Abstract
In kidney nephron, parietal epithelial cells line the Bowman’s capsule and function as a permeability barrier for the glomerular filtrate. Bowman’s capsule cells with proximal tubule epithelial morphology have been found. However, the effects of tubular metaplasia in Bowman’s capsule on kidney function remain poorly understood. Sodium-glucose cotransporter 2 (SGLT2) plays a major role in reabsorption of glucose in the kidney and is expressed on brush border membrane of epithelial cells in the early segment of the proximal tubule. We hypothesized that SGLT2 is expressed in tubularized Bowman’s capsule and used our novel antibody to test this hypothesis. Immunohistochemical analysis was performed with our SGLT2 antibody on C57BL/6 mouse kidney prone to have tubularized Bowman’s capsules. Cell membrane was examined with periodic acid-Schiff stain. The results showed that SGLT2 was localized on brush border membrane of the proximal tubules in young and adult mice. Bowman’s capsules were lined mostly with normal brush border-less parietal epithelial cells in young mice while they were almost completely covered with proximal tubule-like cells in adult mice. Regardless of age, SGLT2 was expressed on brush border membrane of the tubularized Bowman’s capsule but did not co-localize with nephrin in the glomerulus. SGLT2-expressing tubular cells expanded from the urinary pole towards the vascular pole of the Bowman’s capsule. This study identified the localization of SGLT2 in the Bowman’s capsule. Bowman’s capsules with tubular metaplasia may acquire roles in reabsorption of filtered glucose and sodium.
Keywords: SGLT2, proximal tubule, Bowman’s capsule, parietal epithelial cells, brush border membrane, glomerulotubular junction
Introduction
Nutrients and electrolytes that are filtered through the glomerulus of the kidney are reabsorbed mainly in the proximal tubule region of nephron (Nielsen et al. 2012). Parietal epithelial cells line the glomerular capsule (Bowman’s capsule) and function as a permeability barrier for the glomerular filtrate. At the glomerulotubular junction, the Bowman’s capsule cell layer abruptly ends and the proximal tubule epithelium begins. This transition can be easily distinguished by light microscopy as parietal epithelial cells appear flat and lack brush border membrane while proximal tubule epithelial cells are tall and cuboidal and have well-developed brush border membrane (Nielsen et al. 2012). Bowman’s capsules with proximal tubule-like cell layer were first observed several decades ago in kidneys of mice (Crabtree 1940) and later on were found in the human kidney (Rosenheim 1953). Tubular metaplasia in Bowman’s capsule has been seen in kidneys of patients with diabetes, lupus (Finckh and Joske 1954), acute glomerular nephritis (Hutt et al. 1958), and acute tubular necrosis (Solez et al. 1979). More recently, scanning electron microscopic examination of nephrectomy samples showed tubularized Bowman’s capsules in up to 18% of nephrons (Lindop et al. 2002). The cellular structures of human, mouse, and rat tubularized Bowman’s capsules are well studied and shown to be indistinguishable from that of the proximal tubule cells (Ahmadizadeh et al. 1984; Finckh and Joske 1954; Lee et al. 1993; Jakowski 1982; Ward 1970). However, so far, little is known about the cause of tubular metaplasia in Bowman’s capsule or its effects on kidney function.
Daily, about 180 g of glucose is filtered through the glomerulus but only 0.5 g is excreted in the urine (Bakris 2009). It is believed that the bulk of filtered glucose is reabsorbed in the early segment of the proximal tubule by low-affinity high-capacity sodium-glucose cotransporter, SGLT2, while the remaining glucose is reabsorbed in the late proximal tubule by high-affinity low-capacity transporter, SGLT1 (Wright et al. 2011). The crucial role of SGLT2 in reabsorption of glucose in the kidney is evident in patients with familial renal glucosuria. These patients have mutations in the gene encoding SGLT2 and develop severe glucosuria (Lee et al. 2012; Lee 2013; Santer and Calado 2010) with urinary excretion of glucose as high as 160 g per day (Bakris 2009). Studies in mouse showed SGLT2 localization on the brush border membrane of the early proximal tubule and revealed its role in reabsorption of 97% of the filtered glucose (Sabolic et al. 2012; Vallon et al. 2011). We hypothesized that SGLT2 is expressed in proximal tubule-like cells in the Bowman’s capsule. Here, we tested our hypothesis by immunohistochemical analysis on kidneys of male C57BL/6 mice naturally prone to have tubularized Bowman’s capsules (Yabuki et al. 1999).
The lack of commercial resources for well-characterized specific antibodies against SGLTs has been recognized (Wright et al. 2011). We previously showed that the N-terminus region of mouse SGLT2 contains amino acid sequences that were divergent from other SGLTs (Tabatabai et al. 2003), and selected the sequence of the first 16 residues as antigenic peptide (Blodgett et al. 2011). A polyclonal antibody was raised against this peptide in rabbit and was affinity-purified with the antigenic peptide. Western blot analysis on cell lysate proteins from COS-7 cells over-expressing mouse SGLT1 or SGLT2 showed that this antibody hybridized only to SGLT2 (Blodgett et al. 2011). In the same study, fluorescence immunocytochemistry with SGLT2 antibody showed strong signals only in COS-7 cells that over-expressed the protein further indicating that our SGLT2 antibody is specific. In the present study, immunohistochemical analysis with this novel and specific antibody demonstrated for the first time the localization of SGLT2 expression in tubularized Bowman’s capsules in kidneys of C57BL/6 mice.
Materials and Methods
Animals
Male C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Mice had free access to food and water, and were anesthetized with isoflurane. All procedures had been approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin (MCW).
Western blot analysis on kidney proteins
Kidneys were collected from 4–6 week old (wk) mice and were used to prepare total kidney lysate proteins as we described before (Kothinti et al. 2012), or to prepare brush border membrane (BBM) proteins by following a polyethylene glycol precipitation method (Basivireddy and Balasubramanian 2003). Protein concentration was determined with DC Protein Assay (Bio-Rad; Hercules, CA). Western blot analysis was performed with our SGLT2 antibody on kidney homogenate (25 µg) and BBM (2 µg) proteins using chemiluminescence detection assay as described before (Kothinti et al. 2012; Tabatabai et al. 2005). As control, experiments were performed with peptide-blocked antibody.
Periodic acid-Schiff (PAS) staining, immunohistochemistry, and confocal microscopy
Left kidneys were collected from 4wk, 14wk, and 22wk mice and bisected sagittally. Tissues were fixed overnight in buffered zinc formalin, dehydrated through graded alcohols, and embedded in paraffin. Prior to staining, embedded tissues were cut into 4 µm sections, placed on Superfrost Plus slides (Thermo Scientific, Rockford, IL), and dried at 45 °C. Tissue sections were then deparaffinized with xylene and rehydrated to water.
