Abstract
Donor safety in living liver donation is of paramount importance, however, information on long-term outcomes is limited by incomplete follow-up. We sought to ascertain factors that predict post-donation follow-up in 456 living liver donors in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL). Completed donor follow-up was defined as physical, phone, or laboratory contact at a given time point. Univariate and multivariable mixed effects logistic regression models were developed to predict completed follow-up using donor and recipient demographic and clinical data and donor quality of life data. 90% of donors completed follow-up in the first three months, 83% at year 1; completed follow-up ranged from 57% to 72% in years 2–7 and from 41% to 56% in years 8–10. The probability of completed follow-up in the first year was higher for white donors (odds ratio (OR)=3.27, 95% confidence interval (CI)=1.25–8.58), but lower for donors whose recipients had hepatitis C virus or hepatocellular carcinoma (OR=0.34, 95% CI=0.17–0.69). After the first year, older age at donation predicted more complete follow-up. There were significant center differences at all time points (OR range 0.29–10.11), with center variability in both return for in-center visits and in the use of phone/long distance visits. Donor follow-up in the first year post-donation was excellent but decreased with time. Predictors of follow-up varied based on the time since donation. Adapting center best practices, enhanced by using telephone and social media to maintain contact with donors, represents a significant opportunity to gain valuable information about long-term donor outcomes.
Keywords: clinical practice, donor outcomes, donation, follow-up, quality of life
Donor safety is of paramount importance in assessing the success of living donor liver transplantation (LDLT). To date, most data have focused on short-term outcomes including death and surgical complications such as bile leakage (1–4). A wide range of complication rates has been reported in donors after LDLT. Overall, reported complication rates have ranged from 0%- 67%, with an overall crude complication rate in a meta-analysis and the large National Institutes of Health -funded Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) experience of approximately 38% (5–7). Studies assessing donor health-related quality of life (HRQOL) after LDLT also yield variable findings, although most demonstrate that HRQOL (assessed with generic instruments not specific to LDLT) is at or above United States (US) norms (8). These studies also consistently find that nearly all donors state that they would donate again, irrespective of recipient outcomes (8,9–12). Nevertheless, 71% of donors reported abdominal symptoms several months post-operatively that they attributed to the donation surgery,(10) and it is clear that psychological distress is relatively common in both liver and kidney donors (13).
Longer-term follow-up is particularly important and may identify areas that may be sources of stress and concern to donors, including finances (14) and the incidence of long-term psychiatric disturbances (15). To accurately determine the effect of donation on living liver donors, it is important for transplant centers to maintain contact with donors long-term. Currently, 2 years of follow-up are mandated for the United Network for Organ Sharing (UNOS) reporting requirements, but may inadequately capture the impact on long-term donor HRQOL and health. In order to acquire these data, state of the art methods need to be implemented to ensure continued donor follow-up and complete data. These methods have not been implemented in most prior studies. One of the barriers to obtaining high-quality long-term data from donors is variable practice patterns for centers following donors after living donation regarding the frequency and duration of visits after LDLT (16). In addition, years after LDLT, donors may be lost to follow-up or difficult to contact due to a real or perceived lack of need for medical care, insurance and financial barriers, or access to care. The issues that determine whether donors will or will not pursue long-term follow-up are likely multifactorial and include donor, recipient, and center factors, but these issues have not been systematically studied.
Using the multi-center A2ALL cohort study we investigated the factors associated with donor contact with their transplant center for the purpose of A2ALL study follow-up. The objective of this study was to assess the characteristics that make donors more likely to maintain contact with their transplant center, with the goal of improving donor follow-up in future clinical care as well as research settings. This is particularly timely as UNOS considers mandating new thresholds for complete follow-up for living donors in the future.
Methods
Data Collection
The A2ALL study collected prospective data on living liver donors and their recipients enrolled at nine US transplant centers between 2004 and 2009. Prospective living liver donors as well as previous living liver donors who donated after January 1, 1998 were eligible for enrollment. According to the clinical A2ALL protocol, donors were scheduled to return to the transplant center for post-donation follow-up at 1 week, 1 month, 3 months, 12 months, and annually thereafter. For donors who enrolled after donation, protocol study visits began at the corresponding post-donation time point; thus, the expected number of visits per donor varied based upon when they were enrolled. The duration of A2ALL follow-up was beyond the standard clinical follow-up protocol for donors at most centers. If the donor was unable to return to the transplant center for a study visit, coordinators attempted to contact subjects via phone and mail, and donors were asked to send local lab results to the transplant center. These methods of remote follow-up are similar to allowable methods of follow-up for the Organ Procurement and Transplantation Network data collection.
For this analysis, completed follow-up was defined as the collection of any data on the donor’s status by any method since the last prospective assessment. The information could be obtained by clinical visit or by telephone, email, post mail or other means if a visit was not possible. Further categorization was made based on whether data were collected during an actual clinic visit versus other methods only. The expected follow-up visit dates were calculated from the transplant date, and visit window endpoints were halfway between adjacent expected visit dates. Expected visits were only included in the analysis if the donor was consented to the study prior to the start of the window and had not withdrawn consent or died prior to the end of the window. This rule was followed regardless of whether or not a visit occurred, because it was too difficult to determine if a visit should be expected if the subject was not enrolled for the entire window. For example, there were 4 protocol visits in the first year (at 1 week, 1 month, 3 months, and 1 year), however, a donor that enrolled in the month 1 window would only have 2 expected visits in the first year due to the timing of enrollment.
For each donor, recipient information, including diagnoses and dates of re-transplant and death, was obtained from the A2ALL recipient database. When such information was not available, this study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
HRQOL surveys, including the SF-36 (v.2) were administered to donors pre-donation. The SF-36 was additionally administered at 3 and 12 months post-donation, and annually thereafter. Donors who enrolled after donation were administered the SF-36 at the first post-donation study visit after enrollment. The HRQOL surveys were administered in several formats (on tablet computers, via paper forms either by mail or at the clinical center, by telephone, or by web-based format) to maximize response. The mental (MCS) and physical component score (PCS) calculated from the SF-36 survey were standardized to the US population distribution (average = 50, standard deviation (SD) = 10).
The study was approved by the Institutional Review Boards and Privacy Boards of the University of Michigan Data Coordinating Center and each of the nine participating transplant centers. All subjects provided written informed consent to this study.
