Abstract
Sleep disturbances are commonly reported in youth with chronic pain. We examined whether online cognitive-behavioral therapy (CBT) for pain management would impact youth’s sleep. Subjective sleep quality and actigraphic sleep were evaluated in 33 youth (M=14.8 years; 70% female) with chronic pain participating in a larger randomized controlled trial of online-CBT. The Internet treatment condition (n=17) received 8-10 weeks of online-CBT + standard care and the wait-list control condition (n=16) continued with standard care. Although pain improved with online-CBT, no changes were observed in sleep outcomes. Shorter pre-treatment sleep duration was associated with less improvement in post-treatment functioning. Findings underscore the need for further development in psychological therapies to more intensively target sleep loss in youth with chronic pain.
Keywords: adolescent, chronic pain, sleep, randomized controlled trial, cognitive-behavioral therapy
Introduction
Chronic pain is a complex condition that pervades many aspects of an adolescent’s daily life by impacting mood, behavior, and activity participation (Palermo, 2000). Sleep problems are also common among children and adolescents with chronic pain. Youth with chronic pain conditions frequently report short sleep duration, poor sleep quality, and frequent night-wakings (Gilman, Palermo, Kabbouche, Hershey, & Powers, 2007; Haim et al., 2004; Meltzer & Mindell, 2006; Palermo, Wilson, Lewandowski, Toliver-Sokol, & Murray, 2011). Recent studies that include self-report and objective assessments of sleep patterns suggest that while youth with chronic pain and healthy youth demonstrate similar sleep latency, sleep efficiency, and sleep duration, youth with chronic pain report greater insomnia symptoms and spend more time sleeping during the day (Haim et al., 2004; Law, Dufton, & Palermo, 2012; Lewandowski Palermo, De la Motte, & Fu, 2010; Meltzer, Logan, &Mindell, 2005; Palermo, Toliver-Sokol, Fonareva, &Koh, 2007;Palermo, Law, Churchill, & Walker, 2012; Palermo, Wilson, Lewandowski, Toliver-Sokol & Murray, 2011; Tsai et al., 2008).
Adolescence is a particularly important time to consider sleep patterns and sleep quality because of the many physiological and social changes that influence sleep during this developmental stage (Carskadon, 2002; Meltzer & Mindell, 2006). As youth make the transition from childhood to adulthood, their sleep time steadily declines; by the time they reach their senior year in high school, youth report sleeping an average of only 6.9 hours per night (National Sleep Foundation, 2006). This sleep quantity is drastically less than the optimal developmental sleep requirements for adolescents, which have been estimated at 9.2 hours per night (Carskadon, Harvey, Duke, Anders, Litt, & Dement, 1980). Adolescence is also a time when problems with insomnia may emerge. In population-based studies, 12 to 16% of adolescents are reported to have clinically significant insomnia (Ohayon, Caulet, & Lemoine, 1998; Roberts, Lee, Hemandez, & Solari, 2004). Similarly, insomnia is common in adolescents with chronic pain (e.g., Palermo, et al., 2011).
Sleep disturbances are particularly problematic for youth with chronic pain because they appear to exacerbate other symptoms; greater sleep disturbances are associated with depressive symptoms, poorer quality of life, greater pain-related disability, and higher pain intensity (LaPlant, Adams, Haftel, & Chervin, 2007; Lewandowski et al., 2010; Long, Krishnamurthy, & Palermo, 2008; Neut, Fily, Cuvellier, & Vallee, 2012; Palermo & Kiska, 2005; Palermo, Fonareva, & Janosy, 2008; Palermo et al., 2011). Recent data suggest that sleep difficulties among youth with chronic pain are not transient and may be associated with worse outcomes over time. In a longitudinal investigation of insomnia symptoms in youth with chronic pain, Palermo and colleagues (2012) found that difficulties with sleep onset and sleep maintenance persisted over a one-year period. Further, persistent insomnia symptoms were associated with greater health service use and lower quality of life.
The association between sleep and pain is complex. Lewin and Dahl (1999) hypothesized a bidirectional relationship between pain and sleep, whereby uncontrolled pain can cause sleep disruptions, and in turn, disturbed sleep can enhance pain sensitivity, leading to increased disability and a worsening of quality of life. Research has examined both directions of this theory, finding some support for this vicious cycle between chronic pain and disrupted sleep patterns (see Smith & Haythornthwaite, 2004, for a review). Moreover, laboratory studies have demonstrated that restricted sleep enhances certain types of pain sensitivity and pain inhibition processing (Smith, Edwards, McCann, & Haythornthwaite, 2007). However, most of this literature has focused on cross-sectional associations between pain and sleep, and it is unclear whether the same associations exist over time as pain symptoms change.
Sleep disturbances might be expected to diminish over time due to effects of therapies aimed at the reduction or amelioration of painful symptoms. For example, prevailing clinical opinion is that psychological or pharmacological therapies that reduce pain may consequently improve secondary sleep problems. In addition, certain characteristics of therapies (either nonspecific effects or side effects) aimed at pain reduction may specifically change quality or quantity of sleep. For example, cognitive-behavioral therapies that focus on teaching methods of decreasing physiological arousal (e.g., relaxation strategies) may subsequently improve the individual’s ability to settle to sleep and thereby enhance their sleep quality or quantity. Further, cognitive strategies targeting maladaptive beliefs regarding stress and pain may generalize to maladaptive sleep-related beliefs (Babson, Feldner, & Badour, 2010; Edinger & Means, 2005).
Sleep has been identified by a consensus group, PedIMMPACT, as a relevant treatment outcome domain for clinical trials of pain management interventions for youth (McGrath et al., 2008), with the rationale that pain interventions alone may produce a positive effect on sleep outcomes in youth. Cognitive-behavioral therapy (CBT) has emerged as a promising modality to treat children and adolescents with chronic pain, however sleep has not yet been a part of routine assessment in randomized controlled trials evaluating this treatment approach (Eccleston et al., 2012). In a recent systematic review of psychological therapies for pain management, only one of the 37 pediatric trials examined sleep as an outcome domain (Eccleston et al., 2012; Kashikar-Zuck et al., 2012). In their study, Kashikar-Zuck and colleagues reported negligible improvement in self-reported sleep quality from pre- to post-treatment for youth with fibromyalgia, regardless of whether adolescents were assigned to an education group or a cognitive-behavioral intervention. This null finding is intriguing, particularly as robust improvements in functional disability and depression were observed for youth in both conditions, most remarkably for youth assigned to CBT, and highlights the important need for further data on sleep outcomes in the context of CBT pain interventions.
