Abstract
Background
Bisphosphonate therapy for osteoporosis has been associated with atypical femoral fractures. To date, there have been few reports in the literature regarding the preoperative and postoperative courses of patients who have sustained bisphosphonate-associated complete atypical femur fractures.
Objectives/purposes
The purposes of this study were to (1) characterize the preoperative course of patients who eventually presented with bisphosphonate-associated complete atypical femur fractures (duration of bisphosphonate treatment, pain history, risk of converting a nondisplaced fracture to a complete fracture); (2) evaluate the percentage of patients who achieved radiographic union of those fractures after treatment; and (3) determine the patients’ recovery of function using the Short Musculoskeletal Functional Assessment.
Methods
Thirty-three patients with 41 atypical, low-energy femur fractures associated with ≥ 5 years of bisphosphonate use were treated with intramedullary nailing between 2004 and 2011 at one center. The main outcome measurements were Short Musculoskeletal Functional Assessment for function and radiographic evaluation for fracture healing. Patients had been treated with bisphosphonates for an average of 8.8 years (range, 5–20 years) before presentation.
Results
Patients reported a mean of 6 months of pain before presentation (range, 1–8 months). Sixty-six percent of patients with surgically treated complete fractures became pain-free and 98% were radiographically healed by 12 months. Sixty-four percent of patients who underwent intramedullary nailing reported a functional return to baseline within 1 year. Patients who reported major functional limitations at latest followup listed pain and apprehension as the major causes of their limitation.
Conclusions
Patients with surgically treated bisphosphonate-associated complete femur fractures achieved generally reliable although delayed fracture healing if malaligned, and nearly two-thirds of patients returned to self-reported baseline function within 1 year.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteoporosis is a major health concern with over 200 million people having the disease worldwide [16]. The lifelong risk of sustaining an osteoporotic fracture is approximately one in every two women and one in every four men [23]. The economic costs, morbidity, and mortality of osteoporosis are substantial [7, 10, 15]. Each year, 325,000 patients sustain a hip fracture; this devastating, common complication of osteoporosis costs an estimated USD 40,000 per patient to treat [7]. The incidence of osteoporosis-related fractures in the United States is projected to continue increasing from now until 2025 [7].
Alendronate was first approved by the US Food and Drug Administration in 1995, and after that, three other bisphosphonates—risedronate, ibandronate, and zoledronic acid—have been approved for use. The mechanism of action of the drugs is to inhibit the mevalonate pathway of cholesterol synthesis and thus interfere with protein prenylation and induce osteoclast apoptosis [3, 17]. A number of clinical trials have shown this class of medication decreases bone resorption and turnover, increases bone mineral density, and ultimately reduces the risk of vertebral fractures in osteoporotic patients; all the bisphosphonates, except ibandronate, have also been shown to reduce the risk of hip and nonvertebral fractures [6, 10, 27]. With a concomitant reduction in associated costs and healthcare use, bisphosphonates have become widely recognized as a mainstay of treatment for osteoporosis [5, 31].
However, in 2005, a case report suggested a link between long-term bisphosphonate use and atypical nonspinal fractures [24, 31]. Since then, with the publication of many additional case reports and small case series, the association between atypical femoral fractures and long-term bisphosphonate use has now been accepted [3, 12, 18, 22]. It has been suggested that severe and prolonged suppression of bone turnover may impair the ability of the bone to remodel, eventually leading to an accumulation of microdamage and diminution of bone strength [6, 11, 29]. Animal models have provided additional evidence of a potential link, showing alendronate to inhibit normal repair of microdamage from oversuppression of bone turnover [19–21].
The atypical subtrochanteric and diaphyseal femur fractures share distinct radiological characteristics and distinct clinical and radiographic fracture patterns uncommon in patients with osteoporosis (Fig. 1) [14]. Patients who sustain these fractures typically report prodromal thigh pain weeks to months before sustaining fractures. Radiographs before fracture completion and displacement show focal lateral cortical thickening with or without a visible, incomplete fracture line [4, 26]. In addition, these injuries are secondary to low-energy or minimal to no trauma mechanism, unlike other proximal femoral fractures, which are typically associated with major trauma [4]. When complete and displaced, these atypical subtrochanteric and diaphyseal femoral fractures have characteristic radiographic features such as the presence of a transverse or short oblique fracture line, medial spike, focal lateral cortical thickening, and relative lack of comminution, that differ from typical femur fractures but are usually treated with a similar surgical procedure as used for treating conventional femoral fractures [4].
