Abstract
Background
Randomized trials of coronary artery bypass graft (CABG) surgery and percutaneous coronary intervention (PCI) suggest that patient characteristics modify the effect of treatment on mortality.
Objective
To assess whether clinical characteristics modify the comparative effectiveness of CABG versus PCI in an unselected, general patient population.
Design
Observational treatment comparison using propensity score matching and Cox proportional hazards models.
Setting
United States, 1992 to 2008.
Patients
Medicare beneficiaries aged 66 years or older.
Intervention
Multivessel CABG or multivessel PCI.
Measurements
The CABG–PCI hazard ratio (HR) for all-cause mortality, with prespecified treatment-by-covariate interaction tests, and the absolute difference in life-years of survival in clinical subgroups after CABG or PCI, both over 5 years of follow-up.
Results
Among 105 156 propensity score–matched patients, CABG was associated with lower mortality than PCI (HR, 0.92 [95% CI, 0.90 to 0.95]; P < 0.001). Association of CABG with lower mortality was significantly greater (interaction P ≤ 0.002 for each) among patients with diabetes (HR, 0.88), a history of tobacco use (HR, 0.82), heart failure (HR, 0.84), and peripheral arterial disease (HR, 0.85). The overall predicted difference in survival between CABG and PCI treatment over 5 years was 0.053 life-years (range, −0.017 to 0.579 life-years). Patients with diabetes, heart failure, peripheral arterial disease, or tobacco use had the largest predicted differences in survival after CABG, whereas those with none of these factors had slightly better survival after PCI.
Limitation
Treatments were chosen by patients and physicians rather than being randomly assigned.
Conclusion
Multivessel CABG is associated with lower long-term mortality than multivessel PCI in the community setting. This association is substantially modified by patient characteristics, with improvement in survival concentrated among patients with diabetes, tobacco use, heart failure, or peripheral arterial disease.
Primary Funding Source
National Heart, Lung, and Blood Institute.
Coronary artery bypass graft (CABG) surgery and percutaneous coronary intervention (PCI) are among the most common major procedures performed worldwide. Either can be used to treat multivessel coronary artery disease, and their comparative effectiveness has been assessed in several randomized trials (1–7). A recent collaborative analysis of 10 trials comparing CABG with PCI showed that overall mortality risk was slightly, but not significantly, reduced by CABG and that the effect of CABG treatment on mortality was significantly modified by diabetes mellitus and age (8).
Randomized trials are accepted as the reference standard for comparing treatments but are difficult to perform and usually enroll patients selectively. For example, only about 10 000 patients have been randomly assigned to CABG or PCI, a tiny fraction of the millions of patients worldwide who have had one of these procedures. Although the high-quality data from randomized trials can be leveraged into treatment recommendations, it has been increasingly recognized that patients enrolled in clinical trials are generally younger and healthier than those seen in everyday practice. Furthermore, these trials are generally underpowered to examine variation in the treatment effect according to clinical characteristics of the patient. Analysis of Medicare claims provides the opportunity to compare treatments overall and in targeted subgroups by using readily available data from nationally representative patients treated in real-world practices.
The purpose of this study was to compare the mortality rates of Medicare beneficiaries who had multivessel CABG or multivessel PCI and to assess whether the comparative effectiveness of these procedures was modified by patient characteristics.
Methods
The study population comprised fee-for-service Medicare beneficiaries who had multivessel CABG or multivessel PCI between 1992 and 2008. To permit a 1-year look-back period and document the presence of comorbid conditions, we restricted the study population to individuals aged 66 years or older who had Part A and Part B Medicare coverage and were not enrolled in a Medicare HMO. The index coronary revascularization procedures were identified from the 20% random sample of Part A data. Comorbid conditions were defined by using diagnosis and procedure codes (available on request) found in Part A and Part B data (a 5% random sample from 1992 to 1997 and a 20% random sample from 1998 to 2008). We excluded patients with procedures done before 1992 because of the unavailability of some necessary data during that period, but we looked back to 1986 to exclude patients with prior CABG or PCI during that period. This study was approved by the Stanford University Institutional Review Board.
We identified patients by using procedure codes for multivessel CABG (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM], codes 36.12, 36.13, 36.14, 36.16, or 36.11 plus 36.15) and multivessel PCI (before October 2005: ICD-9-CM code 36.05; after October 2005: ICD-9-CM code 00.66 plus 00.41, 00.42, or 00.43 or Current Procedural Terminology code 92981 or 92984). We excluded patients if they had single-vessel PCI or CABG, had concomitant cardiac procedures (such as valve replacement) at the time of CABG or PCI, were of unknown race, or had end-stage renal disease and were receiving long-term dialysis.
We defined comorbid conditions by using outpatient and inpatient encounters in the year before the index procedure. We considered a comorbid condition to be present if it was recorded as a primary or secondary diagnosis code at either encounter.