For PAS staining, tissue slides were treated with 0.5% periodic acid, stained with Schiff reagent, and then counterstained with hematoxylin. For immunohistochemistry, antigen retrieval was performed on tissue sections using citrate buffer (pH 6) (Dako, Carpinteria, CA). After blocking the endogenous peroxidase, non-specific binding to biotin/avidin, and protein, tissue sections were hybridized with our SGLT2 antibody or with nephrin (N-20) antibody from Santa Cruz Biotechnology (Santa Cruz, CA) and then incubated with biotinylated donkey anti-rabbit or rabbit anti-goat IgG secondary antibody, respectively (Jackson ImmunoResearch Laboratories, West Grove, PA & Vector Laboratories, Burlingame, CA). Next, slides were incubated with HRP-conjugated streptavidin (Dako), treated with 3,3'-diaminobenzidine (DAB) (Dako), and then counterstained with hematoxylin. Control experiments were performed with peptide-blocked antibodies or in the absence of the primary antibodies. Finally, whole slides were scanned with NanoZoomer system.
For co-localization studies, tissue sections were double stained with nephrin and SGLT2 antibodies and were respectively detected with Alexa Fluor 647 and Cy3 conjugated secondary antibodies (Life Technologies, Grand Island, NY & Jackson ImmunoResearch). After counterstaining with 4',6-Diamidino-2-Phenylindole (DAPI), slides were covered with ProLong Gold mounting medium (Life Technologies). Images were captured with AIM 4.2 software controlling Zeiss LSM510 confocal microscope (Jena, Germany) using C- Apochromat 63×/1.45 objective. Images were documented in 8bit, multi track mode with DAPI, Cy3 and Alexa-647 dyes excited with 405nm, 561nm and 633nm lasers and recorded through BP420–480nm, BP-575–630nm and LP-650nm filters, respectively.
Quantification of SGLT2-expressing renal corpuscles
Slides from immunohistochemistry with SGLT2 antibody on kidney sections from 6–7 mice at each 4, 14, and 22 weeks of age were used to quantify the number of glomeruli with or without SGLT2-stained Bowman’s capsules, and the percentage of each in total glomeruli per slide was calculated. The mean values ± standard errors are presented. One Way ANOVA was performed with SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA).
Glomerular injury
Glomerular injury (glomerulosclerosis and/or mesangial expansion) was assessed in PAS stained kidney sections. A minimum of 20 randomly selected images (40× magnification) were evaluated in each specimen (Solberg Woods et al. 2010). Glomerular injury was assessed for individual glomeruli on a 0 to 4 scale: grade 0, no changes; grade 1, lesions involving < 25%; grade 2, lesions affecting 25– 50%; grade 3, lesions affecting 50–75%; and grade 4, lesions affecting > 75%.
Results
SGLT2 expression in kidneys of young and adult mice
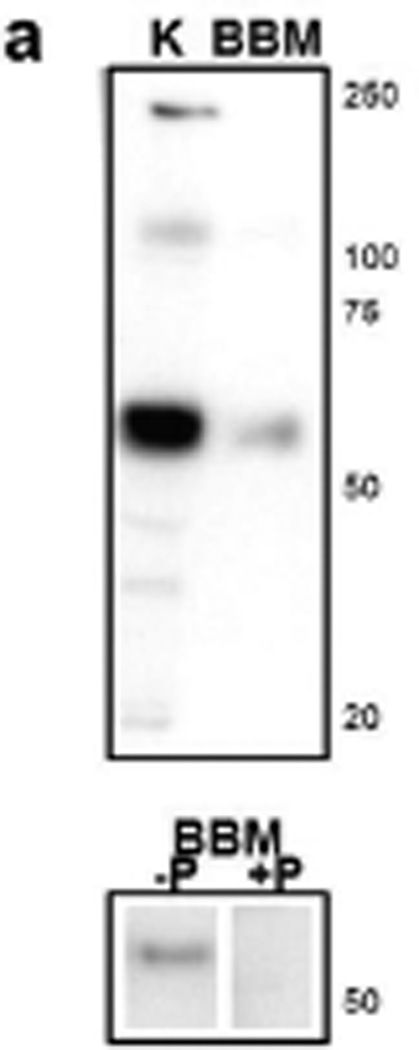
We examined the expression of SGLT2 in kidneys of young and adult male C57BL/6 mice using our polyclonal antibody against mouse SGLT2 peptide. First, Western blot analysis was performed with this antibody on total kidney homogenate and renal brush border membrane proteins (Fig. 1a). The predicted MW size of mouse SGLT2 is 73 kDa, but SGLTs may run faster than their predicted MWs on the SDS/PAGE gels due to incomplete denaturation of the protein (Wright et al. 2011). The results show that our SGLT2 antibody strongly hybridized to a band with molecular weight (MW) size of ~60 kDa in whole kidney homogenate proteins. A single band with similar MW size was observed in brush border membrane proteins, and this signal was abolished when peptide-blocked antibody was used. These results showed that this antibody recognizes SGLT2 from mouse kidney.
Fig. 1.
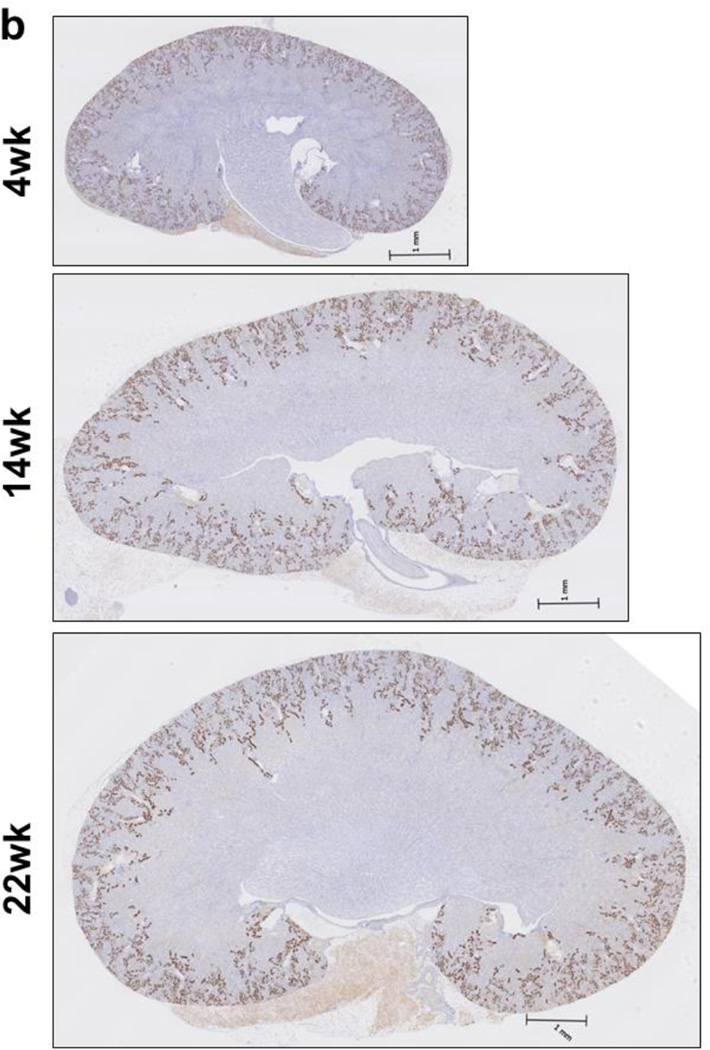
SGLT2 expression in the kidneys of young and adult C57BL/6 mice. (a) Western blot analysis with custom antibody against mouse SGLT2 peptide shows a common 60 kDa band in total kidney lysate (K) and renal brush border membrane (BBM) protein samples. This band is abolished when peptide-blocked SGLT2 antibody is used. Positions of the molecular weight size markers are shown to the right. −P, SGLT2 antibody; +P, peptide-blocked SGLT2 antibody. (b) Representative digital images from immunohistochemistry with SGLT2 antibody on kidney tissues from 4wk, 14wk, 22wk mice and detection with DAB are shown. SGLT2 is localized in the cortex. Ages of the mice are shown to the left.