Therefore, the purpose of this study was to evaluate sleep outcomes in a randomized controlled trial of online family CBT for management of chronic pain in youth. In our previous publication (Palermo, Wilson, Peters, Lewandowski, & Somhegyi, 2009), we reported on primary treatment outcomes of pain and activity limitations, finding that youth receiving online family CBT had significant improvements in both outcome domains compared to youth receiving standard care only. In a subset of the trial participants, we conducted the present study to assess changes in sleep quality and quantity. Treatment outcome data on pain and sleep in the context of intervention offers an opportunity for examining changes in the relationship between pain and sleep over time. In this study, we examine whether online CBT for pain management leads to improved sleep quality or quantity. We hypothesized that CBT for pain management would positively affect adolescent sleep such that youth receiving this treatment were expected to show increased total sleep time, less wake time during the night, and higher subjective ratings of sleep quality in comparison to youth in the wait-list control condition. In addition, we hypothesized that reductions in pain and disability over the course of treatment would correspond with positive changes in sleep. Lastly, we examined baseline sleep as a predictor of adolescent treatment response. Based on literature concerning the detrimental effects of excessive daytime sleepiness on youths’ attention, concentration, mood, and behavior (Fallone, Owens, & Deane, 2002; Gozal, 1998; Soffer-Dudek, Sadeh, Dahl, & Rosenblat-Stein, 2011; Wolfson & Carskadon, 1998), we hypothesized that poorer sleep at baseline would be associated with less improvement in pain and disability post-treatment, even after controlling for baseline pain characteristics, due to decreased ability to engage in CBT strategies.
Methods
This study was approved by our Institutional Review Board. Parents provided informed consent, and adolescents gave assent prior to any research activity.
Procedures
Study participants were recruited from a multidisciplinary pediatric pain clinic, pediatric gastroenterology clinic, and pediatric neurology clinic at an academic medical center in the Pacific Northwest. The study period was approximately 16 months occurring from spring of 2007 to fall of 2008. Participants were a subset of youth from a larger randomized controlled trial of Internet-delivered family cognitive-behavioral therapy for chronic pain (Palermo et al., 2009).
Once referral information was received, a research assistant contacted potential participants by phone to describe the study, screen for eligibility and obtain informed consent. After enrollment into the larger RCT (but prior to randomization), adolescents were informed about the present study involving a sleep assessment that they could opt to participate in. Participants were given an ID number and then completed pre-treatment assessments at home, including an online daily pain and activity limitations diary for seven days, questionnaire measures, a sleep log, and actigraphy. Study materials were returned via postal mailing. Following receipt of all pre-treatment assessments, participants were randomly assigned to either the treatment or control group. Group assignment was determined using a fixed allocation randomization scheme, the details of which are described in full in Palermo and colleagues (2009). The randomization sequence was generated by an online random number generator, with blocks of ten specified to ensure equal treatment allocation within each block. The allocation sequence was concealed in sequentially numbered and sealed envelopes. Following receipt of baseline assessment measures, envelopes were opened by the research assistant to reveal group assignment. Post-treatment assessments were completed eight to ten weeks after enrollment. Participants again completed daily diaries for seven days monitoring their pain and activity limitations, paper-and-pencil questionnaires, a sleep log, and actigraphy.
Sample
Inclusion criteria for study enrollment included age between 11 and 17 years, presence of chronic pain (defined as occurring for at least 3 months), current frequency of pain of at least once per week, and absence of a comorbid chronic disease (e.g., cancer, arthritis). Table 1 shows the clinical characteristics of the sample combined and by treatment conditions. As can be seen, the sample was predominantly female and Caucasian. Most of the adolescents in the sample were experiencing moderate to severe intensity pain from headache, abdominal, or musculoskeletal pain that was occurring daily.
Table 1.
Descriptive characteristics of the combined sample and by treatment condition.
| Combined Sample n (%) | Standard Care n (%) | Internet Treatment n (%) | |
|---|---|---|---|
|
| |||
| Gender | |||
| Male | 10 (30.3%) | 6 (37.5%) | 4 (23.5%) |
| Female | 23 (69.7%) | 10 (62.5%) | 13 (76.5%) |
|
| |||
| Race/Ethnicity | |||
| White/Non-Hispanic | 30 (90.9%) | 16 (100%) | 14 (82.3%) |
| White/Hispanic | 2 (9.1%) | 0 | 2 (11.8%) |
| Other | 1 (3.0%) | 0 | 1 (5.9%) |
|
| |||
| Pain condition | |||
| Headache | 6 (18.2%) | 4 (25.0%) | 1 (5.9%) |
| Abdominal | 16 (48.5%) | 9 (56.3%) | 8 (47.1%) |
| Musculoskeletal | 11 (33.3%) | 3 (18.8%) | 8 (47.1%) |
|
| |||
| Pain Frequency | |||
| 1 - 2 x/week | 2 (6.0%) | 4 (25.0%) | 2 (11.8%) |
| 3 - 6 x/week | 9 (27.3%) | 2 (12.5%) | 3 (17.6%) |
| Daily | 22 (66.7%) | 10 (62.5%) | 12 (70.6%) |
|
| |||
| Pain Frequency | |||
| 1 - 2 x/week | 2 (6.0%) | 4 (25.0%) | 2 (11.8%) |
| 3 - 6 x/week | 9 (27.3%) | 2 (12.5%) | 3 (17.6%) |
| Daily | 22 (66.7%) | 10 (62.5%) | 12 (70.6%) |
|
| |||
| Combined M(SD) | Standard Care M(SD) | Internet Treatment M(SD) | |
|
| |||
| Pain Intensity1 (0-10 NRS) | 5.12(2.04) | 5.00(1.46) | 5.24(2.51) |
|
| |||
| Pain-related Disability1 (0-32) | 6.12(4.91) | 6.21(5.14) | 6.04(4.83) |
Averaged over a 7-day reporting period
Of the 48 participants in the larger trial, 37 were invited to participate in this additional investigation of sleep based on availability of an actiwatch device at the time they were enrolled. Of the 37 youth who were invited to participate, 89% (33 patients) agreed to complete further evaluation with actigraphy. Four participants refused participation due to lack of interest in wearing the device (see Figure 1). Participants and non-participants did not differ on any demographic characteristics.
Figure 1.
