Abstract
The effect of normothermic machine perfusion (NMP) on post-reperfusion hemodynamics and extrahepatic biliary duct histology of donors after cardiac death (DCD) livers after transplantation has not been addressed thoroughly and represented the object of this study. Ten livers (n=5/group) with 60’ of warm ischemia were preserved by cold storage (CS) or sanguineous NMP for 10 hours, and then reperfused for 24 hours with whole blood in an isolated perfusion system to simulate transplantation. In our experiment, arterial and portal venous flows were stable in NMP group during the entire simulated reperfusion, while decreased dramatically in CS group after 16 hours post-reperfusion (P<.05), findings consistent with severe parenchymal injury. Similarly, significant differences existed between CS and NMP group on hepatocellular enzyme release, bile volume produced, and enzyme released into bile (P<.05). On histology CS livers presented with diffuse hepatocyte congestion, necrosis, intraparenchymal hemorrhage, denudated biliary epithelium and submucosal bile duct necrosis, while NMP liver showed very mild injury in liver parenchyma and biliary architecture. Most importantly, Ki67 staining in extrahepatic bile duct showed biliary epithelial regeneration. Our findings advance the knowledge of post-reperfusion events that characterize DCD livers and propose NMP as a beneficial preservation modality able to improve biliary regeneration after a major ischemic event, which may prevent in clinical transplantation the development of ischemic cholangiopathy.
Keywords: Liver transplantation, Warm ischemia, Preservation, Ischemia-reperfusion injury, Ischemic cholangiopathy
INTRODUCTION
Over 16,000 candidates are currently waiting to receive a liver transplant in the United States, with waiting list mortality rates approaching 20% in certain regions of the country [1]. Increasing the donor pool by utilizing expanded criteria grafts may be an effective way to decrease the discrepancy between organ supply and demand [2]. Livers procured from Donors after Cardiac Death (DCDs) represent the most underutilized potential resource of the donor pool [3;4]. However, national data indicates an increasing reluctance of transplant programs to accept these grafts [5]. One of the primary obstacles inhibiting the widespread utilization of DCD livers is the high rate of post-transplant ischemic cholangiopathy (IC) [3;4]. IC is a devastating complication characterized by diffuse and severe intrahepatic and extrahepatic biliary strictures which often results in graft loss, significant morbidity, and higher health care costs [6].
Several factors are involved in the injury of cholangiocytes [6]. The warm ischemia (WI) that characterizes the process of DCD donation causes a dramatic drop of intracellular adenosine triphosphate (ATP) long before organ flushing and cooling. The graft thereafter enters the cold ischemia (CI) phase with a profound energy debt during cold storage (CS). Due to the lack of oxygen during storage, intracellular energy stores are further depleted. The lack of ATP, along with the direct inhibition caused by cold temperature, impairs the function of the Na/K pump, a key enzyme involved in the prevention of cell swelling and death. Cell death is further amplified after reperfusion. [7]. Severe injury of biliary epithelial cell followed by insufficient regeneration of biliary epithelial cells has been proposed as a potential factor in the development of biliary strictures after clinical liver transplantation [8].
Normothermic machine perfusion (NMP), a promising preservation method may be a potential solution to this problem. NMP functions to keep the liver graft at physiological temperature and provides adequate oxygen supply to maintain aerobic metabolism during preservation [3]. Several animal studies have shown its superiority over CS in the preservation of DCD livers [9-16]. The benefit of NMP preservation to decrease biliary injury could work through maintaining sufficient cholangiocytes regeneration after reperfusion. However this mechanism has yet to be studied and represented the object of our study.
The aim of our study was to evaluate the impact of sanguineous NMP on anatomical and physiological outcomes post-reperfusion in a transplant simulation model on DCD porcine livers. We hypothesized that DCD porcine livers undergoing NMP will have better hemodynamics, less injury and increased regeneration of the biliary epithelium compared to those preserved by CS.
MATERIALS AND METHODS
Subjects
Twenty female Yorkshire pigs were used as blood donors (70-80 kg; n=10) or liver donors (31-38 kg; n=10). The study was approved by the Cleveland Clinic's Institutional Animal Care and Use Committee and all procedures performed in accordance with its guidelines.
Blood collection
After induction of general endotracheal anesthesia and exposure of the right carotid artery, blood donor animals were euthanized by exanguination. Blood was collected sterilly in citrated blood bags, stored at 4°C, and used within 12 hours.
Liver procurement
Liver donor animals underwent a midline laparotomy under anesthesia. A cholecystectomy was then performed and hilar structures isolated. The bile duct was transected and bile allowed to drain freely. Heparin (20,000 U) was administered and cardiac arrest induced with 40 meq of potassium chloride intravenously. Livers were exposed to 1 hour of in-situ “no-touch” WI before being removed and flushed ex-situ [17] through the hepatic artery and the portal vein with 2 liters of histidine tryptophan ketoglutarate solution (HTK) (Essential Pharmaceuticals LLC, Newtown, PA) at 4°C. The bile duct in both groups was not flushed or manipulated. After procurement, livers were randomly assigned to either the Study Group (NMP, n=5) or Control Group (CS, n=5) for a preservation time of 10 hours based on a clinically applicable preservation time [2].
Preservation phase
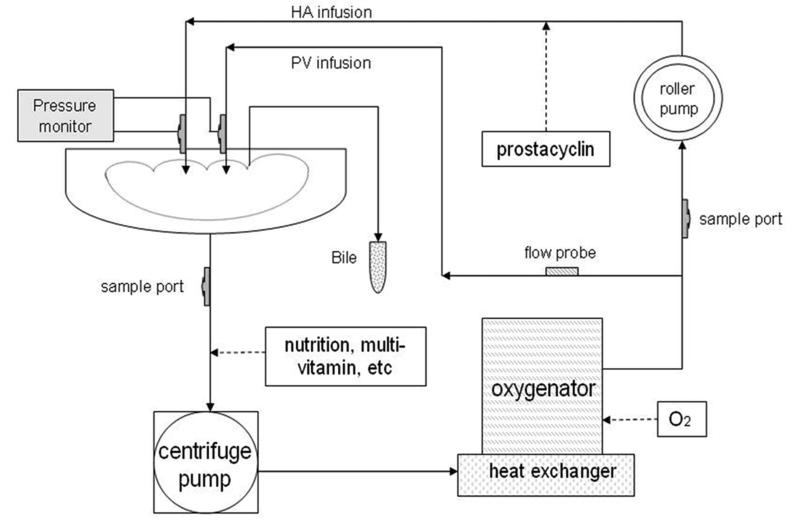
HTK solution was used for CS [18;19]. NMP preservation was performed on a perfusion system consisting of an organ chamber, a centrifugal pump (550 Bio-Console; Medtronic, Minneapolis, MN), a roller pump (3M Sarns; 3M Health Care, Ann Arbor, MI) and an oxygenator/heat exchanger (Affinity; Medtronic, Minneapolis, MN) connected by tubing (Figure 1). Fresh porcine blood (2.5 L) supplemented with heparin, calcium gluconate, sodium bicarbonate, cefotaxime, vancomycin and methylprednisolone was used to prime the system and to perfuse the organ at normothermic temperature (38°C). Nutrients insulin, heparin, multi-vitamins, and trace elements were infused as described by Butler et al [20].