Statistical Analysis
Descriptive statistics included means, standard deviations and proportions. The mean, standard deviation, and interquartile range of the number of expected and completed follow-ups was calculated for the visits from the first week through the first year, every subsequent three year period, and overall. The proportion of donors who completed follow-up at each post-donation study time point was graphed with standard error bars based on the binomial distribution, along with center-specific proportions at each time point for which a given center had at least 5 expected visits. The proportion of donors who completed any type of follow-up (including in-person, phone, or email contact), and the proportion who completed follow-up clinic visits at the transplant center was also graphed by transplant center. Finally, centers were divided into two groups: those who had at least 80% follow-up and those with <80%. The percents of follow-up completed in clinic and by other means were graphed by time point for these two groups.
The probability of completed follow-up was modeled using multivariable repeated measures logistic regression to account for multiple measurements on the same subject over time. Completed follow-up through the first year (month 1, month 3, and year 1) was modeled separately from completed follow-up at year 2 and subsequent annual follow-up (through year 10) because different covariates were important in the earlier vs. later time periods. Week 1 was not included in the models because every subject had completed follow-up at this time. The two highest performing centers were combined because of small cell counts. These centers were both in large cities with ethnically diverse populations. The within-subject correlation across study visits was modeled using a four-parameter Toeplitz covariance structure (16). Variables considered for inclusion included donor demographics (gender, ethnicity, race, age at donation, education level), relationship to recipient, (spouse, immediate family or other), a surrogate for distance from the transplant center (living in the same state or a contiguous state as the transplant center versus a more distant state; only the donor’s state of residence, and not distance from the transplant center, was collected), an indicator variable for each transplant center, transplant center volume (3 year average for the visit year and the two prior years), complications in the first year post-donation (Clavien grade 2 or higher, or Clavien grade 3 or higher), recipient diagnosis (hepatitis C virus (HCV) or hepatocellular carcinoma (HCC), alcohol-related, cholestatic cirrhosis, other), recipient outcomes (graft loss or death in the prior year, or in any previous year), and number of years from donation. To estimate the amount of variation in follow-up explained by center effects we separately modeled the month 3, year 1, and year 2 time points using logistic regression and compared the generalized R-squared statistic between models with and without center effects.
Seven of the nine participating centers (those whose participation continued in the second phase of A2ALL) submitted information on their standard of care follow-up schedule for living liver donors. Indicators for whether follow-up was required at the transplant center beyond one month and two years post-donation were also tested in the models. For the subset of donors for which HRQOL data were available, the PCS and MCS from the SF-36 were tested as potential time-dependent predictors of completed follow-up in a separate logistic regression model. Models were fit using the PCS and MCS from the previous visit to predict completed follow-up at the next visit. In these models, because of the smaller number of donors with HRQOL data and the limited number of usable visits per donor, the within-subject correlation was modeled using the single-parameter compound symmetry covariance structure (16). Variables with p-values less than 0.1 were considered significant for all models described above. All analyses were conducted using SAS 9.2 software (SAS Institute, Inc., Cary, NC).
Results
There were 456 donors included in the analysis, with scheduled post-donation study visits ranging from one week to 10 years post-donation. At donation, mean age was 38 years (range 18–63). The majority was female, white, had education beyond high school, and donated to a spouse or immediate family member (Table 1). The leading diagnoses of the recipients of the living donor grafts included HCV or HCC (45%) and cholestatic cirrhosis (29%). Graft failure (defined as re-transplantation or death) was observed in 25% of recipients during follow-up. Complications occurred in 27% of the donors in the first year post-donation, and 19% had complications with Clavien grade 2 or higher during this time period.
| (a): Characteristics of donors who donated at a center with < 80% or ≥ 80% follow-up (FU) | |||||||
|---|---|---|---|---|---|---|---|
| Overall (n=456) | < 80% FU (n=280) | ≥ 80% FU (n=176) | |||||
| N or Mean |
% or s.d., (range) |
N or Mean |
% or s.d., (range) |
N or Mean |
% or s.d., (range) |
p-value* | |
| Age at Donation | 38.0 | 10.3 (18–63) | 39.2 | 10.4 (18–63) | 36.0 | 9.9 (19–55) | 0.001 |
| Gender | 0.90 | ||||||
| Male | 216 | 47% | 132 | 47% | 84 | 48% | |
| Female | 240 | 53% | 148 | 53% | 92 | 52% | |
| Race | 0.07 | ||||||
| Non-white | 54 | 12% | 27 | 10% | 27 | 15% | |
| White | 402 | 88% | 253 | 90% | 149 | 85% | |
| Ethnicity | 0.05 | ||||||
| Non-Hispanic/Non-Latino | 389 | 85% | 246 | 88% | 143 | 81% | |
| Hispanic/Latino | 67 | 15% | 34 | 12% | 33 | 19% | |
| Total Education | 0.68 | ||||||
| Grade School or High School | 96 | 21% | 59 | 21% | 37 | 21% | |