We used propensity score matching to create the final analysis cohort. We used all of the baseline characteristics listed in the Table as predictors of receiving CABG or PCI, as well as a history of ventricular tachycardia, ventricular fibrillation, other arrhythmias, implantable cardioverter-defibrillator, valvular heart disease, stroke, transient ischemic attack, intracranial hemorrhage, other cerebrovascular disease, fluid and electrolyte abnormalities, gastrointestinal bleeding, anemia, pulmonary vascular disease, hypothyroidism, chronic liver disease, AIDS, systemic cancer, obesity, dementia, depression, psychosis, alcohol use, or drug use. We matched each patient who received a multivessel PCI with one who received a multivessel CABG by using a greedy algorithm (9) that first matched propensity scores at 7 digits, then at 6 digits, and so forth, down to a 2-digit match (that is, agreement at the 0.01 level of propensity score). We also required that patients be matched by year of index procedure, diabetes status, and age within 1 year.
Table.
Baseline Clinical Characteristics
| Characteristic | All Patients, % | Propensity Score–Matched Patients, % | ||
|---|---|---|---|---|
| CABG (n = 194 223) | PCI (n = 57 330) | CABG (n = 52 578) | PCI (n = 52 578) | |
| Age | ||||
| 66–70 y | 31.3 | 29.0 | 30.0 | 29.8 |
| 71–75 y | 31.8 | 27.0 | 27.6 | 27.9 |
| 76–80 y | 24.1 | 22.7 | 23.1 | 23.3 |
| 81–85 y | 10.7 | 14.6 | 14.7 | 14.6 |
| ≥86 y | 2.1 | 6.8 | 4.6 | 4.4 |
| Female | 33.8 | 43.3 | 41.2 | 42.0 |
| Race | ||||
| White | 92.4 | 91.9 | 92.5 | 92.1 |
| Black | 4.3 | 4.6 | 4.4 | 4.6 |
| Other | 3.3 | 3.5 | 3.2 | 3.3 |
| Diabetes | 36.0 | 33.4 | 33.3 | 33.3 |
| Hypertension | 77.6 | 78.7 | 78.5 | 78.6 |
| Hyperlipidemia | 29.1 | 28.0 | 28.0 | 28.5 |
| Tobacco use | 18.1 | 18.6 | 18.1 | 19.0 |
| Chronic kidney disease | 5.1 | 5.7 | 5.4 | 5.5 |
| Peripheral arterial disease | 21.6 | 18.2 | 17.4 | 18.3 |
| Cerebrovascular disease | 22.0 | 16.9 | 16.6 | 16.9 |
| Prior MI | 13.4 | 11.6 | 10.9 | 11.4 |
| Heart failure | 13.1 | 13.9 | 12.5 | 13.2 |
| Unstable angina | 38.4 | 29.5 | 30.4 | 29.9 |
| Atrial fibrillation | 9.5 | 12.2 | 11.2 | 11.6 |
| Primary diagnosis of MI | 21.4 | 29.6 | 28.2 | 28.4 |
| Metropolitan area | 72.4 | 73.0 | 73.0 | 73.0 |
| U.S. Census region | ||||
| New England | 4.4 | 4.3 | 4.2 | 4.4 |
| Middle Atlantic | 13.5 | 11.9 | 12.0 | 12.3 |
| South Atlantic | 21.7 | 21.6 | 21.5 | 21.4 |
| East South Central | 8.9 | 7.1 | 7.1 | 7.3 |
| West South Central | 12.1 | 11.8 | 11.8 | 11.8 |
| East North Central | 19.9 | 19.6 | 20.2 | 20.1 |
| West North Central | 8.4 | 10.1 | 10.3 | 9.9 |
| Mountain | 3.7 | 4.9 | 4.6 | 4.6 |
| Pacific | 7.3 | 8.6 | 8.4 | 8.3 |
| Year of procedure | ||||
| 1992–1994 | 7.6 | 3.2 | 2.6 | 2.6 |
| 1995–2003 | 58.9 | 46.8 | 47.9 | 47.9 |
| 2004–2008 | 33.6 | 50.0 | 49.5 | 49.5 |
CABG = coronary artery bypass graft; MI = myocardial infarction; PCI = percutaneous coronary intervention.
In the primary analysis, we compared the propensity score–matched cohort of CABG and PCI patients over the first 5-year follow-up by using Cox proportional hazards models. We tested the treatment-by-covariate interaction with key baseline factors and reported the interaction P value. We repeated the interaction test after additional adjustment for the baseline covariates in the Table. The modifiers of potential treatment effect analyzed included factors tested previously (8) in the pooled analysis of randomized trials (age, sex, diabetes, hypertension, hyperlipidemia, smoking, unstable angina, prior myocardial infarction [MI], heart failure, and peripheral arterial disease), as well as prespecified additional factors of interest that were not available in the previous analysis (race, chronic kidney disease, cerebrovascular disease, and atrial fibrillation).
