Abstract
Background
Results of treatment for acute knee dislocations and multiligament knee injuries may be influenced by a multitude of patient- and injury-related factors, including neurologic function, vascular status, ipsilateral fractures, and joint stability. The development of heterotopic ossification (HO) may nullify any benefits of reconstruction, because it can cause stiffness and discomfort. Identifying factors associated with HO after knee dislocation may help identify patients who might benefit from prophylaxis.
Questions/purposes
The purposes of this study were (1) to identify specific risk factors for the development of HO in patients with knee dislocation; and (2) to elucidate the relationship between the presence of absence of HO and postoperative range of motion.
Methods
Between 2005 and 2010, we performed 101 multiligament reconstructions for patients with knee dislocations, of which 91 (90%) in 91 patients were available for followup at a minimum of 6 months (mean, 18 months; range, 6–44 months), and were reviewed here. AP and lateral radiographs were reviewed for all patients and HO was classified according to the Mills and Tejwani classification system. This knee dislocation cohort was separated into two groups based on the presence or absence of HO for comparison. Using a significance level of p < 0.05 for factors in the univariate analyses, we identified potential variables for a multivariate logistic regression model to identify risk factors predicting development of HO in patients with multiligament knee injuries; multivariate analysis then was performed to mitigate the influence of potentially confounding variables. Thirty patients (34%) developed HO after multiligament knee injury in our series.
Results
Posterior cruciate ligament reconstruction was the only independent predictor of HO that we identified (odds ratio, 6.3; 95% confidence interval, 1.2–34.6). Patients who developed HO were more likely to develop stiff knees and undergo surgery (50%; 15 of 30 patients) versus those without HO (12%; seven of 58 patients) to attempt to restore functional range of motion (p < 0.001).
Conclusions
HO is a common complication after knee dislocation and can diminish range of motion and cause patients to undergo further surgery. Posterior cruciate ligament reconstruction is an independent risk factor for the development of HO. Strategies to identify risk factors for, and safe prevention of, HO after multiple ligament injury and surgery should be investigated going forward.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Heterotopic ossification (HO) is the formation of bone in soft tissue or any area outside the human skeleton where it normally does not exist. Its development is thought to stem from an alteration in normal skeletogenesis [4, 11] and can occur in the context of musculoskeletal trauma, spinal cord injury, burns, or traumatic brain injury [1–3, 6, 9].
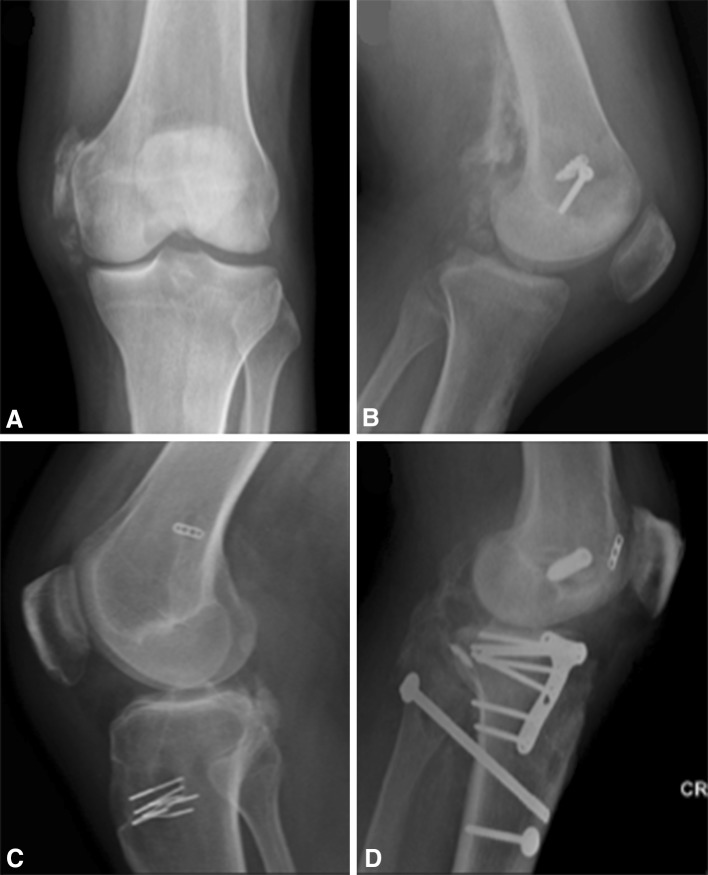
HO is a recognized complication of knee dislocation. Patton and Tew [8] described three patients with knee dislocations after high-energy trauma who all underwent early ligament reconstructive surgery. All three patients showed radiographic evidence of HO at approximately 6 weeks postoperatively with two of the three patients requiring additional surgery in the form of arthroscopic lysis of adhesions and manipulation under anesthesia (MUA) to improve ROM. Both the timing of surgery and posterior cruciate ligament (PCL) reconstruction were theorized to be contributing factors for the development of HO. Two series have described the prevalence of HO after knee dislocation with incidence rates varying from 26% to 43% [5, 13]. Neither of these studies used a multivariate regression model to identify independent risk factors for the development of HO. In a series of 35 patients, Mills and Tejwani [5] determined a high degree of injury, as reflected by an Injury Severity Score > 26, to be a significant risk factor for the development of ankylosing-type HO after surgical treatment for knee dislocation, whereas timing and type of surgery had no statistical influence on the degree of HO formation (Fig. 1).
