Abstract
Pre-eclampsia is a pregnancy-specific hypertensive disorder that may lead to serious maternal and fetal complications. It is a multisystem disease that is commonly, but not always, accompanied by proteinuria. Its cause(s) remain unknown, and delivery remains the only definitive treatment. It is increasingly recognized that many pathophysiological processes contribute to this syndrome, with different signaling pathways converging at the point of systemic endothelial dysfunction, hypertension, and proteinuria. Different animal models of pre-eclampsia have proven utility for specific aspects of pre-eclampsia research, and offer insights into pathophysiology and treatment possibilities. Therapeutic interventions that specifically target these pathways may optimize pre-eclampsia management and may improve fetal and maternal outcomes. In addition, recent findings regarding placental, endothelial, and podocyte pathophysiology in pre-eclampsia provide unique and exciting possibilities for improved diagnostic accuracy. Emerging evidence suggests that testing for urinary podocytes or their markers may facilitate the prediction and diagnosis of pre-eclampsia. In this review, we explore recent research regarding placental, endothelial, and podocyte pathophysiology. We further discuss new signaling and genetic pathways that may contribute to pre-eclampsia pathophysiology, emerging screening and diagnostic strategies, and potential targeted interventions.
Keywords: endothelial dysfunction, placenta, podocyturia, pre-eclampsia
INTRODUCTION
Pre-eclampsia, a hypertensive disorder unique to pregnancy, remains a leading cause of fetal and maternal morbidity and mortality worldwide.1 Unlike other hypertensive pregnancy disorders, pre-eclampsia is a systemic disease with multi-organ involvement, which is commonly, but not always, accompanied by either sudden onset or worsening of pre-existing proteinuria. It is estimated that 5% of otherwise uncomplicated pregnancies will be affected by pre-eclampsia, and that as many as 25% of pregnant women with preexisting hypertension will develop superimposed pre-eclampsia. Pre-eclampsia is commonly viewed as one of the hypertensive pregnancy disorders, which cover a spectrum of clinical presentations from chronic hypertension (i.e. hypertension occurring prior to 20 weeks of gestation) and gestational hypertension (hypertension occurring after 20 weeks of gestation) to more severe forms, including pre-eclampsia, eclampsia (its convulsive form), and HELLP syndrome (Hemolysis, Elevated Liver enzymes, and Low Platelets). The rationale to treat these disorders as a continuum comes from clinical evidence demonstrating that either chronic or gestational hypertension may progress to pre-eclampsia (commonly evidenced by new-onset or worsening of proteinuria), while pre-eclampsia may progress to more severe forms, such as eclampsia or HELLP syndrome. An alternative approach views pre-eclampsia as a separate disease entity. Either way, it is recognized that pre-eclampsia is a heterogeneous disease. Different clinical subtypes may reflect distinct underlying pathological mechanisms.2 For example, it is common in clinical practice to subcategorize pre-eclampsia as early versus late (before and after 34 weeks of gestation, respectively),3 and mild versus severe,4 based on the absence/ presence of severe hypertension, defined as a blood pressure ≥160/110 mm Hg, proteinuria ≥ 5 gr/24-hours, neurological/renal/cardiac impairment, or signs of HELLP. Recent evidence suggests that women with early severe pre-eclampsia, who are at particularly high risk for adverse pregnancy outcomes, may have a more pronounced anti-angiogenic imbalance than those with late pre-eclampsia and more favorable outcomes.5 Active research in this field may delineate the mechanisms of the subtypes of pre-eclampsia, commonly referred to as placental versus maternal pre-eclampsia, based on their etiologies and origins.6, 7
Renal pathology in pre-eclampsia in the form of endotheliosis has long been recognized, and the kidney manifestations of pre-eclampsia form the basis for a “nephrocentric” view in the research and clinical arenas.8 In contrast, obstetric literature questions the importance of kidney injury (as demonstrated by proteinuria) in the diagnosis of pre-eclampsia, suggesting that a subclass of “non-proteinuric pre-eclampsia” should be added,9 or that detection of proteinuria should not be mandatory for a pre-eclampsia diagnosis.10 However, similar to other renal diseases, proteinuria in pre-eclampsia may represent a late marker of renal injury. Our recent data suggest that podocyturia i.e., the urinary loss of viable glomerular epithelial cells (podocytes), may occur prior to the clinical features of pre-eclampsia, potentially representing an earlier marker of renal injury than proteinuria. These findings set the stage for future studies of podocyturia in women who meet all of the clinical criteria, for the diagnosis of pre-eclampsia, except proteinuria.
The main objective of this review is to discuss emerging theories regarding pre-eclampsia pathophysiology, focusing on the different causal pathways that translate into different subtypes (clinical phenotypes) of pre-eclampsia; highlight animal models that may advance the understanding of the roles of specific mechanisms in pre-eclampsia; examine emerging evidence indicating that different signaling pathways may converge at the point of podocyte damage, which may be at the core of renal injury and ultimately lead to proteinuria; and discuss their possible implications for pre-eclampsia diagnosis and management.
ENDOTHELIAL DYSFUNCTION IN PRE-ECLAMPSIA
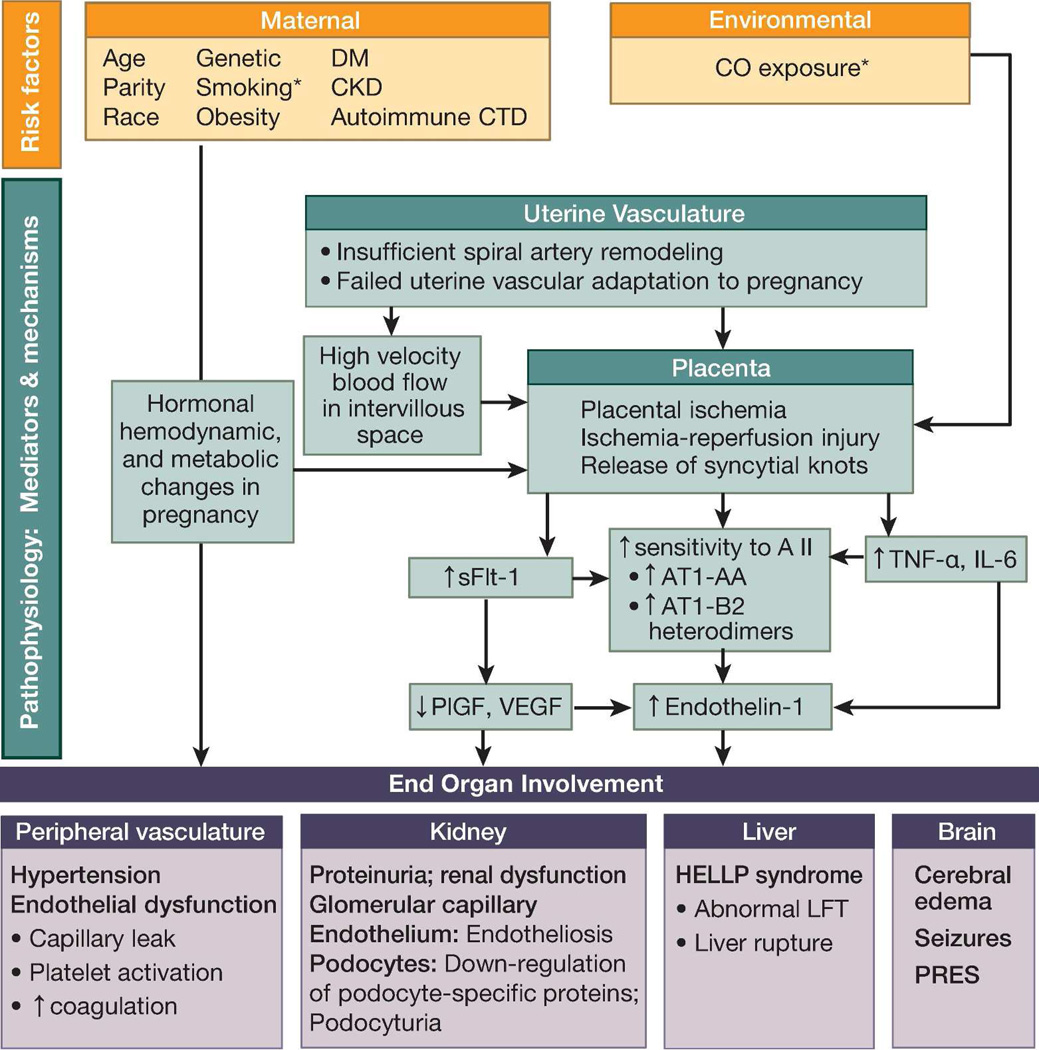
The prediction and treatment of preeclampsia is complicated by the fact that many pathophysiological processes may contribute to this syndrome. These causal pathways are believed to converge at the point of systemic endothelial dysfunction, which leads to hypertension and proteinuria (Figure 1). The fact that hypertension rapidly resolves upon the removal of the fetus and placenta has led to several theories implicating structural and/or functional changes in the developing placenta as factors causing pre-eclampsia. Placental hypoxia is frequently viewed as an early event that may cause placental production of soluble factors leading to endothelial dysfunction.11 Over the last decade, pre-eclampsia has been associated with elevated levels of the soluble receptor for vascular endothelial growth factor (VEGF) of placental origin. This soluble receptor, commonly referred to as soluble fms-like tyrosine kinase receptor-1 (sFlt-1), may bind and neutralize VEGF, and thus limit the availability of free VEGF for fetal and placental angiogenesis. Several rodent models simulate pre-eclampsia by exogenous sFlt-1 administration. In the most direct model, intraperitoneal sFlt-1 injections produce short-term elevations of sFlt-1.12 In the hours after sFlt-1 injection, animals develop hypertension, proteinuria, and altered podocyte protein expression, but do not develop glomerular endotheliosis, the classical renal lesion of pre-eclampsia. Administration of an adenoviral vector encoding sFlt-1 leads to longer-term sFlt-1 exposure in rats.13 This model reproduces the findings of hypertension, proteinuria, and glomerular endotheliosis. Elevated levels of another anti-angiogenic factor, soluble endoglin, have been subsequently implicated in neutralizing transforming factor-β and the resultant vascular damage in pre-eclampsia and HELLP syndrome.14 These anti-angiogenic factors are commonly viewed as the missing link between abnormal placentation and the maternal syndrome. However, these factors are likely a consequence, rather than the cause, of placental ischemia in pre-eclampsia. As such, they likely play an important role in “placental” pre-eclampsia, in which placental ischemia is present, but not in “maternal” pre-eclampsia, which occurs in the absence of placental ischemia,7 or in postpartum pre-eclampsia15 which occurs after delivery in the absence of the placenta.