After testing the hypothesis that the comparative effectiveness of CABG and PCI varied according to prespecified baseline patient characteristics, we estimated the difference in length of survival between these treatments as a function of patient characteristics. We calculated the life-years added by CABG compared with PCI during 5 years of follow-up by using a Cox proportional hazards model. To develop a practical but individualized prediction of survival benefit, we used 13 patient characteristics as predictors and included selected interaction terms. For each patient in the study population, we entered their baseline characteristics into the model to generate a predicted survival curve after treatment with CABG. We then numerically integrated this curve to estimate the life-years of survival over 5 years. We repeated this process with the treatment variable set to PCI. We then subtracted the life-years of survival predicted after PCI from those predicted after CABG to produce an individualized estimate of the effect of CABG compared with PCI for each patient in the study population. We used SAS, version 9.3 (SAS Institute, Cary, North Carolina), to perform all statistical analyses.
Role of the Funding Source
The National Heart, Lung, and Blood Institute sponsored the study but had no role in its design or conduct; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Results
We identified 611 729 initial CABG and PCI procedures between 1992 and 2008 in the 20% sample of Medicare beneficiaries aged 66 years or older with available Part B data. We excluded 48 707 patients because of concomitant cardiac procedures performed at the time of CABG or PCI, 300 378 who had single-vessel revascularization, 2982 because the number of treated vessels could not be measured, and 8109 with end-stage renal disease. The eligible population comprised 251 553 patients; 194 223 of whom had multivessel CABG and 57 330 of whom had multivessel PCI.
In the propensity score analysis, the strongest predictors of receiving CABG rather than PCI were calendar year, age, sex, diabetes, cerebrovascular disease, peripheral arterial disease, atrial fibrillation, and region. The propensity score model had an overall c-statistic of 0.673. We matched 92% of the patients who had multivessel PCI with patients who had multivessel CABG; 10 080 patient pairs were matched on 5 to 7 digits of propensity score, 16 677 on 4 digits, 20 767 on 3 digits, and 5054 on 2 digits.
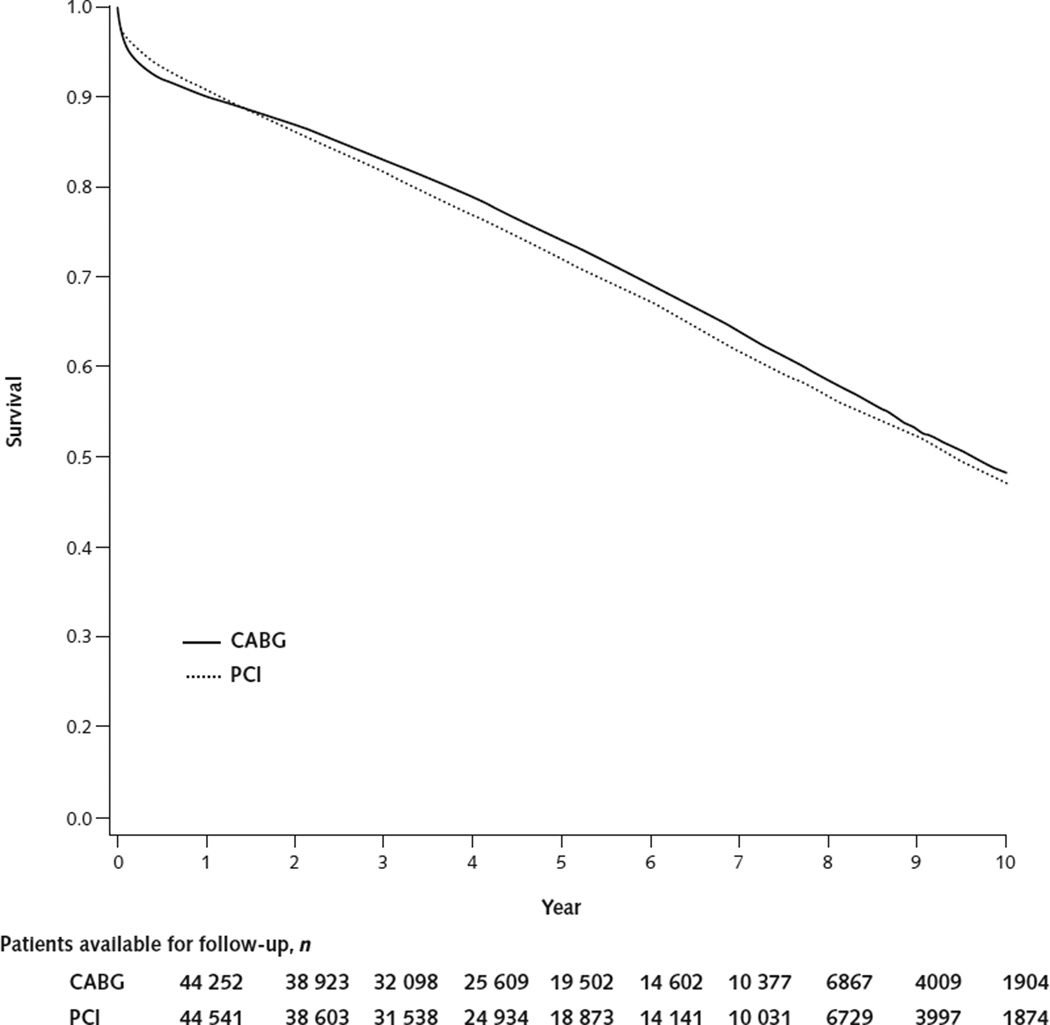
The baseline clinical characteristics of the matched population were similar between patients who received CABG and those who received PCI (Table). The typical patient was a 75-year-old white man with hypertension, and the median follow-up was 4.3 years. Overall, survival at 5 years was 74.1% after CABG and 71.9% after PCI (Figure 1), with a CABG–PCI hazard ratio (HR) for all-cause mortality of 0.92 (95% CI, 0.90 to 0.95).