Next, immunohistochemical analysis was performed with our SGLT2 antibody on kidney tissues from 4, 14, and 22 week old mice. The slides were then scanned and their digital images were used for analysis. The results showed that while the kidney size increased with age, the strongest SGLT2 signals were mainly localized in the cortex in kidneys of 4wk, 14wk and 22wk mice (Fig. 1b). Tissues were not stained in control experiments with peptide-blocked SGLT2 antibody or in the absence of primary antibody (not shown) supporting that SGLT2 signals were specific. These results showed for the first time localization of SGLT2 in kidneys of young mice.
Localization of SGLT2 in the Bowman’s capsule
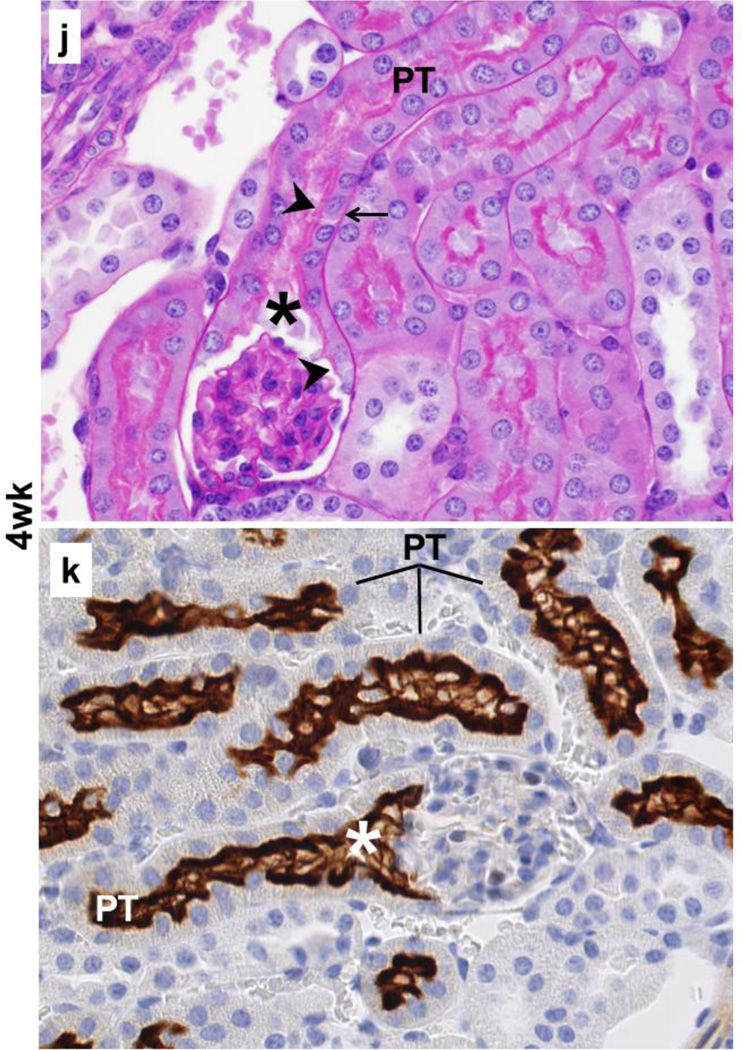
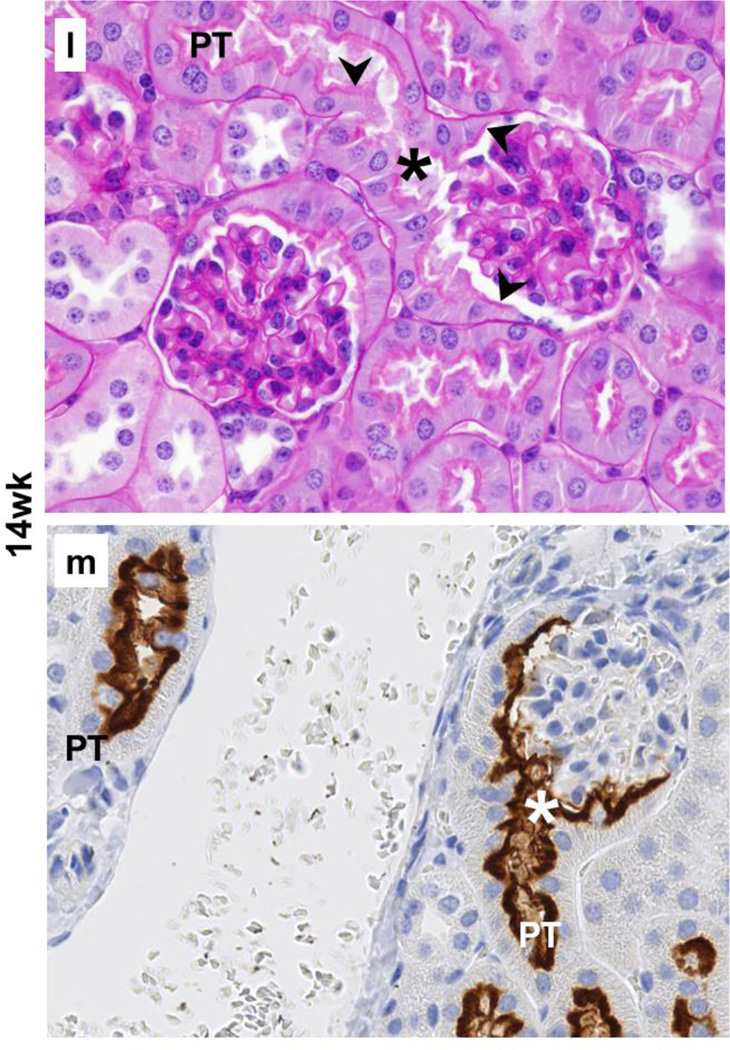
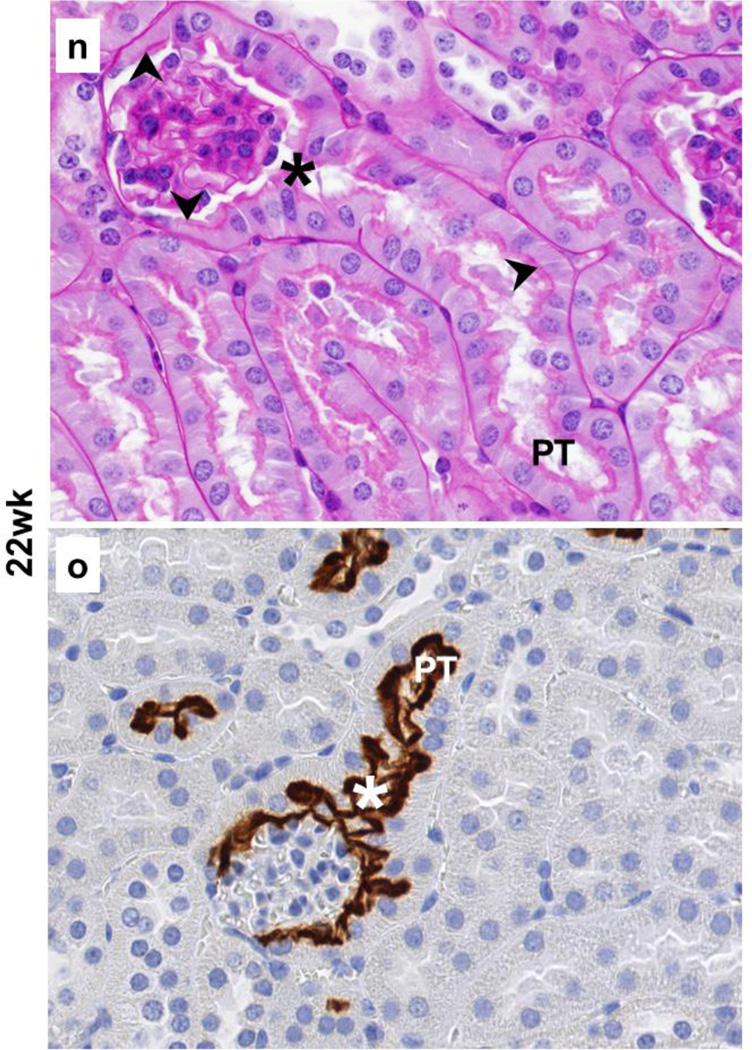
The results from immunohistochemistry with SGLT2 antibody on kidney tissues from 4wk, 14wk, and 22wk mice were used to investigate whether SGLT2 was expressed in the Bowman’s capsule while the presence or absence of the brush border membrane was examined with periodic acid-Schiff staining. The results from these experiments identified the localization of SGLT2 expression on the luminal brush border membrane of the proximal tubule-like cells in the Bowman’s capsules in kidneys of young and adult C57BL/6 mice (Fig. 2).