Figure 1. Etiologies and pathophysiology of pre-eclampsia.
Several different signaling pathways may play a role, ultimately converging at the point of systemic endothelial dysfunction, hypertension, and proteinuria.
Abbreviations: AT1-AA, autoantibodies to the angiotensin II type 1 receptor; AT1-AA-B2 heterodimers, angiotensin II type 1 receptor-bradykinin type 2 receptor heterodimers; carbon monoxide; CKD, chronic renal disease; CTD, connective tissue disease; DM, diabetes mellitus; HELLP, hemolysis, elevated liver enzymes, low platelet count; IL-6, interleukin 6; LFT, liver function tests; PlGF, placental growth factor; PRES, posterior reversible encephalopathy syndrome; sFlt-1, soluble fms-like tyrosine kinase 1; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
* Reduced risk for pre-eclampsia
Placental preeclampsia is characterized by the failure of placental spiral arteries to lose their musculoelastic layers, ultimately leading to decreased placental perfusion.16, 17 Failed adaptation of the uterine spiral arteries supplying the placenta may lead to placental ischemia,18 recurrent episodes of ischemia and reperfusion,19 or high-velocity blood flow that damages placental villous tissue.19 Ultimately, the damaged placenta may release one or more factors into the maternal circulation that cause systemic endothelial dysfunction.18
Several animal models attempt to emulate the incomplete adaptation of the maternal spiral arteries that supply the placenta. The reduced uterine perfusion pressure (RUPP) model surgically reduces uterine blood flow by partial or complete ligation of the aorta or uterine artery (ies). An excellent review of the RUPP model was recently published.20 The RUPP model causes hypertension and proteinuria, but not glomerular endotheliosis; hence, it may not be a good model for studying renal pathophysiology in pre-eclampsia. However, the RUPP model has proven valuable for studying the effects, rather than the causes, of fetal-placental ischemia. Elevated levels of tumor necrosis factor α (TNFα); interleukin (IL)-6; autoantibodies to the angiotensin II type I receptor (AT1-AA); the potent vasoconstrictor endothelin-1; sFlt-1; and soluble endoglin reported in pre-eclampsia are also seen in the RUPP model.21, 22
The baboon utero-placental ischemia model (UPI) is similar to the RUPP rat model, but results in more specific utero-placental ischemia.23 This technique involves uterine artery angiography followed by unilateral uterine artery ligation. Compared with sham animals, pregnant UPI baboons display clinically significant hypertension and proteinuria, with renal biopsies showing endotheliosis similar to human pre-eclampsia. Of the animal models, the baboon UPI model may most closely resemble the human condition. However, the inherent complexities of using non-human primates in research limit its availability. Similar to the RUPP model,24 sFlt-1 levels are elevated in this model, indicating that they are a consequence, rather than the cause, of placental ischemia.
Maternal pre-eclampsia is not associated with abnormal placentation and decreased perfusion. This form occurs in women with vascular dysfunction prior to pregnancy (commonly due to hypertension, diabetes, or obesity), in whom pregnancy acts as a physiological stress test that exacerbates preexisting endothelial dysfunction. This maternal vs. fetal preeclampsia classification is somewhat artificial and simplistic, as both processes likely play a role, but with varying contributions.6 The placental versus maternal form is increasingly interpreted in the context of early versus late preeclampsia, as poor placentation, the pathological substrate for fetal growth restriction, commonly occurs with early disease,25 which, compared to late pre-eclampsia, is characterized by different biochemical markers, heritability, and worse pregnancy outcomes.3 In addition, particularly severe maternal complications have been associated with postpartum pre-eclampsia, which occurs in 6% of all pre-eclamptic pregnancies15 and in up to 30% of those with HELLP syndrome.26 Regardless of the origins/etiologies of the initial insults, systemic endothelial dysfunction appears to be the converging point of different causal pathways, ultimately leading to the cardinal clinical features of pre-eclampsia.
GENETIC STUDIES
Genetic studies of pre-eclampsia suggest heritability of this condition, but have not consistently identified the responsible genetic variants using either candidate gene or genome scanning approaches.27 The discussion that follows presents recent genetic studies.
Notch signaling pathway
The Notch pathway consists of four Notch receptors and five Notch ligands.28 Transcriptional activation of Notch targeting genes leads to proliferation, differentiation, and apoptotic programs, all of which are essential for proper placentation.
Animal studies have shown that abnormal Notch signaling may lead to abnormal cytotrophoblast invasion characteristic of pre-eclampsia.29–31 Preliminary clinical data support the role of impaired Notch signaling in pre-eclampsia and abnormal placentation. Notch protein expression is decreased in pre-eclamptic placental tissue compared to healthy controls.32, 33 VEGF induces expression of Notch1 and its ligand,34 suggesting that decreased Notch signaling in pre-eclampsia may be a consequence of VEGF down-regulation in placental tissue.
Transcription factor storkhead box 1 (STOX1)
Recent studies suggest that this transcription factor may contribute to aberrant placentation in pre-eclampsia. In a Dutch study of families with 2 or more sisters afflicted with pre-eclampsia, a specific mutation of STOX1, Y153H, was identified in pre-eclamptic patients, although with incomplete penetrance.35 While these findings were not replicated in larger studies,36, 37 a recent animal study supported the possible involvement of STOX1 in pre-eclampsia. Transgenic mice overexpressing human STOX1 developed hypertension, proteinuria, and elevated levels of sFlt-1 and sEng.38 Although the renal histology is similar to that seen in pre-eclampsia, hypertension begins before placental formation, suggesting that the pathophysiology might be different than that seen in human disease. These data indicate that STOX1 may contribute to some cases of pre-eclampsia, but it is unlikely to be a common cause of the disorder.
Epigenetic studies
Altered DNA methylation contributes to the control of proliferative, invasive, and immune tolerance in oncogenesis,39 a disease process with many parallels to normal pregnancy. These conditions share the common goal of providing a nutrient supply and immune tolerance to a growing tumor or fetus, respectively. While epigenetic mechanisms are studied in tumor pathology, little is known about the role of DNA methylation in mediating maternal adaptations crucial for normal pregnancy. We recently demonstrated that normal early pregnancy (< 20 gestational weeks) is a transient state of epigenetic change favoring hypomethylation.40 This process may be impaired in pre-eclampsia. Genome-wide methylation profiles in maternal leukocyte DNA at the time of delivery show more methylation in women with pre-eclampsia compared to matched controls with an uncomplicated pregnancy.41 Future research should examine whether differences in methylation contribute to the differential expressions of markers that are associated with pre-eclampsia. New biomarkers and pathways in pre-eclampsia could also be identified by differences in methylation.