Fig. 1A–C.
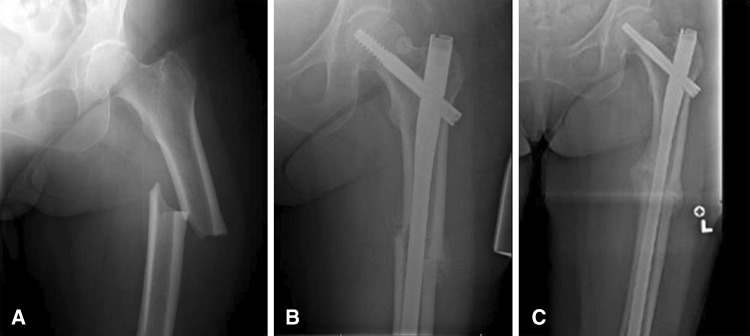
(A) The AP radiograph at presentation of a 78-year-old woman treated with bisphosphonate therapy for 15 years shows radiographic characteristics of a complete bisphosphonate-associated fracture. (B) The AP radiograph of the same patient at 2 months status postintramedullary fixation shows early signs of healing. (C) The AP radiograph at 6 months status postfixation shows a healed fracture.
The purposes of this study were to (1) characterize the preoperative course of patients who eventually presented with bisphosphonate-associated complete atypical femur fractures (duration of bisphosphonate treatment, pain history, and risk of displacing a fracture that was nondisplaced at presentation); (2) evaluate the percentage of patients who achieved radiographic union of those fractures after treatment; and (3) determine the patients’ recovery of function using the Short Musculoskeletal Functional Assessment.
Patients and Methods
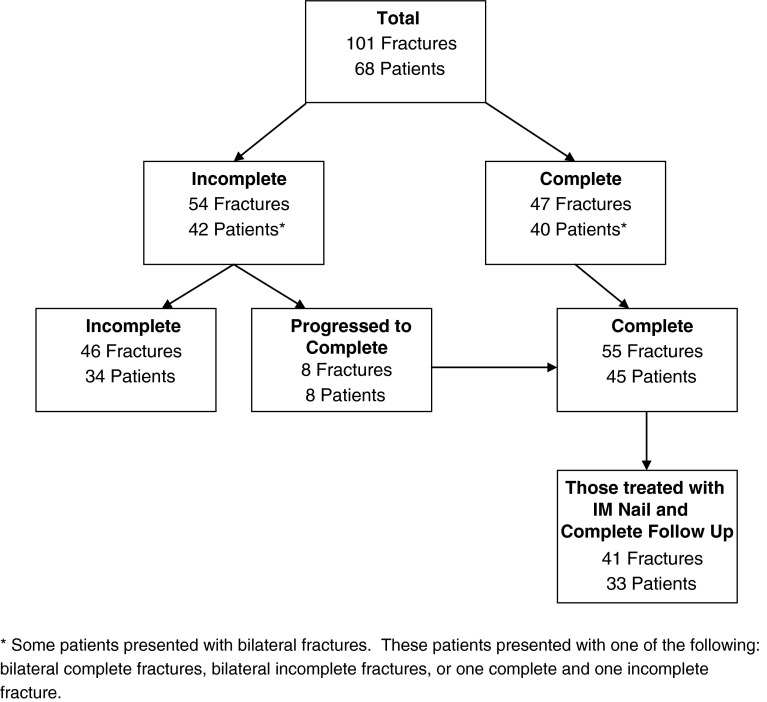
This was an institutional review board-approved, retrospective HIPAA-compliant study for which informed consent was waived. We identified and reviewed the records of all 68 patients with 101 bisphosphonate-associated femoral fractures who were treated at our academic medical center between 2004 and 2011. We included patients in the study who (1) presented with complete bisphosphonate-associated atypical femoral fractures; or (2) presented with incomplete bisphosphonate-associated atypical femoral fractures that progressed to complete fractures while being treated conservatively (Fig. 1). We defined atypical complete femoral fractures by radiographic evidence of a transverse or short oblique fracture line, medial spike, focal lateral cortical thickening, and relative lack of comminution. Incomplete bisphosphonate-associated atypical femoral fractures were defined by radiographic evidence of focal lateral cortical thickening with or without an incomplete fracture line [25].