| Attended College/Technical School | 122 | 27% | 78 | 28% | 44 | 25% | |
| Associate, Bachelor, or Post-college Graduate Degree | 183 | 40% | 122 | 44% | 61 | 35% | |
| Unknown | 55 | 12% | 21 | 8% | 34 | 19% | |
| Relationship to Recipient | 0.09 | ||||||
| Spouse | 53 | 12% | 39 | 14% | 14 | 8% | |
| Immediate Family (Parent, Child, Full-sibling) | 251 | 55% | 145 | 52% | 106 | 60% | |
| Other | 152 | 33% | 96 | 34% | 56 | 32% | |
| Distance from Center | 0.87 | ||||||
| Within One U.S. State of Transplant Center | 367 | 80% | 226 | 81% | 141 | 80% | |
| Greater than One U.S. State from Transplant Center | 89 | 20% | 54 | 19% | 35 | 20% | |
| Complication in the First Year | 124 | 27% | 83 | 30% | 41 | 23% | 0.14 |
| Complication Grade 2 or Higher (in the first year) | 86 | 19% | 61 | 22% | 25 | 14% | 0.04 |
| Complication Grade 3 or Higher (in the first year) | 2 | 0% | 2 | 1% | 0 | 0% | 0.53 |
| Recipient Died | 77 | 17% | 50 | 18% | 27 | 15% | 0.49 |
| Recipient Graft Failure | 114 | 25% | 73 | 26% | 41 | 23% | 0.51 |
| Recipient Diagnosis** | |||||||
| HCV/HCC | 204 | 45% | 115 | 41% | 89 | 51% | 0.05 |
| Alcohol-related Liver Disease | 52 | 11% | 34 | 12% | 18 | 10% | 0.53 |
| Cholestatic Cirrhosis | 130 | 29% | 84 | 30% | 46 | 26% | 0.37 |
| Other Diagnosis | 222 | 49% | 144 | 51% | 78 | 44% | 0.14 |
| (b): Quality of life data for donors who donated at a center with < 80% or ≥ 80% follow-up (FU) | |||||||
|---|---|---|---|---|---|---|---|
| Overall (n=456) | < 80% FU (n=280) | ≥ 80% FU (n=176) | |||||
| N or Mean |
% or s.d., (range) |
N or Mean |
% or s.d., (range) |
N or Mean |
% or s.d., (range) |
p-value* | |
| Number of Subjects with Eligible* SF-36 Forms | 265 | 58% | 151 | 54% | 114 | 65% | 0.02 |
| Average Number of SF-36 Forms per Donor | 2.2 | 1.2 (1–5) | 1.8 | 0.9 (1–4) | 2.6 | 1.3 (1–5) | <0.0001 |
| Average Physical Component Scoreδ | 54.1 | 7.7 (21–70) | 54.1 | 6.8 (23–63) | 54.2 | 8.4 (21–70) | 0.92 |
| Average Mental Component Scoreδ | 52.0 | 9.5 (−1–68) | 52.6 | 8.9 (12–64) | 51.4 | 10.0 (−1–68) | 0.15 |
T-test for continuous variables, chi-square or Fisher’s exact test for categorical variables. Tests compare subjects at centers with <80% FU versus ≥80% FU.
Recipients could have more than one diagnosis so percents may not add up to 100.
Eligible SF-36 forms include forms completed at post-donation visits where the subject had at least one more expected post-donation visit after the visit during which the SF-36 form was completed.
T-test for continuous variables, chi-square or Fisher’s exact test for categorical variables. Tests compare subjects at centers with <80% FU versus ≥80% FU.
The scores are normed to the US population mean and standard deviation (50 and 10, respectively).
Three centers comprising 176 donors had at least 80% follow-up (high follow-up centers) and the remaining 6 centers comprising 280 donors had less than 80% follow-up (low follow-up centers) (Table 1). We chose 80% as a cutoff because it is suggested in the OPTN guidance document for living donor follow-up.17 Donors at high follow-up centers were significantly younger than those at low follow-up centers (mean age was 36.0 years versus 39.2 years, respectively, p=0.001) and had fewer grade 2 or higher complications in the first year (14% versus 22%, p=0.04).
As stated above, donors were expected to attend in-person clinic visits at post-donation times specified by the protocol; any follow-up with the donor, including phone contact, was considered completed follow-up in this analysis. Donors could enroll at any time pre- or post-donation, so the number of expected visits varied by donor, due to differing entry and exit points in the study. Donors had an average of 3.52 completed and 4.45 expected visits (mean 79%) during the time they were enrolled in the study (interquartile ranges 3–5 for both completed and expected) (Table 2). Donors with expected visits in the first year post-donation (n=283) had on average 3.35 completed and 3.56 expected visits (mean 94%) (interquartile ranges both 3–4). In post-donation years 2–4, 5–7, and 8–10 the average numbers of completed divided by expected visits per donor were 1.31/1.97 (mean 66%), 1.42/2.16 (mean 64%), and 0.96/1.80 (mean 51%), respectively.
Table 2.
Distribution of expected visits and completed follow-upδ
| Mean per Subject |
Standard Deviation |
25th Percentile |
75th Percentile |
|
|---|---|---|---|---|
| Overall (n=456 donors) | ||||
| Expected Visits | 4.45 | 1.43 | 3.00 | 5.00 |
| Completed Follow-up | 3.52 | 1.63 | 3.00 | 5.00 |
| Mean of Donor % of Expected Follow-up Completed | 79% | 26% | 60% | 100% |
| Up Through Year 1 (n=283 donors*) | ||||
| Expected Visits | 3.56 | 0.79 | 3.00 | 4.00 |
| Completed Follow-up | 3.35 | 0.88 | 3.00 | 4.00 |
| Mean of Donor % of Expected Follow-up Completed | 94% | 14% | 100% | 100% |
| Years 2–4 (n=239 donors*) | ||||
| Expected Visits | 1.97 | 0.83 | 1.00 | 3.00 |
| Completed Follow-up | 1.31 | 0.94 | 1.00 | 2.00 |
| Mean of Donor % of Expected Follow-up Completed | 66% | 40% | 33% | 100% |
| Years 5–7 (n=180 donors*) | ||||
| Expected Visits | 2.16 | 0.82 | 1.00 | 3.00 |
| Completed Follow-up | 1.42 | 1.02 | 1.00 | 2.00 |
| Mean of Donor % of Expected Follow-up Completed | 64% | 39% | 33% | 100% |
| Years 8–10 (n=89 donors*) | ||||
| Expected Visits | 1.80 | 0.81 | 1.00 | 2.00 |
| Completed Follow-up | 0.96 | 0.95 | 0.00 | 2.00 |
| Mean of Donor % of Expected Follow-up Completed | 51% | 45% | 0% | 100% |
Number of donors who had at least one expected visit in the given time point range.
Donors could consent at any time post-donation, so the number of expected visits within a year for a given donor did not necessarily equal the total number of possible expected protocol study visits within that same year.