Figure 1. Diagram of the liver perfusion system.
Our normothermic machine perfusion (NMP) system consisted of an organ chamber, a centrifugal pump, a roller pump, and an oxygenator/heat exchanger to maintain physiological temperature and oxygen content in perfusate to perfuse liver via portal vein (PV) and hepatic artery (HA). Nutrition was added into perfusate. Prostacyclin was supplied in HA infusion. Bile duct was drained externally.
Organ perfusion was regulated by an algorithm that primarily took into consideration of pressures. NMP livers were connected to the manually operated machine immediately after organ procurement and without flushing HTK out [21]. The organs were perfused at physiologic pressures (HA mean: 70-105 mmHg; PV: 3-5 mmHg). Within these pressure ranges flows were maintained as closely as possible to physiologic values of 0.25 ml/min/gr tissue for the hepatic artery (HA) and 0.75 ml/min/gr tissue for the portal vein (PV) [22]. Such algorithm (see supplemental material) was used to avoid from one hand hypoperfusion and from the other the shear stress-induced endothelial cell injury caused by hyperperfusion [23]. Prostacyclin (Flolan; GlaxoSmithKline LLC, Philadelphia, PA) was infused as a vasodilator (9-30μg/hr) through the HA to decrease vascular resistance and modulate flows (within the target pressure range). The outflow was drained via infra-and supra-hepatic vena cava freely in the organ receptacle and recirculated. Oxygen (100%) ran through the oxygenator to maintain the arterial partial oxygen pressure above 350 mmHg.
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) were measured in the perfusate. Mean HA and PV pressures and flows were recorded at hours 1, 4, 8, and 10 (Figure 2).
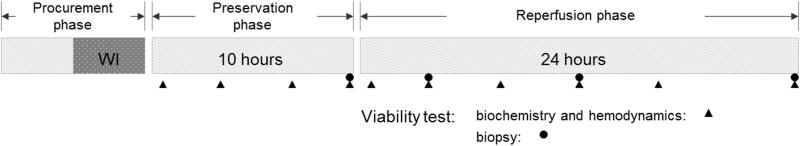
Figure 2. Diagram of the experiment.
All livers underwent 60 mins warm ischemia (WI) before cold flush and procurement. After 10 hours of preservation phase using NMP or cold storage (CS), all livers were reperfused for 24 hours with whole blood at physiologic temperature and pressure. Samples of perfusate and liver tissue, and hemodynamic outcomes, were measured at different time points.
Simulated Reperfusion phase
After CS and NMP preservation, all livers were flushed with 1 liters of normal saline solution at 21°C and connected to the perfusion system for a reperfusion phase of 24 hours to simulate transplantation [24]. The apparatus was rinsed with 3 liters of saline solution and primed with fresh whole blood as perfusate to replicate as closely as possible the events occurring in clinical transplantation. Additives were added and hemodynamic settings were similar to those used in the NMP preservation phase except for the following: 1) PV pressures between 5-15 mmHg were accepted to replicate the higher PV pressure encountered in cirrhotic patients; 2) prostacyclin infusion was maintained at a constant rate of 9μg/hr.
Outcome measures
Sampling was performed at time zero before connecting the liver to the system and thereafter at hours 1 and 4 then every four hours after starting reperfusion to assess organ metabolisms and injury markers (Figure 2).
Primary Outcomes
Hemodynamics
Mean HA and PV pressures and flows were recorded continuously. Flows were indexed to the weight of the graft (ml/min per 100 gr of liver). Arterial and venous resistances were calculated by dividing pressure by flow per 100 g liver tissue.
Biliary injury and epithelial regeneration
LDH, gamma glutamyl transpeptidase (GGT) and bicarbonate at post-reperfusion hour 6 and 18 were measured in the bile as markers of biliary epithelium injury and function respectively [25]. A biopsy was taken of the bile duct at the bifurcation to the left and right hepatic lobe at 24 hours post-reperfusion and stained with hemotoxylin and eosin (H&E). The injury was evaluated in light microscopy by a pathologist (A.B.) who was blinded for the group assignment using a semi-quantitative scoring system (Table 1) reported by Hansen et al [26] and supplemented with analysis on peribiliary glands (PBGs) integrity (superficial and deep) by Op den Dries et al [27]. Bilary epithelium regeneration in the bile duct as well as in the PBGs was assessed using immunohistochemical staining (rabbit antibody: Novocastra, Richmond, VA) on Ki67 protein, a cellular marker strictly associated with cell proliferation [28]. Von Willebrand factor (vWF) staining (rabbit anti-vWF: Dako, Carpenteria, CA) was used to assess platelet activation [29] at the level of the peribiliary vascular plexus.
Table 1.
Histological scoring system on bile duct
| 0 | 1 | 2 | 3 | 4 | Control group | Study group | p- value | |
|---|---|---|---|---|---|---|---|---|
| Mucosal loss | Absent | ≤50% | >50% | 2 (2-2) | 1 (0-2) | .053 | ||
| Bleeding | Absent | ≤50% | >50% | 2 (1-2) | 1 (0.5-1.5) | .166 | ||
| Thrombi | Absent | Present | 1 (0.5-1) | 1 (0.5-1) | 1 | |||
| Vascular lesion | Absent | ≤50% | >50% | 2 (1-2) | 1 (0.5-1) | .074 | ||
| Arteriol- onecrosis | Absent | ≤50% | >50% | 2 (1.5-2) | 1 (0.5-1) | .015 | ||
| Superficial duct necrosis | Absent | ≤25% | 25-50% | 50-75% | >75% | 4 (4-4) | 1 (0.5-3) | .005 |
| Trans-mural duct necrosis | Absent | ≤25% | 25-50% | 50-75% | >75% | 4 (1.5-4) | 0 (0-0.5) | .032 |
| Inflammation -leukocytes per high power field 0.159mm2 | Absent | At least 10 | At least 50 | 0 (0-0.5) | 0 (0-0.5) | 1 | ||
| Inflammation -lymphocytes per high power field 0.159mm2 | Absent | At least 10 | At least 50 | 1 (0.5-1.5) | 1 (0-1.5) | .650 | ||
| Loss of periluminal peribiliary glands (PBGs) | Absent | ≤50% | >50% | 1 (1-2) | 0 (0-1) | 0 (0-1) | ||
| Loss of deep PBGs | Absent | ≤50% | >50% | 1.5 (0.25-2) | 0 (0-0) | .046 | ||
| Total | 19 (14.5-22) | 6 (3-13) | 0.028 |
Secondary Outcomes
Metabolic biomarkers
Oxygen consumption was calculated using partial oxygen pressure, hemoglobin saturation, and blood flow following the formula of Tolboom et al [15]. The pH, glucose and lactate were measured in the effluent. Total glucose consumption was calculated based on the decreased level and infused amount. The ability of the liver to metabolize lidocaine to its metabolite (monoethylglycinexylidide, MEGX test) was conducted after 7 hours post-reperfusion by injecting 4 ml of 1% lidocaine solution into the perfusate and taking samples after 15 minutes. The test was performed by mass spectrometer after separation by high performance liquid chromatography to assess hepatic function [30].