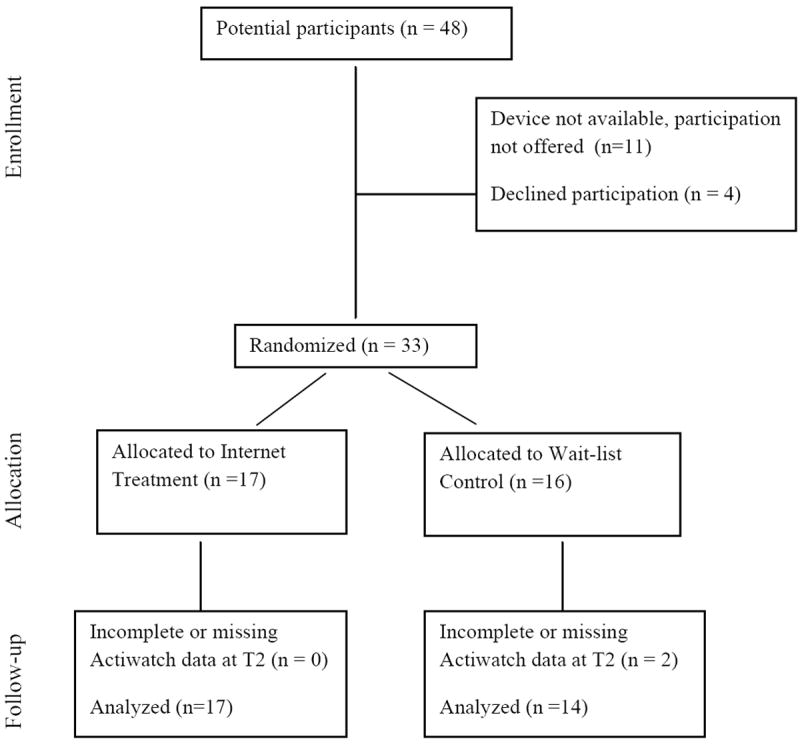
Study Participant Flow.
The thirty-three adolescents who agreed to participate as part of the procedures of the larger trial, were randomized to a wait-list control group receiving standard care alone (n = 16) or online family pain CBT (n = 17) to supplement standard care (Internet treatment group) over 8-10 weeks. Two cases from the wait-list control group had missing or incomplete actigraphy data at Time 2.
Measures
Pain
Pain intensity was assessed with an 11-point numerical rating scale (NRS), ranging from 0= no pain to 10= worst pain, which was recorded using an online daily diary at the pre- and post-treatment assessment periods. Mean pain intensity across each 7-day assessment period was used in subsequent analyses. Change in pain intensity scores across the two time points was also calculated (post-treatment pain subtracted from baseline pain).
Activity limitations
Each day during the 7-day pre- and post-treatment monitoring periods, adolescents completed the prospective version of the Child Activity Limitations Interview (CALI; Palermo, Lewandowski, Long, &Burant, 2008; Palermo, Witherspoon, Valenzuela, & Drotar, 2004) to assess daily activity limitations (pain-related disability). Participants select eight out of the twenty-one listed activities that are most challenging or bothersome due to their chronic pain and then rate the difficulty in performing each activity daily during the assessment period. Ratings of difficulty are from (0) not very difficult to (4) extremely difficult, with total scores ranging from 0-32. Adolescents completed daily prospective ratings on the online diary. Averaged scores across each 7-day period were used in analyses. Change in CALI scores (post-treatment disability subtracted from baseline disability) were also calculated. The diary version of the CALI has consistently demonstrated excellent reliability and validity in pediatric chronic pain samples (Palermo et al., 2009; Wilson, Samuelson, & Palermo, 2010).
Sleep
Objective sleep parameters were measured with wrist actigraphy (Actiwatch 64, Phillips Respironics/MiniMitter Company Inc., Bend, OR), following standardized practice parameters for actigraphy assessment (Morgenthaler et al., 2007). The Actiwatch was worn at home by each adolescent on their non-dominant wrist for seven consecutive days. Upon going to bed and awaking, a button (i.e., event marker) on the Actiwatch was pushed by the wearer, registering as an individual marker at that moment in time. Sleep-wake patterns were extracted from the activity data using the Actiware Sleep version 5.5, which bases its algorithm on the amplitude and frequency of detected movements, which were scored in one-minute epochs using the medium sensitivity/wake threshold. This software was developed and previously validated by Webster and colleagues (1982). Actigraphy has previously been validated in the assessment of pediatric sleep patterns (Meltzer, Montgomery-Downs, Insana, & Walsh, 2012; Sadeh, 2008), and has been used successfully to monitor sleep patterns in adolescents with a variety of painful conditions such as musculoskeletal pain (Tsai et al., 2008), functional recurrent abdominal pain (Haim et al., 2004), and in mixed samples of youth with chronic painful conditions (Palermo et al., 2007).
Actigraphic variables were calculated to be consistent with the recommended definitions for pediatric sleep research (Meltzer et al., 2012); event markers and a corresponding sleep log were used to help identify potential artifact and set sleep intervals. In the present study, sleep onset was defined as the first 10 min segment with no more than one epoch of any recorded activity and sleep offset was defined as the last 10 min segment with no more than one epoch of any recorded activity. The sleep period reflects the total duration between sleep onset and sleep offset. Two sleep variables were computed and used in analyses: total sleep time and sleep percent. Total sleep time is the total amount of time scored as sleep (in minutes) during the sleep period. Sleep percent was calculated as the ratio of estimated total sleep time divided by the sleep period multiplied by 100, with values closer to 100% indicating the most amount of sleep and the least amount of wake time during the night. Sleep percent incorporates minutes of wake after sleep onset (WASO) in its calculation; thus, we did not include WASO as a unique variable in our analyses.
Sleep Quality
In addition, each participant completed a corresponding daily sleep log that was used to verify and validate the actigraphy data, and to provide a subjective rating of sleep quality. The log was used to report bedtimes, times when the actiwatch was removed, night awakenings, sleep latency, and sleep quality. Sleep quality was subjectively reported each morning using an 11-point NRS that ranged from 0= extremely poor sleep to 10= extremely good sleep. Complete sleep quality ratings were only available for 23 participants.
All participants had at least five complete nights of actigraphy and diary data at each time point (minimum of 10 total nights, including weekdays and weekends), which were averaged to obtain mean scores for analyses. Mean scores were used because we were interested in assessing typical sleep, rather than day-to-day variation in sleep. Adolescents were monitored for a total of fourteen days, seven days before randomization and then another seven days immediately post-treatment (after the 8-10 week treatment phase). Intraclass correlation coefficients across the seven-day baseline monitoring period revealed high stability of sleep quality ratings (ICC = .71, 95% CI .57-.84), moderate stability of sleep percent (ICC = .43 (95% CI .57-.84) and low stability of total sleep time (ICC = .29 (95% CI .15-.49).
