Abstract
JAK2V617F is the most common mutation found in Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs). Although a majority of MPN patients carry heterozygous JAK2V617F mutation, loss of heterozygosity (LOH) on chromosome 9p involving the JAK2 locus has been observed in ~30% of MPN patients. JAK2V617F homozygosity via 9pLOH has been associated with more severe MPN phenotype. However, the contribution of 9pLOH in the pathogenesis of MPNs remains unclear. To investigate the roles of wild-type JAK2 (JAK2 WT) and JAK2V617F alleles in the development of MPNs, we have utilized conditional Jak2 knock-out and Jak2V617F knock-in mice and generated heterozygous, hemizygous and homozygous Jak2V617F mice. Whereas heterozygous Jak2V617F expression results in a polycythemia vera-like MPN in mice, loss of Jak2 WT allele in hemizygous or homozygous Jak2V617F mice results in markedly increased white blood cells, neutrophils, reticulocytes and platelets in the peripheral blood, and significantly larger spleen size compared with heterozygous Jak2V617F mice. Hemizygous or homozygous Jak2V617F mice also exhibit accelerated myelofibrosis compared with mice expressing heterozygous Jak2V617F. Together, these results suggest that loss of Jak2 WT allele increases the severity of the MPN. Thus, the Jak2 WT allele functions as a negative regulator of MPN induced by Jak2V617F.
Keywords: Myeloproliferative neoplasms, myelofibrosis, JAK2, JAK2V617F
INTRODUCTION
Philadelphia chromosome (Ph)-negative myeloproliferative neoplasms (MPNs) are a group of clonal stem cell-derived hematologic malignancies characterized by overproduction of myeloid lineage cells. A somatic mutation (V617F) in the JAK2 tyrosine kinase was found in a majority of patients with Ph-negative MPNs, including ~95% cases of polycythemia vera (PV) and 50–60% of patients with essential thrombocythemia (ET) and primary myelofibrosis (PMF).1–5 Although most patients with MPNs harbor heterozygous JAK2V617F mutation, a significant portion (~30%) of MPN patients exhibits loss of heterozygosity (LOH) on chromosome 9p (9pLOH) involving the JAK2 locus.1–4, 6 The 9pLOH results in the loss of JAK2 WT allele and concomitant duplication of the JAK2V617F mutant allele via mitotic recombination.1–4 Interestingly, 9pLOH is more common in PMF and PV than in ET.1–4
It has been found that the MPN patients with 9pLOH or homozygous JAK2V617F mutation have significantly increased white blood cell (WBC) counts and larger spleen size.7–9 In addition, 9pLOH or homozygous JAK2V617F mutation has been associated with increased risk of developing myelofibrosis,8, 9 suggesting that loss of JAK2 WT allele might contribute to the severity of MPN. However, the role of JAK2 WT allele in the pathogenesis of MPNs induced by JAK2V617F has remained unclear.
Previously, we and other groups have generated Jak2V617F knock-in mouse models of MPN.10–13 Expression of heterozygous mouse Jak2V617F is sufficient to induce a PV-like MPN in Jak2V617F knock-in mice.10–12 Thus, heterozygous Jak2V617F plays a causal role in induction of PV. To determine the role of JAK2 WT in the development of MPN induced by JAK2V617F, we have crossed our conditional Jak2V617F knock-in mice10 with floxed Jak2 mice14 and generated heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) and homozygous (Jak2VF/VF) Jak2V617F mice. Phenotypic characterization of these mice indicates that loss of Jak2 WT allele increases the severity of the MPN and accelerates myelofibrosis in mice expressing Jak2V617F. Thus, our results suggest that Jak2 WT may act as a suppressor of MPN induced by Jak2V617F.
MATERIALS AND METHODS
Mice
Conditional Jak2V617F knock-in mice were generated as previously described.10 The Jak2 floxed mouse14 was kindly provided by Dr. Wagner (University of Nebraska, USA). MxCre mouse15 (purchased from the Jackson Laboratory) was crossed to Jak2V617F and Jak2 floxed mice to generate MxCre;Jak2VF/+ (heterozygous), MxCre;Jak2VF/− (hemizygous) and MxCre;Jak2VF/VF (homozygous) mice. All mice used in this study were in a C57BL/6 background. Cre expression was induced by intraperitoneal injection of 3 doses of 300 µg of polyinosine-polycytosine (pI-pC, GE Healthcare, Piscataway, NJ, USA). All animal studies were approved by the Institutional Animal Care and Use Committee of SUNY Upstate Medical University.
Real-time quantitative PCR and allelic ratio
RNA was isolated from the BM using RNeasy Mini Kit (Qiagen, CA, USA) followed by cDNA synthesis using a reverse transcription kit (Qiagen, CA, USA). Real-time quantitative PCR was performed using the SYBR Green PCR Master mix (Qiagen, CA, USA) and a set of primers that amplify a 182-bp segment of Jak2 cDNA including Jak2V617F, as described previously.10 18S was used for normalization of Jak2 expression level. Real-time quantitative PCR was performed using a LightCycler 480 (Roche Applied Science, Indianapolis, IN, USA) and analyzed with associated software. Relative expression values were calculated by the ΔΔCT method using BM samples from wild type mice as the calibrator. The allelic ratio of mutant Jak2V617F to wild-type Jak2 in heterozygous Jak2V617F mice was determined by the T/G ratio as we described previously.10 The T peak identifies the Jak2V617F mutant allele whereas the G peak identifies the wild-type Jak2 allele.
Blood and tissue analysis
Peripheral blood cell counts were determined using Hemavet 950FS (Drew Scientific, Oxford, CT, USA). Blood smears were stained with Wright-Giemsa, and reticulocytes were enumerated by New Methylene Blue staining. For histopathologic analysis, mouse tissue specimens were fixed in 10% neutral-buffered formalin and embedded in paraffin. Tissue sections (4 µm) were stained with hematoxylin and eosin (H&E) and reticulin stains.
Flow cytometry
Bone marrow (BM) and spleen cells were stained with either PE- or APC-conjugated monoclonal antibodies specific for Ter119, CD71, CD61, CD41, Mac-1, Gr-1, B220 or Thy-1 (purchased from eBioscience or Biolegend, San Diego, CA, USA) for 20 min on ice. Flow cytometry was performed with an LSRII (Beckton-Deckinson, San Diego, CA, USA) and analyzed by using FlowJo software (TreeStar, Ashland, OR, USA). For stem/progenitor analysis, BM or spleen cells were stained with antibodies against c-Kit, Sca-1, CD34, CD16/32 (FcγR II/III), and antibodies against lineage (Lin) markers including CD3, CD4, CD8α, CD19, B220, Gr-1, CD127 and Ter119 (from eBioscience or Biolegend, San Diego, CA, USA) for 1 hour on ice followed by flow cytometric analysis.