Hepatocellular injury and bile production
AST, ALT, and LDH were measured in the perfusate. Bile production was collected from a tube inserted in the common bile duct and recorded hourly.
Liver Histology
Samples of liver parenchyma were taken before reperfusion, at 4, 12, and 24 hours reperfusion and stained with H&E to assess architecture. The injury on liver parenchyma was evaluated in light microscopy using a semi-quantitative scoring system (Table 2) as previously reported [12].
Table 2.
Histological scoring system on liver parenchyma
| 0 | 1 | 2 | 3 | |
|---|---|---|---|---|
| Portal hemorrhage | Absent | Focal | Diffuse | |
| Lobular hemorrhage | Absent | Mild (pericentral) | Moderate | Severe (pan lobular) |
| Necrosis | Absent | Mild (pericentral) | Moderate | Severe (pan lobular) |
| Sinusoidal dilatation | Absent | Mild | Moderate | Severe |
| Sinusoidal congestion | Absent | Present | ||
| Cholestasis | Absent | Present |
Liver Weight
Livers were weighted after procurement and at the end of the experiment. The percentage of weight change to the initial weight was compared between the two groups.
Statistical analysis
Data were presented as mean ± standard deviation on the parameters analyzed by parametric statistics. Repeated measures were used on the data with more than two time points. The data were analyzed by linear mixed models with log-transformation when normality and homogeneous variance assumptions were not met. Data were presented as median and interquantile range on parameters analyzed by Wilcoxon's rank sum test when data had only one or two time points. All analyses were done using R software (version 3.0.1, R Core Team, Vienna, Austria). A p value <0.05 was considered significant.
RESULTS
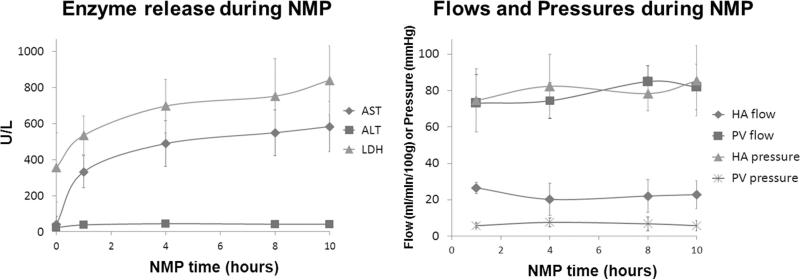
The enzyme levels in the perfusate during NMP increased mainly in the first hour and then gradually reached plateau. At the end of NMP, AST was 583±138, ALT was 41±7, and LDH was 840±190 U/L. The mean HA and PV pressures and flows were stable during entire NMP [Figure 3].
Figure 3. Enzyme release and hemodynamics during NMP.
The enzymes in the perfusate increased mainly in the early phase of NMP and then gradually reached plateau. The mean HA and PV pressures and flows were stable during entire NMP phase.
Primary Outcomes
Hemodynamics
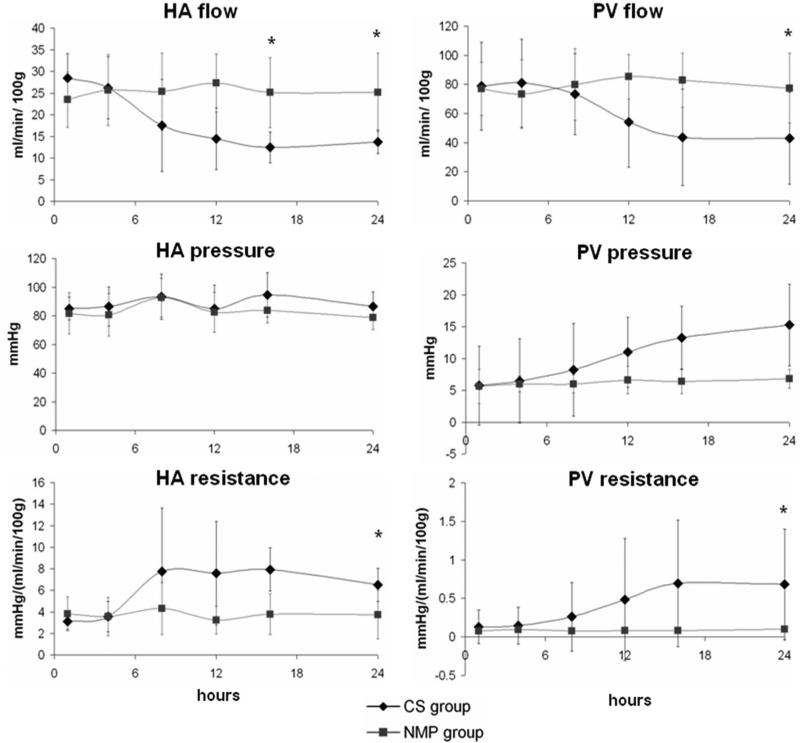
HA flow was preserved and comparable in both groups for the first 6 hours after the simulated reperfusion. The flow gradually decreased in the control group with the final flow being almost half compared to the final arterial flow in the study group (13±3 vs. 23±7 ml/min/100gr liver; P=.04). A similar trend was observed in the PV flow of the control and study groups (39±29 vs. 72±21 ml/min/gr liver at 24hr reperfusion; P=.04). The control group had significantly increased PV pressure (P=.015). The difference between groups on both arterial and venous resistances was also statistically significant during the simulated reperfusion (P=.048 and .008 respectively) (Figure 4).
Figure 4. Hemodynamic outcomes during reperfusion.
HA flow indexed by liver weight (per 100 gr liver tissue) was comparable between groups in the early stage of reperfusion, started to decrease in the CS group after 8 hours, and differed significantly (* P<.05) between groups after 16 hours; PV flow had a similar HA flow pattern. HA pressure of the two groups was consistent; PV pressure increased in the CS group after 8 hours reperfusion. CS group had higher HA and PV resistance compared to NMP group after the early stage of reperfusion.
Biliary injury and epithelial regeneration
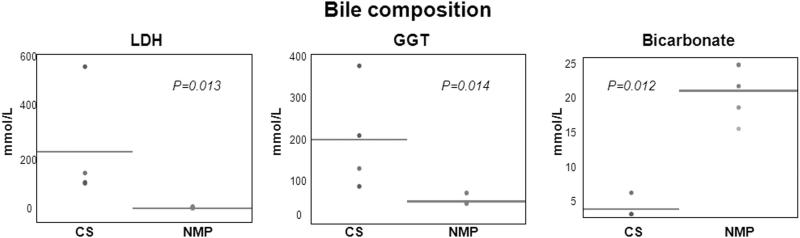
The LDH and GGT in bile of the control group were significantly higher than those in the study group (P=.01 and .01 respectively) at post-reperfusion hour 6, and was not compared in the late phase due to absent bile production in the control group. Similarly the bile content of bicarbonate was significantly lower in the control compared to the study group (P=.01) (Figure 5).
Figure 5. Primary outcomes on biliary injury.
CS group had statistically significant higher levels of lactate dehydrogenase (LDH), gamma glytamyl transpeptidase (GGT), and bicarbonate in bile than NMP group.