Fig. 2.
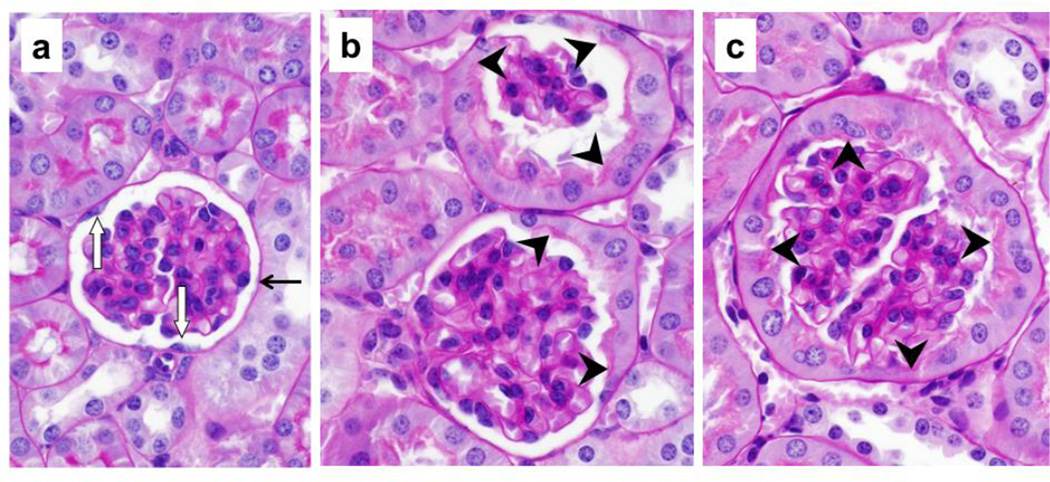
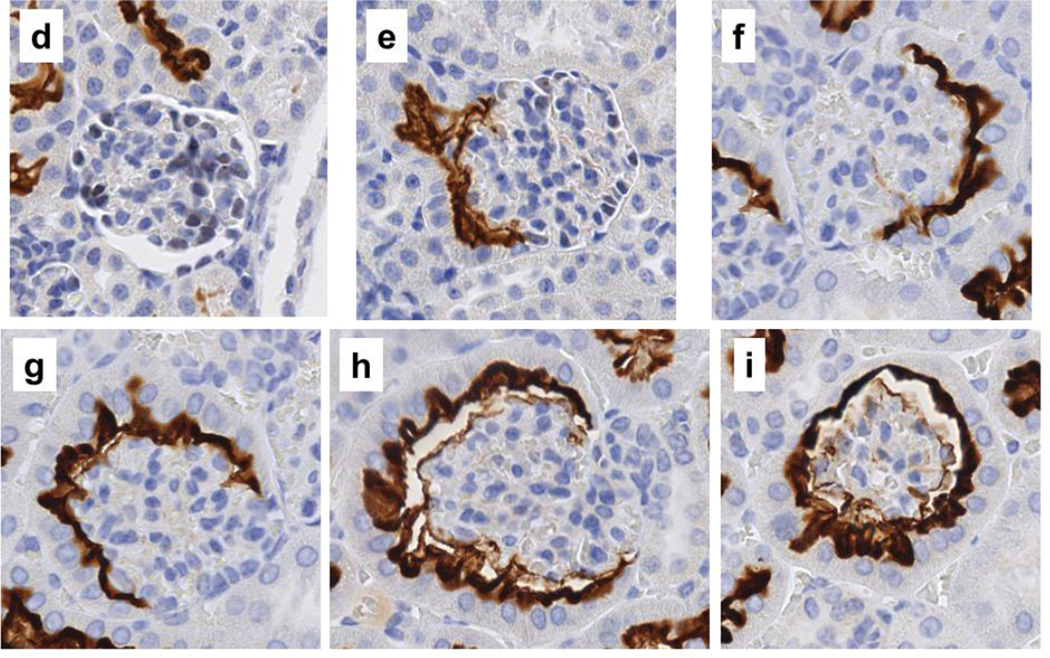
Localization of SGLT2 on brush border membrane of the Bowman’s capsule proximal tubule-like cells. Representative images from the kidney tissue slides from 4wk, 14wk, 22wk mice are shown. (a–c,j,l,n) PAS staining, 40× magnification; (d–i,k,m,o), immunohistochemistry with SGLT2 antibody, 40× magnification. (a–c) With age, the normal brush border-less squamous parietal epithelial cells with oval nuclei in the Bowman’s capsules are replaced with cuboidal cells with brush border membrane. (d–i) SGLT2 is not expressed in parietal epithelial cells but is expressed on the luminal brush border membrane of the proximal tubule-like cells in the Bowman’s capsule. (j,l,n) Proximal tubule-like cells first appear near the urinary pole and then expand on both sides towards the vascular pole, and are continuous with the proximal tubule. (k,m,o) SGLT2 expression in proximal tubule-like cells in the Bowman’s capsule is continuous with that of in the proximal tubule. Expression of SGLT2 is not observed in the distal tubules, the collecting ducts, or other structures. PT, proximal tubule; asterisk, urinary pole; arrowhead, luminal brush border membrane; arrow, basolateral or basement membrane.