DYSREGULATION OF ANGIOGENESIS
Over the last decade, the pathway receiving the most attention involves the imbalance between the pro-angiogenic VEGF and placental growth factor (PlGF), and the anti-angiogenic sFlt-1 and soluble endoglin. Excessive production of anti-angiogenic sFlt-1 and soluble endoglin reduces the bioavailability of pro-angiogenic PlGF and VEGF. While reduced VEGF signaling is central to the sFlt-1 hypothesis, several lines of evidence suggest that this may be insufficient to cause hypertension and proteinuria when PlGF is present. Pregnant rats develop hypertension and proteinuria following adenoviral expression of sFlt-1, but not sFlk-1 (a type 2 VEGF receptor which only binds VEGF).13 In contrast, adenoviral expression of both sFlt-1 or sFlk-1 causes hypertension and proteinuria in non-pregnant rats, which have very low PlGF concentrations.13 On the clinical side, higher blood pressures early in pregnancy and more preterm deliveries were reported in pre-eclamptic women with low PlGF from 15 weeks gestation to term, compared to pre-eclamptic women with normal or high PlGF from 15 weeks gestation to term.2 This suggests that low versus normal/high PlGF levels may underpin two different clinical subtypes of pre-eclampsia.2 Some researchers have suggested redefining pre-eclampsia by using placenta-derived biomarkers, which link placental pathology (abnormal placentation) to impaired angiogenesis (low PlGF levels) and subsequent clinical phenotype (early, severe preeclampsia).6 While this classification may improve the reliability and reproducibility of outcomes assessment in pre-eclampsia, wider application is critically dependent on future studies to establish the cause-effect relationships among these events. An improved understanding of the complex interactions between anti-angiogenic and proangiogenic factors in normal and preeclamptic pregnancies is also needed, but, at present, may be hindered by the analytical limitations of current angiogenic marker assays.
Therapeutic Implications
In humans, sFlt-1 may contribute directly to the pathogenesis of pre-eclampsia. Its removal by apheresis was associated with reduced hypertension and proteinuria in pre-eclamptic women.42 However, the dextran columns used for apheresis remove many substances from the circulation; hence it is not clear whether sFlt-1 was the causative agent.
Other hypothesized mechanisms for increased sFlt-1 in pre-eclampsia include dysregulation of cystathionine γ-lyse (CSE).43 Placental CSE expression is reduced in pre-eclampsia, leading to reduced plasma levels of the pro-angiogenic gaseous vasodilator, hydrogen sulfide (H2S).43 The CSE/H2S may serve as another therapeutic target, pending additional studies to elucidate its protective mechanisms and bioavailability.
MEDIATORS OF ENDOTHELIAL DYSFUNCTION IN PRE-ECLAMPSIA
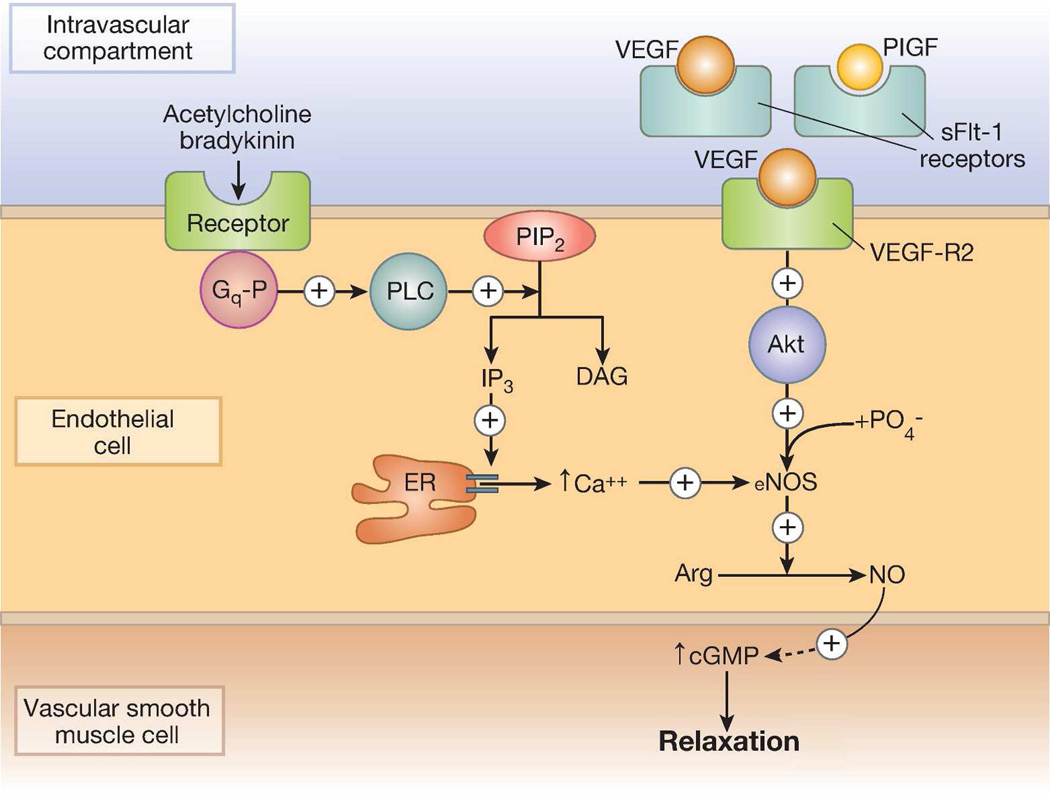
Blood pressure decreases in normal pregnancy due to generalized peripheral vasodilation. This decrease in peripheral vascular resistance is multifactorial. Contributing factors include an increased resistance to angiotensin II44 and an increased ratio between vasodilatory prostacyclin and vasoconstrictive thromboxane, and activation of nitric oxide (NO), a potent vasodilator that mediates endothelium-dependent relaxation (Figure 2). An imbalance between prostacyclin and thromboxane in favor of the latter leaves thromboxane’s vasoconstriction and platelet aggregation activities unopposed.45 This latter observation led to the hypothesis that aspirin might prevent pre-eclampsia.46 While clinical trials reported conflicting results, a meta-analysis of 59 trials involving 37,560 women found that low doses of aspirin reduced the absolute risk pre-eclampsia in women who were at high risk and concluded that future studies are required to assess which women are most likely to benefit, when treatment is best started, and at what dose.47
Figure 2. Regulation of nitric oxide (NO) synthesis.
Left side: calcium-dependent, mediated by acetylcholine and bradykinin. Right side: calcium-independent, stimulated by vascular endothelial growth factor (VEGF). In pre-eclampsia, elevated levels of soluble fms-like tyrosine kinase 1 (sFlt-1) may act as a non-signaling decoy, thus interfering with VEGF-dependent phosphorylation/stimulation of endothelial NOS (eNOS).
Abbreviations: Akt, protein kinase B; Arg, arginine; cGMP, cyclic guanosine monophosphate; DAG, diacylglycerol; ER, endoplasmic reticulum; Gq-P, phosphorylated Gq protein; IP3, inositol triphosphate; PIP2, phosphatidylinositol biphosphate; PLC, phospholipase C.
There are several potential mechanisms of increased reactivity to angiotensin II in pre-eclampsia. These include the presence of agonistic autoantibodies for angiotensin II type 1 (AT1) receptors,48 and heterodimerization between the AT1-receptor and the B2-receptor for the depressor, bradykinin. The AT1-B2-heterodimer mediates increased responsiveness to angiotensin II, and also confers resistance to AT1-receptor inactivation by reactive oxygen species.49
The discussion that follows does not aim to present the complex interactions among a myriad of vasoactive molecules that may have a role in pre-eclampsia,50 but rather to discuss more recent discoveries and their potential roles in identifying new therapeutic targets.