Treatment Conditions
Routine medical care, as recommended by the treating subspecialty team, was continued in both groups throughout the study and involved on average 0 to 2 clinic visits.
Standard care wait-list control group
Adolescents were provided access to the study’s web site but only for completion of daily diary entries at pre- and post-treatment assessments. Upon completion and receipt of post-treatment (8-10 weeks) assessments, all families in this group were given the option to access the Internet program, which is described below.
Internet CBT pain treatment group
After randomization and pre-treatment assessments, participants (adolescents and parents) were granted access to the study’s Internet program, WebMAP, for 8-10 weeks of cognitive-behavioral pain treatment. Development and usability testing (Long & Palermo, 2009), treatment participation (Law, Murphy, & Palermo, 2012), and treatment efficacy for primary outcomes (Palermo et al., 2009) have previously been reported.
The Internet program has three main site portals: Passport (home) page, treatment modules, and daily diary. A travel-theme was incorporated, providing eight “travel destinations” on specific topics of psychoeducation and cognitive-behavioral skills that were completed weekly during the treatment program. At the end of each treatment module, participants completed a homework assignment that was reviewed by an online coach who provided feedback and encouraged continued skills practice. Participants logged on to the web program to complete one treatment module and homework assignment each week. Approximately nine hours of treatment was received by families (4 hours of adolescent treatment, 4 hours of parent treatment, and 1 hour therapist time on assignments).
Each of the eight adolescent modules were core components of CBT for pain management: 1) education about chronic pain, 2) recognizing stress and negative emotions, 3) deep breathing and relaxation, 4) distraction, 5) cognitive skills, 6) sleep hygiene and lifestyle, 7) staying active, and 8) relapse prevention. Lesson 6 in the adolescent program focused on lifestyle and sleep, including education about sleep needs, exercise and physical activity, and nutritional habits. Specifically, the sleep content included education about sleep and relevance to chronic pain management, and educational tips for improving sleep hygiene (e.g., keeping a consistent schedule, developing a bedtime routine, limiting electronics in the bedroom; Mindell & Owens, 2003).
The eight parent modules covered adaptive communication and interaction patterns focused on improving adolescent functioning and coping: 1) education about chronic pain, 2) recognizing stress and negative emotions, 3) operant strategies I, 4) operant strategies II, 5) modeling, 6) sleep hygiene and lifestyle, 7) communication, and 8) relapse prevention. For a complete description of the program, see Palermo et al., 2009.
Statisical Analysis
Descriptive statistics were computed to examine sample characteristics and sleep variables. To test the hypothesis that CBT for pain management would positively impact adolescent’s sleep, analysis of covariance (ANCOVA) procedures testing treatment group differences were performed controlling for pre-treatment values on three sleep variables: total sleep time, sleep percent, and subjective sleep quality. We hypothesized large effects for changes in sleep variables from pre- to post-treatment based on previous research that found large effect size differences (Cohen’s d) in actigraphic sleep measures among adolescents with chronic pain compared to healthy adolescents (Palermo, Toliver-Sokol, Fonareva, & Koh, 2007). We aimed to obtain a sample size of 20 per group (n = 40 total), which would provide 80% power to detect a large effect size (d = 1.0) given an alpha of .05 for a two-tailed test (Cohen, 1988).
To test the second hypothesis regarding whether reductions in pain and disability over the course of treatment were associated with positive changes in sleep, repeated measures analysis of variance (ANOVA) procedures were conducted with time of measurement (baseline vs. follow-up) as a within-subjects factor and change in pain and change in disability as continuous covariates. Finally, to test the hypothesis that pre-treatment (baseline) sleep would predict treatment outcomes, multiple linear regression was used to determine the contribution of sleep patterns (total sleep time, sleep percent) to post-treatment pain and disability after controlling for treatment group and baseline pain and disability.
Results
Descriptive statistics
Demographic and pain characteristics of the sample combined and by treatment condition are shown in Table 1. Participants’ ages ranged from 11-17 years, with a mean of 14.8 years (SD = 2.1); 69.7% were female. Abdominal pain was the most common pain type and accounted for approximately half of the complaints, subsequently followed by musculoskeletal and headache pain. Treatment groups were not significantly different on any demographic or clinical characteristics.
Sleep characteristics of the sample
Pre- and post-treatment sleep variables for the treatment and control conditions are found in Table 2. On average, adolescents at pre-treatment achieved approximately 7 hours of sleep. Nearly a third of the sample (N=10) demonstrated an average sleep time under 6.5 hours per night, as measured by actigraphy. Sleep percent averaged 84.6% (SD = 4.78%). Adolescents reported moderate subjective sleep quality (M = 5.33, SD = 1.97).
Table 2.
a. Sleep values at pre- and post-treatment
| Pre-treatment - Mean (SD) | Post-treatment - Mean (SD) | ||||
|---|---|---|---|---|---|
| Control (n=16) | Treatment (n=17) | Control (n=14) | Treatment (n=17) | ANCOVA resultsb | |
| Total Sleep Time (in minutes) | 412.9 (55.6) | 444.3 (51.7) | 398.4 (56.9) | 434.1 (44.5) | F(1, 30) = 1.20, p = .28, ηp2 =.038 |
| Sleep Percent (%) | 84.7 (5.3) | 84.5 (4.4) | 85.8 (4.7) | 84.1 (5.2) | F(1, 30) = 1.95, p = .17, ηp2 =.061 |
| Sleep Quality 0-10 NRSa | 5.01 (1.5) | 5.51 (2.2) | 5.33 (1.2) | 5.54 (1.6) | F(1, 19) = .21, p = .66, ηp2 =.011 |
n = 23
Treatment group effects after controlling for pre-treatment sleep variable
Pearson product moment correlations and intraclass correlation coefficients were conducted on the sleep variables to assess the reliability and stability of these measures over time. Results indicated a high level of association and stability in actigraphy sleep measures, with strong correlations between baseline and post-treatment total sleep time (r = .69, p < .001; ICC = .70, 95% CI .46-.84) and sleep percent (r = .80, p < .001, ICC = .81, 95% CI .64-.90). Adolescent’s baseline and post-treatment subjective sleep quality ratings showed weaker associations (r = .38, p = .12, ICC = .39, 95% CI - .07-.71).