In specific, in the renal corpuscles from 4wk mice, most Bowman’s capsules were lined with normal brush border-less squamous parietal epithelial cells (Fig. 2a); however, tall cuboidal cells with brush border membrane were also occasionally observed (not shown). In contrast, the Bowman’s capsule in renal corpuscles from 14wk and 22wk mice was either partially or completely lined with proximal tubule-like cells (Fig. 2b,2c) but the fully tubularized Bowman’s capsules were most abundant in 22wk mice. The morphology of the tubular Bowman’s capsule cells was indistinguishable from that of the cells in the proximal tubules. In general, the SGLT2 antibody did not hybridize to parietal epithelial cells; however, regardless of age, strong SGLT2 signals were observed on the luminal side of the tubularized regions of the Bowman’s capsules. In 4wk mice, most Bowman’s capsules did not express SGLT2 (Fig. 2d) and SGLT2 stain was only occasionally observed (Fig. 2e). Unlike in 4wk mice, partially or fully SGLT2-expressing Bowman’s capsules were abundant in kidneys of 14wk and 22wk mice (Fig. 2f–2i) but the prevalence of glomeruli completely encapsulated by SGLT2-expressing layer was highest in 22wk mice (Fig. 2i).
The proximal tubule is connected to the renal corpuscle at the urinary pole of the Bowman’s capsule (Nielsen et al. 2012). Consistently, our results showed proximal tubule cells at the urinary pole of the Bowman’s capsules in kidneys of 4wk (Fig. 2j), 14wk (Fig. 2l), and 22wk (Fig. 2n) mice. SGLT2 expression was localized on the apical side of the proximal tubule cells at the glomerulotubular junction as well as in the rest of the early proximal tubule in 4wk (Fig. 2k), 14wk (Fig. 2m), and 22wk (Fig. 2o) mice kidneys. In Bowman’s capsules from 4wk mice, the proximal tubule-like cells were mainly