evidence that young children exhibit a stronger tendency for morningness than school-age children and adolescents. Future research using representative diverse longitudinal samples is needed to understand the distribution, cultural/ethnic differences, inter-individual variability, and development of chronotype in early childhood and how it may change across the lifespan.
The optimal timing of young children’s sleep is a common concern of parents and educators, as well as a frequent topic of the media. In this study, our measures of both extrinsic parent-influenced bedtimes and intrinsic child-determined sleep onset times were associated with chronotype. Such distinctions are important because unlike older teens and adults who choose their own sleep schedules, caregivers largely influence the sleep timing of toddlers. From a child-oriented approach guided by the “goodness-of-fit” framework (Jenni and O’Connor 2005), optimal sleep schedules may be best identified when taking children’s intrinsic individual circadian characteristics, such as chronotype, into consideration (Jenni and LeBourgeois 2006). This perspective is supported by our recent findings indicating that dissonance between toddler’s melatonin phase and bedtimes influenced evening settling difficulties: children put to bed at a time too close to their DLMOs were more likely to exhibit parent-reports of bedtime resistance and to show longer sleep onset latencies as estimated by actigraphy (LeBourgeois et al. 2013b). Furthermore, midpoint of sleep on weekends may serve as a real-world proxy for the MSF chronotype measure used in this and other studies. For example, children with later weekend sleep midpoints are more likely to be obese/overweight and at-risk (Spruyt et al. 2011) for poor academic success as reflected in their scores on a standardized assessment of school readiness (Crosby et al. 2006).
The determinants and outcomes of chronotype represent a complex interplay between circadian physiology, genetics, and behavior in the context of an individual’s social and solar clocks (Roenneberg et al. 2003). Based upon the adolescent and adult literature (Duffy et al. 2001; Laberge et al. 2000; Wright et al. 2013), we expected individual differences in chronotype would be associated with the timing of toddlers’ circadian clocks. Our findings supported this hypothesis: children rated as more evening types had later melatonin phases. Light, the strongest zeitgeber of the human circadian system, likely plays a key role in this bidirectional relationship (Roenneberg et al. 2003). In modern society, light exposure may be “gated” by a host of behaviors or environmental factors (e.g., school start time, sleep times, electric lighting, screen time). Indeed, in a recent study of Japanese preschoolers, it was found that 30% of children slept in bedrooms with blackout shades, and that those using shades exhibited a stronger evening preference as rated by their parents (Nakade et al. 2012). Evening types were also reportedly exposed to light for shorter durations in the morning between wake time and school arrival. The later bedtimes and wake times of evening types in our study suggest they may be exposed to more evening light after sunset and less light during the early-morning hours. This pattern corresponds to ideal conditions for later circadian phases, as predicted by the adult phase-response curve to light (Khalsa et al. 2003) and as demonstrated experimentally in response to increased exposure to natural light during the daytime and reduced exposure to light at night (Wright et al. 2013). Further evidence for the latter comes from a study showing individuals without electric lighting in the home have earlier bedtimes (Peixoto et al. 2009). Finally, diurnal sensitivity to light is thought to undergo maturational changes, with sensitivity declining with age (Carskadon et al. 2002). If true, the early years of life may represent a sensitive period in determining chronotype that may track across the lifespan.
Though this research was not designed specifically to evaluate the psychometric properties of the CCTQ, our results provide further construct validity for parents reporting on their child’s chronotype. To date, the youngest age assessed with the CCTQ was 4 years, and validity was initially evaluated with parental sleep diaries and actigraphic estimates of sleep timing (Werner et al. 2009). We found concordance rates between the three CCTQ measures that were similar to those reported in school-age children (Werner et al. 2009). Additionally, our findings showing parent-reported chronotype was not only associated with objective sleep timing but also with endogenous circadian phase in a younger population help to establish the utility of the CCTQ as a convenient, cost-effective tool that may be used in epidemiologic, clinical, and experimental studies with young children. However, similar to the findings of others (Giannotti et al. 2002; Werner et al. 2009), our analysis of associations between chronotype and sleep timing is inherently subject to the issue of multicolinearity. This is a particularly important consideration when utilizing MSF as a measure of chronotype, as it is computed based upon reported bedtime, sleep onset latency, and wake time. The circular nature of sleep timing and chronotype is also a concern when utilizing multi-item morningness-eveningness questionnaires because they include items about individual’s preferred sleep timing (Carskadon et al. 1993; Horne and Ostberg 1976). Although we employed actigraphy as an objective measure of sleep, a comprehensive understanding of sleep-chronotype links necessitates the collection of physiological data. For example, we recently reported moderate-to-strong associations between circadian phase and actigraphic estimates of sleep timing, such that toddlers with later DLMOs were more likely to go to bed, fall asleep, and wake up at later times (LeBourgeois et al. 2013a). Such findings compliment the current data indicating relationships between chronotype, circadian physiology, and sleep timing.