Figure 1. Kaplan–Meier survival curves for multivessel CABG and multivessel PCI among Medicare beneficiaries matched on propensity score.
CABG = coronary artery bypass graft; PCI = percutaneous coronary intervention.
Treatment Effect Modification
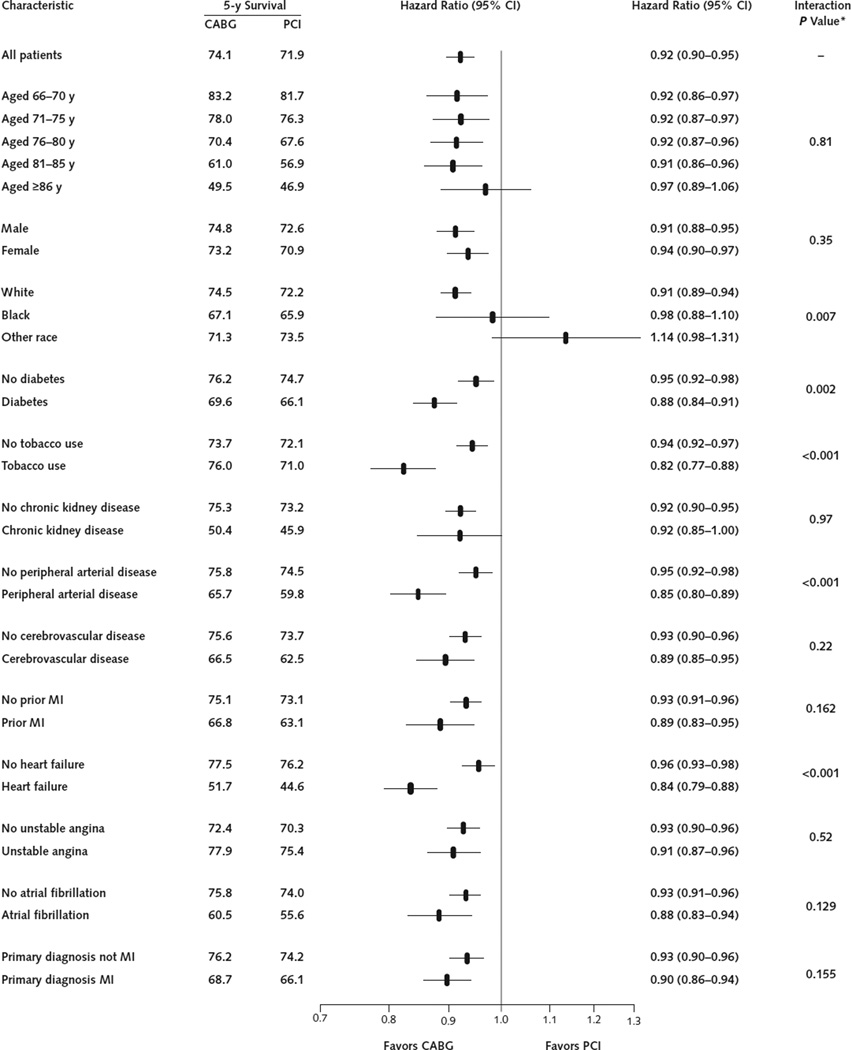
Compared with PCI, the association of CABG with all-cause mortality over the 5-year follow-up was significantly modified by race, diabetes, tobacco use, peripheral arterial disease, and heart failure (Figure 2). Each of these interactions remained significant after additional adjustment for the baseline characteristics listed in Figure 2 (interaction P = 0.047 for diabetes, 0.004 for tobacco use, <0.001 for heart failure, <0.014 for peripheral arterial disease, and <0.014 for race). Interaction of race and treatment was primarily due to patients of “other race” because the difference between white and black patients was not significant either without (interaction P = 0.20) or with (interaction P = 0.057) additional adjustment for baseline characteristics, whereas the difference between white patients and those of other races was significant both without (interaction P = 0.003) and with (interaction P = 0.025) additional adjustment.
Figure 2. Five-year Kaplan–Meier survival estimates and CABG–PCI hazard ratios and associated 95% CIs in subgroups of the propensity score–matched cohort.
CABG = coronary artery bypass graft; MI = myocardial infarction; PCI = percutaneous coronary intervention.
* P values for the interaction with treatment are from models that adjusted only for CABG treatment, the covariate, and their interaction but not for additional baseline covariates.