Forty patients with 47 complete and displaced atypical femoral fractures presented to the emergency departments and outpatient offices of our academic medical center. In addition, eight patients, who had initially presented with eight incomplete atypical femoral fractures (15% of the incomplete cohort) progressing to complete fractures, were also identified; three of these patients sustained contralateral complete fractures and overlap with the initial cohort of 40 patients. Thus, a total of 45 patients with 55 complete displaced bisphosphonate-associated femur fractures were identified for potential inclusion. Patients who had surgical stabilization elsewhere, patients treated with a plate and screw construct, and patients with incomplete followup were excluded (Fig. 2).
Fig. 2.
The flow diagram of patients shows how the final study cohort was determined from our registry of bisphosphonate-associated femoral fracture cases.
The final cohort included 33 patients with 41 fractures (Fig. 2). Of the 33 patients with 41 fractures, 18 patients with 22 fractures were treated with a cephalomedullary nail (Gamma, Stryker, Mahwah, NJ, USA; Biomet, Warsaw, IN, USA), 14 patients with 17 fractures were treated with a standard antegrade femoral nail (Zimmer, Warsaw, IN, USA; Synthes, Paoli, PA, USA), and one patient with nonsimultaneous bilateral fractures was treated with a cephallomedullary nail on one side and a standard nail on the other (Table 1).
Table 1.
Fracture treatment summary for the final cohort
| Patient ID | Sex | Age (years) | Laterality | Site | Implant | Reduction* |
|---|---|---|---|---|---|---|
| 1 | F | 73 | L | Shaft | CMN | Anatomic |
| 2 | F | 66 | R | Shaft | Standard | Slight varus |
| 3 | F | 55 | L | Subtrochanteric | CMN | Anatomic |
| 53 | R | Subtrochanteric | CMN | Anatomic | ||
| 4 | F | 60 | L | Subtrochanteric | Standard | Anatomic |
| 60 | R | Subtrochanteric | Standard | Slight varus | ||
| 5 | F | 76 | L | Shaft | Standard | Anatomic |
| 6 | F | 69 | L | Subtrochanteric | CMN | Varus |
| 69 | R | Subtrochanteric | CMN | Slight varus | ||
| 7 | F | 83 | R | Shaft | CMN | Anatomic |
| 8 | F | 61 | R | Shaft | Standard | Anatomic |
| 9 | F | 74 | R | Shaft | Standard | Anatomic |
| 10 | F | 59 | R | Subtrochanteric | CMN | Anatomic |
| 11 | F | 72 | R | Subtrochanteric | CMN | Slight varus |
| 12 | F | 58 | R | Subtrochanteric | CMN | Anatomic |
| 13 | F | 63 | R | Subtrochanteric | CMN | Varus |
| 14 | F | 62 | L | Subtrochanteric | CMN | Slight varus |
| 15 | F | 58 | L | Subtrochanteric | CMN | Varus |
| 56 | R | Subtrochanteric | CMN | Varus | ||
| 16 | F | 58 | L | Subtrochanteric | CMN | Anatomic |
| 59 | R | Subtrochanteric | CMN | Anatomic | ||
| 17 | F | 58 | R | Subtrochanteric | Standard | Varus |
| 18 | F | 77 | L | Shaft | Standard | Anatomic |
| 19 | F | 65 | L | Shaft | Standard | Anatomic |
| 65 | R | Shaft | Standard | Anatomic | ||
| 20 | F | 58 | L | Shaft | Standard | Anatomic |
| 58 | R | Subtrochanteric | CMN | Varus | ||
| 21 | F | 58 | R | Shaft | Standard | Slight varus |
| 22 | F | 75 | L | Subtrochanteric | CMN | Anatomic |
| 23 | F | 73 | R | Shaft | Standard | Anatomic |
| 24 | F | 69 | R | Shaft | CMN | Anatomic |
| 25 | F | 62 | L | Shaft | Standard | Anatomic |
| 26 | F | 62 | R | Subtrochanteric | CMN | Anatomic |
| 27 | M | 63 | L | Subtrochanteric | CMN | Varus |
| 28 | F | 70 | L | Subtrochanteric | Standard | Anatomic |
| 70 | R | Shaft | Standard | Varus | ||
| 29 | F | 46 | L | Subtrochanteric | CMN | Anatomic |
| 30 | M | 68 | R | Subtrochanteric | CMN | Anatomic |
| 31 | F | 60 | L | Subtrochanteric | Standard | Anatomic |
| 32 | F | 56 | L | Shaft | Standard | Varus |
| 33 | F | 69 | R | Subtrochanteric | CMN | Anatomic |
* Slight varus = malreduction up to 10°; varus = malreduction greater than 10°; F = female; M = male; L = left; R = right; CMN = cephalomedullary nail.