Colony-forming assay
BM and spleen cells were plated in duplicate in complete methylcellulose medium (MethoCult M3434; StemCell Technologies, Vancouver, Canada). Burst-forming units-erythroid (BFU-E) and granulocyte-macrophage colony-forming unit (CFU-GM) colonies were scored on day 7. To detect erythropoietin (EPO)-independent or EPO-dependent colony-forming unit-erythroid (CFU-E) colonies, BM or spleen cells were plated in duplicate in methylcellulose medium (MethoCult M3234; StemCell Technologies, Vancouver, Canada) in the absence of cytokine or in the presence of EPO (3U/ml). CFU-E colonies were counted after 2 days by staining with benzidine solution (Sigma-Aldrich, St. Louis, MO, USA). To detect colony-forming unit megakaryocyte (CFU-Mk), BM cells were plated in collagen-based media (MegaCult; StemCell Technologies, Vancouver, Canada) in the presence of IL-3, IL-6, IL-11 and TPO, and colonies were scored after 8 days by staining with acetylcholiniodide and ferricyanide solutions according to the manufacturer’s protocol.
Primary cell culture and immunoblotting
For megakaryoblast culture, BM lineage negative cells from control (Jak2+/+), heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) and homozygous Jak2V617F (Jak2VF/VF) mice were enriched using a lineage depletion kit (Miltenyi Biotec, Auburn, CA, USA) and were cultured in StemPro medium containing 20 ng/ml stem cell factor (SCF) and 50 ng/ml thrombopoietin (TPO) for 4–5 days as previously described.16 Primary erythroblasts were generated from the BM as previously described.10 For signaling studies, megakaryoblasts or erythroblasts were starved for 6 hr in IMDM medium containing 0.5% BSA at 37°C and cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer (50mM Tris-HCl, 150mM NaCl, 1% Triton X-100, 1% Sodium deoxycholate, 0.1% SDS, 2mM Na3VO4, 5mM NaF, 100 µg/ml PMSF and protease inhibitor cocktail [Sigma-Aldrich, St. Louis, MO, USA]). Immunoblotting was performed using phospho-specific antibodies against Stat5, Akt, or Erk1/2 (Cell signaling Technology, MA), or antibodies against total Jak2, Stat5, Akt or Erk2 (Cell signaling Technology, MA or Santa Cruz Biotechnology, CA). β-actin was used as a loading control.
Statistical analysis
Results are expressed as mean ± SEM, and data were analyzed by the two-tailed Student’s t-test. P <0.05 was considered to be statistically significant.
RESULTS
Loss of Jak2 WT allele increases the severity of MPN in Jak2V617F knock-in mice
To investigate the role of Jak2 WT in MPN induced by Jak2V617F mutation, we generated heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) or homozygous (Jak2VF/VF) Jak2V617F mice. First, we assessed the expression of total Jak2 mRNA in control (Jak2+/+), Jak2VF/+, Jak2VF/− and Jak2VF/VF mice using quantitative real-time PCR. As expected, expression of total Jak2 mRNA was comparable between control, Jak2VF/+ and Jak2VF/VF animals, whereas Jak2 expression level in hemizygous (Jak2VF/−) mice was almost half of that observed in control, heterozygous (Jak2VF/+) or homozygous (Jak2VF/VF) mice (Figure 1a). We also assessed the level of expression of Jak2 WT and Jak2V617F alleles in different hematopoietic compartments of heterozygous Jak2V617F knock-in mice. We observed almost equal levels of expression of Jak2 WT and Jak2V617F alleles in early hematopoietic progenitors (LSK, MEP) as well as in myeloid (Gr-1), erythroid (Ter119), megakaryocytic (CD41) and lymphoid (B220, Thy-1) precursors of heterozygous Jak2V617F knock-in mice in a pure C57BL/6 background (Supplementary Figure S1). This is in contrast to the 129Sv/C57BL/6 mixed background heterozygous Jak2V617F mice in which the expression of Jak2V617F mutant allele was almost half of the Jak2 WT allele as reported previously.10
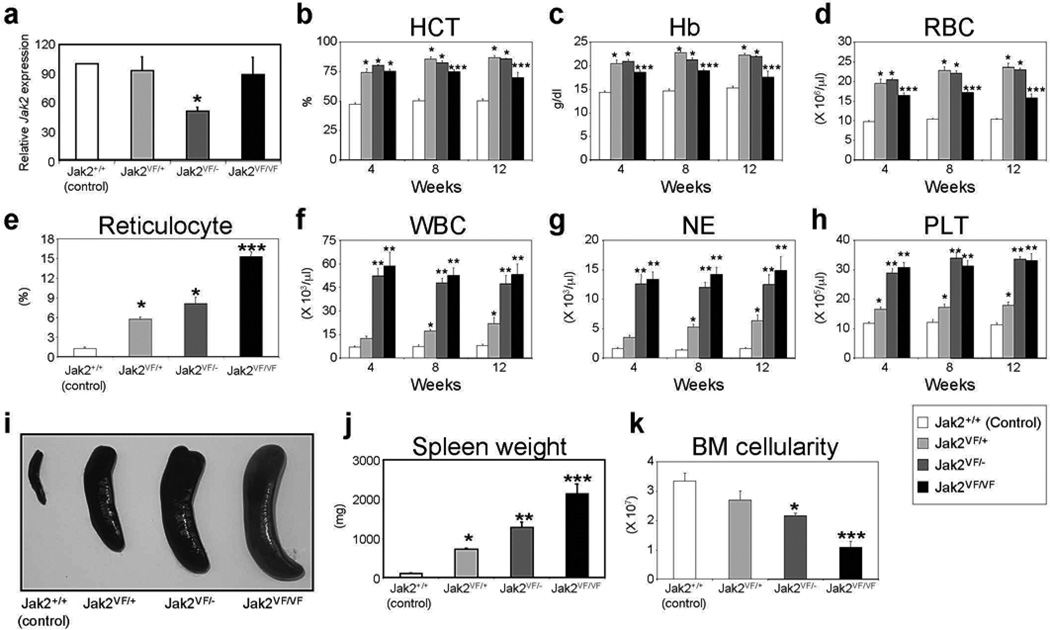
Figure 1. Deletion of Jak2 WT allele increases the severity of MPN in Jak2V617F knock-in mice.
(a) Quantitative real-time PCR analysis of total Jak2 expression in the BM of control (Jak2+/+), heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) or homozygous (Jak2VF/VF) mice. Peripheral blood hematocrit (b), hemoglobin (c), RBC (d), reticulocyte (e), WBC (f), neutrophil (g) and platelet (h) counts were assessed at 4, 8, and 12 weeks after pI-pC induction in control (Jak2+/+), heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) or homozygous (Jak2VF/VF) mice; (n = 10). Spleen size (i) and weight (j) were significantly increased in mice expressing heterozygous Jak2V617F. Deletion of Jak2 WT allele further enhanced splenomegaly in hemizygous (Jak2VF/−) or homozygous (Jak2VF/VF) mice (n = 10). (k) BM cellularity (total BM cell count in femur and tibia) was significantly reduced in hemizygous and homozygous Jak2V617F mice at 12 weeks after pI-pC induction. Asterisks indicate significant differences (p < 0.05) (* indicates significance when compared with controls; ** indicates significance when compared with controls and heterozygous Jak2VF/+; *** indicates significance when compared with controls, heterozygous Jak2VF/+ and hemizygous Jak2VF/−).