Fig. 1A–D.
Classification of HO is demonstrated (Mills and Tejwani [5]): (A) Type I: medial and/or lateral collateral or capsular HO; (B) Type II: medial and/or lateral collateral or capsular HO and posterior femoral HO; (C) Type III: medial and/or lateral collateral or capsular HO and posterior tibial HO; (D) Type IV: knee spanning medial, lateral, or posterior ankylosing HO.
The development of HO can impair a patient’s recovery and rehabilitation after a traumatic knee dislocation and can also nullify the expected benefits of subsequent reconstructive surgery primarily through associated stiffness and discomfort. Furthermore, development of HO postoperatively may cause patients to undergo additional surgery, further compromising the patient’s rehabilitative course and prolonging their return to work and activity. Potentially modifiable factors include the timing of surgery, the type of reconstruction or repair, surgical approach, and external fixator application in the setting of acute dislocation. A more comprehensive understanding of the risk factors for the development of HO in the setting of knee dislocation may help guide the treating physician in the management of these complicated injuries and aid in the decision to implement HO prophylaxis.
We therefore sought to (1) identify specific risk factors for the development of HO in patients with knee dislocation; and (2) elucidate the relationship between the presence of absence of HO and postoperative ROM.
Patients and Methods
This study was approved by the research ethics board at our institution. Between 2005 and 2010, we performed 101 multiligament reconstructions for patients with knee dislocations, of which 91 of 101 (90%) in 91 patients were available for followup at a minimum of 6 months (mean, 18 months; range, 6–44 months), and were reviewed here. Patient records were extracted from a knee dislocation database of prospectively collected data at a single academic trauma center in Toronto, Ontario, Canada. Inclusion criteria were the presence of a knee dislocation or multiligament knee injury (defined as the concomitant disruption of at least one cruciate and collateral ligament) as identified on clinical examination and/or radiographs at initial presentation. Patients without radiographic evidence confirming a dislocation at the time of presentation (such as those patients referred to our institution after a reduction at another hospital) were confirmed to have a multiligament knee injury by MRI and clinical examination. Ten patients were lost to followup before a minimum of 6 months, and three patients were excluded from our analysis for a total of 88 cases. Reasons for exclusion included severe head injury that prevented appropriate followup and clinical examination (two patients) or death shortly after the injury (one patient). All clinical notes were reviewed and the following data were extracted: patient age, sex, mechanism of injury, presence of a closed head injury, associated fractures, ligamentous injuries, vascular injuries, fibular head fractures, application of a joint-spanning external fixator, and the type and timing of reconstructive surgery, if performed. The mechanism of injury was stratified into low- and high-energy mechanisms. Injuries that occurred as the result of (1) motor vehicle or motorcycle collisions; (2) pedestrian struck by a moving vehicle; or (3) collision sports were considered high-energy. All other injury mechanisms (eg, falls) were classified as low-energy. Ligamentous injuries were classified according to the system for knee dislocations developed by Schenck [10] and was based on the individual ligament disruptions detected on clinical examination and MRI. In this classification, knee dislocation (KD) I describes a dislocation with one of the cruciate ligaments intact (for example, a torn PCL, posteromedial corner, and posterolateral corner [PLC] with an intact anterior cruciate ligament [ACL]); KD II is a bicruciate rupture with collaterals intact; in KD III, the ACL, PCL, and one of the collateral ligaments are disrupted (“KD IIIL” for lateral and “KD IIIM” for medial); and KD IV describes an injury where all four major ligament groups are torn (both cruciates and both collaterals). Subsequent modifications to the original classification scheme have resulted in the addition of a further class, KD V, to describe fracture dislocations [12]. For the purposes of this study, we have used KD VI to refer to knee dislocations with an associated vascular injury.