It is important to note that the current findings are from a cohort of healthy children with “regular” sleep schedules and no history of sleep problems, which may limit their generalizability. Thus, it is likely that obtaining data from a large community or population-based sample would produce more variability in chronotype ratings, especially evening types. Additionally, the small difference (6 min) between midsleep time on “free” days as compared to those when children’s sleep schedules were influenced by structured activities (e.g., school, daycare, parental work schedules) may not be representative of the general population. Furthermore, the overestimation of wakefulness during sleep when assessed by actigraphy is an important consideration for future research studying associations between sleep quality and chronotype in children.
Associations between chronotype and outcomes were not examined in this study; however, our findings may have significant implications for children’s health and development. Even in older children and adolescents, evening chronotype is related to negative outcomes, including dysregulated mood and poor academic achievement (Gelbmann et al. 2012; Goldstein et al. 2007). Because early childhood is a sensitive period in the development of many basic skills and functions (e.g., emotion regulation, expressive and receptive language, executive function) predictive of future health, research targeting individual difference factors placing children at-risk for poor outcomes is critical. Additionally, the developmental trajectory of chronotype starting in the early years, including the tracking of individual standing, is an unexplored research area. Given the well-documented circadian phase delay during puberty (Carskadon et al. 2002; Carskadon et al. 1993), eveningness in the early years may represent a vulnerable phenotype for a more severe maturational phase delay and potential development of sleep and mood disorders in the emerging teen years (Gelbmann et al. 2012). Furthermore, co-sleeping is a common arrangement that varies across cultures and socio-economic statuses (Jenni and O’Connor 2005). Sleeping with someone at a different stage of sleep-wakefulness development may be an important factor to consider in understanding children’s sleep timing and chronotype. Finally, large-scale studies indicate that about 25% of young children suffer from sleep disturbance, including bedtime resistance and prolonged sleep onset delay (Owens 2008). Such early sleep problems commonly persist into late childhood and are independently associated with concurrent and future poor health outcomes in adolescence (Gregory et al. 2005). Although some have suggested that behavioral sleep problems may occur in part due to dissonance between children’s individual chronotype and social demands, data supporting this hypothesis are scarce (Jenni and O’Connor 2005). In summary, our findings provide evidence of links between parental reports of chronotype and circadian physiology and objectively measured sleep timing, supporting the concept of chronotype and its assessment by questionnaire in young children.
Acknowledgments
We are most grateful to the children and families for their generosity, time, and effort in making this study possible. We also appreciate all Brown University and the University of Colorado Boulder undergraduate students and research assistants who collected these data. Hannah LeBourgeois assisted in the scoring and management of actigraphy data. This research was primarily supported with funds from the National Institute of Mental Health (K01-MH074643, R01-MH08656). Supplemental funds were provided from the following grants: Sepracor, Inc. ESRC026, HHMI to the Biological Sciences Initiative at the University of Colorado Boulder, and NIH/NCRR Colorado CTSI UL1-TR000154.
Footnotes
Financial/Conflict of Interest Disclosure: The authors have nothing to declare.