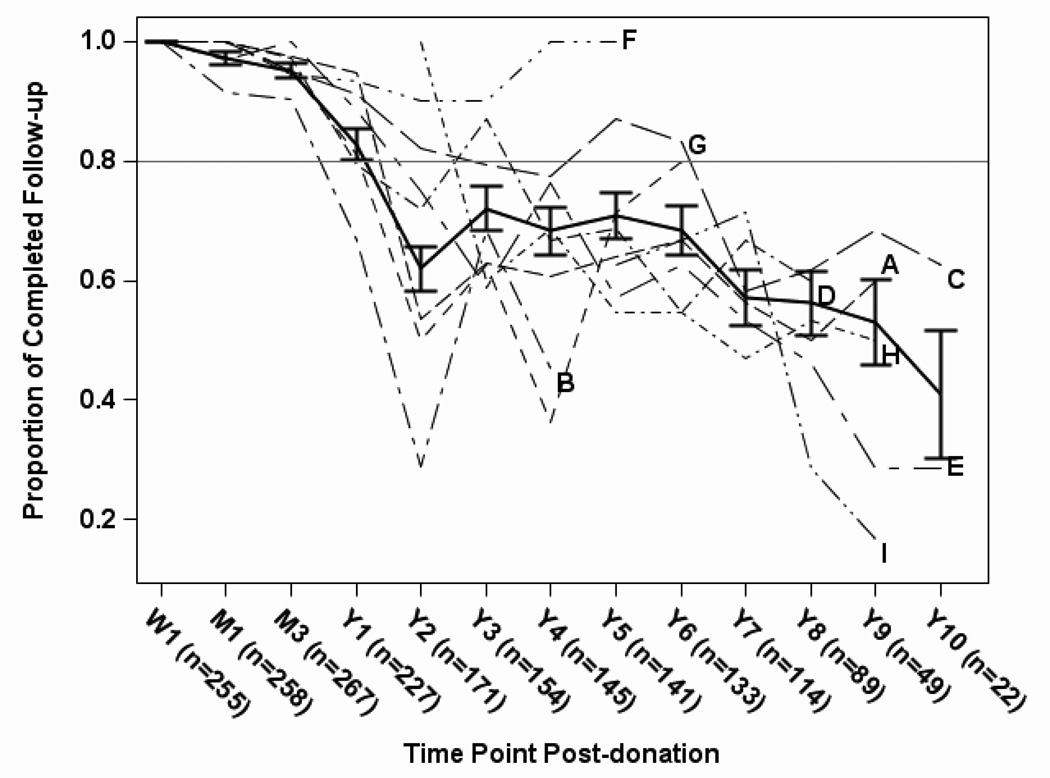
Completed follow-up occurred in more than 90% of protocol study visits in the first several months post-donation, and then dropped to 83% at one year and 62% at two years post-donation (Figure 1). The percent of follow-up completed remained steady between 65% and 75% until 7 years post donation and then decreased steadily until 10 years post-donation. Although there was some variability among the 9 centers, the trends were similar over time.
Figure 1.
Overall proportion of donors with completed follow-up, among those with expected follow-up, by time point (solid line), with ±1 standard error bars. Proportion of donors with completed follow-up by center (dashed or dotted lines) is also shown for the 9 A2ALL centers (labeled A-I) at time points with at least 5 expected visits. Time points post-donation are Week 1 (W1), Month 1 (M1), Month 3 (M3), and Years 1 through 10 (Y1 – Y10).
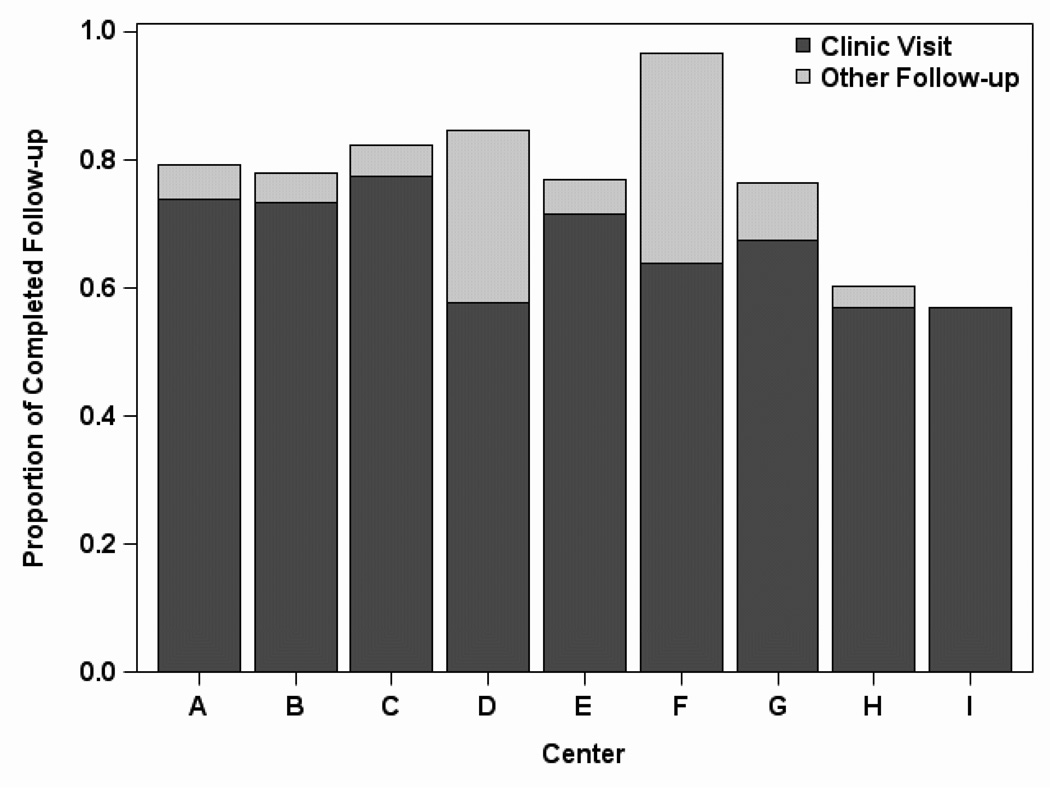
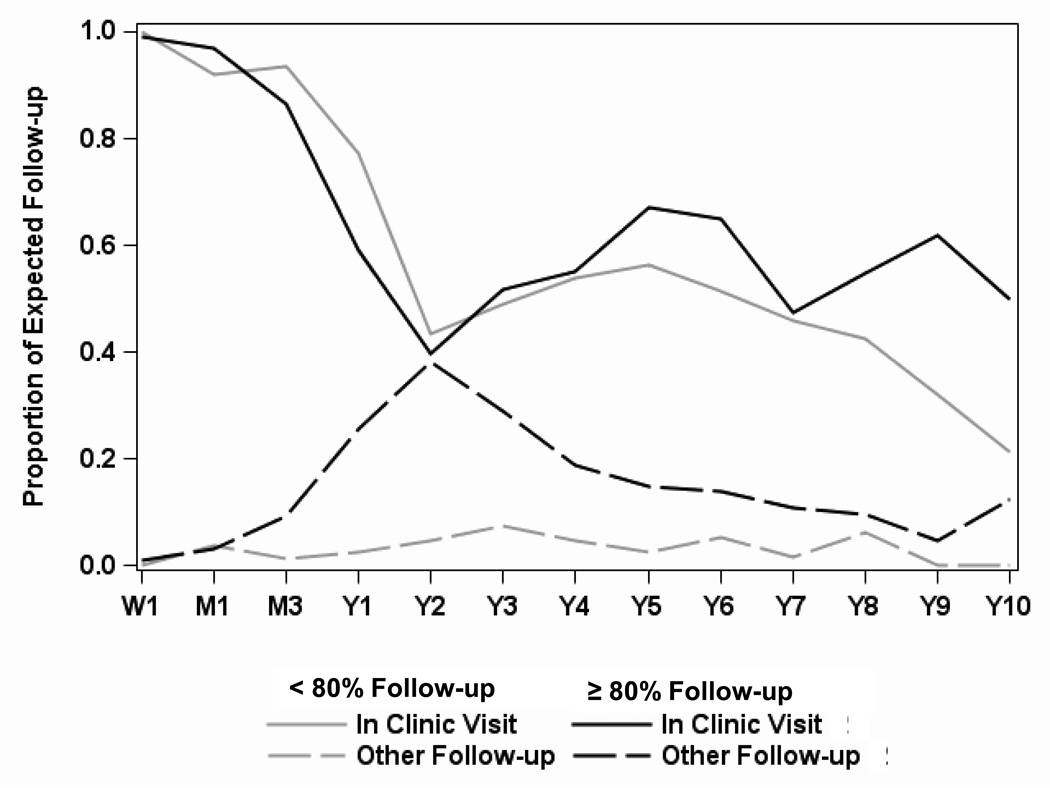
The overall percent of completed follow-up (clinic visits and other follow-up) varied greatly by center, ranging from 57% to 97% (Figure 2). The percent of completed clinic visits was less varied, ranging from 57% to 77%. High and low follow-up centers had a similar proportion of completed in-clinic visits up to year 2, after which high follow-up centers had slightly higher rates of in-clinic follow-up (Figure 3). The proportion of other follow-up rose between month 1 and year 2 for high follow-up centers, compensating for the decreasing in-clinic follow-up, while for low follow-up centers, the proportion of other follow-up remained at about 5% for the duration of the study. Differences in the use of long-distance visits made the biggest contribution to center variability in achieving donor follow-up. The high follow-up centers were able to increase their proportion of follow-up achieved by 10–30% through the use of other methods of follow-up.
Figure 2.