CBT treatment outcome: pre-post group differences on sleep outcomes
ANCOVAs controlling for pre-treatment values were conducted on the sleep outcome variables to examine whether pain management treatment led to any change in sleep quality or quantity. Contrary to hypotheses, results indicated that treatment group was not related to significant improvements in any of the sleep variables (see Table 2). Both the Internet CBT group and the wait-list control condition had similar post-treatment total sleep time, sleep percent, and subjective sleep quality, with no significant changes observed in either group over time. Effect sizes were trivial (ηp2, ranging from .01 to .05). Although the final sample size (n=33) was smaller than our planned sample size (n=40), post hoc power analyses indicated that this sample size would have been sufficiently powered (80%) for detecting large effect sizes.
Changes in pain, sleep, and activity limitations over time
ANOVAs with time of measurement (baseline vs. follow up) as a within-subjects factor were conducted for two sleep outcomes, total sleep time and sleep percent, with change in pain and change in activity limitations scores entered as covariates. The main effect of time of measurement was not significant for total sleep time [F (1, 27) = .48, p = .49, ηp2 = .018] or for sleep percent [F (1, 27) = .353, p = .56, ηp2 = .013]. Changes in pain did not contribute to changes in total sleep time [F (1, 27) = .91, p = .35, ηp2 = .033] or to changes in sleep percent [F (1, 27) = 2.59, p = .12, ηp2 = .088]. Similarly, changes in activity limitations did not contribute to changes in total sleep time [F (1, 27) = 2.68, p = .12, ηp2 = .089] or to changes in sleep percent, [F (1, 28) = .243, p = .63, ηp2 = .009]. In sum, sleep did not significantly change over time in the entire sample, and positive changes in pain and activity limitations (improvements) did not account for changes in sleep.
Baseline sleep as a predictor of pain treatment response
In regression analyses controlling for treatment condition and baseline scores on each outcome, baseline actigraphy sleep variables (grand mean centered total sleep time and sleep percent) were examined as predictors of post-treatment pain intensity and activity limitations. As shown in Table 3, total sleep time and sleep percent at baseline were not predictive of post-treatment pain intensity. However, in the regression model predicting post-treatment activity limitations (R2 = .67, F (4, 28) = 14.02, p< .001; see Table 4), after controlling for treatment group and baseline activity limitations, total sleep time was a significant predictor of activity limitations (β= -.29, p < .03). Shorter sleep duration at baseline predicted higher levels of post-treatment activity limitations, indicating less improvement in pain-related disability. Sleep percent at baseline did not contribute to predicting treatment outcomes.
Table 3.
Hierarchical multiple regression predicting post-treatment pain from total sleep time and sleep percent at baseline
| Variable | R2Δ | Adj R2 | B | SE B | β | t | F | |
|---|---|---|---|---|---|---|---|---|
| Model 1 | .57 | .54 | 19.97** | |||||
| Treatment Groupa | -1.15 | .57 | -.24 | -2.02t | ||||
| Baseline Pain | .86 | .14 | .73 | 6.09** | ||||
| Model 2 | .01 | .52 | 9.68** | |||||
| Treatment Groupa | -1.08 | .61 | -.23 | -1.76 | ||||
| Baseline Pain | .87 | .15 | .74 | 5.95** | ||||
| Total Sleep Time | -.002 | .01 | -.05 | -.34 | ||||
| Sleep Percent | .05 | .06 | .11 | .83 |
coded as control group = 0, treatment group = 1; all predictor variables were centered
p<.06
p<.05
p<.001
Table 4.
Hierarchical multiple regression predicting post-treatment disability from total sleep time and sleep percent at baseline
| Variable | R2Δ | Adj R2 | B | SE B | β | t | F | |
|---|---|---|---|---|---|---|---|---|
| Model 1 | .59** | .56 | 21.66** | |||||
| Treatment Groupa | -1.76 | .90 | -.23 | -1.97t | ||||
| Baseline Disability | .58 | .09 | .73 | 6.25** | ||||
| Model 2 | .08 | .62 | 14.02 | |||||
| Treatment Groupa | -1.08 | .88 | -.15 | -1.28 | ||||
| Baseline Disability | .53 | .09 | .66 | 5.88** | ||||
| Total Sleep Time | -.02 | .01 | -.29 | -2.39* | ||||
| Sleep Percent | -.01 | .09 | .02 | .14 |
coded as control group = 0, treatment group = 1; all predictor variables were centered
p<.06
p<.05
p<.001
Discussion
Our preliminary findings indicate that, contrary to our hypothesis, CBT for pain management did not result in improvements in youth’s sleep. These findings provide some of the only available data to empirically test the prevailing clinical hypothesis that pain reduction will be associated with improved sleep, which was also not supported. We found that reductions in pain intensity and functional disability with CBT pain treatment were not associated with changes in sleep outcomes. These findings are hypothesis-generating, suggesting that youth with chronic pain may experience disrupted sleep not only due to pain but due to a variety of other factors.
As previously noted, the relationship between pain and sleep is complex. Although pain may be a precipitating factor for poor sleep, over time other sleep-related behaviors and cognitions may develop that perpetuate sleep problems independent from the effects of pain. From within the four-factor model of insomnia (Perlis, Giles, Mendelson, Bootzin, & Wyatt, 1997; Perlis, Smith, & Pigeon, 2005), poor sleep that initially occurs as a function of pain may persist even after pain abatement if compensatory sleep behaviors and conditioned arousal have not been addressed in treatment. As such, sleep problems in this population may require separate and more intensive sleep-specific management strategies that address the psychological, environmental, clinical, and behavioral factors that might perpetuate sleep problems in this special population. Potential targets may include physiological and cognitive arousal at bedtime (Palermo, Wilson, Lewandowski, Toliver-Sokol, & Murray, 2011; Palermo, Law, Churchill, & Walker, 2012), poor sleep hygiene (Bruni, Galli, &Guidetti, 1999; Valrie, Gil, Redding-Lallinger, &Daeschner, 2007), medication use, and daytime napping (Law, Dufton, & Palermo, 2012). Further research is needed to better characterize the nature and etiology of adolescent sleep disturbances and adolescent coping strategies that may impact on sleep (e.g., self-medication) in the context of chronic painful conditions.