Compared with littermate control animals, heterozygous Jak2V617F mice exhibited increased hematocrit, hemoglobin, red blood cells (RBC), reticulocytes, white blood cells (WBC), neutrophils, and platelets in the peripheral blood within 4 weeks after pI-pC injection and those were sustained for over 12 weeks (Figures 1b-h), as we previously observed.10 However, hemizygous and homozygous Jak2V617F mice showed significantly more reticulocytes, WBC, neutrophils and platelets compared with heterozygous Jak2V617F animals (Figures 1e-h). Notably, deletion of one allele of wild-type Jak2 in heterozygous Jak2 mice (Jak2+/−) did not cause significant alteration in peripheral blood counts (Supplementary Figure S2), suggesting that one allele of wild-type Jak2 is sufficient to maintain normal hematopoiesis.
Heterozygous Jak2V617F mice exhibited significantly larger spleen size/weight compared with control animals (Figures 1i and j), as we also previously observed.10 Hemizygous and homozygous Jak2V617F mice showed significantly greater splenic enlargement, with 1275 ± 136 mg and 2127 ± 247 mg for Jak2VF/− and Jak2VF/VF mice respectively, compared with 715 ± 20 mg for heterozygous Jak2VF/+ mice at 12 weeks after pI-pC induction (Figures 1i and j). Conversely, BM cellularity (total BM cell counts within femur and tibia) was significantly reduced in hemizygous and homozygous Jak2V617F mice compared with control or heterozygous Jak2V617F animals (Figure 1k).
We also observed increased incidence of deaths in hemizygous and homozygous mice. Whereas control and heterozygous Jak2V617F mice survived for long periods of time with occasional death in the heterozygous group, a significant proportion (~50%) of hemizygous or homozygous mice died within 9–12 weeks after induction (data not shown), suggesting that loss of Jak2 WT allele increases fatality in Jak2V617F knock-in mice. The cause of increased deaths in hemizygous or homozygous mice is, however, not clear. Overall, these results suggest that loss of Jak2 WT allele increases the severity of MPN.
Deletion of Jak2 WT allele causes further expansion of erythroid and megakaryocytic precursors in Jak2V617F knock-in mice
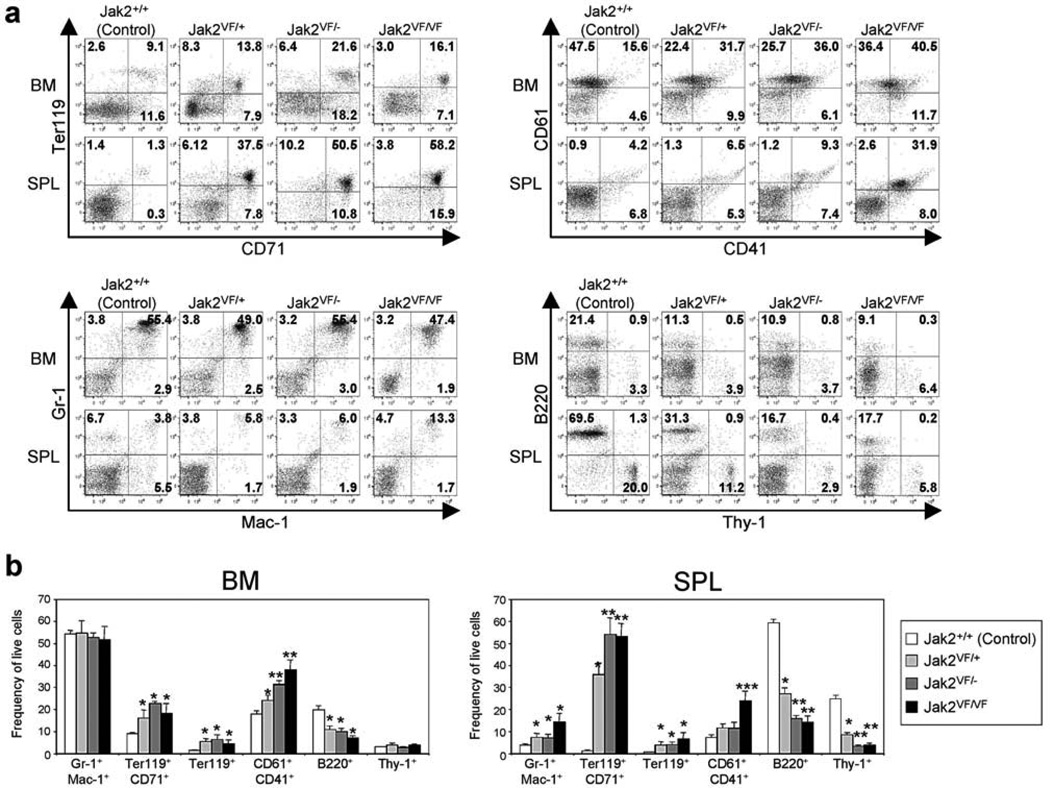
Flow cytometric analysis of the BM and spleens from heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) and homozygous (Jak2VF/VF) mice demonstrated marked increases in erythroid (Ter119/CD71) and megakaryocytic (CD41/CD61) precursors compared with control (Jak2+/+) animals (Figures 2a and b). However, the expansion of Ter119/CD71 and CD41/CD61 populations was much greater in the spleens of hemizygous and homozygous mice compared with that in heterozygous Jak2V617F animals (Figures 2a and b). B-cell (B220+) and T-cell (Thy1+) populations were proportionately reduced in the BM and spleens of heterozygous mice compared with control animals, and those were further reduced in hemizygous and homozygous Jak2V617F mice (Figures 2a and b). Overall, these data suggest that expression of hemizygous or homozygous Jak2V617F gives rise to greater increase in erythroid and megakaryocytic precursors in the BM and spleens.
Figure 2. Flow cytometric analysis of BM and spleens from heterozygous, hemizygous and homozygous Jak2V617F knock-in mice.
(a) Dot plots demonstrate significant increase in erythroid (Ter119+/CD71+) and megakaryocytic (CD61+/CD41+) precursors in the BM and/or spleens of heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) and homozygous (Jak2VF/VF) mice compared with control (Jak2+/+) animals. (b) Percentages of granulocyte/monocyte (Gr-1+/Mac-1+), erythroid (Ter119+/CD71+) and megakaryocytic (CD61+/CD41+) precursors as well as B-cells (B220+) and T-cells (Thy-1+) are shown in bar graphs as mean ± SEM. Data from five independent experiments are presented. Asterisks indicate significant differences (p < 0.05) (* indicates significance when compared with controls; ** indicates significance when compared with controls and heterozygous Jak2VF/+; *** indicates significance when compared with controls, heterozygous Jak2VF/+ and hemizygous Jak2VF/−). Note that deletion of Jak2 WT allele results in further expansion of megakaryocytic (CD61+/CD41+) and erythroid (Ter119+/CD71+) precursors in the BM and/or spleens of hemizygous and homozygous Jak2V617F mice.