Proportion of expected follow-up achieved by clinic visits and other follow-up methods, by center, for all time points combined. Other follow-up included contact with the donor via phone, email, post mail, or local laboratory testing. Centers are ordered by total living donor volume (descending) during 1998–2010.
Figure 3.
Percent of in-person (top lines) and other (e.g., phone, email; bottom lines) follow-up by groups of centers that averaged < 80% (grey lines) versus ≥ 80% (black lines) of expected follow-up. Time points post-donation are Week 1 (W1), Month 1 (M1), Month 3 (M3), and Years 1 through 10 (Y1 – Y10).
Predictors of completed follow-up in the first year (corresponding to study time points at month 1, month 3, and 1 year post-donation) included race, diagnosis of HCV or HCC, indicators for months since donation, and transplant center (Table 3a). Donors of white race were more likely to complete follow-up during this early time period (odds ratio (OR)=3.27, 95% confidence interval (CI): 1.25–8.58), while donors whose recipients had diagnoses of either HCV or HCC were less likely to complete follow-up (OR=0.34, 95% CI: 0.17–0.69). Donors were more likely to complete follow-up at the first and third months post-donation, compared to the first year post-donation, and follow-up during this early time period varied by center (Table 3a). Centers with higher living donor volumes (in the absence of transplant center indicators) had a significantly lower probability of completed follow-up (OR=0.31 per 10 case higher volume, 95% CI: 0.15–0.66). For the centers where the information was available, there was no statistical evidence that a clinical standard of requiring follow-up beyond one month resulted in donors being more likely to return for follow-up. These estimates remained largely unchanged when prior recipient death and re-transplant were added to the model. Although not significant, prior recipient death and re-transplant were associated with a higher probability of completed follow-up (death OR= 1.33, 95% CI: 0.44–3.97, re-transplant OR=2.29, 95% CI: 0.46–11.46). The results were similar when recipient graft failure (defined as first of death or re-transplant) was included in the model (OR=1.74, 95% CI: 0.64–4.70).