Surgeries were performed on a radiolucent flat or fracture table and the nails were generally inserted closed and locked using a large lag screw that was placed into the femoral head and neck or from greater to lesser trochanter depending on nail type. In some cases, we performed an open fracture reduction and in all cases, distal locking screws were inserted. Postoperatively, patients were allowed to be weightbearing as tolerated and were followed at standard followup intervals of 1 month, 3 months, 6 months, and 1 year by the treating surgeons.
We retrospectively documented patient demographics, initial radiographic diagnosis, treatment modality, time to healing, and self-reported functional status. Clinical healing was defined as a resolution of pain, whereas radiographic healing was defined as bridging across three or four cortices and/or loss of a visible fracture line based on standard AP and lateral femoral radiographs. Determination of fracture healing was made by the treating surgeon in conjunction with a musculoskeletal radiologist (KAE, NCT, ZSR).
Patients were contacted by telephone at a mean of 33 months (range, 6–85 months) from the date of fracture completion to complete the Short Musculoskeletal Functional Assessment (SMFA). We excluded patients who could not be contacted for followup questions from functional analysis. Functional status as well as clinical data were analyzed with Student’s t-test and Fisher’s exact test.
Results
The final patient cohort of 33 patients and 41 complete and displaced atypical bisphosphonate-associated femoral fractures had been treated with bisphosphonate therapy for an average of 8.8 years (range, 5–20 years) before presentation. This patient cohort reported a mean of 6 months of prodromal pain before initial presentation (range, 1–8 months) see Table 2 for demographics.
Table 2.
Patient demographics summary
| Demographic | Male (n = 2) | Female (n = 31) |
|---|---|---|
| Age distribution (years) | ||
| 40–49 | 0 | 1 |
| 50–59 | 0 | 9 |
| 60–69 | 2 | 12 |
| 70–79 | 0 | 8 |
| 80+ | 0 | 1 |
| Laterality | ||
| Left, isolated | 1 | 9 |
| Right, isolated | 1 | 14 |
| Bilateral | 0 | 8 |
| Length of Fosamax use | ||
| Mean years | 5.0 | 9.1 |
| SD | 0 | 4.1 |
Forty of 41 (98%) fractures in the final cohort united after the index surgery at a mean of 8.3 months (range, 2–18 months). Critical analysis of radiographs revealed that varus malreduction at the fracture site had a negative impact on healing. Twenty-six fractures were reduced anatomically, whereas 15 fractures were reduced nonanatomically (Table 1). The mean healing time for the anatomically reduced group was 7.1 months (range, 2–12 months; SD 3.6 months); the nonanatomically reduced fractures healed in 10.8 months (range, 2–18 months; SD 4.3 months). One patient went on to nonunion resulting from a technical error and united after revision surgery, which included removal of the intramedullary nail and compression plating with a blade plate and bone graft at 13.5 months after initial injury and 2.5 months after revision surgery. The treating surgeon used a short cephalomedullary nail as a result of previous distal hardware placement. The distal locking bolt was placed through a slot instead of a hole, which allowed for varus displacement at the fracture site and nonunion (Fig. 3A). Although this patient went on to achieve fracture union (Fig. 3B), the radiographic healing time for this patient was excluded from statistical analyses.