AP and lateral radiographs were reviewed and classified according to the HO classification scheme developed by Mills and Tejwani [5]: Type 0, no HO; Type I, medial and/or lateral collateral or capsular HO; Type II, medial and/or lateral collateral or capsular HO and posterior femoral HO; Type III, medial and/or lateral collateral or capsular HO and posterior tibial HO; and Type IV, knee spanning medial, lateral, or posterior ankylosing HO.
Of the 88 patients whose records were analyzed, 69 of 88 patients (78%) were males and the average age was 33 years (range, 17–57 years). Injury occurred by a high-energy mechanism in 49 of 88 patients (55%). Two of 88 patients (2%) had a significant closed head injury and 23 of 88 patients (26%) had an associated fracture. The most common ligamentous injury pattern was KD IIIL, affecting 27 of 88 patients (31%). Six of 88 patients (7%) had an associated vascular injury requiring surgery and 15 of 88 patients (17%) had a fibular head fracture. Sixteen of 88 patients (18%) had application of an external fixator as part of the acute management after their injury. Sixty-eight of 88 patients (77%) went on to have ligament reconstructive surgery at an average of 242 days (range, 2–2006 days) after the initial injury, whereas 20 of 88 patients (23%) were managed nonoperatively (Table 1).
Table 1.
Patient demographics and univariate analysis
| Variable | Knee dislocation cohort (n = 88) | No HO cohort (n = 58) | HO cohort (n = 30) | p value |
|---|---|---|---|---|
| Age (mean [SD] in years) | 33.4 (10.5) | 31.8 (9.9) | 36.7 (10.7) | 0.031* |
| Sex (male) | 69 (78.4%) | 44 (76%) | 25 (83.3%) | 0.586 |
| Head injury | 2 (2.3%) | 1 (1.7%) | 1 (3.3%) | 1.00 |
| High-energy mechanism | 49 (55.7%) | 30 (51.7%) | 19 (63.3%) | 0.368 |
| Associated fractures | 23 (26.1%) | 14 (24.1%) | 9 (30%) | 0.612 |
| External fixator applied | 16 (18.2%) | 6 (10.3%) | 10 (33.3%) | 0.017† |
| Injury classification (Schenck) | ||||
| KD I | 19 (21.6%) | 16 (27.6%) | 3 (10%) | 0.099 |
| KD II | 2 (2.2%) | 1 (1.7%) | 1 (3.3%) | 1.00 |
| KD IIIL | 27 (30.7%) | 17 (29.3%) | 10 (33.3%) | 0.808 |
| KD IIIM | 22 (35%) | 18 (31%) | 4 (13.3%) | 0.076 |
| KD IV | 12 (13.6%) | 4 (6.9%) | 8 (25.7%) | 0.018 |
| KD V | 6 (6.8%) | 2 (3.4%) | 4 (13.3%) | 0.175 |
| Vascular injury requiring reconstruction | 6 (6.8%) | 2 (3.4%) | 4 (13.3%) | 0.175 |
| Fibular head fracture | 15 (17%) | 9 (15.5%) | 6 (20%) | 0.766 |
| Reconstructive surgery | 68 (77.4%) | 44 (76%) | 24 (80%) | 0.791 |
| PMC reconstruction or repair | 25 (28.4%) | 17 (29.3% | 8 (25.7%) | 1.00 |
| PLC reconstruction or repair | 39 (44.3%) | 21 (36%) | 18 (60%) | 0.043† |
| PCL reconstruction | 47 (53.4%) | 26 (44.8%) | 21 (70%) | 0.042† |
| ACL reconstruction | 53 (60.2%) | 36 (62.1%) | 17 (56.7%) | 0.652 |
| Time from injury to reconstruction (median [IQR] in days) | 242 (357.9) | 311.3 (411.4) | 136.2 (200.5) | 0.046‡ |
* Independent samples t-test; †Fisher’s exact test; ‡Wilcoxon rank sum test; HO = heterotopic ossification; KD = knee dislocation; PMC = posteromedial corner; PLC = posterolateral corner; PCL = posterior cruciate ligament; ACL = anterior cruciate ligament; IQR = interquartile range.