| (a): Probability of completed follow-up in the first year, modeled using repeated measures logistic regression | ||||
|---|---|---|---|---|
| Predictor | Odds Ratio |
Lower 95% Confidence Limit |
Upper 95% Confidence Limit |
P-value* |
| White Race (Ref=Non-white) | 3.27 | 1.25 | 8.58 | 0.0160 |
| Recipient Diagnosis of HCV or HCC (Ref=All other diagnoses) | 0.34 | 0.17 | 0.69 | 0.0028 |
| Time Post-donation (Ref=Year 1) | ||||
| Month 1 | 9.23 | 4.21 | 20.24 | <.0001 |
| Month 3 | 4.84 | 2.55 | 9.16 | <.0001 |
| Centerβ (Ref=overall mean) | ||||
| A/H | 3.31 | 1.05 | 10.45 | 0.0412 |
| B | 0.21 | 0.11 | 0.40 | <.0001 |
| C | 1.26 | 0.54 | 2.95 | 0.5931 |
| D | 0.88 | 0.35 | 2.24 | 0.7883 |
| E | 1.73 | 0.57 | 5.29 | 0.3349 |
| F | 1.36 | 0.23 | 8.13 | 0.7334 |
| G | 0.55 | 0.20 | 1.54 | 0.2549 |
| (b): Probability of completed follow-up in years 2–10, modeled using repeated measures logistic regression | ||||
|---|---|---|---|---|
| Predictor | Odds Ratio |
Lower 95% Confidence Limit |
Upper 95% Confidence Limit |
P-value* |
| Donor Age at Donation (per 10 years) | 1.20 | 1.02 | 1.41 | 0.0310 |
| Time Post-donation (Ref=Year 2) | ||||
| Year 3 | 1.42 | 0.88 | 2.30 | 0.1528 |
| Year 4 | 1.13 | 0.68 | 1.88 | 0.6302 |
| Year 5 | 1.11 | 0.65 | 1.90 | 0.6911 |
| Year 6 | 0.93 | 0.55 | 1.57 | 0.7924 |
| Year 7 | 0.47 | 0.28 | 0.80 | 0.0052 |
| Year 8 | 0.48 | 0.27 | 0.86 | 0.0138 |
| Year 9 | 0.39 | 0.20 | 0.77 | 0.0064 |
| Year 10 | 0.24 | 0.10 | 0.59 | 0.0018 |
| Center (Ref=overall mean) | ||||
| A | 0.69 | 0.45 | 1.05 | 0.0818 |
| B | 0.29 | 0.17 | 0.47 | <.0001 |
| C | 1.76 | 1.19 | 2.60 | 0.0049 |
| D | 1.13 | 0.71 | 1.78 | 0.6102 |
| E | 0.80 | 0.50 | 1.30 | 0.3694 |
| F | 10.11 | 3.19 | 32.00 | 0.0001 |
| G | 0.49 | 0.27 | 0.89 | 0.0195 |
| H | 0.77 | 0.55 | 1.08 | 0.1259 |
| I | 0.84 | 0.60 | 1.17 | 0.3068 |
One center was excluded from this model because it did not have any new donors during the prospective era.
Predictors of completed follow-up beyond the first year included age at donation, indicators for the number of years post-donation, and transplant center (Table 3b). Older donors were more likely to complete follow-up (OR=1.20 per 10 years, 95% CI: 1.02–1.41). The probabilities of completed follow-up in the third, fourth, and fifth year post-donation were similar to the second year, but from the sixth year on, follow-up became less and less likely. Again, completed follow-up varied greatly by transplant center (OR range 0.29–10.11 compared to the overall mean). Unlike follow-up in the first year, however, center living donor volume was not significantly associated with completed follow-up when tested in the absence of center indicators (OR=0.86 per 10 cases higher volume, 95% CI: 0.64–1.17). An indicator for whether the center clinical standard required follow-up beyond two years was also tested, and again showed no relationship between this clinical standard and higher rates of follow-up. Similar to the early post-donation analysis, the addition of prior recipient death and re-transplant did not substantively change the estimates. Recipient death was associated with no change in the probability of completed follow-up (OR=1.00, 95% CI: 0.60–1.66) and recipient re-transplant was associated with a non-significantly higher probability of completed follow-up (OR=1.44, 95% CI: 0.84–2.49). The results were also similar for recipient graft failure (OR=1.10, 95% CI: 0.73–1.67).
Because center effects were highly significant in both early and late time point models, we investigated the amount of variation explained by center effects compared to other variables, separately by time point in logistic models for three month, one year, and two year time points. In the three month model, the generalized R-squared values with and without center effects were 0.07 and 0.05, respectively. The corresponding values at the year one time point were 0.13 and 0.03, and for the two year time point were 0.22 and 0.02. These results demonstrate an increasing effect of individual center practice as time from donation increases. By two years, center effects were tenfold the effect of all other covariates.
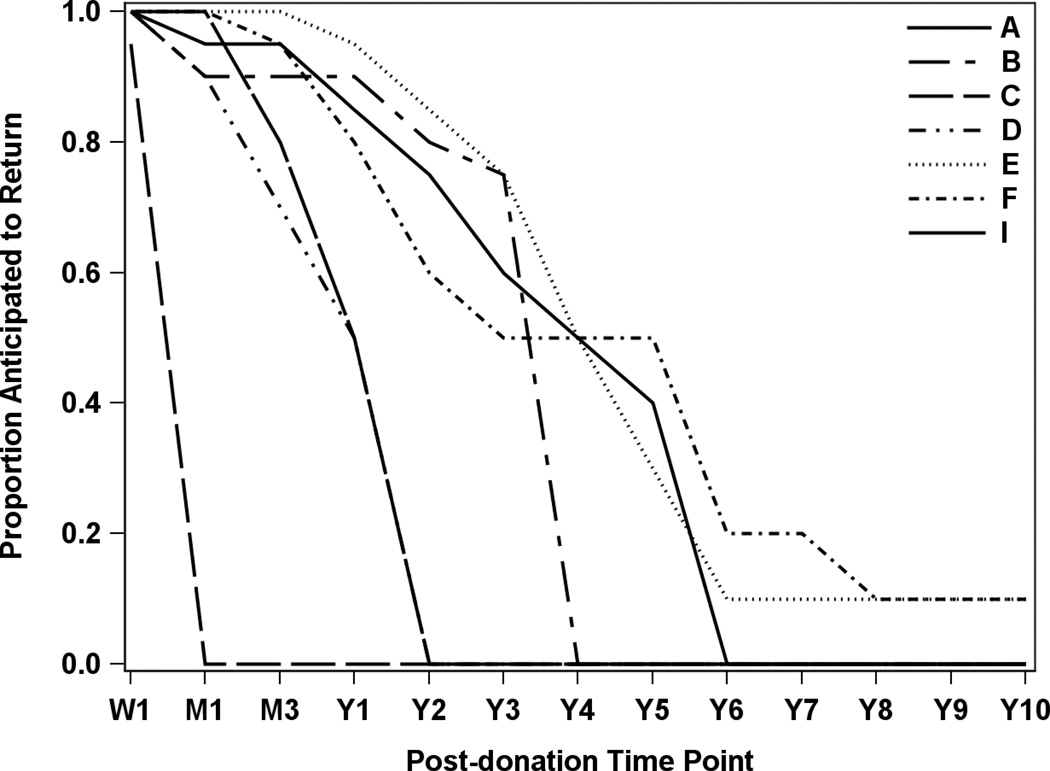
Because A2ALL was an observational study, we postulated that center variation in donor follow-up might be related to differences in the standard of care for follow-up of nonA2ALL donors. Though all centers and donors agreed to follow the A2ALL protocol, the degree of rigor and resources used to achieve donor follow-up might vary according to the center standard of care. Thus, the centers were surveyed to determine their standard of care in long-term donor follow-up and seven centers responded. Figure 4 also demonstrates that center practice differed in how long donors were expected to return to the clinic, with one center expecting drop-off after the first week while others expected donors to return out to ten years post-donation. Although the highest performing center (Center F) expected annual follow-up as part of their clinical standard of care through 10 years post-donation, even centers whose standard of care did not follow donors beyond two years were able to achieve around 80% overall follow-up in the A2ALL study. Thus standard of care practice did not fully explain center variability in A2ALL follow-up success.