Differences in Life Expectancy
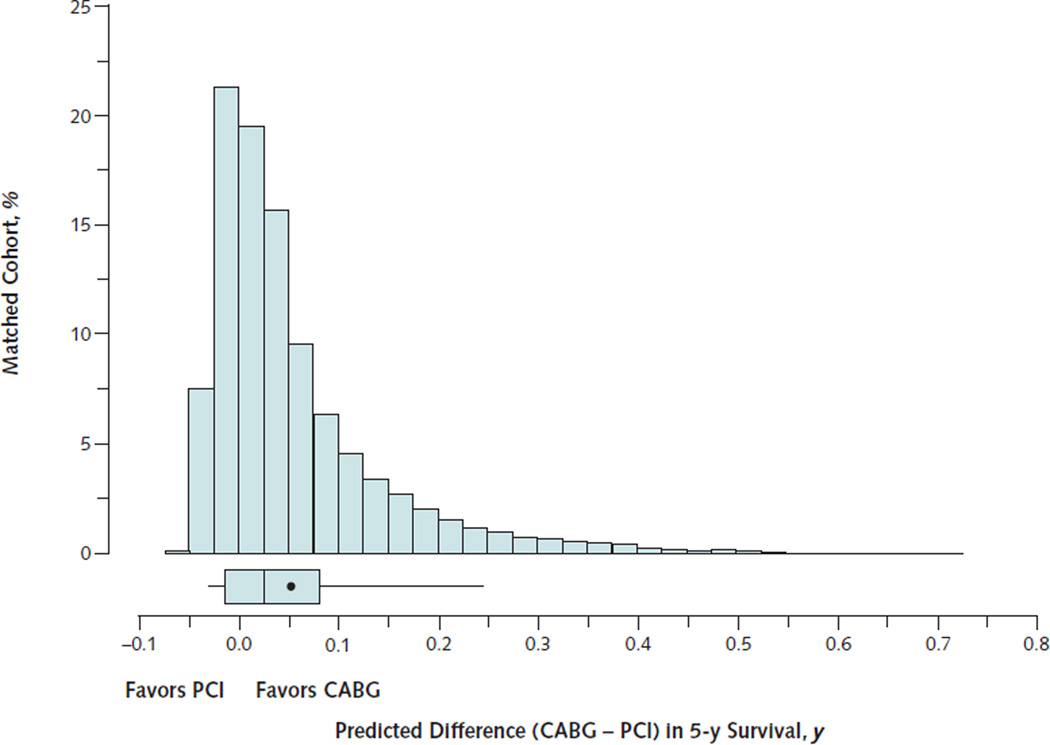
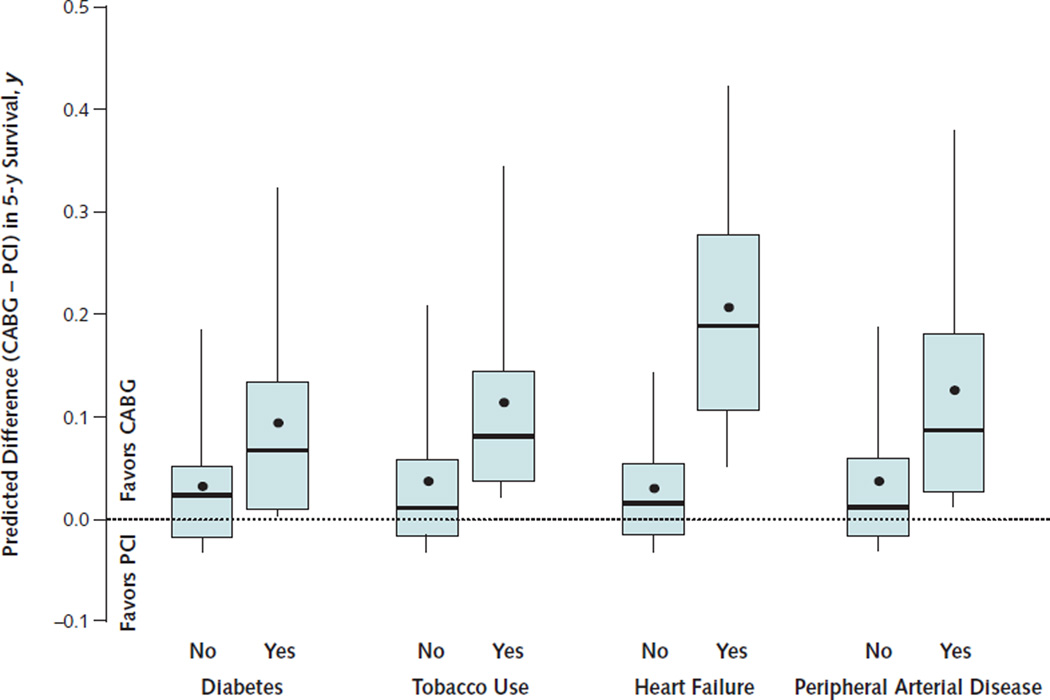
Based on individualized predictions of survival in the study population, the mean increase in life expectancy from CABG compared with PCI in the 5 years after coronary revascularization was 0.053 life-years (P < 0.001). Estimated life-years added by CABG varied widely across the 105 156 patients in the, however (Figure 3). Median predicted change in life expectancy from CABG was 0.029 life-years, but the 5th percentile was −0.006 life-year (that is, favored PCI) and the 95th percentile was 0.235 lifeyears. Patients with diabetes, heart failure, tobacco use, or peripheral arterial disease had higher predicted differences in their life expectancy when treated with CABG (Figure 4). A substantial proportion of patients (41%) were predicted to have better survival after PCI than after CABG. These patients were characterized by the absence of diabetes, heart failure, tobacco use, peripheral vascular disease, and a primary diagnosis of MI on hospitalization. Examples of individualized estimates of the CABG–PCI survival difference as a function of baseline clinical characteristics are provided in the Appendix (available at www.annals.org).