Fig. 3A–B.
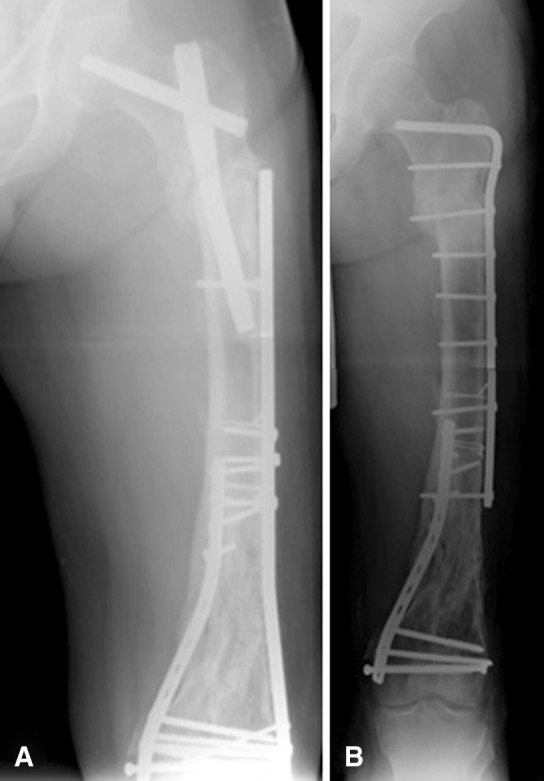
Radiographs of the patient who went on to nonunion. (A) In this radiograph, the distal locking bolt was placed through a slot instead of a hole, which allowed for varus displacement at the fracture site and nonunion. (B) The patient went on to achieve fracture union after revision surgery.
Of the 33 patients with 41 complete fractures, 22 patients with 27 fractures (66% [27of 41fractures]) became pain-free and 21 patients with 26 fractures (64% [21of 33 patients]) self-reported a return to baseline functional status within 1 year. Twelve patients with 15 fractures (37% [12 of 33 patients]), who reported major functional limitations at latest followup, listed pain and apprehension as the major causes of their limitation. Major functional limitations were determined by a comparison to normative SMFA values from the general population [13]. A comparison of reported SMFA scores between patients reduced anatomically and nonanatomically did not yield significant differences.
Discussion
Osteoporosis continues to be a health concern for the growing aging population, whereas long-term bisphosphonate therapy, used to treat osteoporotic patients, has been associated with atypical femoral fractures. There have been few reports in the literature regarding the preoperative and postoperative courses of patients who have sustained bisphosphonate-associated complete atypical femur fractures. In our review of clinical data, we have been able to characterize the prodromal course of patients who eventually presented with bisphosphonate-associated complete atypical femur fractures, evaluate and compare the percentage of patients who achieved radiographic union with differences in reduction quality, and determine their ultimate functional outcomes based on their SMFA questionnaire results in conjunction with clinical observations.
The limitations of our study are inherent in a retrospective case series. First, clinical indices such as serum vitamin D levels, bone densitometry scores, and other contributing medical conditions were not available for all patients, making it unclear whether other factors may have influenced our functional results. Second, a small group of patients had also sustained incomplete or complete fractures on the contralateral side or sustained bilateral complete fractures; the SMFA questionnaire is limited in that it cannot distinguish the contribution of the contralateral side, which may ultimately influence the outcome. In addition, a small number of patients were treated with teriparatide, which may potentially positively affect the outcome.
Our final patient cohort was characterized by bisphosphonate use for a mean of 8.8 years and prodromal pain for a mean of 6 months. These observations are consistent with the belief that the presence of such pain, associated with a history of bisphosphonate therapy, may serve as an indicator of impending fracture [28]. Radiographs of these atypical femoral fractures have also shown consistent characteristics such as transverse or short oblique fracture, focal lateral cortical thickening, the presence of a medial beak, defined as a spiked appearance of the medial femoral cortex, and relative lack of comminution [4]. Those who display clinical or radiographic symptoms of incomplete bisphosphonate-associated femur fractures should be counseled about the potential risk of sustaining a complete fracture and prophylactic measures to prevent fracture completion [8].
