Abstract
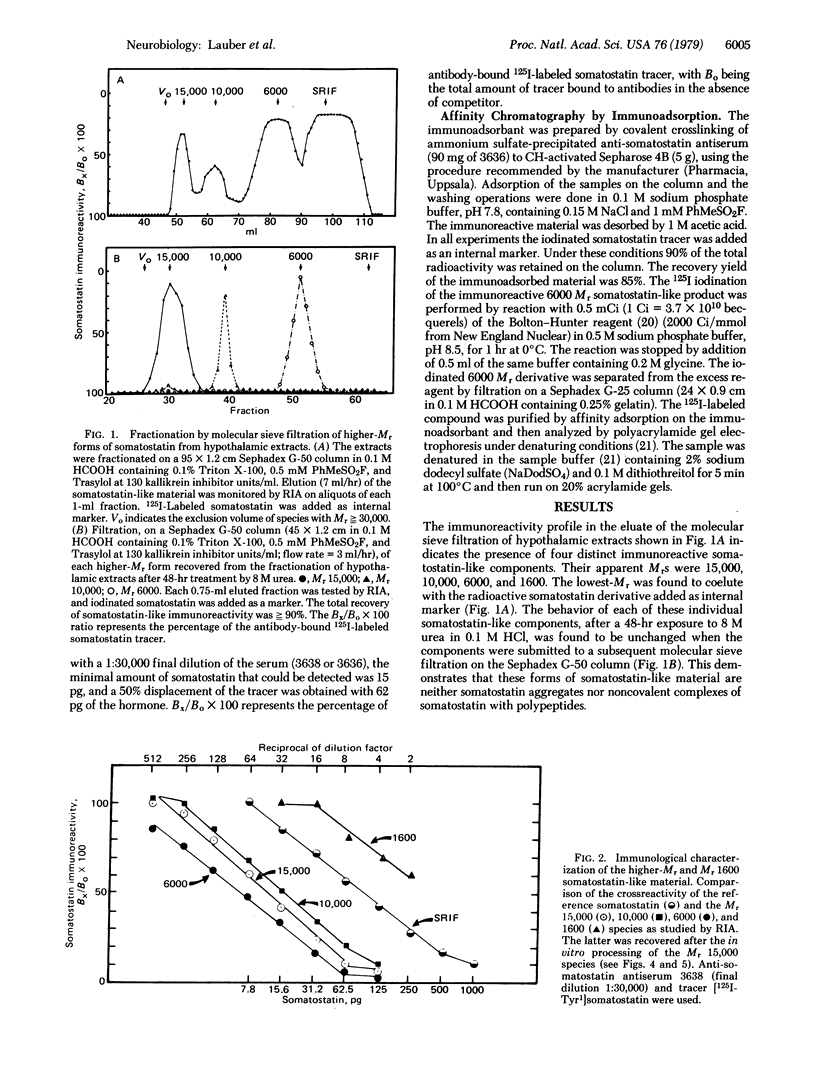
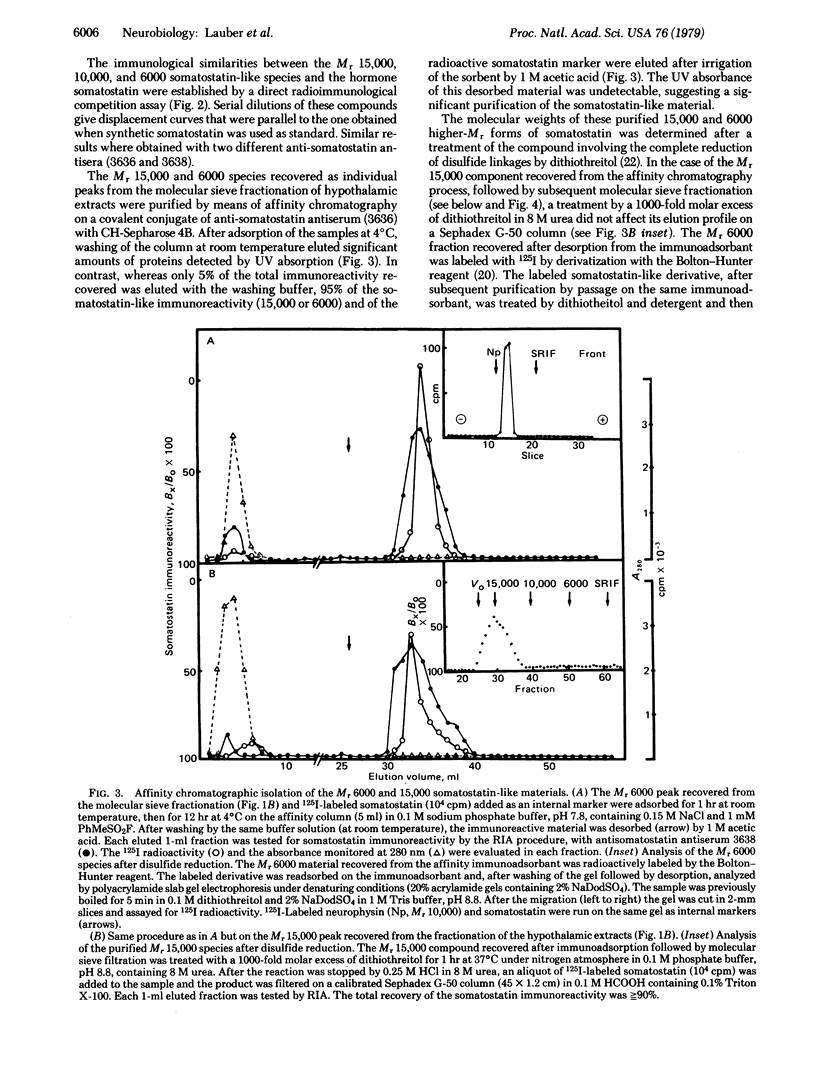
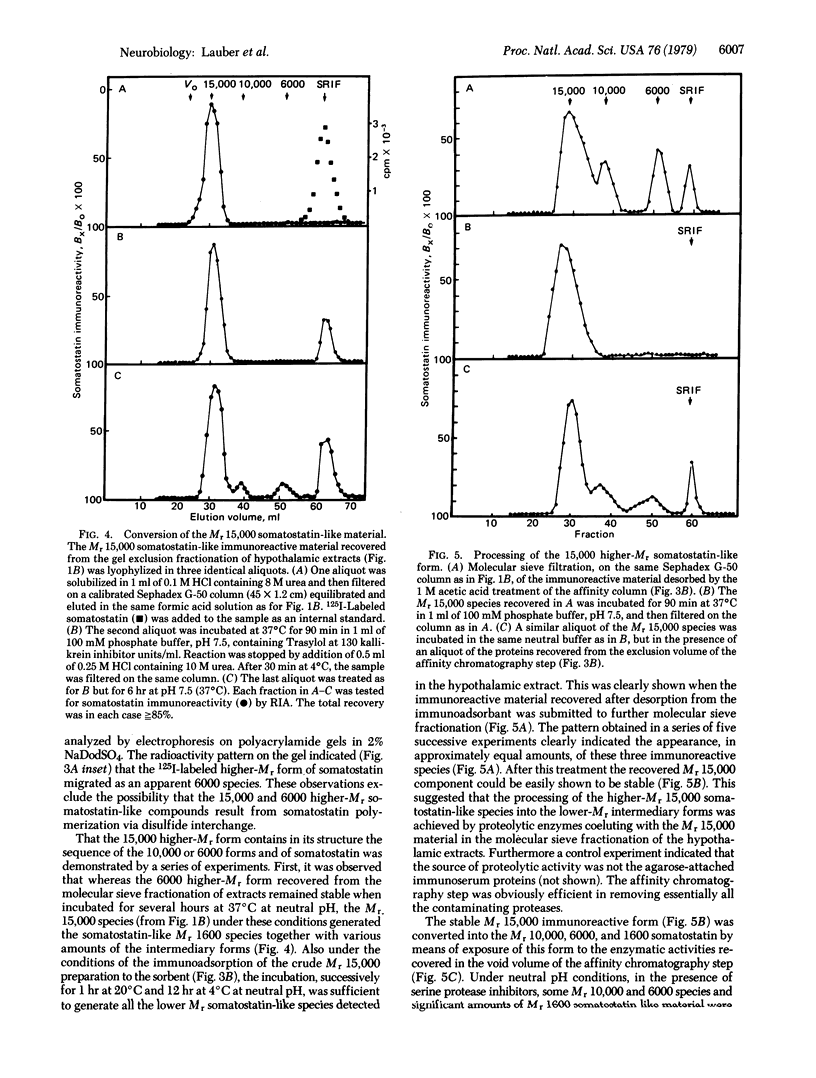
Extracts of mouse hypothalamus made in acid/urea containing protease inhibitors were analyzed for somatostatin immunoreactivity after molecular sieve filtration on Sephadex G-50. Higher molecular weight (higher-Mr) somatostatin-like forms with apparent molecular weights of 15,000, 10,000, and 6000 could be identified, besides the molecular weight 1600 somatostatin. Immunological identities with somatostatin were unambiguously demonstrated by the analysis of the displacement curves in the radioimmunoassay. The Mr 15,000, 6000, and 1600 species were purified by affinity chromatography on an anti-somatostatin immune serum covalent conjugate with Sepharose used as immunoadsorbant. After disulfide reduction by dithiothreitol, the size of the Mr 15,000 and 6000 somatostatin-like species was assessed either by molecular sieve filtration or by polyacrylamide gel electrophoresis. The results indicated that the higher-Mr somatostatin-like species isolated from the hypothalamus did not result from hormone polymerization by means of disulfide interchange. The processing in vitro of the 15,000 higher-Mr form of somatostatin was achieved by proteolytic enzymes coeluted with this species during the fractionation of hypothalamic extracts. Under neutral pH conditions the intermediary higher-Mr forms were generated together with the Mr 1600 somatostatin-like species. This processing activity could be either strongly inhibited at acidic pH or in acid/urea medium or else eliminated by selective immunoadsorption of the 15,000 higher-Mr form. Neither trypsin nor the γ subunit of 7S nerve growth factor was able to produce this processing, suggesting that enzymes with other kinds of specificity may be involved. It is concluded that somatostatin biosynthesis in the mouse hypothalamus may occur via a high-Mr precursor that is processed into intermediary forms leading to the tetradecapeptide hormone.