Figure 3. Distribution of the estimated life-years of survival added by CABG compared with PCI over 5-y follow-up.
The percentage of the study population is indicated on the vertical axis, and the life-years of survival added over 5-y follow-up are shown on the horizontal axis. The box-and-whisker plot indicates the 50th percentile as the line within the box; the 25th and 75th percentiles as the left and right edges of the box, respectively; and the 5th and 95th percentiles as the left and right whiskers, respectively. The mean is indicated by the point within the box.
CABG = coronary artery bypass graft; PCI = percutaneous coronary intervention.
Figure 4. Distribution of the estimated life-years of survival added by CABG compared with PCI over 5 y in clinical subgroups.
The box-and-whisker plots indicate the 50th percentile as the line within the box; the 25th and 75th percentiles as the bottom and top edges of the box, respectively; and the 5th and 95th percentiles as the bottom and top whiskers, respectively. The mean is indicated by the point within the box.
CABG = coronary artery bypass graft; PCI = percutaneous coronary intervention.
Discussion
Comparative effectiveness research aims to provide information needed by decision makers, particularly physicians and their patients, to choose among alternative approaches to clinical management. Therefore, it is important to understand not only how treatments affect an “average patient” but also how their effectiveness may vary among specific patients with different characteristics. Randomized clinical trials are well-suited to compare treatment efficacy for the average patient, but because of their narrow eligibility criteria and limited sample sizes, they are less suitable for examining variations in clinical effectiveness across patient populations treated in typical practice settings.
In our study, we evaluated the treatment effectiveness of coronary revascularization among a large population of real-world patients and providers. Coronary artery bypass grafting was associated with significantly lower mortality than PCI in the overall patient population, with an HR of 0.92 (CI, 0.90 to 0.95; P < 0.001). This finding is similar to the overall HR of 0.91 found in a prior analysis of pooled data from 10 randomized trials (8) and is consistent with the results of a recent study comparing CABG and PCI on the basis of data from clinical registries linked with Medicare (10). This body of evidence suggests that the use of CABG rather than PCI is likely to reduce mortality for the average patient with multivessel coronary disease.
Although the expected outcomes for an average patient are clearly important, outcomes for individual patients may differ. In this study, we found that several patient characteristics significantly modified the comparative effectiveness of CABG and PCI such that the expected survival difference between the procedures varied widely among individuals (Figure 3). Patients with a history of peripheral arterial disease, tobacco use, diabetes, or heart failure had a particular survival advantage from CABG (Figures 2 and 4). Conversely, patients with none of these characteristics had slightly better survival with PCI. This variation in the comparative effectiveness of CABG and PCI underscores the importance of individualizing treatment.
The comparative effectiveness of treatments is usually measured in relative terms by using HRs, risk ratios, or odds ratios. These relative measures are well-suited to assess whether the treatment “works” in a consistent manner across studies and patient subgroups. However, even with the same relative risk reduction, the absolute risk difference from treatment is larger among high-risk patients. In this study, we calculated the expected absolute difference in survival over 5 years to illustrate the substantial variation among patients in the comparative effectiveness of CABG and PCI (Figure 3). The number of life-years added by CABG over 5 years is an underestimate of the lifetime effect of treatment because the CABG and PCI survival curves are separated for up to 10 years (Figure 1) and subsequent follow-up is limited. Although a lifetime estimate of the life-years added by CABG would be preferable, it would require many additional extrapolations. Nevertheless, our finding that the variations in the comparative effectiveness of CABG and PCI further amplify the differences in expected survival among patient subgroups (Figure 4) is an important insight for clinical decision making.
Because patients with diabetes have a higher risk and a disproportionate treatment effect from CABG relative to PCI (Figure 2), they have a particularly large difference in survival if treated with CABG rather than PCI (Figure 4). The strong effect of CABG on survival compared with PCI among patients with diabetes and multivessel coronary disease was recently confirmed by a large randomized trial of 1900 patients (11).
Coronary artery bypass grafting was also associated with a particularly lower mortality than PCI among patients with a history of peripheral arterial disease (Figures 2 and 4). A similar trend was present in the pooled data from 10 randomized trials, but only 665 patients in that study had peripheral arterial disease and the interaction test was not significant (interaction P = 0.33). Our study includes more than 18 000 patients with a diagnosis of peripheral arterial disease, so it had sufficient statistical power to detect a difference in the treatment effect in this subgroup. Peripheral arterial disease may serve as a marker for patients with more extensive coronary atherosclerosis, for whom CABG may provide a greater survival advantage, but data on coronary anatomy were not available in this study to evaluate this possibility.