Heme oxygenase and carbon monoxide
Heme oxygenase (HO) converts heme to biliverdin in a process that releases carbon monoxide (CO). Biliverdin reductase then rapidly converts biliverdin to bilirubin. Both biliverdin and bilirubin are powerful anti-oxidants, and CO may also have beneficial effects. HO activity appears to be crucial for proper placental vascular development.51, 52 HO-1 knockout mice display abnormal placentation, intrauterine growth restriction (IUGR), and fetal demise. Heme administration produces a similar phenotype in wild-type mice. Interestingly, inhaled CO reverses these adverse effects.53 A study of non-smoking women during late gestation (>31 weeks), demonstrated that women with pre-eclampsia, compared to those without, have significantly reduced end-tidal CO levels, indicating reduced HO-1 activity.54
Studies demonstrate dose-dependent protection against pre-eclampsia for women residing in areas with higher ambient CO.55 These observational data are supported by in vitro studies, where CO protects placental villous explants against hypoxia/reoxygenation injury through anti-apoptotic mechanisms.56 Human umbilical vein endothelial cells (HUVEC) treated with CO-releasing molecule-2 (CORM-2) release less sFlt-1, likely through decreased VEGF-E mediated tyrosine phosphorylation of VEGFR-2.57 These studies provide a mechanism for the observed protective effect of CO exposure on pre-eclampsia risk.
Nitric oxide
Nitric oxide (NO), a potent vasodilator that mediates endothelium-dependent relaxation, has been linked to endothelial dysfunction in pre-eclampsia.58 As NO is regulated by VEGF,59 it was hypothesized that down-regulation of VEGF in pregnancy may result in a suboptimal increase in NO and endothelial dysfunction in pre-eclampsia (Figure 2). However, data concerning NO production in pre-eclampsia have been conflicting. Investigators have reported lower,60 unchanged,61 and higher62 production compared to normal controls, even after controlling for diet, medications and urinary excretion that can affect serum nitrate levels. It appears that reduced NO bioavailability, rather than changes in absolute NO levels, may alter endothelial function. Elevated intracellular levels of NO and superoxide react to produce an excess of peroxynitrate, the presence of which is confirmed by nitrotyrosine formation. Endothelial dysfunction results from the decreased abilities of specific proteins to undergo tyrosine phosphorylation once peroxynitrate-induced tyrosine nitration is completed.63 Several studies have observed elevated peroxynitrate levels in pre-eclampsia, as measured by nitrotyrosine formation.64, 65 NO induces vasodilation, not only through its direct effect on endothelial cells, but also by inhibiting the potent vasoconstrictors endothelin-166 and thromboxane,67 and by stimulating production of a vasodilatory prostacyclin.68 Based on these observations, some authors have suggested that all aspects of the pathophysiology of pre-eclampsia are related to the combined effects of a relative NO deficiency coupled with a peroxynitrate excess.58
Therapeutic Implications
CO, NO and H2S belong to the category of endogenously generated small molecules, gasotransmitters. These molecules are emerging as potential therapeutics for several disease entities, including cardiovascular disease and pre-eclampsia. Therapeutic use of CO gas and CO-releasing molecule was studied in preclinical animal models, which demonstrated anti-inflammatory properties, cardiovascular protective effects, and superior preservation of organs for transplantation compared to cold storage procedures.69 At present, the use of gasotransmitters in clinical practice is limited by their instability, potential toxicities, and the lack of appropriate delivery systems.
MATERNAL INNATE AND ADAPTIVE IMMUNE RESPONSES IN PRE-ECLAMPSIA
Pre-eclampsia has long been considered an immune-mediated syndrome. Pre-eclamptic women display elevated inflammatory cytokines and autoantibodies. Autoantibodies to the AT1 receptor are present in pre-eclampsia and may contribute to hypertension.48 AT1-AA bind to and activate AT1 receptors on vascular cells; their binding can be blocked by AT1 receptor blockers, such as losartan. Hypertension in RUPP rats is associated with elevated AT1-AA; hence placental ischemia may be an important stimulus for AT1-AA production.70 AT1-AA infusion in pregnant rats induces hypertension and mitigates impaired vasodilatory responses to acetylcholine in renal interlobar arteries.71 In addition, recent evidence suggests that elements of innate immunity, such as TNFα and interleukin 6 (IL-6), may induce a pre-eclamptic syndrome by generation of AT1-AA,70, 72 and that activation of Toll-like receptors, also involved in innate immunity, may cause a pre-eclampsia-like syndrome in rats.73 Additional studies are needed to determine the exact role of AT1-AA in the pathophysiology of pre-eclampsia and to explore toll-like receptors as potential therapeutic targets.
PODOCYTE PATHOPHYSIOLOGY IN PRE-ECLAMPSIA
Proteinuria is the hallmark of pre-eclampsia that differentiates it from other hypertensive disorders of pregnancy, despite the controversy surrounding its usefulness in diagnosing pre-eclampsia. Proteinuria is thought to be due to endothelial cell swelling and disruption of fenestrae. Over the last six years, our research has focused on derangements of podocytes and podocyte-specific proteins (such as nephrin, synaptopodin, podocin, and podocalyxin), and their roles in the mechanism(s) of proteinuria in pre-eclampsia.
Studies of human tissue show that the expressions of podocyte-specific proteins are severely affected by pre-eclampsia. A study comparing renal sections from women with pre-eclampsia, compared to those from women with either normotensive or chronic hypertensive pregnancies, reported reduced expressions of podocyte-associated proteins, nephrin, glomerular epithelial protein 1, GLEPP-1, and ezrin in their renal tissue sections.74 Decreased glomerular expressions of nephrin and synaptopodin were seen in renal tissue sections from women who died from pre-eclampsia compared to those of women with normal pregnancies who died from other causes.75 Podocin expression, however, was relatively unchanged. The degree of podocyte dysfunction required for such dramatic changes in nephrin and synaptopodin expressions might be expected to cause changes in multiple other proteins important to the integrity of the glomerular filtration barrier and, possibly, podocyte attachment.
The detection of podocyte products and live podocytes in the urine (podocyturia) suggests that podocyte pathology is more severe than might be inferred from renal biopsy studies. Various methods have been developed to detect urinary podocyte products.76–86 Culturing of urinary podocytes increases specificity by removing dead and non-specific cells, but is difficult and time consuming. Cytospin techniques, while quicker and possibly more amenable to automation, suffer from low sensitivity and specificity due to the large amount of cellular debris. More sensitive techniques using reverse transcriptase-polymerase chain reaction (RT-PCR) and mass spectrometry remain in development. Studies utilizing these methods are outlined below and summarized in Table 1.
Table 1.
Studies of podocyturia and urinary podocyte markers in pre-eclampsia
| Author and year |
Study groups | Time point(s) | Sample preparation | Podocyte detection Method |
Results |
|---|---|---|---|---|---|
| Garovic et al (2007)76 | 15 PE 16 NL |
< 24 hours before delivery | Podocyte culture | IF for podocin, nephrin, podocalyxin, and synaptopodin | Podocin staining present in 15/15 PE and absent in 16/16 NL Other 3 less sensitive and specific |
| Aita et al (2009)77 | 11 PE 45 NL |
35 weeks 4 days post 1 month post |
Cytospin | IF for podocalyxin | Podocyturia at 35 weeks and 4 days post in PE Almost no podocyturia at 1 month post delivery 9 of 45 controls showed podocyturia 4 days postdelivery Correlation between podocyturia and BP, but not proteinuria |
| Zhao et al (2011)78 | 16 severe PE 3 mild PE 7 NL 7 NP with nephrotic syndrome |
3rd trimester | Podocyte culture | IF for nephrin | Podocyturia present in all cases of severe PE and nephrotic syndrome Podocyturia absent in all NL and all 3 mild PE cases |
| Jim et al (2012)79 | 29 PE 9 GHTN and HTN 9 NL |
< 24 hours before delivery | Cytospin | IF for synaptopodin | Podocyturia in 11 of 29 (38%) of PE, 3 of 9 (33%) with HTN, and 0 of 9 NL Sensitivity = 38%, Specificity = 70% |
| Facca et al (2012)80 | 25 NL 14 PE |
3rd trimester | Cytospin | IF for nephrin | Mean total number of podocytes 0.9 ± 1.6 for NL versus 9.3 ± 16.8 for PE (P=0.212) |
| Kelder et al (2012)81 | 35 PE 5 GHTN 34 NL 12 NP |
31 to 36 weeks gestation | Urine centrifugation TRIzol RNA isolation | RT-PCR for nephrin, podocin, VEGF | Elevated mRNA for nephrin, podocin, VEGF in PE compared to NL and NP Positive correlation between nephrin and VEGF mRNA in PE (r=0.82, P<0.0001) |
| Wang et al (2012)82 | 20 PE 6 HTN 8 NL |
3rd trimester | ELISA of frozen urine supernatant | ELISA for nephrin, podocalyxin, Big-h3, and VEGF | Urinary nephrin, podocalyxin, and Big-h3 levels are increased in PE Urinary Big-h3 levels correlate with levels of nephrin and podocalyxin |
| Chen et al (2013)83 | 14 PE 14 GHTN 13 NL |
< 1 week before delivery | Cytospin | IF for podocalyxin | Number of podocytes was higher in PE compared to GHTN (P<0.05) and NL (P<0.001) |
| Son et al (2013)84 | 43 Severe PE 30 NL |
< 24 hours before delivery | ELISA of frozen urine supernatant | ELISA for nephrin | Urine nephrin higher in severe PE than in NL Urine nephrin correlated with proteinuria, diastolic BP, and renal dysfunction |
| Craici et al (2013)85 | 15 PE 15 GHTN 44 NL |
Late second trimester (median 27 weeks) | Podocyte culture | IF for podocin | Podocyturia present in 15/15 PE, absent in 15/15 GHTN, and absent in 44/44 NL Podocyturia in second trimester was more sensitive and specific for later PE than any combination of angiogenic factors |
| Garovic et al (2013)86 | 13 PE 6 PE/HELLP 4 NL |
< 24 hours before delivery | Trypsin digestion of the urinary sediment | LC-MS/MS | Podocin-specific tryptic peptide significantly higher in PE/HELLP compared to NL |
BP, blood pressure; GHTN, gestational hypertension; HTN, hypertension; IF, immunofluorescence; NL, normal pregnancy; NP, not pregnant; PE, pre-eclampsia; VEGF, vascular endothelial growth factor; HELLP, hemolysis, elevated liver enzymes, low platelet count; LC-MS/MS, liquid chromatography coupled with tandem mass spectrometry.