Keywords: prohormones, radioimmunoassay, affinity chromatography, posttranslational cleavage, proteolytic enzymes
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimura A., Sato H., Dupont A., Nishi N., Schally A. V. Somatostatin: abundance of immunoreactive hormone in rat stomach and pancreas. Science. 1975 Sep 19;189(4207):1007–1009. doi: 10.1126/science.56779. [DOI] [PubMed] [Google Scholar]
- Berger E. A., Shooter E. M. Evidence for pro-beta-nerve growth factor, a biosynthetic precursor to beta-nerve growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3647–3651. doi: 10.1073/pnas.74.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A. E., 3rd, Sanchez-Franco F., Spencer E., Patel Y. C., Jackson I. M., Reichlin S. Characterization of hypophysiotropic hormones in porcine hypothalamic extracts. Endocrinology. 1978 Oct;103(4):1075–1083. doi: 10.1210/endo-103-4-1075. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Brownstein M., Arimura A., Sato H., Schally A. V., Kizer J. S. The regional distribution of somatostatin in the rat brain. Endocrinology. 1975 Jun;96(6):1456–1461. doi: 10.1210/endo-96-6-1456. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Chihara K., Arimura A., Schally A. V. Immunoreactive somatostatin in rat hypophyseal portal blood: effects of anesthetics. Endocrinology. 1979 May;104(5):1434–1441. doi: 10.1210/endo-104-5-1434. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Zyznar E., Vale W., Unger R. H. Multiple forms of somatostatin-like immunoreactivity in canine pancreas. FEBS Lett. 1978 Oct 15;94(2):327–330. doi: 10.1016/0014-5793(78)80968-5. [DOI] [PubMed] [Google Scholar]
- Dubois M. P. Immunoreactive somatostatin is present in discrete cells of the endocrine pancreas. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1340–1343. doi: 10.1073/pnas.72.4.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Analysis of the common precursor to corticotropin and endorphin. J Biol Chem. 1978 Aug 25;253(16):5732–5744. [PubMed] [Google Scholar]
- Gillioz P., Giraud P., Conte-Devolx B., Jaquet P., Codaccioni J. L., Oliver C. Immunoreactive somatostatin in rat hypophysial portal blood. Endocrinology. 1979 May;104(5):1407–1410. doi: 10.1210/endo-104-5-1407. [DOI] [PubMed] [Google Scholar]
- Guillemin R. Peptides in the brain: the new endocrinology of the neuron. Science. 1978 Oct 27;202(4366):390–402. doi: 10.1126/science.212832. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Efendić S., Hellerström C., Johansson O., Luft R., Arimura A. Cellular localization of somatostatin in endocrine-like cells and neurons of the rat with special references to the A1-cells of the pancreatic islets and to the hypothalamus. Acta Endocrinol Suppl (Copenh) 1975;200:5–41. [PubMed] [Google Scholar]
- Kemmler W., Peterson J. D., Steiner D. F. Studies on the conversion of proinsulin to insulin. I. Conversion in vitro with trypsin and carboxypeptidase B. J Biol Chem. 1971 Nov 25;246(22):6786–6791. [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Potts J. T., Jr, Rich A. Proparathyroid hormone: identification of a biosynthetic precursor to parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Mar;69(3):643–647. doi: 10.1073/pnas.69.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber M., Camier M., Cohen P. Immunological and biochemical characterization of distinct high molecular weight forms of neurophysin and somatostatin in mouse hypothalamus extracts. FEBS Lett. 1979 Jan 15;97(2):343–347. doi: 10.1016/0014-5793(79)80118-0. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Chu L. L., Cohn D. V. Conversion of proparathyroid hormone to parathyroid hormone by a particulate enzyme of the parathyroid gland. J Biol Chem. 1976 Nov 10;251(21):6711–6716. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar R. P. Somatostatin immunoreactive peptides of higher molecular weight in ovine hypothalamic extracts. J Endocrinol. 1978 Jun;77(3):429–430. doi: 10.1677/joe.0.0770429. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Noe B. D., Fletcher D. J., Bauer G. E., Weir G. C., Patel Y. Somatostatin biosynthesis occurs in pancreatic islets. Endocrinology. 1978 Jun;102(6):1675–1685. doi: 10.1210/endo-102-6-1675. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: identification of beta-lipotropin peptides and their arrangement relative to corticotropin in the precursor synthesized in a cell-free system. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5300–5304. doi: 10.1073/pnas.74.12.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schally A. V. Aspects of hypothalamic regulation of the pituitary gland. Science. 1978 Oct 6;202(4363):18–28. doi: 10.1126/science.99816. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman P. M., Tushinski R. J., Bancroft F. C. Pregrowth hormone: product of the translation in vitro of messenger RNA coding for growth hormone. Proc Natl Acad Sci U S A. 1976 Jan;73(1):29–33. doi: 10.1073/pnas.73.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]