localized adjacent to the glomerulotubular junction (Fig. 2j), but these cells had expanded towards (Fig. 2l) or had reached (Fig. 2n) the vascular pole in Bowman’s capsules from 14wk and 22wk mice. This directional transition in the Bowman’s capsule cell layer morphology paralleled with the appearance of SGLT2 expression near the urinary pole in 4wk mice (Fig. 2k) and the expansion of its expression on both sides (Fig. 2m) towards the vascular pole (Fig. 2o) in 14wk and 22wk mice. In all three age groups, the proximal tubule-like cell layer and the SGLT2 signals in Bowman’s capsules were both continuous with that of the epithelial cells in the proximal tubules.
Glomerulus analysis
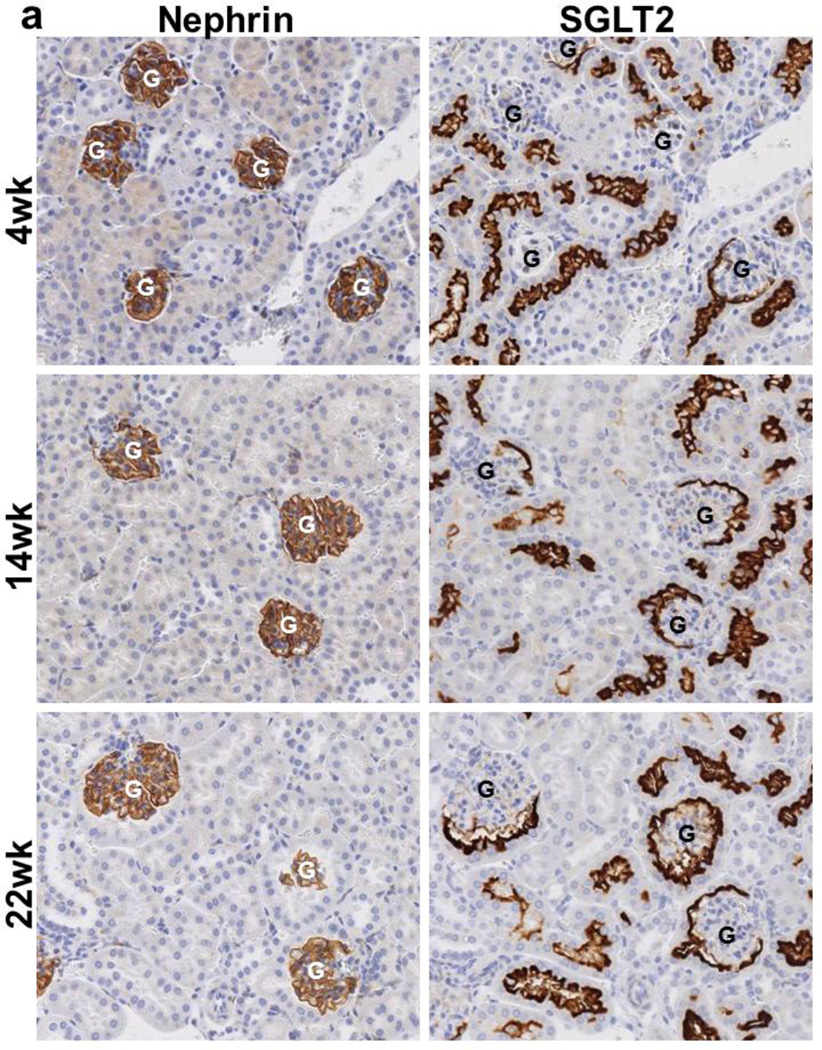
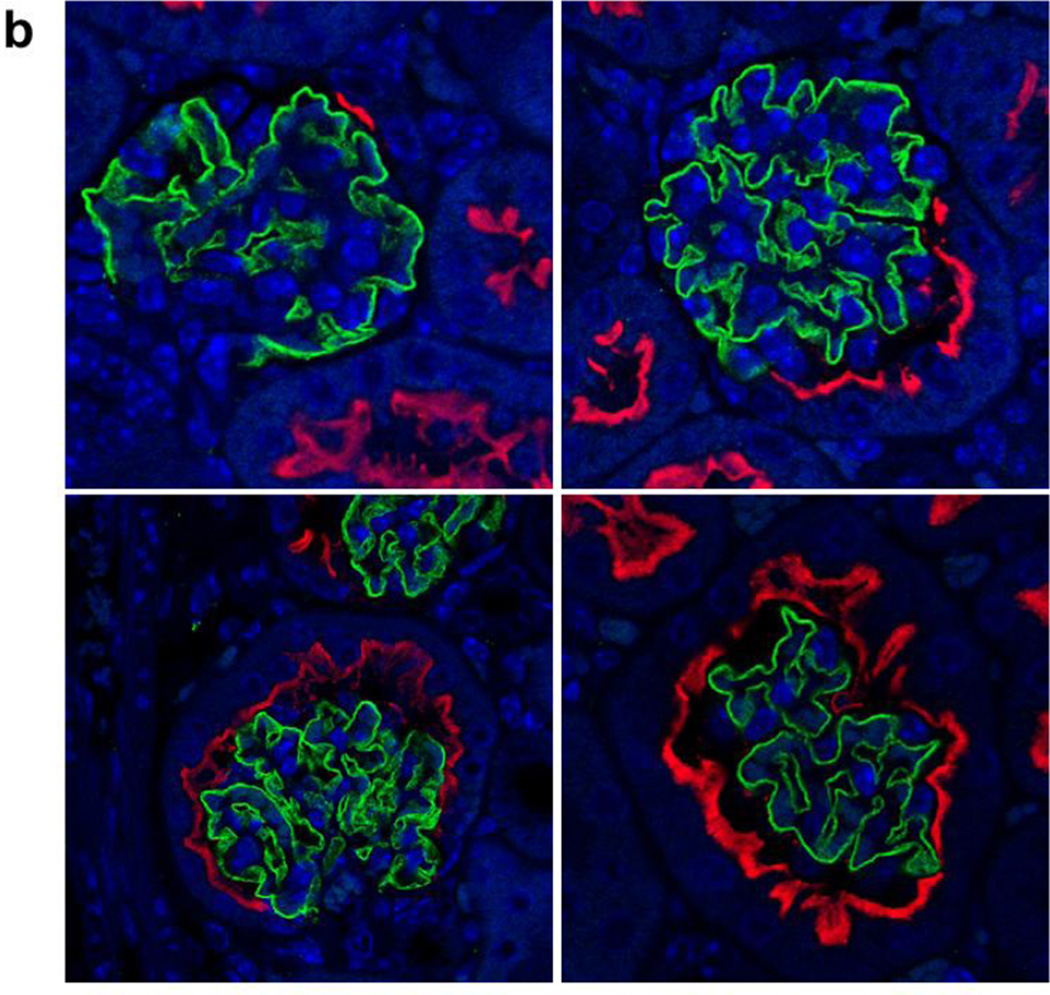
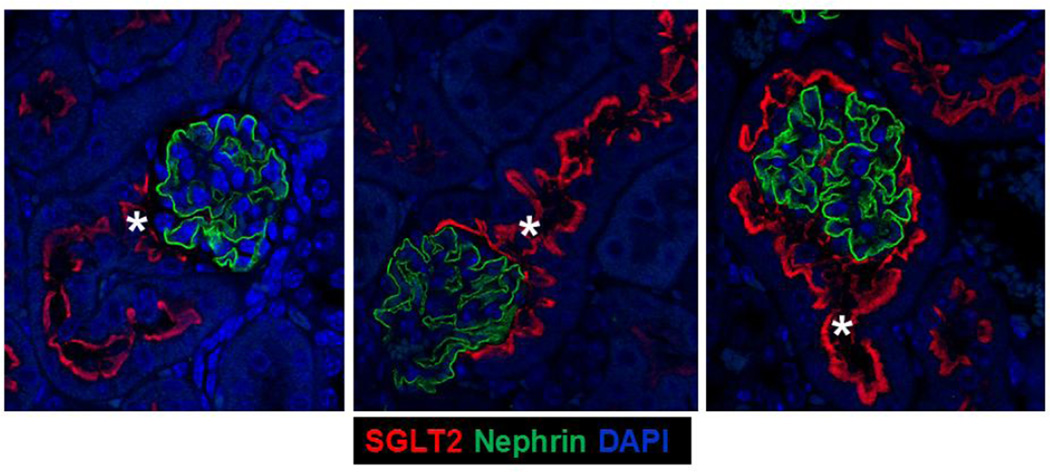
We investigated SGLT2 expression in the visceral epithelial cells (podocytes) in kidneys of 4wk, 14wk, and 22wk mice using nephrin as a podocyte marker (Nielsen et al. 2012). Immunohistochemical analysis with SGLT2 and nephrin antibodies on serially sectioned tissues showed that while nephrin antibody stained all glomeruli, SGLT2 antibody only occasionally stained the Bowman’s capsules in the adjacent tissue sections from kidneys of 4wk mice (Fig. 3a). There were substantially more glomeruli with than without SGLT2-expressing Bowman’s capsules in 14wk mice while almost all glomeruli had SGLT2-stained Bowman’s capsules in tissues from 22wk mice. Immunofluorescence analysis on tissues double stained with SGLT2 and nephrin antibodies showed that SGLT2-expressing tubular cells developed in the Bowman’s capsule without significant changes in nephrin expression in the glomerulus (Fig. 3b). Despite strong SGLT2 and nephrin signals, co-localization was not observed in tissues of 4wk, 14wk, or 22wk mice.
Fig. 3.