Using staining for podocin to detect live podocytes, we have shown 100% sensitivity and specificity in diagnosing pre-eclampsia at the time of delivery.76 Synaptopodin, nephrin, and podocalyxin were also useful markers for urinary podocytes, but lacked sensitivity and specificity compared to podocin. This is in agreement with autopsy studies showing that podocin expression is preserved in podocytes from pre-eclamptic patients compared with nephrin and synaptopodin.75 Podocyturia appears before the onset of proteinuria, and the number of podocytes positively correlates with the degree of proteinuria, suggesting a cause-effect relationship between ongoing podocyte loss and the onset and severity of proteinuria,85 i.e., that these are mechanistically related.
Several lines of evidence support the associations between dysregulated pro-angiogenic factors, hypertension, and podocyte injury. Most convincing is the observation that bevacizumab, an anti-VEGF antibody that decreases VEGF signaling in a manner similar to sFlt-1, causes hypertension and proteinuria in non-pregnant individuals. Renal findings in patients treated with bevacizumab, endotheliosis and thrombotic microangiopathy,87 are similar to those found in pre-eclampsia and its severe form, HELLP syndrome. Podocyturia is also seen in patients treated with bevacizumab, although less consistently than with pre-eclampsia.88
The usefulness of podocyturia for early diagnosis of pre-eclampsia remains an active research topic. Other groups have confirmed that podocyturia is specific to the diagnosis of pre-eclampsia, using both podocalyxin and nephrin staining.77, 78 A recent study using synaptopodin staining of urinary cytospins questioned the usefulness of this technique, finding only 38% sensitivity and 70% specificity for diagnosing pre-eclampsia.79 Previous research indicates, however, that synaptopodin is a marker of intact, well-differentiated podocytes, and that its expression may be altered in proteinuric diseases.89, 90 Urinary sediments obtained from cytospin techniques are also contaminated with non-specific cellular and noncellular debris that may significantly affect the performance characteristics of the test.80, 91 Reduced sensitivity and specificity in this case may, therefore, be the result of technical aspects of podocyte detection rather than the actual presence or absence of podocyturia.
More novel methods for detecting urinary podocytes and their products may also prove useful for the early diagnosis of pre-eclampsia. Mass spectrometry and RT-PCR offer standardized, reproducible techniques for the detection of podocyte products.81, 92 These methods are operator-independent and highly reproducible, and may facilitate large-scale studies that will determine the clinical usefulness of podocyturia in larger patient populations that are more broadly representative of pregnant women. Critical clinical questions that remain un-answered include the ability of podocyturia to differentiate between pre-eclampsia, other complications of pregnancy (such as gestational diabetes mellitus), and other proteinuric diseases that either predate pregnancy or occur during pregnancy.
Endothelin 1: a possible final common pathway for endothelial and podocyte dysfunction
Recent data suggest that endothelin-1, one of the most powerful human vasoconstrictors, may act through the endothelin type A (ETA) receptor to provide a bridge between placental ischemia and the clinical signs of pre-eclampsia, both hypertension and podocyte damage/proteinuria. Endothelin-1 may act both in an autocrine or paracrine manner, therefore, systemic levels do not necessarily reflect local tissue expression or effects. Endothelin-1 mediates hypertension in pregnant rats after infusion of either TNFα70 or AT1-AA,93 whereas antagonism of the ETA receptor has resulted in blood pressure improvement in animal models of pre-eclampsia, both in the RUPP94, 95 and mouse models of adenoviral sFlt-1 overexpression.96 With respect to podocytes, there is strong in vitro evidence supporting the role of endothelin-1 in podocyte dysfunction and subsequent proteinuria. Pre-eclamptic sera are not directly toxic to cultured podocytes. However, endothelial cells exposed to sera from pre-eclamptic women produce compounds that alter nephrin expression and cause extracellular nephrin cleavage in cultured podocytes.97 These effects can be replicated with purified endothelin-1 and are prevented by ETA blockade. These findings suggest that pre-eclamptic sera induce proteinuria by affecting the glomerular capillary endothelium, and that endothelin-1 may cause podocyte dysfunction via the ETA receptor. This is further supported by both in vivo and in vitro studies indicating that i) endogenous endothelin contributes to glomerulosclerosis and proteinuria, as these changes are reversible by endothelin-1 inhibition, and ii) podocyte apoptosis and structural damage, induced by puromycin aminoglycoside, may be reduced by blocking endothelin receptors.98
Therapeutic implications
Podocyturia has been shown to decrease with blood pressure control and modulation of the renin-angiotensin-aldosterone system, by either angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists in proteinuric disorders, such as IgA nephropathy,99 and in animal models of progressive proteinuric disease.100 In pregnancy, both ACE inhibitors and angiotensin II receptor antagonists are contraindicated. As podocyte detachment in pre-eclampsia may represent an end-event where different dysregulated pathways converge, future studies focusing on the mechanism(s) of podocyte injury and detachment may identify novel therapeutic targets. With respect to endothelin as a possible therapeutic target, animal studies demonstrate fetal malformations in both ETA receptor knockout mice101 and with ETA receptor blockade.102 There may be safe “windows” for the use of ETA blockade in mid and late gestation, where prolonging pregnancy for even a few weeks might reduce fetal morbidity and mortality.102 Additional research is needed to determine whether maternal ETA blockade in late gestation might be safe and efficacious, especially in view of recent clinical studies showing a significant improvement in proteinuria in diabetic nephropathy, but raising safety concerns due to an increased risk for cardiovascular events.103
PRE-ECLAMPSIA, PODOCYTE INJURY, AND FUTURE CHRONIC RENAL DISEASE
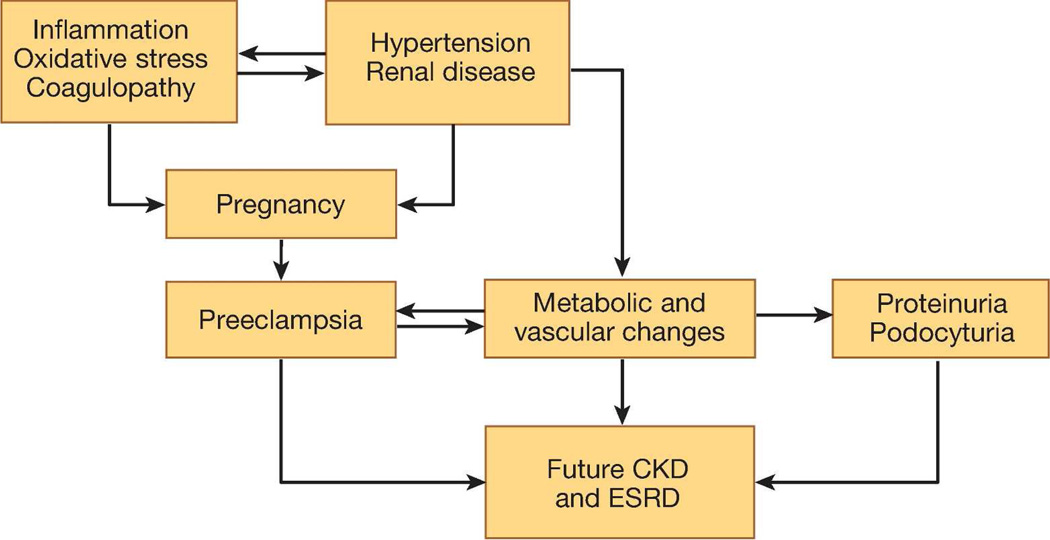
In addition to its potential diagnostic utility, podocyte damage and shedding may affect renal function years after pre-eclamptic pregnancies. Clinical studies support that women with a history of pre-eclampsia have an increased future risk of albuminuria, chronic kidney disease (CKD), and end stage renal disease (ESRD).104–108 It is unclear whether this association is independent of risk factors that may be common to pre-eclampsia and renal disease (Figure 3). In a mouse model of selective podocyte depletion using diphtheria toxin,109 a single episode of podocyte injury resulted in glomerular destabilization and persistent podocyte loss. Podocyturia is seen in patients with focal segmental glomerular sclerosis (FSGS),110 which, in turn, has been identified as a dominant histopathological lesion in the renal biopsies from women with persistent proteinuria after pre-eclamptic pregnancies.111 Taken together, these data raise a testable hypothesis that women with pre-eclamptic pregnancies and persistent proteinuria postpartum may have an underlying lesion of FSGS characterized by ongoing podocyte loss, which may contribute to their increased risks for proteinuria, CKD, and ESRD later in life. However, the cause-effect relationship between podocyturia and FSGS needs to be confirmed in appropriately designed longitudinal studies.