Data acquisition was performed by two authors (APD, TT) and was done so in compliance with institutional ethical requirements. All data were reviewed and verified independently by the two authors. The knee dislocation cohort was separated into two groups, based on the presence or absence of HO, for comparison. Means and SDs were calculated for age; comparison was performed with an independent sample Student’s t-test. The median and interquartile range were calculated for time to reconstruction because of the nonnormal distribution; comparison was performed using the Wilcoxon rank sum test. Frequencies were calculated for categorical variables (sex, mechanism of injury, injury classification, fibular head fracture, and vascular injury) and compared using Fisher’s exact test.
Radiographic evidence of HO on standard knee radiographs was present in 30 of 88 patients (34%). Seventeen of 30 patients developed Type 1 HO (57%), four of 30 patients developed Type II HO (13%), eight of 30 patients developed Type III HO (27%), and one of 30 patients developed Type IV HO (3%) according to the Mills classification scheme [5]. Further characteristics of the HO cohort are presented (Table 1).
In an exploratory univariate analyses (and using a threshold significance level of p < 0.05), we identified potential variables for subsequent inclusion in a definitive multivariate logistic regression model to identify independent risk factors for development of HO. The predictor variables we initially considered were age, sex, mechanism of injury (by energy of trauma), and type of knee dislocation (as per Schenk classification). If surgery was performed, the time from injury to reconstructive surgery and the specific ligaments that were addressed were also considered as was the use of an external fixator. Other potential risk factors for the development of HO were also considered such as the presence of concomitant head injury, vascular injury, or associated fractures. The choice of predictor variables for the initial univariate model was based on the senior author’s (DBW) experience as well as other investigations, which have suggested these as important [5, 8, 13]. The outcome variable was the presence of HO on standard radiographs.
The preliminary univariate analysis comparing patients with and without HO suggested statistically significant differences between the two groups (HO versus no HO) in age, external fixator application after the initial injury, time from injury to surgical reconstruction, and percentage of surgery involving PLC or PCL reconstruction. These variables were subsequently included in the definitive multivariate regression analysis model. The same preliminary univariate comparison demonstrated the groups were similar with respect to sex, presence of head injury, mechanism of injury, presence of an associated fracture, presence of vascular injury requiring surgery, and presence of fibular head fracture. In terms of injury classification, the only statistically significant difference between the two groups was a higher frequency of KD Type IV injuries in the HO cohort (p = 0.02). The numbers were small for this particular comparison however (eight KD IV in the HO group versus four KD IV patients in the non-HO group) and it was not included on that basis.
The impact of HO on final ROM was also considered. Our indication for MUA and arthroscopic lysis of adhesions was an ROM arc < 90° and/or a flexion contracture > 20° at 3 months followup after the injury or after reconstructive surgery. These groups were compared using Fisher’s exact test. The primary goal of manipulation was to improve ROM. Final ROM arcs were recorded for all patients and comparison between the two groups was performed with an independent sample Student’s t-test.
Results
PCL reconstruction was the only factor found to be an independent predictor of HO in knee dislocation (p = 0.025; odds ratio, 6.59; 95% confidence interval, 1.26–34.4) in the multivariate analysis. Age, external fixator application, PLC reconstruction, and time from injury to surgery did not demonstrate statistical significance in the prediction of HO with other variables considered (Table 2). A secondary exploratory analysis was performed to further analyze the effect of time to surgery with more chronic reconstructions omitted (wait time to surgery > 500 days, n = 13). The time to surgery threshold of 500 days was chosen arbitrarily. The results of this secondary analysis did not differ appreciably from the original in that time to surgery was not found to be an independent risk factor for the development of HO (Table 2).
Table 2.