Deletion of Jak2 WT allele enhances expansion of megakaryocyte-erythroid progenitors (MEP) in Jak2V617F knock-in mice
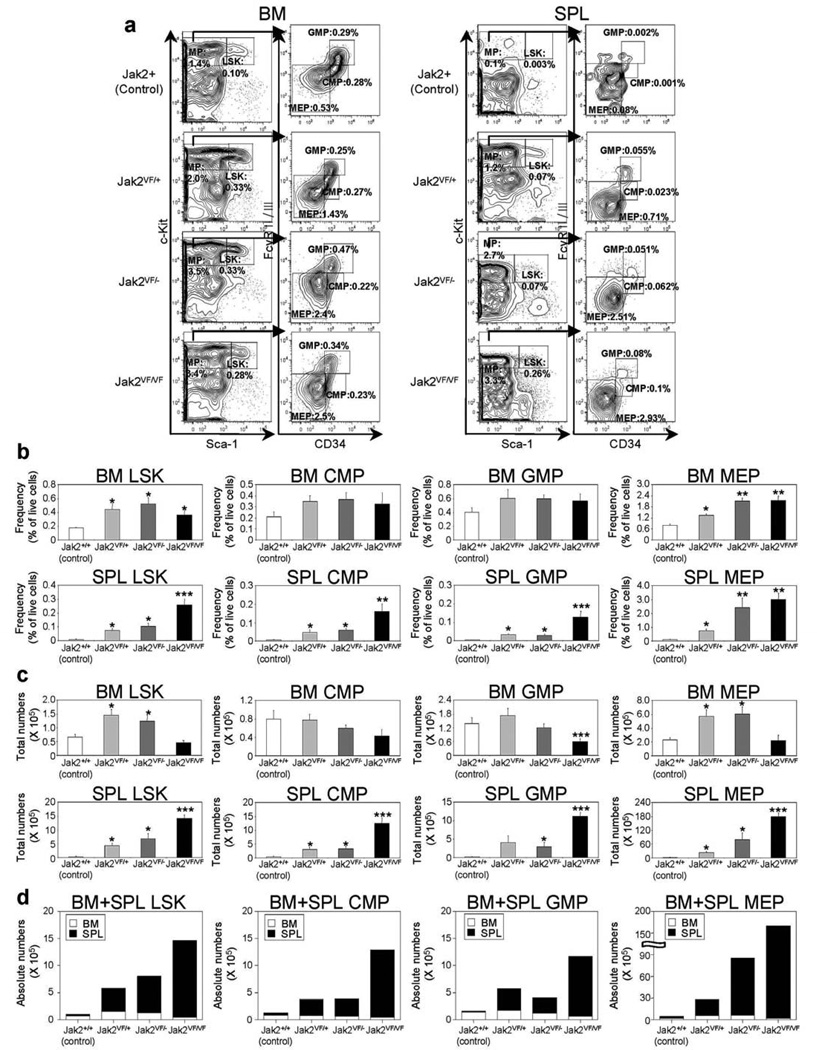
We next compared the effects of heterozygous, hemizygous and homozygous Jak2V617F expression on early hematopoietic progenitors. Expression of heterozygous Jak2V617F resulted in significant increases in Lin−Sca1+c-kit+ (LSK) and megakaryocyte-erythroid progenitor (MEP) compartments in the BM and spleens of heterozygous Jak2V617F mice at 12 weeks after pI-pC induction (Figures 3a and b). Compared with control animals, hemizygous and homozygous Jak2V617F mice also exhibited significant increase in frequency of LSK and MEP in the BM and spleens (Figures 3a and b). Since the BM cellularity was greatly reduced in homozygous Jak2V617F mice at 12 weeks after induction, the total numbers of LSK and MEP were rather decreased in the BM of homozygous Jak2V617F mice (Figure 3c). However, there was a marked increase in the frequency and total numbers of LSK, MEP, CMP and GMP populations in the spleens of homozygous Jak2V617F mice (Figure 3c). Spleens of heterozygous and hemizygous Jak2V617F mice also exhibited significant increase in LSK, MEP, CMP and GMP populations compared with those in control animals, but the increase in LSK, MEP, CMP and GMP was much greater in the spleens of homozygous Jak2V617F mice than in heterozygous or hemizygous Jak2V617F animals (Figures 3b and c). Overall, we observed a significant increase in absolute numbers of LSK and MEP in hemizygous or homozygous Jak2V617F mice compared with heterozygous Jak2V617F mice (Figure 3d).
Figure 3. Deletion of Jak2 WT increases megakaryocyte-erythroid progenitor (MEP) compartment in Jak2V617F knock-in mice.
(a) Flow cytometric analysis of LSK (Lin−Sca-1+c-Kit+) and subsets of myeloid progenitors including CMP (Lin−Sca-1−c-Kit+CD34+FcγRII/IIIlo), GMP (Lin−Sca-1−c-Kit+CD34+FcγRII/IIIhigh), and MEP (Lin−Sca-1−c-Kit+CD34−FcγRII/III−) in the BM and spleens from control (Jak2+/+), heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) or homozygous (Jak2VF/VF) Jak2V617F mice. Representative contour plots from five independent experiments are shown. (b) Percentages of LSK, CMP, GMP, and MEP are shown in histograms as mean ± SEM. Data is presented as percentage of live cells. (c) Total numbers of LSK, CMP, GMP and MEP in BM (femur plus tibia) or whole spleens are shown in histograms as mean ± SEM. (d) Absolute numbers (BM plus spleens) of LSK, CMP, GMP and MEP are shown in histograms as mean ± SEM. Asterisks indicate significant differences (p < 0.05) (* indicates significance when compared with controls; ** indicates significance when compared with controls and heterozygous Jak2VF/+; *** indicates significance when compared with controls, heterozygous Jak2VF/+ and hemizygous Jak2VF/−). Note that deletion of Jak2 WT allele increases absolute numbers of LSK and MEP in hemizygous and homozygous Jak2V617F mice.