Sleep problems are relatively common during adolescence, with approximately 25-40% of youth endorsing some sleep-related concern (Meltzer & Mindell, 2006; Ohayon, Roberts, Zulley, Smirme, & Priest, 2000). Sleep loss in youth is associated with a wide range of negative sequela, including academic impairment (Meijer, Habekothe, van den Wittenboer, 2009; Pilcher & Huffcutt, 1996), emotional lability, (Dagys, McGlinchey, Talbot, Kaplan, Dahl, & Harvey, 2012), and impulsivity (Catrett & Gaultney, 2009; Holm, Forbes, Ryan, Phillips, Tarr, & Dahl, 2009). For youth with chronic pain, poor sleep creates additional problems due to its links with increased pain and disability (Palermo & Kiska, 2005). Although the youth with chronic pain in our sample reported relatively short sleep duration and only moderate quality sleep, it is important to note that their sleep patterns were commensurate with that of otherwise healthy youth. Thus, in order to achieve optimal sleep, an effective sleep intervention for adolescents with chronic pain may require improving sleep beyond what is considered developmentally normative. On a related note, an alternative interpretation of our null findings is that our sample may not have had clinically significant problems with sleep at baseline or pain management regimens (e.g., medications) may have influenced sleep, thus limiting our ability to make notable improvements. Future research would benefit from inclusion of methods to assess for clinically significant sleep disturbance and careful assessment of medication use during the treatment period. It remains to be seen whether a CBT intervention for pain would result in improvements in sleep for youth who identify both pain and sleep as problematic, and the extent to which pain medication use may change as a function of treatment.
Consistent with our hypothesis, shorter total sleep time at baseline was predictive of higher levels of functional disability at follow up, suggesting that adolescents who have comorbid sleep problems may accrue less benefit from cognitive-behavioral therapy for pain management. It is possible that sleep disruption may heighten vigilance toward somatic sensations and may alter pain processing. Adults with primary insomnia have been shown to have lower pain thresholds and attenuation in pain inhibition, as assessed with an experimental conditioned pain modulation paradigm, when compared to controls (Haack et al., 2012). Further research is needed to understand the mechanisms that may underlie this association, and to determine whether providing behavioral intervention specifically targeting sleep disturbance can enhance the effects of behavioral pain management treatment on pain and functional disability outcomes for youth with chronic pain.
In adults with chronic pain, CBT for insomnia has been evaluated in several case series and randomized controlled trials resulting in promising changes in patients’ sleep quantity and quality (Currie, Wilson, Pontefract, & deLaplante, 2000; Jungquist et al., 2010; Morin, Kowatch, & Wade, 1989; Sánchez et al., 2012; Vitiello, Rybarczyk, Von Korff, &Stepanski, 2009). There is some suggestion that CBT insomnia strategies not only change sleep patterns (see also Cheng & Dizon, 2012, for a review) but also results in improvements in pain in adults (Vitiello, et al., 2009). Such innovative approaches to the complex relationship between pain and sleep will require examination of the optimal order and timing of interventions to answer the question of whether for example, CBT for insomnia is more effective when delivered prior to or following CBT for pain management. Treatment development in the area of sleep is critically needed in the population of adolescents with chronic pain.
Because sleep has rarely been assessed as an outcome in trials of psychological therapies for chronic pain, our findings contribute to this important gap in the literature. Although this was a small sample and we were limited in our available power to the detection of only large effects, our preliminary findings demonstrated that neither sleep duration nor sleep percent changed in the hypothesized, positive direction. It is possible that CBT for pain may result in improvement in sleep-relevant variables that were not captured by the present study’s methodology. For example, it is possible that reduction in pain contributed to reduced sleep onset latency, which actigraphy is not designed to capture. Our intervention may also have resulted in reductions in pain medication usage, which may have influenced sleep; however, we did not assess medication use during the treatment period. Finally, a larger sample size would have permitted evaluation of improvements in sleep stability through more sophisticated data analytic strategies, including multilevel modeling. These limitations should be considered and addressed in future research. Our online family pain CBT program contained core cognitive and behavioral strategies to decrease pain and improve youth’s activity participation as these were our primary treatment outcomes (similar to other CBT programs for pain management). Although we included basic education about sleep and sleep habits in one module, we did not intend to deliver sleep intervention to address significant sleep problems (such as insomnia), which would require more focused and intensive intervention strategies. Basic sleep hygiene instruction has been studied in only one previous investigation in youth with chronic pain (Bruni, Galli, & Guidetti, 1999), and sleep patterns at post-treatment were not assessed in that trial.
Interestingly, our null findings contribute to a growing body of research that suggests that cognitive behavioral therapy targeting other conditions does not improve sleep symptoms. Residual sleep disturbances are indeed common after CBT for depression (Carney, Harris, Friedman, & Segal, 2011) and PTSD (Belleville, Guay, & Marchand, 2011) in adults, and there is some emerging evidence to suggest that sleep problems persist after treatment of adolescent depression (Clarke & Harvey, 2012; Kennard et al., 2006). These recent findings, in conjunction with our own, suggest that traditional cognitive behavioral therapy is not sufficiently targeting sleep symptoms, even in the face of ‘primary disorder’ symptom abatement. They also underscore that sleep problems are not necessarily a secondary issue, but worthy of attention in their own right (NIH Consensus Science Statements, 2005).
Our study had several strengths including the use of multimodal assessment of sleep that included actigraphic and self-report measures. This represents an advance over prior studies conducted in adult populations where sleep assessments were based entirely on self-report. Objective and subjective assessments of sleep provide complementary information. However, although we chose an objective measure of sleep (actigraphy), even this method is subject to misclassification and may have biased the findings to the null. Our results should be interpreted in light of several limitations including that we had a small sample recruited from one treatment center, which limits generalizability of our findings. In addition, we were unable to thoroughly examine clinical pain variables such as pain diagnosis, pain location, or use of pain medication during the treatment period, and therefore may have overlooked other potential contributors to adolescent sleep outcomes. The potential effect of clinical pain variables on sleep is an important area of future inquiry. These findings should be viewed as preliminary and hypothesis-generating given the study limitations.
Given that many youth with chronic pain experience sleep disturbances, and that these sleep disturbances are associated with greater pain-related disability, there is a clear need to focus attention on sleep in this population. Future research is needed to more clearly identify the etiology of sleep dysfunction in youth with chronic pain. Longitudinal research and assessment of specific sleep disorders (e.g., insomnia) and of specific sleep habits may shed light on this issue and provide more focused targets for sleep interventions. Sleep is an important research priority in adolescent chronic pain management.