Logistic regression modeling HO onto putative predictors (n = 68)*
| Independent factor | OR for development of HO (95% CI) | p value |
|---|---|---|
| Age, per year | 1.04 (0.99–1.08) | 0.162 |
| External fixator applied | 1.88 (0.43–8.27) | 0.406 |
| PLC reconstruction or repair | 2.46 (0.65–9.28) | 0.183 |
| PCL reconstruction | 6.59 (1.26–34.4) | 0.025 |
| Time from injury to reconstruction, per week | 0.99 (0.97–1.01) | 0.159 |
| After omitting individuals with a surgical waiting period > 500 days (n = 13 outliers)† | ||
| Age, per year | 1.05 (0.99–1.11) | 0.123 |
| External fixator applied | 1.82 (0.40–8.28) | 0.439 |
| PLC reconstruction or repair | 2.33 (0.57–9.56) | 0.241 |
| PCL reconstruction | 6.30 (1.17–34.06) | 0.033 |
| Time from injury to reconstruction, per week | 1.00 (0.97–1.04) | 0.867 |
* Omnibus likelihood ratio test: χ2(5) = 20.9, p = 0.001, c-statistic = 0.82; †omnibus likelihood ratio test: χ2(5) = 13.7, p = 0.018, c-statistic = 0.77; HO = heterotopic ossification; OR = odds ratio; CI = confidence interval; PLC = posterolateral corner; PCL = posterior cruciate ligament.
Overall, 22 of 88 patients (25%) required surgery to address knee stiffness (Table 3). Patients in the HO cohort were more likely to undergo manipulation and/or arthroscopic débridement of arthrofibrosis (15 of 30 in HO cohort [50%] versus seven of 58 in the non-HO group [12%]; p < 0.001) (Table 3). The preoperative ROM arc in the 15 patients undergoing operative intervention to improve their ROM was 61° (range, 15°–120°; SD = 31°), which improved to 109° (range, 70°–140°; SD = 19°) postoperatively at final followup. Despite further operative intervention intended to improve ROM in these 15 patients, the final ROM arcs were still lower in the HO group than in the non-HO group at last followup (123°; range, 85°–140°; SD = 15° in the non-HO cohort versus 112°; range, 70°–140°; SD = 14° in the HO cohort; p < 0.05; Table 3).
Table 3.
Heterotopic ossification and ROM
| Variable | Knee dislocation cohort (n = 88) | No HO cohort (n = 58) | HO cohort (n = 30) | p value |
|---|---|---|---|---|
| Stiffness requiring surgical intervention (MUA and arthroscopic lysis of adhesions) | 22 (25%) | 7 (12.1%) | 15 (50%) | < 0.001* |
| Final ROM arc | 118.6 (15.8%) | 123.2 (14.9) | 111.7 (14.5) | < 0.001† |
* Fisher’s exact test; †independent samples t-test; HO = heterotopic ossification; MUA = manipulation under anesthesia.
Discussion
HO is an important complicating factor to consider when managing patients after knee dislocation, but to our knowledge, risk factors for its development have not been characterized in large series. We were able to identify and quantify an exhaustive list of possible risk factors for the development of HO in a large cohort of patients with knee dislocation. A preliminary univariate analysis identified potentially significant variables for inclusion in a multiple regression analysis. When we considered age, time to surgery, use of a fixator, and whether a PLC or PCL reconstruction was performed for multiligament knee injury, a multivariate regression analysis demonstrated that concomitant PCL reconstruction was the sole independent predictor for the development of HO.
Our retrospective cohort study has a number of notable limitations. Many of the patients in our cohort were referred to our Level I trauma center from other tertiary care institutions. This selection bias may have resulted in a disproportionately higher number of complicated knee dislocations with associated injuries. However, the rate of development of HO in our cohort (34%; 30 of 88 patients) is comparable to the rates described by Mills and Tejwani (43%) [5] and Stannard et al. (26%) [13]. Our study is actually the largest cohort specifically looking at HO. Despite our large numbers for a relatively uncommon event, we are likely still underpowered given that the optimal statistical model would include at least 10 events for each variable of interest in the multivariate regression model. Furthermore, we have considered HO as a binary, “yes or no” event. In reality, the severity of the condition must also be considered for its true impact to be realized. This would undoubtedly take far more patients than were available to us at a single center. Thirteen patients were lost as a result of exclusions and issues with followup. This rate of loss, although regrettable, is likely consistent with the traumatic nature of the injury and followup in general for such patients [7].
Patton and Tew [8] theorized a link between PCL reconstruction and posterior capsular ossification in their series of three patients who developed HO after knee dislocation. Of note, the patients in this series underwent surgical treatment at 16, 7, and 10 days after the injury, further implicating the timing to surgery as a potential factor in the development of HO after injury. Our analysis, albeit potentially statistically underpowered, did not confirm time from injury to surgery as an independent risk factor for postoperative HO development.