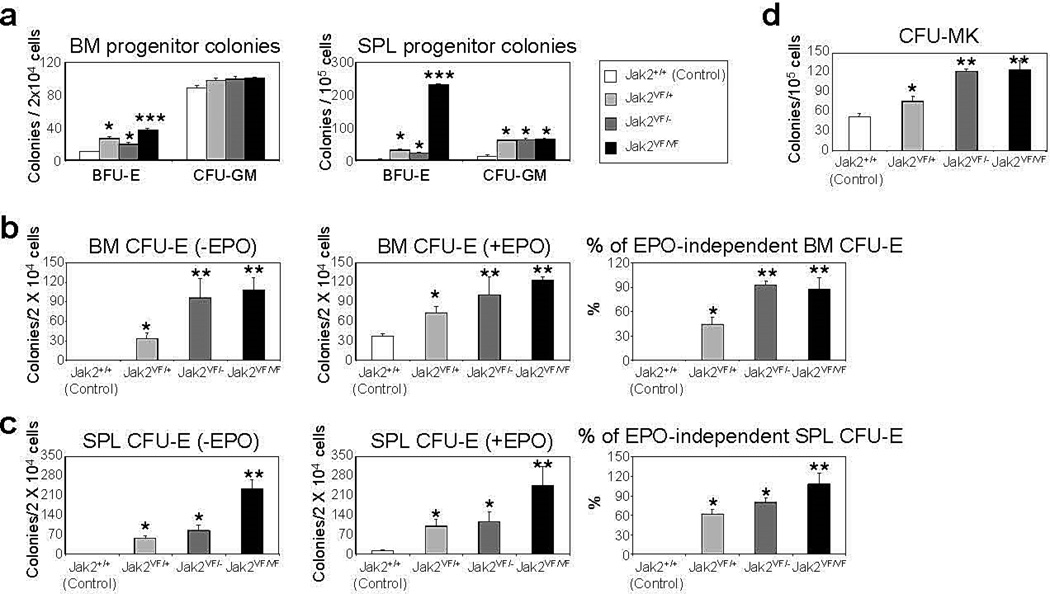
Hematopoietic progenitor colony assays showed significant increase in hematopoietic progenitor colonies in the BM and spleens of heterozygous, hemizygous and homozygous Jak2V617F mice compared with control animals (Figures 4a-d). The BFU-E colonies were significantly increased in the BM and spleens of heterozygous, hemizygous and homozygous Jak2V617F mice (Figure 4a). However, homozygous Jak2V617F mice BM and spleens exhibited greater numbers of BFU-E colonies compared with those in heterozygous and hemizygous mice (Figure 4a). No significant difference was observed in CFU-GM colonies among heterozygous, hemizygous and homozygous Jak2V617F mice (Figure 4a). In contrast to control animals, BM and spleens from heterozygous, hemizygous and homozygous Jak2V617F mice exhibited EPO-independent CFU-E colonies (Figures 4b and c). Hemizygous and homozygous Jak2V617F mice BM and spleens showed significantly larger number of EPO-independent CFU-E colonies compared with heterozygous Jak2V617F mice (Figures 4b and c). Interestingly, 80–90% of CFU-E colonies in the BM and spleens of hemizygous and homozygous Jak2V617F mice were EPO-independent whereas only 40–60% of CFU-E colonies in the BM and spleens of heterozygous Jak2V617F mice were EPO-independent (Figures 4b and c). We also observed marked increase in the number of megakaryocytic (CFU-Mk) colonies in the BM of hemizygous and homozygous mice compared with heterozygous Jak2V617F animals (Figure 4d). Thus, loss of Jak2 WT allele increases the expansion of megakaryocyte-erythroid progenitors.
Figure 4. Deletion of Jak2 WT increases hematopoietic progenitor colony formation in Jak2V617F mice.
(a) BM and spleen cells from control (Jak2+/+), heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) or homozygous (Jak2VF/VF) mice (n = 4) were plated in complete methylcellulose medium (Methocult M3434) in presence of cytokine cocktail. BFU-E and CFU-GM colonies were scored on day 7. BM (b) or spleen (c) cells from control, heterozygous, hemizygous and homozygous Jak2V617F mice (n = 4) were plated in methylcellulose media (Methocult M3234) in the absence of cytokine or in the presence of EPO (3 U/ml). CFU-E colonies were scored after 2 days. Percentages of EPO-independent CFU-E colonies in the BM and spleens are shown in the right panels. (d) BM cells were plated in collagen-based (MegaCult-C) media and CFU-Mk colonies were scored 8 days after plating. Asterisks indicate significant differences (p < 0.05) (* indicates significance when compared with controls; ** indicates significance when compared with controls and heterozygous Jak2VF/+; *** indicates significance when compared with controls, heterozygous Jak2VF/+ and hemizygous Jak2VF/−). Notably, deletion of Jak2 WT allele increases erythroid (CFU-E) and megakaryocytic (CFU-Mk) colonies in hemizygous and homozygous Jak2V617F mice.
Loss of Jak2 WT allele accelerates myelofibrosis in Jak2V617F knock-in mice
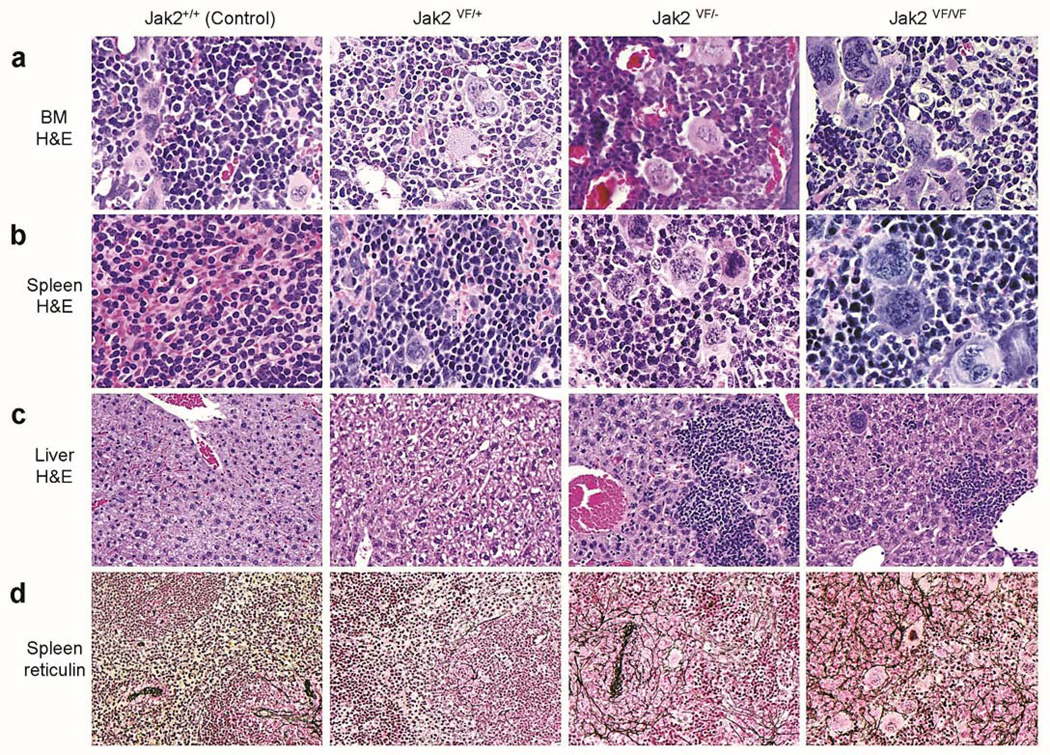
Histopathologic analyses of the BM from heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) and homozygous (Jak2VF/VF) Jak2V617F mice at 12 weeks after induction demonstrated increase in erythroid, myeloid and megakaryocytic cells (Figure 5a). Hemizygous and homozygous mice BM showed significantly more megakaryocytes with atypical nuclear features compared with heterozygous Jak2V617F mice BM (Figure 5a). Spleen sections from heterozygous mice exhibited effacement of normal splenic architecture with trilineage hyperplasia (Figure 5b). Spleens from hemizygous and homozygous mice demonstrated more extensive extramedullary hematopoiesis with increased clusters of dysplastic megakaryocytes showing large nuclei compared with spleens from heterozygous Jak2V617F mice (Figure 5b). Livers from control and heterozygous Jak2V617F mice showed normal hepatic architecture, whereas livers from hemizygous and homozygous mice exhibited significant infiltration of myeloid precursors at 12 weeks after pI-pC induction (Figure 5c). Reticulin staining of the spleens from heterozygous Jak2V617F mice showed very slight fibrosis in the red and white pulps at 12 weeks after pI-pC injection (Figure 5d). Spleens from hemizygous and homozygous mice exhibited extensive reticulin fibrosis in both red and white pulp (Figure 5d). Hemizygous mice exhibited grade 1–2 fibrosis (in a scale of 0 to 4) whereas homozygous mice displayed more advanced myelofibrosis (grade 2–3) at 12 weeks after induction. These results suggest that loss of Jak2 WT allele accelerates myelofibrosis in Jak2V617F knock-in mice.