Acknowledgments
Funding: NIH R01HD053431 and K24HD060068 (TMP)
Contributor Information
Jessica Fales, Seattle Children’s Research Institute, M/S CW8-6, PO Box 5371, Seattle, WA 98145
Tonya M. Palermo, University of Washington and Seattle Children’s Research Institute, M/S CW8-6, PO Box 5371, Seattle, WA 98145
Emily F. Law, University of Washington and Seattle Children’s Research Institute, M/S CW8-6, PO Box 5371, Seattle, WA 98145
Anna C. Wilson, Oregon Health & Science University, 707 SW Gaines, Portland, OR 97239
References
- Babson KA, Feldner MT, Badour CL. Cognitive behavioral therapy for sleep disorders. Psychiatric Clinics of North America. 2010;33(3):629–640. doi: 10.1016/j.psc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Belleville G, Guay S, Marchand A. Persistence of sleep disturbances following cognitive-behavior therapy for posttraumatic stress disorder. Journal of Psychosomatic Research. 2011;70(4):318–327. doi: 10.1016/j.jpsychores.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Bruni O, Galli F, Guidetti V. Sleep hygiene and migraine in children and adolescents. Cephalalgia. 1999;25:57–59. doi: 10.1177/0333102499019s2516. [DOI] [PubMed] [Google Scholar]
- Carney CE, Harris AL, Friedman J, Segal ZV. Residual sleep beliefs and sleep disturbance following Cognitive Behavioral Therapy for major depression. Depression & Anxiety. 2011;28(6):464–470. doi: 10.1002/da.20811. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Factors influencing sleep patterns of adolescents. In: Carskadon MA, editor. Adolescent sleep patterns: Biological, social and psychological influences. Cambridge, U.K.: Cambridge University Press; 2002. [Google Scholar]
- Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2(4):453–460. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- Catrett CD, Gaultney JF. Possible insomnia predicts some risky behaviors among adolescents when controlling for depressive symptoms. Journal of Genetic Psychology. 2009;170(4):287–309. doi: 10.1080/00221320903218331. [DOI] [PubMed] [Google Scholar]
- Cheng SK, Dizon J. Computerised cognitive behavioral therapy for insomnia: A systematic review and meta-analysis. Psychotherapy and Psychosomatics. 2012;81(4):206–216. doi: 10.1159/000335379. [DOI] [PubMed] [Google Scholar]
- Clarke G, Harvey AG. The complex role of sleep in adolescent depression. Child & Adolescent Psychiatric Clinics of North America. 2012;21(2):385–400. doi: 10.1016/j.chc.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Currie SR, Wilson KG, Pontefract AJ, deLaplante L. Cognitive-behavioral treatment of insomnia secondary to chronic pain. Journal of Consulting and Clinical Psychology. 2000;68(3):407–416. doi: 10.1037//0022-006x.68.3.407. [DOI] [PubMed] [Google Scholar]
- Dagys N, McGlinchey EL, Talbot LS, Kaplan KA, Dahl RE, Harvey AG. Double trouble? The effects of sleep deprivation and chronotype on adolescent affect. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2012;53(6):660–670. doi: 10.1111/j.1469-7610.2011.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Palermo T, Williams A, Lewandowski A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Systematic Reviews. 2012;(12) doi: 10.1002/14651858.CD003968.pub3. Art No.: CD003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD007407.pub2. CD007407. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Means MK. Cognitive-behavioral therapy for primary insomnia. Clinical Psychology Review. 2005;25(5):539–558. doi: 10.1016/j.cpr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Fallone G, Owens JA, Deane J. Sleepiness in children and adolescents: clinical implications. Sleep Medicine Reviews. 2002;6(4):287–306. doi: 10.1053/smrv.2001.0192. [DOI] [PubMed] [Google Scholar]
- Gilman DK, Palermo TM, Kabbouche MA, Hershey AD, Powers SW. Primary headache and sleep disturbances in adolescents. Headache. 2007;47(8):1189–1194. doi: 10.1111/j.1526-4610.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102(3):616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- Haack M, Scott-Sutherland J, Santangelo G, Simpson NS, Sethna N, Mullington JM. Pain sensitivity and modulation in primary insomnia. European Journal of Pain. 2012;16(4):522–533. doi: 10.1016/j.ejpain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim A, Pillar G, Pecht A, Lerner A, Tov N, Jaffe M, Hardoff D. Sleep patterns in children and adolescents with functional recurrent abdominal pain: Objective versus subjective assessment. Acta Paediatrica. 2004;93(5):677–680. [PubMed] [Google Scholar]
- Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. Journal of Adolescent Health. 2009;45(4):326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, Lovell DJ, et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: A multisite, single-blind, randomized, controlled clinical trial. Arthritis & Rheumatism. 2012;64(1):297–305. doi: 10.1002/art.30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard B, Silva S, Vitiello B, Curry J, Kratochvil C, Simons A, Hughes J, et al. TADS Team. Remission and residual symptoms after short-term treatment in the Treatment of Adolescents with Depression Study (TADS) Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(12):1404–1411. doi: 10.1097/01.chi.0000242228.75516.21. [DOI] [PubMed] [Google Scholar]
- LaPlant MM, Adams BS, Haftel HM, Chervin RD. Insomnia and quality of life in children referred for limb pain. Journal of Rheumatology. 2007;34(12):2486–2490. [PubMed] [Google Scholar]
- Law EF, Dufton L, Palermo TM. Daytime and nighttime sleep patterns in adolescents with and without chronic pain. Health Psychology. 2012;31(6):830–833. doi: 10.1037/a0026485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law EF, Murphy LK, Palermo TM. Evaluating treatment participation in an internet-based behavioral intervention for pediatric chronic pain. Journal of Pediatric Psychology. 2012;37(8):893–903. doi: 10.1093/jpepsy/jss057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski AS, Palermo TM, De la Motte S, Fu R. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain. 2010;151(1):220–225. doi: 10.1016/j.pain.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin DS, Dahl RE. Importance of sleep in the management of pediatric pain. Journal of Developmental & Behavioral Pediatrics. 1999;20(4):244–252. doi: 10.1097/00004703-199908000-00007. [DOI] [PubMed] [Google Scholar]