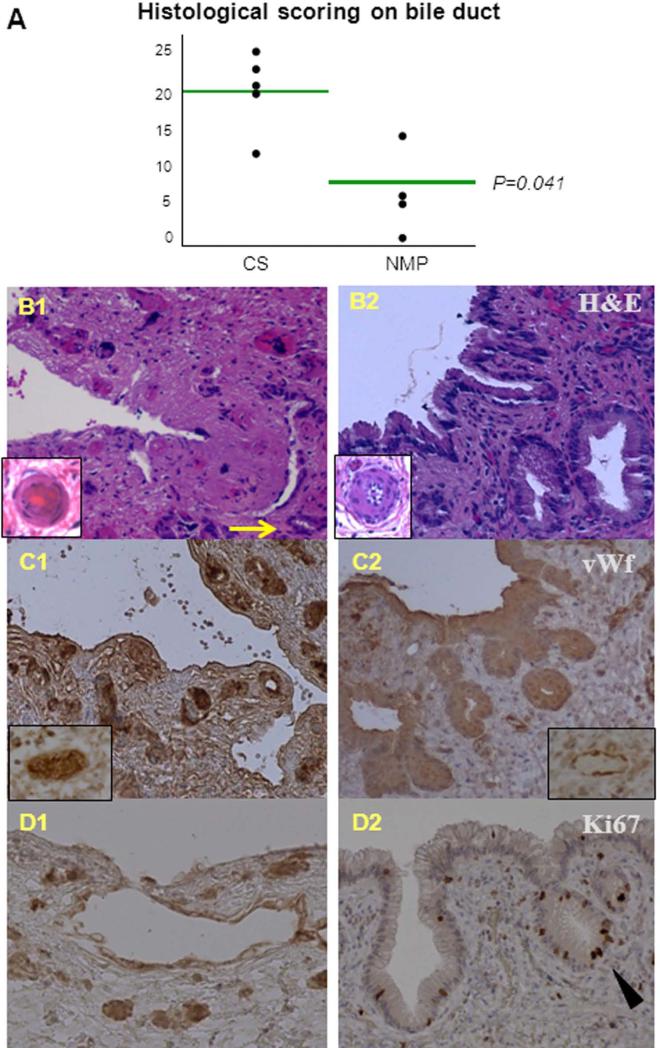
The semi-quantitative scores on biliary injury showed a statistically significant difference between the control and study groups (P=.04) (Figure 6). Remarkable difference between the two groups was present in the degree of ductal necrosis, PBGs injury and arteriolonecrosis (Table 1). The control group presented denudated biliary epithelium, extensive transmural biliary necrosis, diffuse PBGs necrosis, and fibrin deposition in the peribiliary capillary plexus. The biliary epithelium, architecture and the peribiliary plexus of the study group appeared mild injury. Diffuse Ki67 staining was present all throughout the biliary epithelium and PBGs of NMP preserved livers, indicating recent cell proliferation. Conversely, Ki67 staining was virtually absent in the bile ducts and PBGs of the control group. Immunohistological stains for vWF demonstrated increased platelet aggregates in the peribiliary capillary plexus of CS group compared to the NMP group (Figure 6).
Figure 6. Histology of bile duct after 24 hours reperfusion.
(A): The injury on bile duct was significantly different (P=.041) between CS and NMP groups in the histological scoring;
(B): in hemotoxylin and eosin (H&E) staining (20× magnification), the CS-preserved livers (B1) presented biliary epithelial denudation, transmucosal necrosis, peribiliary gland (PBG) necrosis (arrowed), and fibrin deposition in peribiliary capillary plexus, whilst the NMP-preserved livers (B2) had very mild injury on epithelium, architecture, and plexus; the inserts represented the peribiliary arterioles of respective groups in higher magnification (60×) (B1: arteriolonecrotic; B2: normal);
(C): in von Willebrand factor (vWF) staining (20× magnification), the CS-preserved livers (C1) had more platelet aggregation in peribiliary capillary plexus compared to the NMP-preserved livers (C2); the inserts represented peribiliary venules in higher magnification (60×) (C1: platelet vWF stained and aggregated in vessels; C2: platelet not aggregated and vWF staining mostly on endothelium);
(D): diffuse Ki67 staining (20× magnification) was present in the biliary epithelium and PBGs (arrow head) of the NMP-preserved livers, indicating cell proliferation, but was absent in the CS-preserved livers.
Secondary Outcomes
Metabolic biomarkers
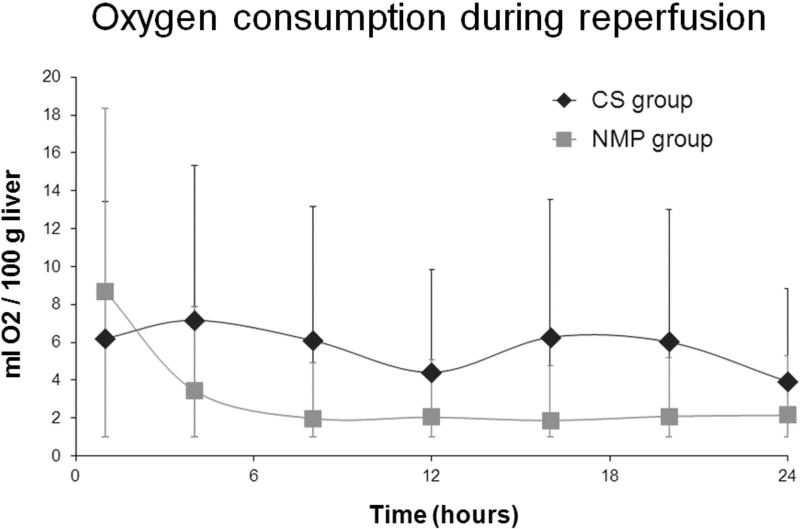
The pH, oxygen consumption, total glucose consumption, and lactate were comparable between the two groups during reperfusion (P>.05) (Figure 7; Table 3). The control group had significantly lower MEGX concentration at 1.7 (1.2-2.8) ng/ml than the study group at 56.0 (36.8-60.0) ng/ml at 15 min after lidocaine injection (P=.008).
Figure 7. Oxygen consumption during reperfusion.
All livers had comparable oxygen consumption during reperfusion. The difference was insignificant.
Table 3.