Figure 4.
Anticipated follow-up based on usual clinical practice as reported by center. Of the nine A2ALL centers, data were not available for two centers that were no longer participating in the study at the time of the analysis.
For the subset of 265 donors who completed at least one SF-36 form post-donation and had a subsequent expected visit, PCS and MCS from the SF-36 were tested as potential predictors of completed follow-up. These donors had a total of 475 forms (average 2.2 per donor, range 1–5). The average PCS and MCS for this subset of post-donation forms were 54.1 and 52.0, respectively (ranges 21 to 70 and −1 to 68), with higher scores indicating better functioning. Although the ranges are wide, the means are above the general population mean of 50, but within one standard deviation (SD = 10). Higher PCS was marginally associated with a lower probability of completed follow-up (OR=0.74 per 10 unit increase, or one SD increase in a normative population, 95% CI: 0.55–1.00). No significant associations between completed follow-up and mental component score were found (OR=0.99 per 10 unit increase, or one SD increase in a normative population, 95% CI: 0.79–1.23).
Discussion
In summary, living liver donors were very compliant with follow-up for the first year, but follow-up decreased over time in a non-linear manner. From years two to seven, the proportion of completed visits was approximately 70%. There were substantial center differences in completed visits. Though every center agreed to follow the A2ALL protocol, which required annual donor visits to the transplant center, the standard clinical protocol for donor follow-up at each center varied, particularly after the first year. Additionally centers varied in their use of methods for obtaining information indirectly, i.e. telephone and distant visits outside the transplant center.
Patients who missed follow-up did not differ in many ways from those who completed follow-up, arguing against a substantial systemic bias in analyzing long-term donor outcomes in those that follow-up. However, there were some differences. Donors who missed follow-up in the long-term were more likely to be younger and have a higher PCS on their SF-36 scores. This suggests that donors may be less likely to follow up if they are healthier and do not perceive a need for care. This would suggest that the data gathered among donors who do follow-up would lead to a lower HRQOL than the overall target population of all donors, which is reassuring. Non-white race also predicted lower rates of completed follow-up, as whites were over three times more likely to follow-up during the first year post donation. Though we did not collect socioeconomic data, this may highlight potential economic or cultural barriers to follow-up. Distance from the transplant center could not be calculated as we only collected state of residence as a variable. When analyzed as same state or contiguous state vs. more than one state from the transplant center, this was not predictive of follow-up. However, distances between states vary widely in the different areas between the A2ALL Centers; thus, actual distance may be an unmeasured factor included in the center differences measured. Recipient diagnosis of HCV or HCC also predicted lower rates of follow-up with an odds ratio of 0.34. The reasons for this three-fold lower odds of donor follow-up are unclear. Though recipient complications and adverse outcomes did not predict follow-up, recipient diagnosis may be a marker for the higher rates of complications due to recurrence in HCV and HCC patients or other unmeasured factors. This phenomenon needs further exploration.
Our data concur with prior data suggesting that QOL was at or above US norms on a general QOL survey (11) and that virtually all were satisfied with their decision to donate, irrespective of recipient outcomes (9,10). Severe psychiatric disturbances have been reported in some living donors from our group, however, suggesting that ongoing long-term follow-up is needed (15). Interestingly centers that achieved ≥ 80% follow-up had donors that were on average younger and had fewer higher grade complications (Table 1); these are all factors associated in individual donors with lower probability of follow-up (Tables 3a and 3b). This suggests that some centers are able to overcome the barriers to follow-up and achieve higher rates of long-term follow-up in spite of covariate mixes that would suggest the opposite. This deserves further investigation.
Our data are limited by the fact that we cannot attribute the reasons for lack of donor follow-up convincingly. Though centers had agreed to pursue follow-up through the study, there was inter- and intra-center variability. It is also not possible to derive the reason for lack of follow-up without contact with those donors (perhaps by an outside party, e.g. UNOS) to assess these reasons. Despite these limitations, quantifying the proportion of donor follow-up is important in understanding our limitations in assessing long-term donor outcomes. As a National Institutes of Health-funded study, it is likely that our efforts at donor follow-up exceed what is currently achievable without a change in incentives at the transplant center level, or centralized follow-up by the OPTN or Department of Health and Human Services. But the ability of some centers to achieve near complete (97%) donor follow-up demonstrates that adoption of best practices can improve donor follow-up and that ≥ 80% can be achieved as suggested in the UNOS guidance document17 with a combination of motivating donors less likely to follow-up (younger, healthier donors) and using telephone (or other long-distance contact) in addition to clinic visits. Though it is likely that physical follow-up at the transplant center provides more valuable information than phone contact, any contact and particularly QOL data is useful and should be encouraged. It is important to note that there was nearly a 1.5 fold range in physical visits between centers, in addition to the nearly 2-fold range in follow-up when phone contact was included. With increased reliability on telemedicine and social networking, this represents a significant opportunity to gain valuable information about long-term donor outcomes and should be included in future research and in OPTN policy. It is important to note that no centers achieved ≥ 80% follow-up with actual physical clinic visits alone after the first year post-donation.
In summary, these data suggest that donor follow-up is excellent in the short run and declines over time. A large portion of the variability in donor follow-up is related to center differences and could be improved with protocols for long-distance donor follow-up, including phone, cooperation with primary care providers and perhaps a telemedicine initiative. The fact that donors who missed follow-up visits were more likely to have higher physical QOL and be of younger age reassures us that we may not be missing substantial complications. However, longer follow-up and improved center protocols for that follow-up are clearly needed to improve both our knowledge of long-term complications post donation, as well as to ensure the long-term health of those donors. Future research should also compare the quality of data and value of long-distance initiatives compared with physical return to the transplant sites as ways to improve the quality of follow-up and decrease the burden on donors and centers.