Figure 3. Possible mechanisms of the association between pre-eclampsia and future renal disease.
Abbreviations: CKD, chronic kidney disease; ESRD, end-stage renal disease.
With respect to future studies of the mechanisms of renal injury later in life, these may be limited by the fact that humans are the only species known to suffer from spontaneous pre-eclampsia. Although many animal models have been developed for the study of this disorder, renal endotheliosis, considered specific for pre-eclampsia, is absent in most of them. Two recently reported mouse models may prove useful for these studies. One is a matrix metalloproteinase-9 (MMP9) null mouse model: pregnant MMP9-null mice bearing null embryos exhibited clinical features of pre-eclampsia, including a reduced percentage of glomeruli with open capillaries (i.e., glomerular endotheliosis).112The second model used IL-10−/− mice injected with 100 µl of sera from pre-eclamptic women. They developed elevated blood pressure, proteinuria, IUGR, glomerular endotheliosis, increased levels of sFlt-1 and sEng, and spiral artery pathology.113 These changes were absent when sera from normal pregnant women were injected, and they were specific to pregnancy, i.e., were absent after injection of non-pregnant IL 10−/− mice.113 These models closely mimic human disease, including the renal pathology, and may provide valuable insight into pre-eclampsia pathophysiology and treatment.
CONCLUSIONS
Over the last decade, our understanding of the pathophysiology of pre-eclampsia and related disorders has increased dramatically. The heterogeneity of both the causal pathways and clinical presentations of pre-eclampsia suggests that therapies acting on a particular pathway will only be effective in patients with aberrations in that particular pathway. Research on pre-eclampsia prevention and treatment should either focus on pathways common to all women with pre-eclampsia, or target the subgroup of women who have abnormalities in the pathway examined. The potential to identify targeted therapies that address the underlying causes of disease in distinct pathophysiological subtypes of pre-eclampsia may improve treatment options for a disease which has seen few therapeutic advances in recent decades.
Acknowledgments
Sources of Support: The project described was supported by the award numbers K08HD051714 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, P50AG44170 from the National Institute on Aging (Vesna D. Garovic), and K12HD065987 from the Office of Women’s Health Research, Building Interdisciplinary Careers in Women’s Health (Tracey L. Weissgerber).
Footnotes
Disclosure
Conflict of interest: Dr. Garovic is the inventor of technology referenced in this article. That technology has been patented by Mayo Clinic, but is currently not licensed.
All other authors report no conflict of interest.
References
- 1.World Health Organization. Geneva: WHO; [Accessed June 15, 2013]. The World Health Report 2005 - Make Every Mother and Child Count. http://www.who.int/whr/2005/en/. [Google Scholar]
- 2.Powers RW, Roberts JM, Plymire DA, et al. Low Placental Growth Factor Across Pregnancy Identifies a Subset of Women With Preterm Preeclampsia: Type 1 Versus Type 2 Preeclampsia? Hypertension. 2012;60:239–246. doi: 10.1161/HYPERTENSIONAHA.112.191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011;66:497–506. doi: 10.1097/OGX.0b013e3182331028. [DOI] [PubMed] [Google Scholar]
- 4.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [No authors listed]. [PubMed] [Google Scholar]
- 5.Rana S, Powe CE, Salahuddin S, et al. Angiogenic Factors and the Risk of Adverse Outcomes in Women With Suspected Preeclampsia. Circulation. 2012;125:911–919. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staff AC, Benton SJ, von Dadelszen P, et al. Redefining Preeclampsia Using Placenta- Derived Biomarkers. Hypertension. 2013;61:932–942. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 7.Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175:1365–1370. doi: 10.1016/s0002-9378(96)70056-x. [DOI] [PubMed] [Google Scholar]
- 8.August P. Preeclampsia: a "nephrocentric" view. Adv Chronic Kidney Dis. 2013;20:280–286. doi: 10.1053/j.ackd.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Homer CS, Brown MA, Mangos G, et al. Non-proteinuric pre-eclampsia: a novel risk indicator in women with gestational hypertension. J Hypertens. 2008;26:295–302. doi: 10.1097/HJH.0b013e3282f1a953. [DOI] [PubMed] [Google Scholar]
- 10.Pettit F, Brown MA. The management of pre-eclampsia: what we think we know. Eur J Obstet Gynecol Reprod Biol. 2012;160:6–12. doi: 10.1016/j.ejogrb.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 11.Genbacev O, Zhou Y, Ludlow JW, et al. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto H, Hamano Y, Charytan D, et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003;278:12605–12608. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 13.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 15.Matthys LA, Coppage KH, Lambers DS, et al. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol. 2004;190:1464–1466. doi: 10.1016/j.ajog.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Khong TY, De Wolf F, Robertson WB, et al. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 17.Meekins JW, Pijnenborg R, Hanssens M, et al. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 18.Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 19.Burton GJ, Woods AW, Jauniaux E, et al. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol. 2012;303:H1–H8. doi: 10.1152/ajpheart.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaMarca B, Wallukat G, Llinas M, et al. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52:1168–1172. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaMarca BD, Gilbert J, Granger JP. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension. 2008;51:982–988. doi: 10.1161/HYPERTENSIONAHA.107.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makris A, Thornton C, Thompson J, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert JS, Babcock SA, Granger JP. Hypertension Produced by Reduced Uterine Perfusion in Pregnant Rats Is Associated With Increased Soluble Fms-Like Tyrosine Kinase-1 Expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 25.Xiong X, Demianczuk NN, Saunders LD, et al. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol. 2002;155:203–209. doi: 10.1093/aje/155.3.203. [DOI] [PubMed] [Google Scholar]
- 26.Sibai BM, Ramadan MK, Usta I, et al. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome)[see comment] Am J Obstet Gynecol. 1993;169:1000–1006. doi: 10.1016/0002-9378(93)90043-i. [DOI] [PubMed] [Google Scholar]
- 27.Jebbink J, Wolters A, Fernando F, et al. Molecular genetics of preeclampsia and HELLP syndrome — A review. Biochim Biophys Acta. 2012;1822:1960–1969. doi: 10.1016/j.bbadis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 28.D'Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afshar Y, Jeong JW, Roqueiro D, et al. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J. 2012;26:282–294. doi: 10.1096/fj.11-184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krebs LT, Xue Y, Norton CR, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 31.Hunkapiller NM, Gasperowicz M, Kapidzic M, et al. A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development. 2011;138:2987–2998. doi: 10.1242/dev.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahin Z, Acar N, Ozbey O, et al. Distribution of Notch family proteins in intrauterine growth restriction and hypertension complicated human term placentas. Acta Histochem. 2011;113:270–276. doi: 10.1016/j.acthis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Cobellis L, Mastrogiacomo A, Federico E, et al. Distribution of Notch protein members in normal and preeclampsia-complicated placentas. Cell Tissue Res. 2007;330:527–534. doi: 10.1007/s00441-007-0511-6. [DOI] [PubMed] [Google Scholar]
- 34.Liu ZJ, Shirakawa T, Li Y, et al. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dijk M, Mulders J, Poutsma A, et al. Maternal segregation of the Dutch preeclampsia locus at 10q22 with a new member of the winged helix gene family. Nat Genet. 2005;37:514–519. doi: 10.1038/ng1541. [DOI] [PubMed] [Google Scholar]