Total glucose consumption and lactate levels in perfusate during reperfusion.
| Reperfusion time | Control group | Study group | P-value |
|---|---|---|---|
| Total glucose consumption (g) | 23.8 (14.5-25.7) | 12.5 (11.4-17.0) | .07 |
| Lactate at 12hr (mmol/L) | 2 (1.6-17.9) | 0.3 (0.2-2.3) | .11 |
| Lactate at 24hr (mmol/L) | 3.1 (1.4-21.3) | 0.7 (0.3-3.4) | .11 |
Hepatocellular injury and bile production
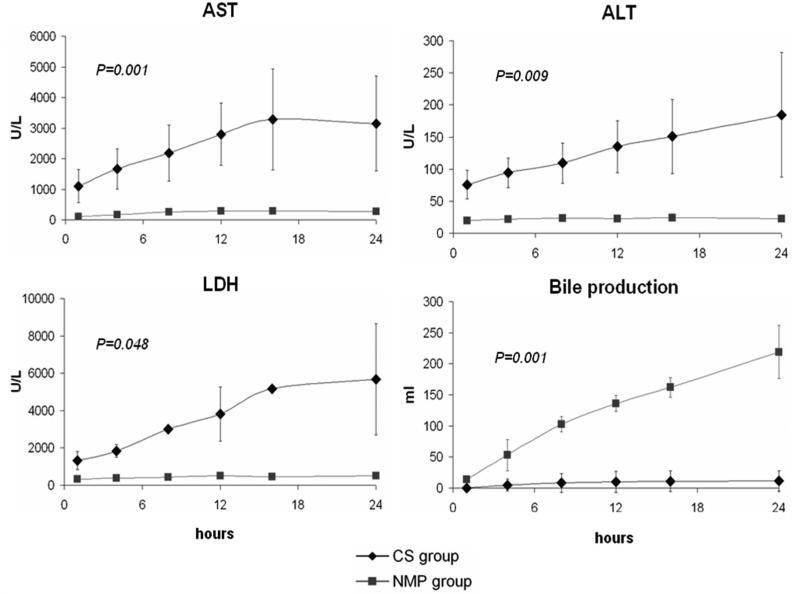
The control group had statistically significant higher AST, ALT, and LDH release compared to the study group at all-time points (P=.001, .009, and .048 respectively) (Figure 8). Bile was steadily produced by the study group during the entire reperfusion phase at a consistent rate of 9.1±1.7 ml/hr (R2>0.93) and reached a total bile production of 219.2±42.5 ml at the end of reperfusion. Most bile production by the control group occurred in the first 12 hours (total production 11.6±16.3 ml). The overall rate of bile production was significantly different between groups (P=.001). Moreover, the total bilirubin in perfusate was also significantly higher in the control group compared to the study group (P=.001).
Figure 8. Outcomes on hepatocellular injury and bile production during reperfusion.
The CS-preserved livers had significantly higher aspartate transaminase (AST), alanine transaminase (ALT), and LDH (P<.05) in the perfusate, and much less bile production, compared to the NMP-preserved livers after reperfusion.
Liver Histology
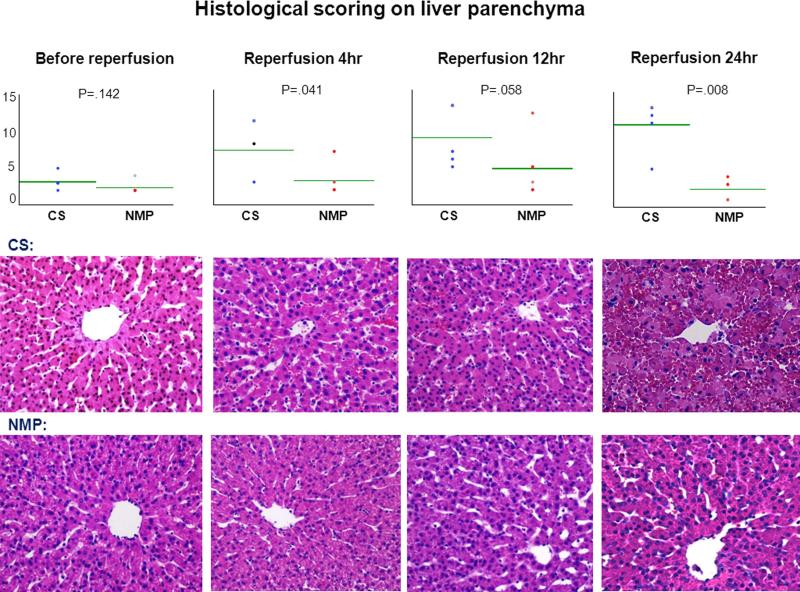
The livers of the two groups looked comparable after preservation phase. The livers in the control group gradually exhibited widespread sinusoidal congestion, patchy necrosis and extensive erythrocyte extravasation after starting reperfusion whereas NMP livers were architecturally intact. The semi-quantitative scores between groups were statistically different only after reperfusion (P=.041, .058, .008 at 4, 12, and 24 hours respectively) (Figure 9).
Figure 9. Histology of liver parenchyma before and during reperfusion.
CS and NMP groups had insignificant difference in the histological scoring on the injury of liver parenchyma before reperfusion (P=.142), but had significant difference after starting reperfusion (P=.041, .058, .008 at 4, 12, and 24 hours respectively); the CS-preserved livers gradually presented sinusoidal congestion, profuse hemorrhage and necrosis during reperfusion; the NMP-preserved livers looked quite intact consistently. (20× magnification; H&E staining)
Liver Weight
Liver weight of the control group increased 15 (9-52) % from procurement to the end of reperfusion consistent with severe edema. On the contrary, the liver weight of the study group decreased 13 (9-19) %, which was statistically significantly different (P=.01).
DISCUSSION
Our study describes, in a large animal liver DCD model, the impact of sanguineous NMP on post-reperfusion hemodynamics and its effect on the extrahepatic bile duct epithelium. The results of our study demonstrate that NMP, as compared to CS, is associated with significantly better HA and PV hemodynamics and biliary epithelium preservation in the 24 hours following simulated graft reperfusion. Most importantly we found that NMP has the ability to promote extrahepatic biliary epithelium and PBGs regeneration.
NMP is an emerging technology with a growing body of literature that describes its potential in liver preservation [31]. Several studies have investigated post-reperfusion hemodynamics in a transplant simulation model after NMP [12;24;32]. However none of these studies assessed the impact of NMP on the extrahepatic bile duct epithelium. While the highly problematic non-anastomotic biliary strictures may occur in both the intra-hepatic and the extrahepatic bile duct after DCD transplantation, they are usually more pronounced in extrahepatic bile ducts [33]. This has been attributed to the fact that unlike intrahepatic bile duct, the extrahepatic bile duct depends entirely on arterial blood supply for oxygenation. Therefore, the extrahepatic bile duct becomes a site extremely susceptible to any hemodynamic change in the post-reperfusion stage.
In our study, the control group presented with relatively desired flows in the early post-reperfusion period. After the sixth hour, however, the HA and the PV flows decreased dramatically to values of less than half compared to the study group. Inversely the HA and PV resistance exhibited a progressive increase starting at 6 hours post-reperfusion. This may be explained by the fact that ischemia-reperfusion injury (IRI) is a complex phenomenon that involves early and late changes [34]. It is likely that during the early stage of IRI, normal hemodynamics may be maintained by the release of potent vasodilators such as nitric oxide [35;36]. These mediators are released in large quantity following a major IRI insult and have the function to facilitate perfusion. In our study, after 24 hours of simulated reperfusion, CS-preserved livers presented with complete disruption of the sinusoidal architecture, intraparenchymal hemorrhage and significant necrosis. It is possible that as IRI progressed, the vasodilatation was replaced by microcirculatory failure characterized by complete disruption of the sinusoids resulting in increased intrahepatic vascular resistance.