Figure 5. Histopathologic analysis of heterozygous, hemizygous and homozygous Jak2V617F knock-in mice.
(a) Hematoxylin and eosin (H&E) staining of the BM sections (500X) from heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) and homozygous (Jak2VF/VF) mice show trilineage hyperplasia. Jak2VF/− and Jak2VF/VF mice BM exhibit significantly more megakaryocytes with atypical nuclear features compared with heterozygous (Jak2VF/+) mice BM. (b) Spleen sections (H&E staining; 500X) from heterozygous, hemizygous, and homozygous Jak2V617F mice exhibit destruction of normal splenic architecture with attenuated white pulp and markedly expanded red pulp, increased numbers of megakaryocytes, and clusters of immature erythroid and granulocyte precursors. Spleens from hemizygous or homozygous Jak2V617F mice show increased numbers of abnormal megakaryocytes with enlarged nuclei. (c) Liver sections (H&E staining; 200X) from hemizygous, and homozygous Jak2V617F mice display infiltration of myeloid precursors. (d) Reticulin staining of the spleen sections (200X) from hemizygous and homozygous Jak2V617F mice show extensive reticulin fibrosis in both red and white pulp at 12 weeks after pI-pC induction. Heterozygous Jak2V617F mice spleens exhibit very slight myelofibrosis at this stage (12 weeks after pI-pC induction).
Loss of Jak2 WT allele enhances constitutive signaling in primary hematopoietic cells expressing Jak2V617F
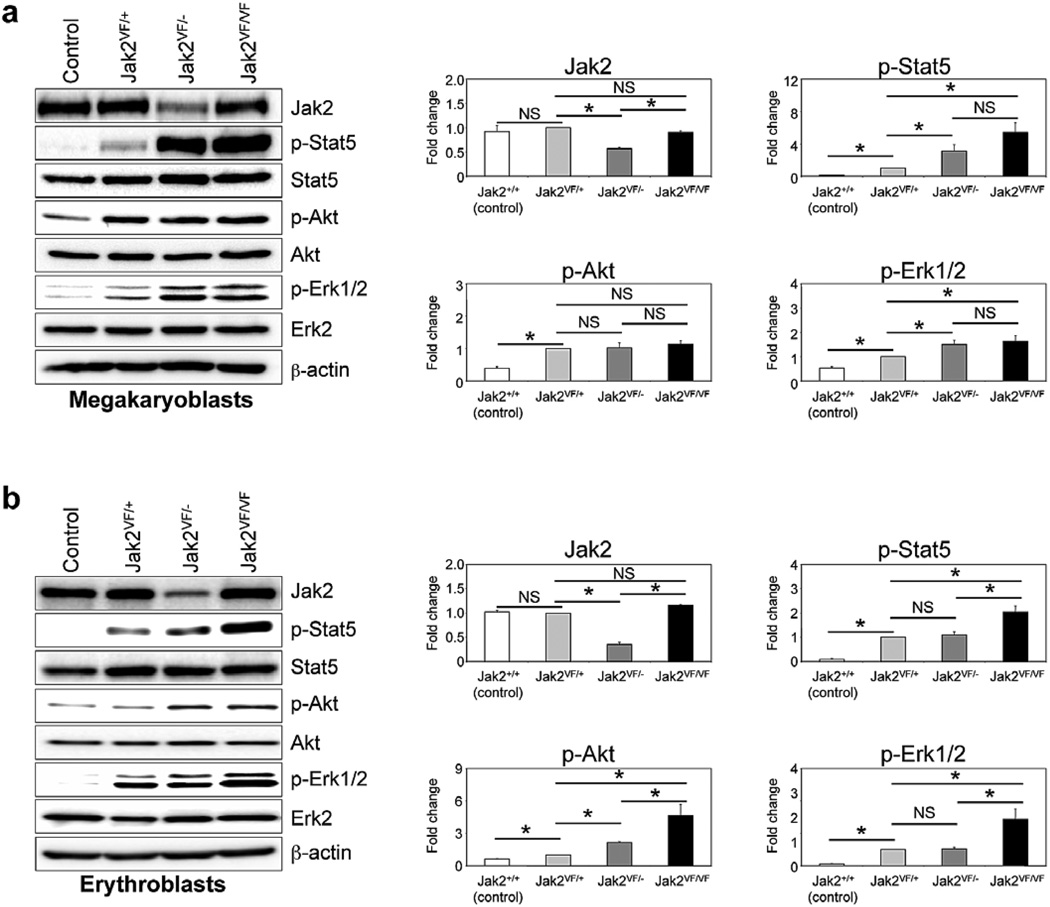
To further confirm that wild-type Jak2 acts as a negative regulator of hematopoietic signaling mediated by Jak2V617F, we compared the effects of heterozygous, hemizygous and homozygous Jak2V617F expression on hematopoietic signaling. Primary megakaryoblasts and erythroblasts derived from the BM of control (Jak2+/+), heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) and homozygous Jak2V617F (Jak2VF/VF) mice were utilized for signaling studies. Whereas heterozygous Jak2V617F expression resulted in constitutive activation of Stat5, Erk1/2 and/or Akt, hemizygous or homozygous Jak2V617F expression further enhanced the constitutive activation of these signaling pathways (Figures 6a and b). Homozygous Jak2V617F-expressing megakaryoblasts/erythroblasts exhibited the highest level of activation of Stat5, Erk1/2 and Akt signaling (Figures 6a and b). Hemizygous Jak2V617F megakaryoblasts and erythroblasts, which harbored only one allele of Jak2 (Jak2V617F) and thus expressed lower levels of total Jak2 protein than in control, heterozygous or homozygous Jak2V617F megakaryoblasts/erythroblasts, showed greater activation of constitutive signaling compared with control or heterozygous Jak2V617F megakaryoblasts/erythroblasts (Figures 6a and b). Notably, activation of Stat5 and Erk1/2 was much greater in megakaryoblasts than in erythroblasts of hemizygous Jak2V617F mice compared with those in heterozygous Jak2V617F mice (Figures 6a and b). Overall, these results suggest that wild-type Jak2 negatively regulates signaling mediated by Jak2V617F.
Figure 6. Deletion of Jak2 WT enhances constitutive signaling in primary hematopoietic cells expressing Jak2V617F.