- 36.Berends AL, Bertoli-Avella AM, de Groot CJ, et al. STOX1 gene in pre-eclampsia and intrauterine growth restriction. BJOG. 2007;114:1163–1167. doi: 10.1111/j.1471-0528.2007.01414.x. [DOI] [PubMed] [Google Scholar]
- 37.Kivinen K, Peterson H, Hiltunen L, et al. Evaluation of STOX1 as a preeclampsia candidate gene in a population-wide sample. Eur J Hum Genet. 2007;15:494–497. doi: 10.1038/sj.ejhg.5201788. [DOI] [PubMed] [Google Scholar]
- 38.Doridot L, Passet B, Mehats C, et al. Preeclampsia-like symptoms induced in mice by fetoplacental expression of STOX1 are reversed by aspirin treatment. Hypertension. 2013;61:662–668. doi: 10.1161/HYPERTENSIONAHA.111.202994. [DOI] [PubMed] [Google Scholar]
- 39.Germenis AE, Karanikas V. Immunoepigenetics: the unseen side of cancer immunoediting. Immunol Cell Biol. 2007;85:55–59. doi: 10.1038/sj.icb.7100006. [DOI] [PubMed] [Google Scholar]
- 40.White WM, Brost BC, Sun Z, et al. Normal early pregnancy: a transient state of epigenetic change favoring hypomethylation. Epigenetics. 2012;7:729–734. doi: 10.4161/epi.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White WM, Brost B, Sun Z, et al. Genome-wide methylation profiling demonstrates hypermethylation in maternal leukocyte DNA in preeclamptic compared to normotensive pregnancies. Hypertens Pregnancy. 2013;32:257–269. doi: 10.3109/10641955.2013.796970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thadhani R, Kisner T, Hagmann H, et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011;124:940–950. doi: 10.1161/CIRCULATIONAHA.111.034793. [DOI] [PubMed] [Google Scholar]
- 43.Wang K, Ahmad S, Cai M, et al. Dysregulation of Hydrogen Sulfide Producing Enzyme Cystathionine gamma-lyase Contributes to Maternal Hypertension and Placental Abnormalities in Preeclampsia. Circulation. 2013;127:2514–2522. doi: 10.1161/CIRCULATIONAHA.113.001631. [DOI] [PubMed] [Google Scholar]
- 44.Gant NF, Daley GL, Chand S, et al. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mills JL, DerSimonian R, Raymond E, et al. Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: A multicenter prospective study. JAMA. 1999;282:356–362. doi: 10.1001/jama.282.4.356. [DOI] [PubMed] [Google Scholar]
- 46.Villa PM, Kajantie E, Räikkönen K, et al. Aspirin in the prevention of pre-eclampsia in high-risk women: a randomised placebo-controlled PREDO Trial and a meta-analysis of randomised trials. BJOG. 2013;120:64–74. doi: 10.1111/j.1471-0528.2012.03493.x. [DOI] [PubMed] [Google Scholar]
- 47.Duley L, Henderson-Smart DJ, Meher S, et al. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007:CD004659. doi: 10.1002/14651858.CD004659.pub2. [DOI] [PubMed] [Google Scholar]
- 48.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.AbdAlla S, Lother H, el Massiery A, et al. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness.[see comment] Nat Med. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- 50.Garovic VD, Hayman SR. Hypertension in pregnancy: an emerging risk factor for cardiovascular disease. Nat Clin Pract Nephrol. 2007;3:613–622. doi: 10.1038/ncpneph0623. [DOI] [PubMed] [Google Scholar]
- 51.Wong RJ, Zhao H, Stevenson DK. A deficiency in haem oxygenase-1 induces foetal growth restriction by placental vasculature defects. Acta Paediatr. 2012;101:827–834. doi: 10.1111/j.1651-2227.2012.02729.x. [DOI] [PubMed] [Google Scholar]
- 52.McCaig D, Lyall F. Inhibitors of heme oxygenase reduce invasion of human primary cytotrophoblast cells in vitro. Placenta. 2009;30:536–538. doi: 10.1016/j.placenta.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Zenclussen ML, Casalis PA, El-Mousleh T, et al. Haem oxygenase-1 dictates intrauterine fetal survival in mice via carbon monoxide. J Pathol. 2011;225:293–304. doi: 10.1002/path.2946. [DOI] [PubMed] [Google Scholar]
- 54.Baum M, Schiff E, Kreiser D, et al. End-tidal carbon monoxide measurements in women with pregnancy-induced hypertension and preeclampsia. Am J Obstet Gynecol. 2000;183:900–903. doi: 10.1067/mob.2000.109047. [DOI] [PubMed] [Google Scholar]
- 55.Zhai D, Guo Y, Smith G, et al. Maternal exposure to moderate ambient carbon monoxide is associated with decreased risk of preeclampsia. Am J Obstet Gynecol. 2012;207:57.e51–57.e59. doi: 10.1016/j.ajog.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 56.Bainbridge SA, Belkacemi L, Dickinson M, et al. Carbon monoxide inhibits hypoxia/reoxygenation-induced apoptosis and secondary necrosis in syncytiotrophoblast. Am J Pathol. 2006;169:774–783. doi: 10.2353/ajpath.2006.060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cudmore M, Ahmad S, Al-Ani B, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 58.Lowe DT. Nitric oxide dysfunction in the pathophysiology of preeclampsia. Nitric Oxide. 2000;4:441–458. doi: 10.1006/niox.2000.0296. [DOI] [PubMed] [Google Scholar]
- 59.Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davidge ST, Stranko CP, Roberts JM. Urine but not plasma nitric oxide metabolites are decreased in women with preeclampsia. Am J Obstet Gynecol. 1996;174:1008–1013. doi: 10.1016/s0002-9378(96)70341-1. [DOI] [PubMed] [Google Scholar]
- 61.Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24-h NO(x) and cGMP during normal pregnancy and preeclampsia in women on a reduced NO(x) diet. Am J Physiol. 1999;277:F48–F57. doi: 10.1152/ajprenal.1999.277.1.F48. [DOI] [PubMed] [Google Scholar]
- 62.Ranta V, Viinikka L, Halmesmaki E, et al. Nitric oxide production with preeclampsia. Obstet Gynecol. 1999;93:442–445. doi: 10.1016/s0029-7844(98)00465-7. [DOI] [PubMed] [Google Scholar]
- 63.Gow AJ, Duran D, Malcolm S, et al. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett. 1996;385:63–66. doi: 10.1016/0014-5793(96)00347-x. [DOI] [PubMed] [Google Scholar]
- 64.Myatt L, Rosenfield RB, Eis AL, et al. Nitrotyrosine residues in placenta. Evidence of peroxynitrite formation and action. Hypertension. 1996;28:488–493. doi: 10.1161/01.hyp.28.3.488. [DOI] [PubMed] [Google Scholar]
- 65.Kossenjans W, Eis A, Sahay R, et al. Role of peroxynitrite in altered fetal-placental vascular reactivity in diabetes or preeclampsia. Am J Physiol Heart Circ Physiol. 2000;278:H1311–H1319. doi: 10.1152/ajpheart.2000.278.4.H1311. [DOI] [PubMed] [Google Scholar]
- 66.Ahlborg G, Lundberg JM. Nitric oxide-endothelin-1 interaction in humans. J Appl Physiol. 1997;82:1593–1600. doi: 10.1152/jappl.1997.82.5.1593. [DOI] [PubMed] [Google Scholar]
- 67.Benyo Z, Gorlach C, Wahl M. Role of nitric oxide and thromboxane in the maintenance of cerebrovascular tone. Kidney Int Suppl. 1998;67:S218–S220. doi: 10.1046/j.1523-1755.1998.06753.x. [DOI] [PubMed] [Google Scholar]
- 68.Wang W, Diamond SL. Does elevated nitric oxide production enhance the release of prostacyclin from shear stressed aortic endothelial cells? Biochem Biophys Res Commun. 1997;233:748–751. doi: 10.1006/bbrc.1997.6548. [DOI] [PubMed] [Google Scholar]
- 69.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 70.LaMarca B, Speed J, Fournier L, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52:1161–1167. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parrish MR, Ryan MJ, Glover P, et al. Angiotensin II type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gend Med. 2011;8:184–188. doi: 10.1016/j.genm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lamarca B, Speed J, Ray LF, et al. Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation. Int J Infereron Cytokine Mediator Res. 2011;2011:65–70. doi: 10.2147/IJICMR.S22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tinsley JH, Chiasson VL, Mahajan A, et al. Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens. 2009;22:1314–1319. doi: 10.1038/ajh.2009.185. [DOI] [PubMed] [Google Scholar]
- 74.Zhao S, Gu X, Groome LJ, et al. Decreased Nephrin and GLEPP-1, But Increased VEGF, Flt-1, and Nitrotyrosine, Expressions in Kidney Tissue Sections From Women With Preeclampsia. Reprod Sci. 2009;16:970–979. doi: 10.1177/1933719109338630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garovic VD, Wagner SJ, Petrovic LM, et al. Glomerular expression of nephrin and synaptopodin, but not podocin, is decreased in kidney sections from women with preeclampsia. Nephrol Dial Transplant. 2007;22:1136–1143. doi: 10.1093/ndt/gfl711. [DOI] [PubMed] [Google Scholar]