Our study build upon a recent paper by Boehnert et al. on NMP in DCD pig livers preserved with Steen solution and reperfused in a transplant simulation model. The authors observed bile duct necrosis and a 50% reduction in HA flow in livers preserved with CS compared to NMP [16]. Their hemodynamic data did not include inquiry into PV flow and vascular resistance, and they used a 12 hours period of post-reperfusion observation, at which time points our two groups did not show statistically significant hemodynamic differences. By extending the simulated reperfusion period to 24 hours our study indicates that virtually all CS preserved livers start to fail from a hemodynamic stand point at 16 hours. Furthermore our hemodynamic findings were correlated semi-quantitatively to extrahepatic bile duct viability.
We did not find any difference in the perfusate in terms of pH, oxygen consumption, glucose consumption and lactate level. The pH finding was not surprising since in both groups perfusate pH was maintained as closely as possible to physiologic values by infusing bicarbonate and adjusting O2 flow in the oxygenator. Interestingly, O2 consumption was comparable in both groups. This is consistent with findings from another study, in which severely injured livers presented with a high O2 consumption rate [12]. Glucose consumption was also comparable between groups and could be the consequence of different metabolic reactions; CS livers enter the transplant simulation phase with a huge energy debt while NMP livers need O2 to maintain physiologic metabolism. Lactate level was also comparable in both groups. It is difficult to explain such finding as previous ex-vivo studies did not present this data [12;16;24]. It may be that in the setting of ex-vivo perfusion the amount of lactic acid produced by the liver is not significant and tissues such as the muscles, which rely on anaerobic metabolism during ischemia, are lacking.
As well, outcomes of NMP of 6 discarded human livers using blood banked packed red blood cells and fresh frozen plasma has been reported after a period of CS [21]. The authors described hemodynamics data and extrahepatic bile duct histology after a preservation time of 6 hours, concluding that NMP is technically feasible and allows viability assessment before transplantation. In our model, by reperfusing livers with whole blood during the transplant simulation phase we were able to more closely mimic the events occurring during clinical transplantation and to analyze the bile duct histology after a longer period of simulated reperfusion.
In the usual course of a liver transplantation, the PV is reperfused first followed by a period of 45-60 minutes before arterial reperfusion. During this interval period the biliary epithelial cells experience a second warm ischemic stress defined as the “secondary warm ischemia time (SWIT)”. An important study by, Zhu and colleagues [37] investigated the impact of different SWITs on the bile duct and peribiliary plexus in a rat autologous liver transplantation model. The authors described a time-dependent relationship between SWIT and pathological injury, with the group exposed to the longest SWIT presenting reduced cholangiocyte proliferation markers, increased apoptosis and thrombosis of the peribiliary plexus. This study suggests that optimal arterial perfusion is crucial for the survival of the biliary epithelial cells in the delicate phase following graft reperfusion.
Because WI time in the procurement of DCD livers is inevitable, many investigators have shifted their focus on finding ways to ameliorate, and potentially even abrogate the SWIT [38]. Our reperfusion model employed simultaneous PV and HA reperfusion, and was therefore different from the classic sequence of revascularization occurring in clinical transplantation. We believe this may have in fact blunted the injury in both experimental groups.
Based on the hemodynamic findings of our study it can be hypothesized that during transplantation of severely injured DCD grafts (long WI, prolonged hypoperfusion during the agonal phase etc.), the liver undergoes not only the well known hypoxic injury intrinsic to the donation process, but also an additional ischemic insult in the first 24 hours post reperfusion. By reestablishing tissue perfusion immediately after organ procurement and by providing better preservation, NMP would therefore transform the injury sequence (WI → CI → SWIT → WI post-reperfusion hypoperfusion) into ischemic preconditioning (WI → oxygenated NMP → SWIT → graft reperfusion) and eliminate two of the three ischemic biliary hits.
The second and perhaps most interesting finding of our study relates to the effect of NMP on biliary epithelium integrity and regeneration. Twenty four hours after simulated reperfusion, CS-preserved livers presented with diffuse epithelial injury involving both the extrahepatic duct and PBGs. This was associated with transmural bile duct necrosis and microcirculatory compromise as demonstrated by peri-biliary capillary plexus arteriolonecrosis, endothelial disruption, fibrin deposition and platelet aggregates. In contrast, NMP-preserved livers presented with mild epithelial injury. More importantly the Ki-67 staining showed active cholangiocytes regeneration in the bile duct lumen and in both superficial and deep PBGs. Ki67 staining was virtually absent in CS bile ducts.
PBGs are tubulo-alveolar glands found within the duct walls [39]. The glands communicate with the bile duct lumens through channels opening into diverticula that occur with regularity around the mucosal surface. Other than mucus production, the role of PBGs in regeneration has only recently been defined [40]. Compelling evidence [41;42] suggests that PBGs host multipotent stem cells able to differentiate into cholangiocytes, contributing to the renewal/repair of biliary epithelium in both physiologic and pathologic conditions. Interestingly, quantitative assessments of the numbers of peribiliary glands and their sizes indicate that the highest numbers are in branching points in the biliary tree such as the cystic duct and common hepatic duct junction or the bifurcation of the left and right hepatic duct [40], a finding consistent with the anatomical location of non-anastomotic biliary strictures observed in DCD liver transplantation.
Op den Dries first postulated that biliary epithelium regeneration following a major ischemic event (such as the one observed during DCD donation) can only be possible if integrity of the microvascular plexus serving the bile duct and more importantly the PBGs is maintained [27]. Our bench data support this hypothesis and are in accordance with the findings of a recent clinical study by Hansen and colleagues. The authors analyzed 93 extrahepatic bile ducts obtained immediately after arterial revascularization of livers from donation after brain death. They assessed morphological injury that could be associated with ischemic-type biliary strictures (observed in 19.4% of their patients), and determined that arteriolonecrosis was the single most important factor involved in the development of ischemic-type biliary strictures. Such injury was almost always associated with bile duct wall necrosis and other signs of microangiopathy (hemorrhage, vascular endothelium edema) [26].
In our study, we observed the presence of platelet aggregates in the peribiliary blood vessels of the control group as demonstrated by vWF immunohistochemistry staining. This is also a novel and important finding. Recently activated platelets [43] have been found to play a crucial role in sinusoidal endothelium activation and leukocytes recruitment, suggesting that inhibition of platelet activation may represent a target of future research in machine perfusion. To this point one could argue that using whole blood as perfusate during the preservation phase of NMP may have not represented the best choice since leukocytes and platelets are considered key players in ischemia-reperfusion injury [34]. In our model the decision to use whole blood was based on several considerations; First most of the experience in ex-vivo pig NMP is with whole blood [12;13;20;24;44]. Friends and colleagues were able to perfuse pig livers for a period of 72 hrs, [20], demonstrating the whole blood perfusion is able to sustain organ function for a prolonged period of time in spite of the presence of leukocytes and platelets. Secondly our experiment primary goal was not to assess the injury produced by leukocytes and platelets, even though this represents an extremely important question that future studies should address.