We found that patients with HO were more likely to undergo further surgery to treat pain and functional limitations and that even after repeat surgery, these patients had less ROM than patients who never developed HO. We are aware of two prior studies of HO after knee dislocation [5, 13]; however, those studies did not specifically address the functional consequences of HO in this setting. We believe this is an important area for future study given how often these patients underwent further surgery to treat the problem.
The risk of HO is an important consideration when treating patients with knee dislocation and/or multiligament knee injuries. HO prophylaxis may be appropriate if PCL reconstructions are considered part of surgical treatment, but before a firm therapeutic recommendation can be made on this point, prospective studies should be performed; small numbers will almost certainly require this work to be a collaborative multicenter trial. Implementation of prophylactic measures such as nonsteroidal medications or radiotherapy must proceed with an understanding of the potential risks involved. Should HO develop after injury or surgery, ROM limitations can be anticipated, and these may not respond to manipulation.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Chen HC, Yang JY, Chuang SS, Huang CY, Yang SY. Heterotopic ossification in burns: our experience and literature reviews. Burns. 2009;35:857–862. doi: 10.1016/j.burns.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Cipriano CA, Pill SG, Keenan MA. Heterotopic ossification following traumatic brain injury and spinal cord injury. J Am Acad Orthop Surg. 2009;17:689–697. doi: 10.5435/00124635-200911000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Citak M, Suero EM, Backhaus M, Aach M, Godry H, Meindl R, Schildhauer TA. Risk factors for heterotopic ossification in patients with spinal cord injury: a case-control study of 264 patients. Spine (Phila Pa 1976). 2012;37:1953–1957. doi: 10.1097/BRS.0b013e31825ee81b. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan FS, Hahn GV, Zasloff MA. Heterotopic ossification: two rare forms and what they can teach us. J Am Acad Orthop Surg. 1994;2:288–296. doi: 10.5435/00124635-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Mills WJ, Tejwani N. Heterotopic ossification after knee dislocation: the predictive value of the injury severity score. J Orthop Trauma. 2003;17:338–345. doi: 10.1097/00005131-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Nauth A, Giles E, Potter BK, Nesti LJ, O’Brien FP, Bosse MJ, Anglen JO, Mehta S, Ahn J, Miclau T, Schemitsch EH. Heterotopic ossification in orthopaedic trauma. J Orthop Trauma. 2012;26:684–688. doi: 10.1097/BOT.0b013e3182724624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pape HC, Zelle BA, Lohse R, Stalp M, Hildebrand F, Krettek C, Panzica M, Duhme V, Sittaro NA. Evaluation and outcome of patients after polytrauma: can patients be recruited for long-term follow-up? Injury. 2006;37:1197–1203. doi: 10.1016/j.injury.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 8.Patton WC, Tew WM. Periarticular heterotopic ossification after multiple knee ligament reconstructions. A report of three cases. Am J Sports Med. 2000;28:398–401. doi: 10.1177/03635465000280032001. [DOI] [PubMed] [Google Scholar]
- 9.Potter BK, Forsberg JA, Davis TA, Evans KN, Hawksworth JS, Tadaki D, Brown TS, Crane NJ, Burns TC, O’Brien FP, Elster EA. Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am. 2010;92(Suppl 2):74–89. doi: 10.2106/JBJS.J.00776. [DOI] [PubMed] [Google Scholar]
- 10.Schenck R. Classification of knee dislocations. Oper Tech Sports Med. 2003;11:193–198. doi: 10.1053/otsm.2003.35918. [DOI] [Google Scholar]
- 11.Shafritz AB, Shore EM, Gannon FH, Zasloff MA, Taub R, Muenke M, Kaplan FS. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med. 1996;335:555–561. doi: 10.1056/NEJM199608223350804. [DOI] [PubMed] [Google Scholar]
- 12.Stannard JP, Sheils TM, Lopez-Ben RR, McGwin G, Jr, Robinson JT, Volgas DA. Vascular injuries in knee dislocations: the role of physical examination in determining the need for arteriography. J Bone Joint Surg Am. 2004;86:910–915. [PubMed] [Google Scholar]
- 13.Stannard JP, Wilson TC, Sheils TM, McGwin G, Jr, Volgas DA, Alonso JE. Heterotopic ossification associated with knee dislocation. Arthroscopy. 2002;18:835–839. doi: 10.1053/jars.2002.32842. [DOI] [PubMed] [Google Scholar]