- 76.Garovic VD, Wagner SJ, Turner ST, et al. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol. 2007;196:e321–e327. doi: 10.1016/j.ajog.2007.02.007. 320. [DOI] [PubMed] [Google Scholar]
- 77.Aita K, Etoh M, Hamada H, et al. Acute and transient podocyte loss and proteinuria in preeclampsia. Nephron Clin Pract. 2009;112:c65–c70. doi: 10.1159/000213083. [DOI] [PubMed] [Google Scholar]
- 78.Zhao S, Gu Y, Coates G, et al. Altered nephrin and podoplanin distribution is associated with disturbed polarity protein PARD-3 and PARD-6 expressions in podocytes from preeclampsia. Reprod Sci. 2011;18:772–780. doi: 10.1177/1933719111398145. [DOI] [PubMed] [Google Scholar]
- 79.Jim B, Jean-Louis P, Qipo A, et al. Podocyturia as a diagnostic marker for preeclampsia amongst high-risk pregnant patients. J Pregnancy. 2012;2012 doi: 10.1155/2012/984630. 984630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Facca TA, Kirsztajn GM, Pereira AR, et al. Renal evaluation in women with preeclampsia. Nephron Extra. 2012;2:125–132. doi: 10.1159/000338271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelder TP, Penning ME, Uh HW, et al. Quantitative polymerase chain reaction-based analysis of podocyturia is a feasible diagnostic tool in preeclampsia. Hypertension. 2012;60:1538–1544. doi: 10.1161/HYPERTENSIONAHA.112.201681. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Zhao S, Loyd S, et al. Increased urinary excretion of nephrin, podocalyxin, and betaig-h3 in women with preeclampsia. Am J Physiol Renal Physiol. 2012;302:F1084–F1089. doi: 10.1152/ajprenal.00597.2011. [DOI] [PubMed] [Google Scholar]
- 83.Chen G, Zhang L, Jin X, et al. Effects of angiogenic factors, antagonists, and podocyte injury on development of proteinuria in preeclampsia. Reprod Sci. 2013;20:579–588. doi: 10.1177/1933719112459227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Son GH, Kwon JY, Lee S, et al. Comparison of serum and urinary nephrin levels between normal pregnancies and severe preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2013;166:139–144. doi: 10.1016/j.ejogrb.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 85.Craici IM, Wagner SJ, Bailey KR, et al. Podocyturia predates proteinuria and clinical features of preeclampsia: longitudinal prospective study. Hypertension. 2013;61:1289–1296. doi: 10.1161/HYPERTENSIONAHA.113.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garovic VD, Craici IM, Wagner SJ, et al. Mass spectrometry as a novel method for detection of podocyturia in pre-eclampsia. Nephrol Dial Transplant. 2013;28:1555–1561. doi: 10.1093/ndt/gfs074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF Inhibition and Renal Thrombotic Microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muller-Deile J, Schiffer M. Renal involvement in preeclampsia: similarities to VEGF ablation therapy. J Pregnancy. 2011;2011:176973. doi: 10.1155/2011/176973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mundel P, Heid HW, Mundel TM, et al. Synaptopodin: An Actin-associated Protein in Telencephalic Dendrites and Renal Podocytes. J Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barisoni L, Kriz W, Mundel P, et al. The Dysregulated Podocyte Phenotype: A Novel Concept in the Pathogenesis of Collapsing Idiopathic Focal Segmental Glomerulosclerosis and HIV-Associated Nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 91.Achenbach J, Mengel M, Tossidou I, et al. Parietal epithelia cells in the urine as a marker of disease activity in glomerular diseases. Nephrol Dial Transplant. 2008;23:3138–3145. doi: 10.1093/ndt/gfn235. [DOI] [PubMed] [Google Scholar]
- 92.Garovic VD, Craici IM, Wagner SJ, et al. Mass spectrometry as a novel method for detection of podocyturia in pre-eclampsia. Nephrol Dial Transplant. 2013;28:1555–1561. doi: 10.1093/ndt/gfs074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.LaMarca B, Parrish M, Ray LF, et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alexander BT, Rinewalt AN, Cockrell KL, et al. Endothelin Type A Receptor Blockade Attenuates the Hypertension in Response to Chronic Reductions in Uterine Perfusion Pressure. Hypertension. 2001;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 95.Tam Tam KB, George E, Cockrell K, et al. Endothelin type A receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. Am J Obstet Gynecol. 2011;204:e331–e334. doi: 10.1016/j.ajog.2011.01.049. 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li F, Hagaman JR, Kim H-S, et al. eNOS Deficiency Acts through Endothelin to Aggravate sFlt–1–Induced Pre-Eclampsia–Like Phenotype. J Am Soc Nephrol. 2012;23:652–660. doi: 10.1681/ASN.2011040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Collino F, Bussolati B, Gerbaudo E, et al. Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol. 2008;294:F1185–F1194. doi: 10.1152/ajprenal.00442.2007. [DOI] [PubMed] [Google Scholar]
- 98.Ortmann J, Amann K, Brandes RP, et al. Role of Podocytes for Reversal of Glomerulosclerosis and Proteinuria in the Aging Kidney After Endothelin Inhibition. Hypertension. 2004;44:974–981. doi: 10.1161/01.HYP.0000149249.09147.b4. [DOI] [PubMed] [Google Scholar]
- 99.Nakamura T, Ushiyama C, Suzuki S, et al. Effects of angiotensin-converting enzyme inhibitor, angiotensin II receptor antagonist and calcium antagonist on urinary podocytes in patients with IgA nephropathy. Am J Nephrol. 2000;20:373–379. doi: 10.1159/000013619. [DOI] [PubMed] [Google Scholar]
- 100.Fukuda A, Wickman LT, Venkatareddy MP, et al. Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int. 2012;81:40–55. doi: 10.1038/ki.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clouthier DE, Hosoda K, Richardson JA, et al. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- 102.Taniguchi T, Muramatsu I. Pharmacological knockout of endothelin ET(A) receptors. Life Sci. 2003;74:405–409. doi: 10.1016/j.lfs.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 103.Mann JFE, Green D, Jamerson K, et al. Avosentan for Overt Diabetic Nephropathy. J Am Soc Nephrol. 2010;21:527–535. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kattah AG, Asad R, Scantlebury DC, et al. Hypertension in Pregnancy Is a Risk Factor for Microalbuminuria Later in Life. J Clin Hypertens (Greenwich) 2013;15:617–623. doi: 10.1111/jch.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang IK, Muo CH, Chang YC, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ. 2013;185:207–213. doi: 10.1503/cmaj.120230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vikse BE, Irgens LM, Karumanchi SA, et al. Familial factors in the association between preeclampsia and later ESRD. Clin J Am Soc Nephrol. 2012;7:1819–1826. doi: 10.2215/CJN.01820212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McDonald SD, Han Z, Walsh MW, et al. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis. 2010;55:1026–1039. doi: 10.1053/j.ajkd.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 108.Vikse BE, Irgens LM, Leivestad T, et al. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 109.Wharram BL, Goyal M, Wiggins JE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 110.Hara M, Yanagihara T, Kihara I. Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron. 2001;89:342–347. doi: 10.1159/000046097. [DOI] [PubMed] [Google Scholar]
- 111.Heaton JM, Turner DR. Persistent renal damage following pre-eclampsia: a renal biopsy study of 13 patients. J Pathol. 1985;147:121–126. doi: 10.1002/path.1711470207. [DOI] [PubMed] [Google Scholar]
- 112.Plaks V, Rinkenberger J, Dai J, et al. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci U S A. 2013;110:11109–11114. doi: 10.1073/pnas.1309561110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kalkunte S, Boij R, Norris W, et al. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am J Pathol. 2010;177:2387–2398. doi: 10.2353/ajpath.2010.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]