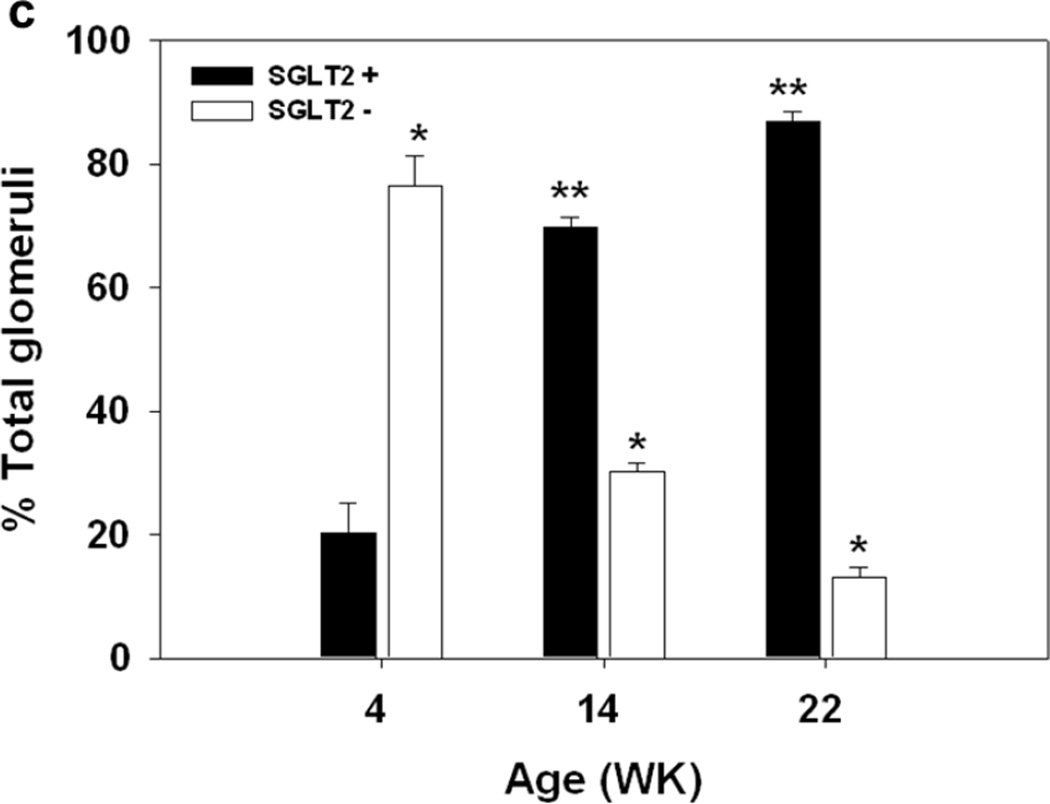
Glomerulus analysis. (a) Representative images from immunohistochemial analysis with nephrin and SGLT2 antibodies on serially sectioned kidney tissues from 4wk, 14wk, and 22wk mice are shown; 10× magnification. Nephrin is expressed in all glomeruli. SGLT2-expressing Bowman’s capsules are more abundant in tissues from adult than young mice. (b) Representative confocal images from kidney tissue sections from 4wk, 14wk, and 22wk mice double stained with SGLT2 and nephrin antibodies and counterstained with DAPI are shown; 63× magnification. In the renal corpuscles, nephrin signals (green) are in the glomeruli while SGLT2 signals (red) are on the luminal side of the tubularized Bowman’s capsule layer. SGLT2 and nephrin signals do not co-localize. Asterisk, urinary pole. (c) The average numbers of the total glomeruli in kidney sections from 4wk, 14wk, and 22wk mice were similar and were counted to be 186, 171, and 161 per slide, respectively. The percentage of glomeruli with SGLT2-expressing Bowman’s capsules significantly increased with age. G, glomerulus; *, P < 0.001 between SGLT2-positive and -negative glomeruli at each age; **, P < 0.001 between SGLT2-positive glomeruli at different ages. Results are from 6–7 mice per age group.
Glomeruli with SGLT2-expressing capsules constituted 20 ± 5% of total glomeruli in 4wk mice but they significantly increased to 70 ± 1% and 87 ± 2% in 14wk and 22wk mice, respectively (Fig. 3c). Assessment of glomerular injury in these tissues showed no signs of glomerular sclerosis or mesangial expansion.
Discussion
Tubular metaplasia in human Bowman’s capsule was described over 60 years ago, but the effects of this transformation on kidney function have not yet been fully understood. The results from early studies on rodent kidneys suggest that tubular Bowman’s capsule cells may have similar functions as the proximal tubule epithelial cells. One study showed that the proximal tubule enzymes, alkaline phosphatase and γ-glutamyl transpeptidase, were also expressed on the brush border membrane of proximal tubule-like cells in the Bowman’s capsules of mouse and rat kidneys (Briere et al. 1983). And, another study demonstrated protein transport capacity in tubular Bowman’s capsule cells by showing the accumulation of horseradish peroxidase, a 40 kDa protein, in these cells one hour after its injection into mouse (Dietert 1967). SGLT2 mediates sodium-dependent reabsorption of glucose in the early proximal tubule and, here, we investigated its expression in tubularized Bowman’s capsule.
It is well established that tubularized Bowman’s capsules are present in the kidneys of adult male C57BL/6 mice (Yabuki et al. 1999). In the present study, tubular metaplasia in Bowman’s capsule was found in kidneys of young and adult mice (Fig. 2). The cuboidal cells with PAS-stained apical brush border member that were found in Bowman’s capsules were indistinguishable from the proximal tubule epithelia (Fig. 2j,2l,2n). SGLT2 was expressed normally in renal cortex and was localized on the apical side of the early proximal tubules in kidneys of young and adult mice (Fig. 1b,2k,2m,2o). Regardless of age, SGLT2 was also expressed on the luminal brush border membrane of the Bowman’s capsule proximal tubule-like cells (Fig. 2e–i). SGLT2-expressing tubular cells first appeared near the glomerulotubular junction in Bowman’s capsules of young mice (Fig. 2k) and then expanded on both sides from the urinary pole towards the vascular pole in older mice (Fig. 2m,2o), but remained continuous with the proximal tubule epithelium.
SGLT2 signal was neither present in parietal epithelial cells (Fig. 2d) nor co-localized with nephrin signal in podocytes (Fig. 3b) suggesting that the renal corpuscle epithelial cells do not express SGLT2. The proportion of glomeruli with SGLT2-expressing Bowman’s capsules increased from 20% in 4wk mice to almost 90% in 22wk mice (Fig. 3a,3c) without significant changes in expression of nephrin (Fig. 3b) or any signs of glomerular injury. These results are consistent with tubular metaplasia in human Bowman’s capsule without other significant abnormalities (Finckh and Joske 1954; Ward 1970).
A new class of anti-diabetes drugs is designed to reduce blood glucose by blocking the SGLT2-mediated reabsorption of glucose in the kidney (Bays 2013). However, the location of SGLT2 expression in the proximal tubule, relative to SGLT1, may play a role in the observed suboptimal effects of SGLT2 inhibitor drugs. It is commonly accepted that SGLT2 mediates the reabsorption of >80% of filtered glucose; however, treatment of diabetic patients with SGLT2 inhibitors causes only 30% to 50% increase in urinary excretion of glucose (Abdul-Ghani et al. 2013). The reason may be that the inhibition of glucose reabsorption in the early proximal tubule increases glucose concentration in the late proximal tubule lumen, which may in turn enhance SGLT1-mediated glucose reabsorption (Abdul-Ghani et al. 2013; Rieg et al. 2013), therefore, reducing the effects of SGLT2 inhibition.
Localization of SGLT2 has also been proposed to play role in regulation of glomerular filtration rate (GFR) in diabetes (Gilbert 2013; Vallon 2011). It was shown that glomerular hyperfiltration developed in streptozotocin-induced diabetic wild-type mice but the diabetic SGLT2 knockout mice were protected (Vallon et al. 2013). It was proposed that this may be due to SGLT2-mediated sodium reabsorption in the early proximal tubule and its downstream effects on sodium concentration in the distal tubule lumen and tubulo-glomerular feedback (Vallon 2011; Vallon et al. 2013).
In summary, this study demonstrates for the first time SGLT2 localization in tubularized Bowman’s capsules in kidneys of C57BL/6 mice. Our results suggest that tubular metaplasia in Bowman’s capsule may lead to reabsorption of filtered glucose and sodium in the renal corpuscle, which in turn can affect their concentrations in the proximal and distal tubules lumen. These changes may ultimately alter the blood glucose and the GFR.
Acknowledgements
This study was supported by funds available to NMT and sources included the National Institutes of Health grants R01DK085031 and R01ES004026, and an award from the Medical College of Wisconsin Research Affairs Committee.