Primary megakaryoblasts (a) and erythroblasts (b) were derived from the BM of control, heterozygous (Jak2VF/+), hemizygous (Jak2VF/−) and homozygous (Jak2VF/VF) Jak2V617F mice. Cells were serum and factor depleted for 6 hours and lysed in RIPA buffer. Immunoblotting was performed using phospho-specific antibodies against Stat5, Akt and Erk1/2. Membranes were re-probed with respective total antibodies. Total Jak2 protein levels were also determined by immunoblotting using anti-Jak2 antibody. β-actin was used as a loading control. Histograms (in the right panels) demonstrate the fold changes in phosphorylation of Stat5, Akt and Erk1/2 when compared to the phosphorylation levels of those proteins in heterozygous Jak2V617F-expressing megakaryoblasts or erythroblasts in the absence of cytokines. Data from three to four independent experiments are shown in histograms as mean ± SEM. Asterisks indicate significant differences (p < 0.05). Notably, deletion of Jak2 WT allele enhanced constitutive phosphorylation/activation of Stat5, Erk1/2 and/or Akt in primary hematopoietic cells from hemizygous and homozygous Jak2V617F mice.
DISCUSSION
Loss of heterozygosity (LOH) in chromosomal regions by acquired uniparental disomy is commonly observed in myeloid malignancies.17 Such LOH results in the loss of normal allele and concomitant duplication of oncogenic mutations in genes including FLT3,18, 19 MPL20 and JAK2.1–4, 6 9pLOH involving the JAK2 locus has been found in a significant proportion (~30%) of MPN cases1–4, 6 and associated with more severe MPN phenotype including more advanced myelofibrosis.7–9 It has been shown that expression of Jak2 WT inhibits cytokine-independent growth of hematopoietic cells mediated by Jak2V617F,1 suggesting a negative role for Jak2 WT in hematopoietic transformation evoked by Jak2V617F. The generation of inducible heterozygous, hemizygous and homozygous Jak2V617F mice enabled us to study the contribution of Jak2 WT and Jak2V617F alleles in the development of MPNs. We demonstrate that deletion of Jak2 WT allele increases WBC, neutrophils, reticulocytes and platelets in the peripheral blood and enhances splenomegaly in Jak2V617F knock-in mice. We also show that deletion of Jak2 WT allele accelerates progression to myelofibrosis in Jak2V617F knock-in mice. Notably, hemizygous Jak2V617F (Jak2VF/−) mice bearing only one allele of Jak2V617F exhibited more myeloid cell expansion, larger spleen size and faster progression to myelofibrosis than the heterozygous Jak2V617F (Jak2VF/+) mice expressing both Jak2 WT and mutant Jak2V617F alleles. Homozygous (Jak2VF/VF) mice expressing two mutant Jak2V617F alleles exhibited the most severe MPN with extensive myelofibrosis. Thus, the Jak2 WT allele serves as a negative regulator of MPN evoked by Jak2V617F.
Consistent with our previous study,10 we observed significant increase in HSC-enriched LSK populations in mice expressing heterozygous Jak2V617F at 12 weeks after pI-pC induction (Figures 3c and d). We also observed a significant increase in LSK in hemizygous or homozygous Jak2V617F mice compared with control animals (Figures 3c and d). Interestingly, the total number of LSK was lesser in the BM but significantly higher in the spleens of hemizygous or homozygous Jak2V617F mice compared with heterozygous Jak2V617F animals (Figure 3). It is possible that a fibrotic BM microenvironment may not be suitable for retention of HSCs. Thus, the HSCs may egress from the BM and mobilize to the spleen in hemizygous or homozygous Jak2V617F mice exhibiting myelofibrosis. Indeed, MF patients have limited numbers of hematopoietic cells in their BM but higher proportion of HSCs in the spleens, and the MF splenic HSCs are capable of self-renewal in vivo.21 We also observed marked increases in the absolute number of MEP in hemizygous and homozygous Jak2V617F mice compared with heterozygous Jak2V617F animals (Figure 3d). Interestingly, the increase in MEP is more prominent in the spleens than in the BM of hemizygous and homozygous Jak2V617F mice. These results clearly suggest that loss of wild-type Jak2 allele enhances the expansion of MEP in mice expressing Jak2V617F.
MEP can differentiate into both erythroid and megakaryocytes. We observed significantly higher percentage of EPO-independent CFU-E colonies in the BM and spleens of hemizygous or homozygous Jak2V617F mice compared with heterozygous Jak2V617F animals (Figures 4b and c). Similarly, we observed greater numbers of reticulocytes and early erythroid precursors in hemizygous or homozygous Jak2V617F mice compared with heterozygous Jak2V617F mice (Figures 1e and 2). Hemizygous or homozygous Jak2V617F expression also resulted in significantly increased fractions of megakaryocytic precursors and larger numbers of CFU-Mk colonies compared with heterozygous Jak2V617F expression (Figures 2a,b and 4d). These findings further suggest that deletion of Jak2 WT allele increases myeloid cell expansion in Jak2V617F knock-in mice.
We have shown previously that heterozygous Jak2V617F knock-in mice developed myelofibrosis at older age (~24 weeks after pI-pC induction).10 In this study, we have analyzed the mice at 12 weeks after pI-pC induction. We show that deletion of Jak2 WT allele accelerates the progression to myelofibrosis in hemizygous or homozygous Jak2V617F knock-in mice. Notably, homozygous Jak2V617F mice exhibited the most extensive myelofibrosis, consistent with recent reports that high JAK2V617F mutant allele burden is associated with faster progression to myelofibrosis in patients with PV.8, 9
Recently, Kubovcakova et al have examined the effects of wild-type Jak2 deletion in a transgenic mouse model of Jak2V617F.22 Deletion of endogenous wild-type mouse Jak2 increased erythropoiesis and enhanced PV phenotype, but did not alter platelet or granulocyte levels in transgenic mice expressing human Jak2V617F.22 In addition, deletion of wild-type Jak2 significantly reduced the survival of Jak2V617F transgenic mice.22 We observed frequent deaths in hemizygous or homozygous mice cohorts with ~50% hemizygous or homozygous Jak2V617F mice dying within 9–12 weeks after induction (data not shown). We also observed significant increases in granulocytes, reticulocytes and platelets in hemizygous or homozygous Jak2V617F knock-in mice (Figure 1e-h). The differences in phenotypes in our Jak2V617F knock-in and the Jak2V617F transgenic model described by Kubovcakova et al could be due to differences in the activity of mouse versus human Jak2V617F protein and/or differences in the strategies used for Jak2V617F expression in these models (knock-in versus transgenic).