Author Contributorship
Study Design: LeBourgeois, Carskadon, Jenni, Wright; Data Collection: LeBourgeois, Simpkin, Garlo, Akacem; Manuscript Preparation: LeBourgeois, Simpkin, Garlo, Akacem, Carskadon, Jenni, Wright
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005;28:1568–1577. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12:278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. 1980. Sleep. 2002;25:453–460. [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Crosby BM, Gryczkowski M, LeBourgeois MK, Olmi D, Rabian B, Harsh J. Mid-sleep time and school readiness in black and white preschool children. Sleep. 2006;30:A78. [Google Scholar]
- Deacon S, Arendt J. Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci Lett. 1994;167:191–194. doi: 10.1016/0304-3940(94)91059-6. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Gelbmann G, Kuhn-Natriashvili S, Pazhedath TJ, Ardeljan M, Wober C, Wober-Bingol C. Morningness: protective factor for sleep-related and emotional problems in childhood and adolescence? Chronobiol Int. 2012;29:898–910. doi: 10.3109/07420528.2012.686946. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11:191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Goldstein D, Hahn CS, Hasher L, Wiprzycka UJ, Zelazo PD. Time of day, intellectual performance, and behavioral problems in morning versus evening type adolescents: is there a synchrony effect? Pers Individ Dif. 2007;42:431–440. doi: 10.1016/j.paid.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Eley TC, Moffitt TE, O’Connor TG, Poulton R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005;33:157–163. doi: 10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- Harada T, Hirotani M, Maeda M, Nomura H, Takeuchi H. Correlation between breakfast tryptophan content and morning-evening in Japanese infants and students aged 0–15 yrs. J Physiol Anthropol. 2007;26:201–207. doi: 10.2114/jpa2.26.201. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- Jenni OG, LeBourgeois MK. Understanding sleep-wake behavior and sleep disorders in children: the value of a model. Curr Opin Psychiatry. 2006;19:282–287. doi: 10.1097/01.yco.0000218599.32969.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni OG, O’Connor BB. Children’s sleep: an interplay between culture and biology. Pediatrics. 2005;115:204–216. doi: 10.1542/peds.2004-0815B. [DOI] [PubMed] [Google Scholar]
- Katzenberg D, Young T, Finn L, Lin L, King DP, Takahashi JS, Mignot E. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21:569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge L, Carrier J, Lesperance P, Lambert C, Vitaro F, Tremblay RE, Montplaisir J. Sleep and circadian phase characteristics of adolescent and young adult males in a naturalistic summertime condition. Chronobiol Int. 2000;17:489–501. doi: 10.1081/cbi-100101059. [DOI] [PubMed] [Google Scholar]
- Lazar AS, Slak A, Lo JC, Santhi N, von Schantz M, Archer SN, Groeger JA, Dijk DJ. Sleep, diurnal preference, health, and psychological well-being: a prospective single-allelic-variation study. Chronobiol Int. 2012;29:131–146. doi: 10.3109/07420528.2011.641193. [DOI] [PubMed] [Google Scholar]
- LeBourgeois MK, Carskadon MA, Akacem LD, Simpkin CT, Wright KP, Jr, Achermann P, Jenni OG. Circadian phase and its relationship to nighttime sleep in toddlers. J Biol Rhythms. 2013a;28:322–331. doi: 10.1177/0748730413506543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBourgeois MK, Wright KP, Jr, LeBourgeois HB, Jenni OG. Dissonance between parent-selected bedtimes and young children’s circadian physiology influences nighttime settling difficulties. Mind Brain Educ. 2013b;7:234–242. doi: 10.1111/mbe.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakade M, Akimitsu O, Wada K, Krejci M, Noji T, Taniwaki N, Takeuchi H, Harada T. Can breakfast tryptophan and vitamin B6 intake and morning exposure to sunlight promote morning-typology in young children aged 2 to 6 years? J Physiol Anthropol. 2012;31:11. doi: 10.1186/1880-6805-31-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. Classification and epidemiology of childhood sleep disorders. Prim Care. 2008;35:533–546. doi: 10.1016/j.pop.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Peixoto CA, da Silva AG, Carskadon MA, Louzada FM. Adolescents living in homes without electric lighting have earlier sleep times. Behav Sleep Med. 2009;7:73–80. doi: 10.1080/15402000902762311. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Roepke SE, Duffy JF. Differential impact of chronotype on weekday and weekend sleep timing and duration. Nat Sci Sleep. 2010;2010:213–220. doi: 10.2147/NSS.S12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnick SL, Goodlin-Jones BL, Anders TF. The use of actigraphy to study sleep disorders in preschoolers: some concerns about detection of nighttime awakenings. Sleep. 2008;31:395–401. doi: 10.1093/sleep/31.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011;127:e345–352. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H, LeBourgeois MK, Geiger A, Jenni OG. Assessment of chronotype in four- to eleven-year-old children: reliability and validity of the Children’s Chronotype Questionnaire (CCTQ) Chronobiol Int. 2009;26:992–1014. doi: 10.1080/07420520903044505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham L. Time-of-day preference for preschool-aged children. Chrestomathy. 2006;5:259–268. [Google Scholar]
- Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, Martin JL. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26:213–216. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]