Acknowledgements
The data reported here have been supplied by Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Disclosure
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531, and U01-DK62536). Additional support was provided by Health Resources and Services Administration (HRSA), and the American Society of Transplant Surgeons (ASTS).
Abbreviations
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation Cohort Study
- HCV
hepatitis C virus
- HCC
hepatocellular carcinoma
- HRQOL
Health-related quality of life
- LDLT
living donor liver transplantation
- MCS
mental component score
- PCS
physical component score
- OPTN
Organ Procurement and Transplantation Network
- UNOS
United Network for Organ Sharing
- OR
odds ratio
- CI
confidence interval
Appendix
The A2ALL Study Group includes Northwestern University, Chicago, IL; University of California – Los Angeles, CA; University of California – San Francisco, CA; University of Colorado Health Sciences Center, Denver, CO; University of North Carolina, Chapel Hill, NC; Epidemiology and Clinical Trials Branch, Division of Digestive Diseases and Nutrition, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD; University of Michigan, Ann Arbor, MI; Department of Surgery, Columbia Presbyterian Medical Center, New York, NY; University of Pennsylvania, Philadelphia, PA; Department of Internal Medicine, University of Virginia, Charlottesville, VA; Virginia Commonwealth University, Richmond, VA.
Footnotes
This study was presented in part at the 12th annual meeting of the American Transplant Congress, Boston, MA, June 2–6, 2011.
References
- 1.Miller C, Florman S, Kim-Schluger L, et al. Fulminant and fatal gas gangrene of the stomach in a healthy live liver donor. Liver Transpl. 2004;10:1315–1319. doi: 10.1002/lt.20227. [DOI] [PubMed] [Google Scholar]
- 2.Brown RS, Jr, Russo MW, Lai M, et al. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818–825. doi: 10.1056/NEJMsa021345. [DOI] [PubMed] [Google Scholar]
- 3.Ghobrial RM, Saab S, Lassman C, et al. Donor and recipient outcomes in right lobe adult living donor liver transplantation. Liver Transpl. 2002;8:901–909. doi: 10.1053/jlts.2002.35548. [DOI] [PubMed] [Google Scholar]
- 4.Trotter JF, Adam R, Lo CM, Kenison J. Documented deaths of hepatic lobe donors for living donor liver transplantation. Liver Transpl. 2006;12:1485–1488. doi: 10.1002/lt.20875. [DOI] [PubMed] [Google Scholar]
- 5.Beavers KL, Sandler RS, Shrestha R. Donor morbidity associated with right lobectomy for living donor liver transplantation to adult recipients: a systematic review. Liver Transpl. 2002;8:110–117. doi: 10.1053/jlts.2002.31315. [DOI] [PubMed] [Google Scholar]
- 6.Ghobrial RM, Freise CE, Trotter JF, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008;135:468–476. doi: 10.1053/j.gastro.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abecassis MM, Fisher RA, Olthoff KM, et al. Complications of Living Donor Hepatic Lobectomy-A Comprehensive Report. Am J Transplant. 2012;12:1208–1217. doi: 10.1111/j.1600-6143.2011.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DuBay DA, Holtzman S, Adcock L, et al. Adult right-lobe living liver donors: quality of life, attitudes and predictors of donor outcomes. Am J Transplant. 2009;9:1169–1178. doi: 10.1111/j.1600-6143.2009.02614.x. [DOI] [PubMed] [Google Scholar]
- 9.Pascher A, Sauer IM, Walter M, et al. Donor evaluation, donor risks, donor outcome, and donor quality of life in adult-to-adult living donor liver transplantation. Liver Transpl. 2002;8:829–837. doi: 10.1053/jlts.2002.34896. [DOI] [PubMed] [Google Scholar]
- 10.Trotter JF, Talamantes M, McClure M, et al. Right hepatic lobe donation for living donor liver transplantation: impact on donor quality of life. Liver Transpl. 2001;7:485–493. doi: 10.1053/jlts.2001.24646. [DOI] [PubMed] [Google Scholar]
- 11.Kim-Schluger L, Florman SS, Schiano T, et al. Quality of life after lobectomy for adult liver transplantation. Transplantation. 2002;73:1593–1597. doi: 10.1097/00007890-200205270-00012. [DOI] [PubMed] [Google Scholar]
- 12.Miyagi S, Kawagishi N, Fujimori K, et al. Risks of donation and quality of donors' life after living donor liver transplantation. Transpl Int. 2005;18:47–51. doi: 10.1111/j.1432-2277.2004.00028.x. [DOI] [PubMed] [Google Scholar]
- 13.Dew MA, Zuckoff A, DiMartini AF, et al. Prevention of poor psychosocial outcomes in living organ donors: from description to theory-driven intervention development and initial feasibility testing. Prog Transplant. 2012;22:280–292. doi: 10.7182/pit2012890. quiz 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verbesey JE, Simpson MA, Pomposelli JJ, et al. Living donor adult liver transplantation: a longitudinal study of the donor's quality of life. Am J Transplant. 2005;5:2770–2777. doi: 10.1111/j.1600-6143.2005.01092.x. [DOI] [PubMed] [Google Scholar]
- 15.Trotter JF, Hill-Callahan MM, Gillespie BW, et al. Severe psychiatric problems in right hepatic lobe donors for living donor liver transplantation. Transplantation. 2007;83:1506–1508. doi: 10.1097/01.tp.0000263343.21714.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beavers KL, Cassara JE, Shrestha R. Practice patterns for long-term follow-up of adult-to-adult right lobectomy donors at US transplantation centers. Liver Transpl. 2003;9:645–648. doi: 10.1053/jlts.2003.50123. [DOI] [PubMed] [Google Scholar]
- 17.Guidance for Developing and Implementing Procedures to Collect Post-Donation Follow-up Data from Living Donors. [Accessed March 31, 2014];2013 Revised and Updated 2013. http://optn.transplant.hrsa.gov/ContentDocuments/Guidance_Post_Living_Donor_Follow_Up.pdf.