Compared with PCI, CABG was associated with lower mortality among patients with a history of tobacco use, but the reasons for this association are uncertain. Previous studies have shown that patients who quit smoking after coronary revascularization have better outcomes than those who continue to smoke (12, 13) and that patients are more likely to quit smoking after CABG than after PCI (14). More patients in this study may have quit smoking after CABG than after PCI, adding to any survival advantage from the procedure itself. Data available in this study did not distinguish between current and former smokers, and we could not identify patients who continued to smoke after revascularization. Further study of the complex relationships among smoking, coronary revascularization, and outcomes is warranted.
Coronary artery bypass grafting was also associated with lower mortality than PCI among patients with a history of heart failure (interaction P < 0.001). Such patients have not been well-represented in clinical trials. Only 3% of patients in the 10 randomized trials had a history of heart failure, compared with 13% in our analysis of Medicare beneficiaries. Consequently, the comparative effectiveness of CABG and PCI in patients with heart failure has not been well-studied. The claims data we used for this study lack important clinical details about these patients, such as measures of left ventricular function, myocardial ischemia, and the extent of coronary artery disease.
This study used nonrandomized data to compare the effectiveness of CABG and PCI. Comparing treatments by using observational data has been controversial because of potential biases related to treatment selection and the lack of data on important clinical factors that might affect outcomes. We used 2 steps to mitigate these obstacles. First, we restricted the population to patients with multivessel PCI or multivessel isolated CABG, which produced a population that more closely resembled patients eligible for randomized trials of these procedures. Second, we used propensity score matching to further control for potential treatment selection bias, which closely balanced observed patient characteristics (Table) that differed greatly in the initial study population. This yielded an estimate of the overall CABG–PCI HR (0.92) that was similar to the one derived from pooling randomized trial data (0.91). Our finding that several baseline clinical characteristics modified the comparative treatment effectiveness of CABG and PCI is also consistent with the results of the earlier pooled analysis (8). The broad agreement between the present study and the results of the pooled randomized trials suggests that carefully conducted analyses of observational data can provide reliable estimates of real-world treatment effectiveness.
The major limitation of this study is that it is based on claims data and lacks detail on many key baseline clinical characteristics and information on medications and behaviors during subsequent follow-up. These limitations may be particularly relevant to our analyses of tobacco use and heart failure, which seem to modify the CABG–PCI treatment effect but may be correlated with other unobserved clinical characteristics. In addition, the claims data used in this study do not contain information on symptom severity or on the functional status or quality of life of patients.
This study provides strong evidence that clinical characteristics modify the comparative effectiveness of CABG and PCI on mortality, especially for diabetes, which had been previously shown (8). Our study suggests that additional factors, particularly smoking, peripheral arterial disease, and heart failure, also modify the CABG–PCI treatment effect. These variations in comparative effectiveness underscore the need to personalize treatment recommendations for coronary revascularization among patients with multivessel coronary disease.
Context.
Although randomized, controlled trials have shown small reductions in mortality with coronary artery bypass graft (CABG) surgery versus percutaneous coronary intervention (PCI) for coronary revascularization, the restrictive enrollment may limit generalizability to patients in real-world practice.
Contribution
This comparative effectiveness study using data from Medicare recipients found that CABG was associated with a small mortality benefit versus PCI and certain patient characteristics modified the association. The expected survival advantage varies widely among individuals. Patients with diabetes, a history of smoking, peripheral arterial disease, and heart failure had a particular benefit; those without these factors had slightly better survival with PCI.
Caution
Clinical details of individual patients could not be assessed.
Implication
Individual clinical variables are associated with differences in the benefits of CABG versus PCI.
—The Editors
Acknowledgments
Grant Support: By grant HL099872 from the National Heart, Lung, and Blood Institute.
Appendix: Examples of Individualized Estimates of Treatment Effectiveness
We estimated individualized changes in life expectancy after CABG rather than PCI by using a Cox proportional hazards model with 13 covariates: age, sex, race, diabetes, tobacco use, chronic kidney disease, peripheral vascular disease, cerebrovascular disease, prior MI, heart failure, unstable angina, atrial fibrillation, and a primary diagnosis of MI at the same hospitalization as the revascularization procedure. Individualized treatment estimates as a function of these variables will be made available on a Web site, the possible format of which can be seen at http://med.stanford.edu/hsr/cabg-pci. Example patients are described below.
Example 1: A 70-year-old black man with diabetes and peripheral vascular disease but no tobacco use or heart failure. The median number of life-years added over 5 years by CABG among 43 similar patients in the data set was 0.079 (range, 0.067 to 0.139 life-years).
Example 2: A 75-year-old white woman with diabetes but without heart failure, peripheral vascular disease, or tobacco use. The median number of life-years added over 5 years by CABG among 2267 similar patients in the data set was 0.007 (range, 0.005 to 0.133 life-years).
Example 3: A 66-year-old white man without diabetes, heart failure, peripheral vascular disease, tobacco use, or a primary diagnosis of acute MI. The median number of life-years added over 5 years by CABG among 2596 similar patients in the data set was −0.014 (range, −0.012 to −0.028 life-years) (that is, the predicted survival was better with PCI).