The excellent technical assistances were from the MCW Children’s Research Institute Histology and Imaging Cores, and NIH funding 8UL1TR000055 supporting MCW core resources. High-resolution slide scanning was performed using a Hamamatsu Nanozoomer HT system operated by the Pediatric BioBank & Analytical Tissue Core, a MACC Fund supported core facility at MCW. ABB’s current affiliation is U.S. Forest Service, Madison, WI.
References
- Abdul-Ghani MA, Defronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes. 2013;62:3324–3328. doi: 10.2337/db13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadizadeh M, Echt R, Kuo CH, Hook JB. Sex and strain differences in mouse kidney: Bowman's capsule morphology and susceptibility to chloroform. Toxicol. Lett. 1984;20:161–171. doi: 10.1016/0378-4274(84)90142-5. [DOI] [PubMed] [Google Scholar]
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–1277. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- Basivireddy J, Balasubramanian KA. A simple method of rat renal brush border membrane preparation using polyethylene glycol precipitation. Int. J. Biochem. Cell Biol. 2003;35:1248–1255. doi: 10.1016/s1357-2725(02)00345-x. [DOI] [PubMed] [Google Scholar]
- Bays H. Sodium Glucose Co-transporter Type 2 (SGLT2) Inhibitors: Targeting the Kidney to Improve Glycemic Control in Diabetes Mellitus. Diabetes Ther. 2013;4:195–220. doi: 10.1007/s13300-013-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett AB, Kothinti RK, Kamyshko I, Petering DH, Kumar S, Tabatabai NM. A fluorescence method for measurement of glucose transport in kidney cells. Diabetes Technol. Ther. 2011;13:743–751. doi: 10.1089/dia.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere N, Petitclerc C, Plante G. Presence of alkaline phosphatase and gamma-glutamyl transpeptidase on the parietal layer of Bowman's capsule. Acta Histochem. 1983;73:237–241. doi: 10.1016/s0065-1281(83)80032-4. [DOI] [PubMed] [Google Scholar]
- Crabtree C. Sex differences in the structure of Bowman’s capsule in the mouse. Science. 1940;91:299. doi: 10.1126/science.91.2360.299. [DOI] [PubMed] [Google Scholar]
- Dietert SC. The columnar cells occurring in the parietal layer of Bowman's capsule. Cellular fine structure and protein transport. J. Cell Biol. 1967;35:435–444. doi: 10.1083/jcb.35.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finckh ES, Joske RA. The occurrence of columnar epithelium in Bowman's capsule. J. Pathol. Bacteriol. 1954;68:646–648. doi: 10.1002/path.1700680243. [DOI] [PubMed] [Google Scholar]
- Gilbert RE. Sodium-glucose linked transporter-2 inhibitors: potential for renoprotection beyond blood glucose lowering? Kidney Int. 2013 doi: 10.1038/ki.2013.451. [DOI] [PubMed] [Google Scholar]
- Hutt MS, Pinniger JL, DE Wardener HE. The relationship between the clinical and the histological features of acute glomerular nephritis. Q. J. Med. 1958;27:265–291. [PubMed] [Google Scholar]
- Jakowski RM. Renal tubular epithelium lining parietal layer of Bowman's capsule in adult Long-Evans rats. Vet. Pathol. 1982;19:212–215. doi: 10.1177/030098588201900215. [DOI] [PubMed] [Google Scholar]
- Kothinti RK, Blodgett AB, North PE, Roman RJ, Tabatabai NM. A novel SGLT is expressed in the human kidney. Eur. J. Pharmacol. 2012;690:77–83. doi: 10.1016/j.ejphar.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Han KH, Park HW, Shin JI, Kim CJ, Namgung MK, Kim KH, Koo JW, Chung WY, Lee DY, Kim SY, Cheong HI. Familial renal glucosuria: a clinicogenetic study of 23 additional cases. Pediatr. Nephrol. 2012 doi: 10.1007/s00467-012-2109-9. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Sparke J, Howie AJ. The mammalian glomerulotubular junction studied by scanning and transmission electron microscopy. J. Anat. 1993;182(Pt 2):177–185. [PMC free article] [PubMed] [Google Scholar]
- Lee YW. Clinical and genetic analysis in a patient with primary renal glucosuria: Identification of a novel mutation in the gene. Exp. Ther. Med. 2013;6:1532–1534. doi: 10.3892/etm.2013.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindop GB, Gibson IW, Downie TT, Vass D, Cohen EP. The glomerulo-tubular junction: a target in renal diseases. J. Pathol. 2002;197:1–3. doi: 10.1002/path.1087. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Kwon T, Fenton RA, Praetorious J. Anatomy of the kidney. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner BM, editors. Brenner and Rector's the Kidney. 9th ed. Elsevier/Saunders; 2012. pp. 31–93. [Google Scholar]
- Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt KA, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacologic SGLT2 inhibition in euglycemia. Am. J. Physiol Renal Physiol. 2013 doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheim ML. Type 2 nephritis relapsing thirty years after onset, with two full-term pregnancies occurring during the course of the disease. Proc. R. Soc. Med. 1953;46:715–716. [PMC free article] [PubMed] [Google Scholar]
- Sabolic I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am. J. Physiol Cell Physiol. 2012;302:C1174–C1188. doi: 10.1152/ajpcell.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin. J. Am. Soc. Nephrol. 2010;5:133–141. doi: 10.2215/CJN.04010609. [DOI] [PubMed] [Google Scholar]
- Solberg Woods LC, Stelloh C, Regner KR, Schwabe T, Eisenhauer J, Garrett MR. Heterogeneous stock rats: a new model to study the genetics of renal phenotypes. Am. J. Physiol Renal Physiol. 2010;298:F1484–F1491. doi: 10.1152/ajprenal.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solez K, Morel-Maroger L, Sraer JD. The morphology of "acute tubular necrosis" in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 1979;58:362–376. [PubMed] [Google Scholar]
- Tabatabai NM, Blumenthal SS, Lewand DL, Petering DH. Mouse kidney expresses mRNA of four highly related sodium-glucose cotransporters: regulation by cadmium. Kidney Int. 2003;64:1320–1330. doi: 10.1046/j.1523-1755.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- Tabatabai NM, Blumenthal SS, Petering DH. Adverse effect of cadmium on binding of transcription factor Sp1 to the GC-rich regions of the mouse sodium-glucose cotransporter 1, SGLT1, promoter. Toxicology. 2005;207:369–382. doi: 10.1016/j.tox.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J. Am. Soc. Nephrol. 2011;22:104–112. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V. Molecular determinants of renal glucose reabsorption. Focus on "Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2". Am. J. Physiol Cell Physiol. 2011;300:C6–C8. doi: 10.1152/ajpcell.00444.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am. J. Physiol Renal Physiol. 2013;304:F156–F167. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AM. Tubular metaplasia in Bowman's capsule. J. Clin. Pathol. 1970;23:472–474. doi: 10.1136/jcp.23.6.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- Yabuki A, Suzuki S, Matsumoto M, Nishinakagawa H. Morphometrical analysis of sex and strain differences in the mouse nephron. J. Vet. Med. Sci. 1999;61:891–896. doi: 10.1292/jvms.61.891. [DOI] [PubMed] [Google Scholar]