We also have provided evidence that wild-type Jak2 negatively regulates signaling mediated by Jak2V617F. Hemizygous Jak2V617F megakaryoblasts or erythroblasts expressing only one allele of Jak2 (Jak2V617F) exhibited greater activation of signaling than the heterozygous Jak2V617F megakaryoblasts/erythroblasts expressing both Jak2 WT and Jak2V617F alleles (Figures 6a and b). Obviously, homozygous Jak2V617F megakaryoblasts/ erythroblasts expressing two Jak2V617F alleles showed the highest level of activation of Stat5, Erk1/2 and/or Akt signaling (Figures 6a and b). The enhanced activation of constitutive signaling caused by the loss of wild-type Jak2 allele may contribute to the severity of MPN in hemizygous or homozygous Jak2V617F animals. Interestingly, the increase in Stat5 and Erk1/2 activation was much greater in megakaryoblasts than in erythroblasts of hemizygous Jak2V617F mice compared with those in heterozygous Jak2V617F animals (Figures 6a and b). It has been shown previously that Erk1/2 plays a critical role in megakaryocyte differentiation and platelet formation.16 Indeed, we observed significantly larger numbers of platelets in the peripheral blood and increased megakaryocytic (CFU-Mk) colonies in the BM of hemizygous or homozygous Jak2V617F mice compared with heterozygous Jak2V617F animals (Figures 1h and 4d).
In this report, we have demonstrated that loss of wild-type Jak2 allele increases myeloid cell expansion and splenomegaly, and accelerates myelofibrosis in Jak2V617F knock-in mice. Whereas Jak2 WT plays an important role in normal hematopoietic development,23–25 mutant Jak2V617F induces abnormal and extramedullary hematopoiesis. Hemizygous Jak2V617F mice, which express only one allele of Jak2 (Jak2V617F), exhibited more severe MPN and faster progression to myelofibrosis than the heterozygous Jak2V617F mice. Homozygous Jak2V617F mice expressing two Jak2V617F alleles displayed the highest degree of myelofibrosis. Thus, loss of wild-type Jak2 or acquisition of Jak2V617F homozygosity contributes to the severity of MPNs. Similar observations were made in FLT3/ITD knock-in mice in which loss of wild-type FLT3 allele enhanced myeloid expansion and led to a more aggressive phenotype.26 In conclusion, our results show that Jak2 WT and Jak2V617F alleles play opposite roles in MPNs. Therefore, specific targeting of the mutant Jak2V617F allele without affecting the Jak2 WT allele would be the appropriate strategy for the treatment of Jak2V617F-positive MPNs.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Kay-Uwe Wagner (University of Nebraska, USA) for providing the Jak2 floxed mouse. This work was supported by the grants from the Leukemia & Lymphoma Society and US National Institute of Health (NIH) (R01 HL095685) awarded to G.M. G.M. is a Scholar of the Leukemia & Lymphoma Society.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Hajime Akada performed research, analyzed data and wrote the manuscript; Saeko Akada performed research; Robert E Hutchison performed histopathologic analysis and revised the manuscript; Golam Mohi designed the research, analyzed data and wrote the manuscript.
Supplementary information is available at Leukemia’s website
REFERENCES
- 1.James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 2.Levine RL, Wadleigh M, cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 3.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 4.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Eng. J. Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 5.Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, et al. Identification of an Acquired JAK2 Mutation in Polycythemia Vera. J. Biol. Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kralovics R, Guan Y, Prchal JT. Acquired uniparental disomy of chromosome 9p is a frequent stem cell defect in polycythemia vera. Exp Hematol. 2002;30:229–236. doi: 10.1016/s0301-472x(01)00789-5. [DOI] [PubMed] [Google Scholar]
- 7.Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, Ponziani V, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952–1959. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- 8.Passamonti F, Rumi E, Pietra D, Elena C, Boveri E, Arcaini L, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24:1574–1579. doi: 10.1038/leu.2010.148. [DOI] [PubMed] [Google Scholar]
- 9.Silver RT, Vandris K, Wang YL, Adriano F, Jones AV, Christos PJ, et al. JAK2(V617F) allele burden in polycythemia vera correlates with grade of myelofibrosis, but is not substantially affected by therapy. Leuk Res. 2011;35:177–182. doi: 10.1016/j.leukres.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. 2010;115:3589–3597. doi: 10.1182/blood-2009-04-215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marty C, Lacout C, Martin A, Hasan S, Jacquot S, Birling MC, et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood. 2010;116:783–787. doi: 10.1182/blood-2009-12-257063. [DOI] [PubMed] [Google Scholar]
- 12.Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17:584–596. doi: 10.1016/j.ccr.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Spensberger D, Ahn JS, Anand S, Beer PA, Ghevaert C, et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood. 2010;116:1528–1538. doi: 10.1182/blood-2009-12-259747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krempler A, Qi Y, Triplett AA, Zhu J, Rui H, Wagner KU. Generation of a conditional knockout allele for the Janus kinase 2 (Jak2) gene in mice. Genesis. 2004;40:52–57. doi: 10.1002/gene.20063. [DOI] [PubMed] [Google Scholar]
- 15.Kühn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 16.Mazharian A, Watson SP, Séverin S. Critical role for ERK1/2 in bone marrow and fetal liver-derived primary megakaryocyte differentiation, motility, and proplatelet formation. Exp Hematol. 2009;37:1238–1249. doi: 10.1016/j.exphem.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Keefe C, McDevitt MA, Maciejewski JP. Copy neutral loss of heterozygosity: a novel chromosomal lesion in myeloid malignancies. Blood. 2010;115:2731–2739. doi: 10.1182/blood-2009-10-201848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61:7233–7239. [PubMed] [Google Scholar]
- 19.Raghavan M, Lillington DM, Skoulakis S, Debernardi S, Chaplin T, Foot NJ, et al. Genome-wide single nucleotide polymorphism analysis reveals frequent partial uniparental disomy due to somatic recombination in acute myeloid leukemias. Cancer Res. 2005;65:375–378. [PubMed] [Google Scholar]
- 20.Szpurka H, Gondek LP, Mohan SR, Hsi ED, Theil KS, Maciejewski JP. UPD1p indicates the presence of MPL W515L mutation in RARS-T, a mechanism analogous to UPD9p and JAK2 V617F mutation. Leukemia. 2008;23:610–614. doi: 10.1038/leu.2008.249. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Prakash S, Lu M, Tripodi J, Ye F, Najfeld V, et al. Spleens of myelofibrosis patients contain malignant hematopoietic stem cells. J Clin Invest. 2012;122:3888–3899. doi: 10.1172/JCI64397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubovcakova L, Lundberg P, Grisouard J, Hao-Shen H, Romanet V, Andraos R, et al. Differential effects of hydroxyurea and INC424 on mutant allele burden and myeloproliferative phenotype in a JAK2-V617F polycythemia vera mouse model. Blood. 2013;121:1188–1199. doi: 10.1182/blood-2012-03-415646. [DOI] [PubMed] [Google Scholar]
- 23.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 24.Neubauer H, Cumano A, Müller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 25.Park SO, Wamsley HL, Bae K, Hu Z, Li X, Choe SW, et al. Conditional deletion of Jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: implications for Jak2 inhibition in humans. PLoS One. 2013;8:e59675. doi: 10.1371/journal.pone.0059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Bailey E, Greenblatt S, Huso D, Small D. Loss of the wild-type allele contributes to myeloid expansion and disease aggressiveness in FLT3/ITD knockin mice. Blood. 2011;118:4935–4945. doi: 10.1182/blood-2011-01-328096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.