Example 4: A 77-year-old white woman with diabetes, peripheral vascular disease, and heart failure but no tobacco use. The median number of life-years added over 5 years by CABG among 176 similar patients in the data set was 0.286 (range, 0.211 to 0.502 life-years).
Footnotes
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M12-1564.
Reproducible Research Statement: Study protocol: The protocol is fully described in the manuscript. Statistical code: Available on request. Data set: Medicare data sets are available to qualified researchers but cannot be released by the investigators.
Author Contributions: Conception and design: M.A. Hlatky, L. Baker, A.S. Go.
Analysis and interpretation of the data: M.A. Hlatky, D.B. Boothroyd, D.S. Kazi, M.D. Solomon, T.I. Chang, D. Shilane, A.S. Go.
Drafting of the article: M.A. Hlatky.
Critical revision of the article for important intellectual content: D.B. Boothroyd, D.S. Kazi, M.D. Solomon, T.I. Chang, D. Shilane, A.S. Go.
Final approval of the article: M.A. Hlatky, D.B. Boothroyd, L. Baker, D.S. Kazi, M.D. Solomon, T.I. Chang, D. Shilane, A.S. Go.
Provision of study materials or patients: L. Baker.
Statistical expertise: M.A. Hlatky, D.B. Boothroyd, D. Shilane.
Obtaining of funding: M.A. Hlatky, A.S. Go.
Administrative, technical, or logistic support: M.A. Hlatky, L. Baker.
Collection and assembly of data: D.B. Boothroyd, L. Baker.
References
- 1.BARI Investigators. The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol. 2007;49:1600–1606. doi: 10.1016/j.jacc.2006.11.048. [PMID: 17433949] [DOI] [PubMed] [Google Scholar]
- 2.King SB, 3rd, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eight-year mortality in the Emory Angioplasty versus Surgery Trial (EAST) J Am Coll Cardiol. 2000;35:1116–1121. doi: 10.1016/s0735-1097(00)00546-5. [PMID: 10758949] [DOI] [PubMed] [Google Scholar]
- 3.Serruys PW, Ong AT, van Herwerden LA, Sousa JE, Jatene A, Bonnier JJ, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46:575–581. doi: 10.1016/j.jacc.2004.12.082. [PMID: 16098418] [DOI] [PubMed] [Google Scholar]
- 4.Booth J, Clayton T, Pepper J, Nugara F, Flather M, Sigwart U, et al. SoS Investigators. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS) Circulation. 2008;118:381–388. doi: 10.1161/CIRCULATIONAHA.107.739144. [PMID: 18606919] [DOI] [PubMed] [Google Scholar]
- 5.Hueb W, Lopes N, Gersh BJ, Soares PR, Ribeiro EE, Pereira AC, et al. Ten-year follow-up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2010;122:949–957. doi: 10.1161/CIRCULATIONAHA.109.911669. [PMID: 20733102] [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez AE, Baldi J, Fernández Pereira C, Navia J, Rodriguez Alemparte M, Delacasa A, et al. ERACI II Investigators. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II) J Am Coll Cardiol. 2005;46:582–588. doi: 10.1016/j.jacc.2004.12.081. [PMID: 16098419] [DOI] [PubMed] [Google Scholar]
- 7.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [PMID: 19228612] [DOI] [PubMed] [Google Scholar]
- 8.Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, Brooks MM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190–1197. doi: 10.1016/S0140-6736(09)60552-3. [PMID: 19303634] [DOI] [PubMed] [Google Scholar]
- 9.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques; Presented at 26th Annual SAS Users Group International Conference; 22–25 April 2001; Long Beach, California. [Google Scholar]
- 10.Weintraub WS, Grau-Sepulveda MV, Weiss JM, O’Brien SM, Peterson ED, Kolm P, et al. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–1476. doi: 10.1056/NEJMoa1110717. [PMID: 22452338] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, et al. FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [PMID: 23121323] [DOI] [PubMed] [Google Scholar]
- 12.van Domburg RT, Meeter K, van Berkel DF, Veldkamp RF, van Herwerden LA, Bogers AJ. Smoking cessation reduces mortality after coronary artery bypass surgery: a 20-year follow-up study. J Am Coll Cardiol. 2000;36:878–883. doi: 10.1016/s0735-1097(00)00810-x. [PMID: 10987614] [DOI] [PubMed] [Google Scholar]
- 13.Hasdai D, Garratt KN, Grill DE, Lerman A, Holmes DR., Jr Effect of smoking status on the long-term outcome after successful percutaneous coronary revascularization. N Engl J Med. 1997;336:755–761. doi: 10.1056/NEJM199703133361103. [PMID: 9052653] [DOI] [PubMed] [Google Scholar]
- 14.Crouse JR, 3rd, Hagaman AP. Smoking cessation in relation to cardiac procedures. Am J Epidemiol. 1991;134:699–703. doi: 10.1093/oxfordjournals.aje.a116146. [PMID: 1951275] [DOI] [PubMed] [Google Scholar]