After surgical treatment, the likelihood of radiographic healing in our cohort was observed to be high: 98% of fractures ultimately united at a mean of 8.3 months (range, 2–18 months). Furthermore, we noticed differences in healing time between fractures reduced anatomically and nonanatomically with anatomically reduced fractures healing 3.7 months faster than those fixed in varus. Healing times of almost 8 months for these fractures are noted to be longer than those for typical femoral fractures, which heal at an average of 3 to 6 months [2, 12, 25].
Despite high rates of radiographic healing, we did observe mixed results in functional outcomes with only 66% of the cohort achieving pain-free status and only 64% self-reporting a return to baseline functional status. Statistical comparison of our patients’ SMFA scores to normative SMFA scores indicates that 37% of our cohort continued to experience major functional limitation after surgery; pain and apprehension are noted as some of the major causes of their limitation [13]. Nonetheless, these findings generally suggest that the intramedullary nail is an effective treatment modality for treating complete bisphosphonate-associated femur fractures. The reduction quality was not observed to significantly affect functional outcome.
Bisphosphonates have been proven to be highly effective as a treatment modality in patients with osteoporosis for the prevention of osteoporotic fractures [2, 9, 30]. Although the association between long-term bisphosphonate therapy and atypical femoral fractures is now accepted, the benefits of bisphosphonate therapy far outweigh the risks, and bisphosphonates continue to be a mainstay of treatment [1]. In general, patients who sustain complete, atypical femoral fractures associated with long-term bisphosphonate use who are surgically treated can expect to heal radiographically by 8 months. A varus malreduction at the fracture site seems to delay healing. Although the majority are likely to return to baseline functional status within 1 year, a notable population may not resolve residual functional limitations within that same timeframe.
Acknowledgments
We thank the NYU Langone Medical Center and the Jamaica Hospital Medical Center for providing the facilities and resources to perform clinical research and contribute to the medical community.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved or waived approval for the reporting of this case and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Audran M, Cortet B, Thomas T. What do we know about atypical femoral fractures? Insights and enigmas. Joint Bone Spine. 2011;78:568–571. doi: 10.1016/j.jbspin.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/S0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 3.Capeci CM, Tejwani NC. Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J Bone Joint Surg Am. 2009;91:2556–2561. doi: 10.2106/JBJS.H.01774. [DOI] [PubMed] [Google Scholar]
- 4.Chan SS, Rosenberg ZS, Chan K, Capeci C. Subtrochanteric femoral fractures in patients receiving long-term alendronate therapy: imaging features. AJR Am J Roentgenol. 2010;194:1581–1586. doi: 10.2214/AJR.09.3588. [DOI] [PubMed] [Google Scholar]
- 5.Chrischilles EA, Dasbach EJ, Rubenstein LM, Cook JR, Tabor HK, Black DM. The effect of alendronate on fracture-related healthcare utilization and costs: the fracture intervention trial. Osteoporos Int. 2001;12:654–660. doi: 10.1007/s001980170065. [DOI] [PubMed] [Google Scholar]
- 6.De Das S, Setiobudi T, Shen L. A rational approach to management of alendronate-related subtrochanteric fractures. J Bone Joint Surg Br. 2010;92:679–686. doi: 10.1302/0301-620X.92B5.22941. [DOI] [PubMed] [Google Scholar]
- 7.Dell R, Greene D. Is osteoporosis diseases management cost effective? Curr Osteoporos Rep. 2010;8:49–55. doi: 10.1007/s11914-010-0009-0. [DOI] [PubMed] [Google Scholar]
- 8.Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma. 2014 Sep 14 [Epub ahead of print]. [DOI] [PubMed]