- Long AC, Krishnamurthy V, Palermo TM. Sleep disturbances in school-age children with chronic pain. Journal of Pediatric Psychology. 2008;33(3):258–268. doi: 10.1093/jpepsy/jsm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AC, Palermo TM. Brief report: Web-based management of adolescent chronic pain: development and usability testing of an online family cognitive behavioral therapy program. Journal of Pediatric Psychology. 2009;34(5):511–516. doi: 10.1093/jpepsy/jsn082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Zeltzer L, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Journal of Pain. 2008;9(9):771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Meijer AM, Habekothe HT, Van Den Wittenboer GL. Time in bed, quality of sleep and school functioning of children. Journal of Sleep Research. 2000;9(2):145–153. doi: 10.1046/j.1365-2869.2000.00198.x. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Logan DE, Mindell JA. Sleep patterns in female adolescents with chronic musculoskeletal pain. Behavioral Sleep Medicine. 2005;3(4):193–208. doi: 10.1207/s15402010bsm0304_2. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Mindell JA. Sleep and sleep disorders in children and adolescents. Psychiatric Clinics of North America. 2006;29:1059–1076. doi: 10.1016/j.psc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Medicine Reviews. 2012;16:463–475. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA, Owens JA. Sleep problems in pediatric practice: Clinical issues for the pediatric nurse practitioner. Journal of Pediatric Health Care. 2003;17(6):324–331. doi: 10.1016/s0891-5245(03)00215-3. [DOI] [PubMed] [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Swick T, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep. 2007;30(4):519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- Morin CM, Kowatch RA, Wade JB. Behavioral management of sleep disturbances secondary to chronic pain. Journal of Behavior Therapy and Experimental Psychiatry. 1989;20(4):295–302. doi: 10.1016/0005-7916(89)90060-8. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. Sleep in America poll 2006 [Google Scholar]
- Neut D, Fily A, Cuvellier J, Vallée L. The prevalence of triggers in paediatric migraine: A questionnaire study in 102 children and adolescents. The Journal Of Headache And Pain. 2012;13(1):61–65. doi: 10.1007/s10194-011-0397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Consensus Science Statements. State-of-the-Science Conference Statement on manifestations and management of chronic insomnia in adults; 2005. pp. 1–30. [PubMed] [Google Scholar]
- Ohayon MM, Caulet M, Lemoine P. Comorbidity of mental and insomnia disorders in the general population. Comprehensive Psychiatry. 1998;39(4):185–197. doi: 10.1016/s0010-440x(98)90059-1. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Roberts RE, Zulley J, Smirne S, Priest RG. Prevalence and patterns of problematic sleep among older adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(12):1549–1556. doi: 10.1097/00004583-200012000-00019. [DOI] [PubMed] [Google Scholar]
- Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: A critical review of the literature. Journal of Developmental & Behavioral Pediatrics. 2000;21(1):58–69. doi: 10.1097/00004703-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Law EF, Churchill SS, Walker A. Longitudinal courase and impact of insomnia symptoms in adolescents with and without chronic pain. Journal of Pain. 2012;13(11):1099–1106. doi: 10.1016/j.jpain.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Fonareva I, Janosy NR. Sleep quality and efficiency in adolescents with chronic pain: Relationship with activity limitations and health-related quality of life. Behavioral Sleep Medicine. 2008;6(4):234–250. doi: 10.1080/15402000802371353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Kiska R. Subjective sleep disturbances in adolescents with chronic pain: Relationship to daily functioning and quality of life. Journal of Pain. 2005;6(3):201–207. doi: 10.1016/j.jpain.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Lewandowski AS, Long AC, Burant CJ. Validation of a self-report questionnaire version of the Child Activity Limitations Interview (CALI): The CALI-21. Pain. 2008;139:644–652. doi: 10.1016/j.pain.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Toliver-Sokol M, Fonareva I, Koh JL. Objective and subjective assessment of sleep in adolescents with chronic pain compared to healthy adolescents. Clinical Journal of Pain. 2007;23(9):812–820. doi: 10.1097/AJP.0b013e318156ca63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Wilson AC, Lewandowski AS, Toliver-Sokol M, Murray CB. Behavioral and psychosocial factors associated with insomnia in adolescents with chronic pain. Pain. 2011;152(1):89–94. doi: 10.1016/j.pain.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Wilson AC, Peters M, Lewandowski A, Somhegyi H. Randomized controlled trial of an Internet-delivered family cognitive-behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146(1-2):205–213. doi: 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Witherspoon D, Valenzuela D, Drotar D. Development and validation of the Child Activity Limitations Interview: A measure of pain-related functional impairment in school-age children and adolescents. Pain. 2004;109(3):461–470. doi: 10.1016/j.pain.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. Journal of Sleep Research. 1997;6(3):179–188. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Smith MT, Pigeon WR. Etiology and pathophysiology of insomnia. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 4. Philadelphia: Elseviers Saunders; 2005. [Google Scholar]
- Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19(4):318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Lee ES, Hemandez M, Solari AC. Symptoms of insomnia among adolescents in the lower Rio Grande Valley of Texas. Sleep. 2004;27(4):751–760. doi: 10.1093/sleep/27.4.751. [DOI] [PubMed] [Google Scholar]
- Sadeh A. Commentary: Comparing actigraphy and parental report as measures of children’s sleep. Journal of Pediatric Psychology. 2008;33(4):406–407. doi: 10.1093/jpepsy/jsn018. [DOI] [PubMed] [Google Scholar]
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain interrelate? Insights from the longitudinal and cognitive-behavioral trials literature. Sleep Medicine Reviews. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Developmental Psychology. 2000;36(3):291–301. doi: 10.1037//0012-1649.36.3.291. [DOI] [PubMed] [Google Scholar]
- Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- Soffer-Dudek N, Sadeh A, Dahl R, Rosenblat-Stein S. Poor sleep quality predicts deficient emotion information processing over time in early adolescence. Sleep. 2011;34(11):1499–1508. doi: 10.5665/sleep.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Labyak SE, Richardson LP, Lentz MJ, Brandt PA, Ward TM, Landis CA. Actigraphic sleep and daytime naps in adolescent girls with chronic musculoskeletal pain. Journal of Pediatric Psychology. 2008;33(3):307–311. doi: 10.1093/jpepsy/jsm117. [DOI] [PubMed] [Google Scholar]
- Valrie CR, Gil KM, Redding-Lallinger R, Daeschner C. Brief report: Sleep in children with sickle cell disease: An analysis of daily diaries utilizing multilevel models. Journal of Pediatric Psychology. 2007;32(7):857–861. doi: 10.1093/jpepsy/jsm016. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. Journal of Clinical Sleep Medicine. 2009;5(4):355–362. [PMC free article] [PubMed] [Google Scholar]
- Webster JB, Kripke DF, Messin S, Mullaney DJ, Wyborney G. An activity-based sleep monitor system for ambulatory use. Sleep. 1982;5(4):389–399. doi: 10.1093/sleep/5.4.389. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Samuelson B, Palermo TM. Obesity in children and adolescents with chronic pain: associations with pain and activity limitations. Clinical Journal of Pain. 2010;26(8):705–711. doi: 10.1097/AJP.0b013e3181e601fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69(4):875–887. [PubMed] [Google Scholar]