The main limitation of our study is the lack of biopsies of the extrahepatic bile duct at different time points. Multiple samples of the extra-hepatic bile duct would have allowed us to observe injury progression in CS livers and to assess cholangiocyte proliferation before, during and after NMP preservation. Future studies should incorporate such analysis as it would contribute to understand the mechanism responsible for the superior bile duct preservation during NMP (i.e. prevention of CS injury vs stimulation of repair/regeneration vs. both).
One potential study limitation is the fact that the study group received an additional dose of steroids during the preservation phase whereas the control group received steroids only after reperfusion. The study was design as such in effort to simulate the method of organ perfusion as it occurs in the clinical setting where steroids are not added inside the HTK solution) [45]. Although it is possible that this extra steroid dose may have further decreased the inflammation observed in the study group, it is also likely that the effect of steroids in a cold metabolically suppressed organ would be minimal, highlighting the theoretical advantages of a preservation modality (NMP) that allows pharmacological intervention during preservation.
The generalizability of our simulated animal model to human organ transplantation that occurs in the clinical setting is another recognized limitation. Elements of the innate and adaptive immune system are major effectors of reperfusion injury with cells mobilized from all compartments such as the spleen, lymph nodes and bone marrow. Yet in our simulated model the only immune cells present were passenger leukocytes from the graft and the leukocytes present in the donor blood. We may have therefore observed a blunted reperfusion injury compared to what would be observed in the human transplant settings. Although our bench model offers the advantage of a highly controlled and reproducible, experimental environment and provides mechanistic insights, further translational research is still needed that can closely simulate the conditions of the clinical setting.
In conclusion our study offers important insights on the benefits of NMP preservation on hemodynamics and biliary regeneration of DCD livers following simulated graft reperfusion and supports the theoretical ability of NMP to prevent ischemic cholangiopathy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the excellent assistance of Biological Research Unit, Lerner Research Institute, Cleveland Clinic Foundation, and the histotechnical expertise of Nina Dvorina. William Baldwin is supported by NIH grant P01AI087586. Histidinetryptophan-Ketoglutarate (HTK) solution used for all cases was provided at no charge by Essential Pharmaceuticals LLC, Newtown, PA. This Project was partially funded by the Ohio Solid Organ Transplantation Consortium.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ATP
Adenosine triphosphate
- CI
Cold ischemia
- CS
Cold storage
- DCD
Donors after cardiac death
- GGT
Gamma glutamyl transpeptidase
- HA
Hepatic artery
- H&E
Hemotoxylin and eosin
- HTK
histidine tryptophan ketoglutarate
- IC
Ischemic cholangiopathy
- IRI
Ischemia-reperfusion injury
- LDH
Lactate dehydrogenase
- MEGX
Monoethylglycinexylidide
- NMP
Normothermic machine perfusion
- PV
Portal vein
- PBG
Peribiliary gland
- SWIT
Secondary warm ischemia time
- vWF
von Willebrand factor
- WI
Warm ischemia
Footnotes
DISCLOSURE
The authors of this manuscript have no conflict of interest to disclose.
REFERENCES
- 1.Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4 Pt 2):961–72. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 2.Durand F, Renz JF, Alkofer B, Burra P, Clavien PA, Porte RJ, et al. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl. 2008;14:1694–707. doi: 10.1002/lt.21668. [DOI] [PubMed] [Google Scholar]
- 3.Reddy S, Zilvetti M, Brockmann J, McLaren A, Friend P. Liver transplantation from non-heart-beating donors: current status and future prospects. Liver Transpl. 2004;10:1223–32. doi: 10.1002/lt.20268. [DOI] [PubMed] [Google Scholar]
- 4.Quintini C, Hashimoto K, Uso TD, Miller C. Is there an advantage of living over deceased donation in liver transplantation? Transplant Int. 2013;26(1):11–9. doi: 10.1111/j.1432-2277.2012.01550.x. [DOI] [PubMed] [Google Scholar]
- 5.2011 Annual Data Report, Organ Procurement and Transplantation Network (OPTN) http://srtr.transplant.hrsa.gov/annual_reports/2011/default.aspx.
- 6.Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253(4):817–25. doi: 10.1097/SLA.0b013e3182104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cursio R, Gugenheim J. Ischemia-reperfusion injury and ischemic-type biliary lesion following liver transplantation. J Transplant. 2012;2012:164329. doi: 10.1155/2012/164329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karimian N, Op den Dries S, Porte RJ. The origin of biliary strictures after liver transplantation: is it the amount of epithelial injury or insufficient regeneration that counts? J Hepatol. 2013;58(6):1065–7. doi: 10.1016/j.jhep.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Schon MR, Kollmar O, Wolf S, Schrem H, Matthes M, Akkoc N, et al. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surg. 2001;233:114–23. doi: 10.1097/00000658-200101000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brockmann J, Reddy S, Coussios C, Pigott D, Guirriero D, Hughes D, et al. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250:1–6. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]
- 11.Fondevila C, Hessheimer AJ, Masthuis MH, Munoz J, Taura P, Calatayud D, et al. Superior preservation of DCD livers with continuous normothermic perfusion. Ann Surg. 2011;254:1000–7. doi: 10.1097/SLA.0b013e31822b8b2f. [DOI] [PubMed] [Google Scholar]
- 12.Imber CJ, St Peter SD, Lopez de Cenarruzabeitia I, Pigott D, James T, Taylor R, et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation. 2002;73:701–9. doi: 10.1097/00007890-200203150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Berendsen T, Kim K, Soto-Gutierrez A, Bertheium F, Yarmush ML, et al. Excorporeal normothermic machine perfusion resuscitates pig DCD livers with extended warm ischemia. J Surg Res. 2012;173:e83–8. doi: 10.1016/j.jss.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellomo R, Suzuki S, Marino B, Starkey GK, Chambers B, Fink MA, et al. Normothermic extracorporeal perfusion of isolated porcine liver after warm ischaemia: a preliminary report. Crit Care Resusc. 2012;14(3):173–6. [PubMed] [Google Scholar]
- 15.Tolboom H, Pouw RE, Izamis ML, Milwid JM, Sharma N, Soto-Gutierrez A, et al. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplantation. 2009;87:170–7. doi: 10.1097/TP.0b013e318192df6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boehnert MU, Yeung JC, Bazerbachi F, Knaak JM, Selzner n, McGilvray ID, et al. Normothermic acellular ex vivo liver perfusion reduces liver and bile duct injury of pig livers retrieved after cardiac death. Am J Transplant. 2013;13(6):1441–9. doi: 10.1111/ajt.12224. [DOI] [PubMed] [Google Scholar]
- 17.Moench C, Heimann A, Foltys D, Schneider B, Minouchehr S, Schwandt E, et al. Flow and pressure during liver preservation under ex situ and in situ perfusion with University of Wisconsin solution and Histidine-Tryptophan-Ketoglutarate solution. European Surgical Research. 2007;39:175–181. doi: 10.1159/000100800. [DOI] [PubMed] [Google Scholar]
- 18.Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK, et al. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors. Liver Transpl. 2008;14(3):365–73. doi: 10.1002/lt.21372. [DOI] [PubMed] [Google Scholar]
- 19.Feng L, Zhao N, Yao X, Sun X, Du L, Diao X, et al. Histidine-tryptophanketoglutarate solution vs. University of Wisconsin solution for liver transplantation: a systematic review. Liver Transpl. 2007;13(8):1125–36. doi: 10.1002/lt.21208. [DOI] [PubMed] [Google Scholar]
- 20.Butler AJ, Rees MA, Wight DG, Casey ND, Alexander G, White DJ, et al. Successful extracorporeal porcine liver perfusion for 72 hr. Transplantation. 2002;73(8):1212–8. doi: 10.1097/00007890-200204270-00005. [DOI] [PubMed] [Google Scholar]
- 21.Op den Dries S, Karimian N, Sutton ME, Westerkamp AC, Nijsten MW, Gouw AS, et al. Ex vivo normothermic machine perufsion and viability testing of discarded human donor livers. Am J Transplant. 2013;13(5):1327–35. doi: 10.1111/ajt.12187. [DOI] [PubMed] [Google Scholar]
- 22.Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastro. 2010;16(48):6046–57. doi: 10.3748/wjg.v16.i48.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlegel A, Rougemont Od, Graf R, Clavien PA, Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013;58(2):278–286. doi: 10.1016/j.jhep.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 24.St Peter SD, Imber CJ, Lopez I, Hughes D, Friend PJ. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg. 2002;89(5):606–16. doi: 10.1046/j.1365-2168.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 25.Vajdova K, Smrekova R, Kukan M, Lutterova M, Wsolova L. Bile analysis as a tool for assessing integrity of biliary epithelial cells after cold ischemia-reperfusion of rat livers. Cryobiology. 2000;41(2):145–52. doi: 10.1006/cryo.2000.2276. [DOI] [PubMed] [Google Scholar]
- 26.Hansen T, Hollemann D, Pitton MB, Heise M, Hoppe-Lotichius M, Schuchmann M, et al. Histological examination and evaluation of donor bile ducts received during orthotopic liver transplantation-a morphological clue to ischemic-type biliary lesion? Virchows Arch. 2012;461(1):41–8. doi: 10.1007/s00428-012-1245-8. [DOI] [PubMed] [Google Scholar]
- 27.Op den Dries S. Ph.D. thesis. University of Groningen; http://irs.ub.rug.nl/ppn/369506979. [Google Scholar]
- 28.Sutton ME, op den Dries S, Koster MH, Lisman T, Gouw AS, Porte RJ. Regeneration of human extrahepatic biliary epithelium: the peribiliary glands as progenitor cell compartment. Liver Int. 2012;32(4):554–559. doi: 10.1111/j.1478-3231.2011.02721.x. [DOI] [PubMed] [Google Scholar]
- 29.Szanto T, Joutsi-Korhonen L, Deckmyn H, Lassila R. New Insights into von Willebrand Disease and platelet function. Semin Thromb Hemost. 2012;38(1):55–63. doi: 10.1055/s-0031-1300952. [DOI] [PubMed] [Google Scholar]
- 30.Oellerich M, Armstrong VW. The MEGX test: a tool for the real-time assessment of hepatic function. Ther Drug Monit. 2001;23(2):81–92. doi: 10.1097/00007691-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Vogel T, Brockmann JG, Friend PJ. Ex-vivo normothermic liver perfusion: an update. Curr Opin Organ Transplant. 2010;15(2):167–72. doi: 10.1097/MOT.0b013e328337349d. [DOI] [PubMed] [Google Scholar]
- 32.Uygun K, Tolboom H, Izamis ML, Uygun B, Sharma N, Yagi H, et al. Diluted blood reperfusion as a model for transplantation of ischemic rat livers: alanine aminotransferanse is a direct indicator of viability. Transplant Proc. 2010;42(7):2463–7. doi: 10.1016/j.transproceed.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, et al. Nonanastomotic biliary strictures after liver transplantation, part 1: radiological features and risk factors for early vs late presentation. Liver Transplantation. 2007;13(5):708–718. doi: 10.1002/lt.21166. [DOI] [PubMed] [Google Scholar]
- 34.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Pina E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147(1):153–9. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pannen BH. New insights into the regulation of hepatic blood flow after ischemia and reperfusion. Anesth Analg. 2002;94(6):1448–57. doi: 10.1097/00000539-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, Culberson C, Korneszczuk K, Clemens MG. Differential mechanisms of hepatic vascular dysregulation with mild vs. moderate ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2008;294(5):g1219–26. doi: 10.1152/ajpgi.00527.2007. [DOI] [PubMed] [Google Scholar]
- 37.Zhu XH, Pan JP, Wu YF, Ding YT. Effects of warm ischemia time on biliary injury in rat liver transplantation. World J Gastroenterol. 2012;18(43):6308–14. doi: 10.3748/wjg.v18.i43.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abou Abbass A, Abouljoud M, Yoshida A, Kim DY, Slater R, Hundley J, et al. Biliary complications after orthotopic liver transplantation from donors after cardiac death: broad spectrum of disease. Transplant Proc. 2010;42(9):3392–8. doi: 10.1016/j.transproceed.2010.07.099. [DOI] [PubMed] [Google Scholar]
- 39.Nakanuma Y, Katayanagi K, Terada T, Saito K. Intrahepatic peribiliary glands of humans. I. Anatomy, development and presumed functions. J Gastroenterol Hepatol. 1994;9(1):75–9. doi: 10.1111/j.1440-1746.1994.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 40.Cardinale V, Wang Y, Carpino G, Mendel G, Alpini G, Gaudio E, et al. The biliary tree--a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol. 2012;9(4):231–40. doi: 10.1038/nrgastro.2012.23. [DOI] [PubMed] [Google Scholar]
- 41.Terada T, Kida T, Nakanuma Y. Extrahepatic peribiliary glands express alpha-amylase isozymes, trypsin and pancreatic lipase: an immunohistochemical analysis. Hepatology. 1993;18(4):803–8. doi: 10.1002/hep.1840180409. [DOI] [PubMed] [Google Scholar]
- 42.Terada T, Nakanuma Y. Expression of pancreatic enzymes (alpha-amylase, trypsinogen, and lipase) during human liver development and maturation. Gastroenterology. 1995;108(4):1236–45. doi: 10.1016/0016-5085(95)90225-2. [DOI] [PubMed] [Google Scholar]
- 43.Lalor PF, Herbert J, Bicknell R, Adams DH. Hepatic sinusoidal endothelium avidly binds platelets in an integrin-dependent manner, leading to platelet and endothelial activation and leukocyte recruitment. Am J Physiol Gastrointest Liver Physiol. 2013;304:G469–78. doi: 10.1152/ajpgi.00407.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy SP, Bhattacharjya S, Maniakin N, Greenwood J, Guerreiro D, Hughes D, et al. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion. Transplantation. 2004;77(9):1328–1332. doi: 10.1097/01.tp.0000119206.63326.56. [DOI] [PubMed] [Google Scholar]
- 45.Erhard J, Lange R, Scherer R, Kox WJ, Bretschneider HJ, Gebhard MM, et al. Comparison of histidine-tryptophan-ketoglutarate (HTK) solution versus University of Wisconsin (UW) solution for organ preservation in human liver transplantation. A prospective, randomized study. Transplant Int. 1994;7(3):177–81. doi: 10.1007/BF00327084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.