- 9.Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, Feldstein A, Haskell WL, Hochberg MC, Torner JC, Lombardi A, Black DM. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res. 2004;19:1259–1269. doi: 10.1359/JBMR.040326. [DOI] [PubMed] [Google Scholar]
- 10.Girgis CM, Seibel MJ. Guilt by association? Examining the role of bisphosphonate therapy in the development of atypical femur fractures. Bone. 2011;48:963–965. doi: 10.1016/j.bone.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169–180. doi: 10.1016/j.bone.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Hollick R, Reid D. Role of bisphosphonates in the management of postmenopausal osteoporosis: an update on recent safety anxieties. Menopause Int. 2011;17:66–72. doi: 10.1258/mi.2011.011014. [DOI] [PubMed] [Google Scholar]
- 13.Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84:208–215. doi: 10.2106/00004623-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Ing-Lorenzini K, Desmeules J, Plachta O, Suva D, Dayer P, Peter R. Low-energy femoral fractures associated with the long-term use of bisphosphonates: a case series from a Swiss university hospital. Drug Safety. 2009;32:775–785. doi: 10.2165/00002018-200932090-00002. [DOI] [PubMed] [Google Scholar]
- 15.Johnell O, Kanis J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 16.Kanis JA. Assessment of Osteoporosis at the Primary Health-Care Level, WHO Technical Report. Sheffield, UK:WHO Scientific Group,World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield; 2007.
- 17.Koval KJ, Zuckerman JD, editors. Fractures in the Elderly. New York, NY, USA: Lippincott-Raven Publishers; 1998. [Google Scholar]
- 18.Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, van der Meulen MC, Lorich DG, Lane JM. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;20:1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Mashiba T, Burr D. Bisphosphonate treatment suppresses not only stochnastic remodeling but also the targeted repair of microdamage. Calcif Tissue Int. 2001;69:281–286. doi: 10.1007/s002230010036. [DOI] [PubMed] [Google Scholar]
- 20.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 21.Mashiba T, Turner C, Hirano T, Forwood M, Johnston C, Burr D. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001;28:524–531. doi: 10.1016/S8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 22.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 23.Nieves JW, Cosman F. Atypical subtrochanteric and femoral shaft fractures and possible association with bisphosphonates. Curr Osteoporos Rep. 2010;8:34–39. doi: 10.1007/s11914-010-0007-2. [DOI] [PubMed] [Google Scholar]
- 24.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg ZS, La Rocca Vieira R, Chan SS, Babb J, Akyol Y, Rybak LD, Moore S, Bencardino JT, Peck V, Tejwani NC, Egol KA. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol. 2011;197:954–960. doi: 10.2214/AJR.10.6262. [DOI] [PubMed] [Google Scholar]
- 26.Sellmeyer DE. Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA. 2010;304:1480–1484. doi: 10.1001/jama.2010.1360. [DOI] [PubMed] [Google Scholar]
- 27.Somford MP, Draijer FW, Thomassen BJ, Chavassieux PM, Boivin G, Papapoulos SE. Bilateral fractures of the femur diaphysis in a patient with rheumatoid arthritis on long-term treatment with alendronate: clues to the mechanism of increased bone fragility. J Bone Miner Res. 2009;24:1736–1740. doi: 10.1359/jbmr.090408. [DOI] [PubMed] [Google Scholar]
- 28.Wang K, Moaveni A, Dowrick A, Liew S. Alendronate-associated femoral insufficiency fractures and femoral stress reactions. J Orthop Surg (Hong Kong). 2011;19:89–92. doi: 10.1177/230949901101900121. [DOI] [PubMed] [Google Scholar]
- 29.Watts N, Diab D. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–1565. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 30.Weil YA, Rivkin G, Safran O, Liebergall M, Foldes AJ. The outcome of surgically treated femur fractures associated with long-term bisphosphonate use. J Trauma. 2011;71:186–190. doi: 10.1097/TA.0b013e31821957e3. [DOI] [PubMed] [Google Scholar]
- 31.Yoon RS, Hwang JS, Beebe KS. Long-term bisphosphonate usage and subtrochanteric insufficiency fractures. J Bone Joint Surg Br. 2011;93:1289–1295. doi: 10.1302/0301-620X.93B10.26924. [DOI] [PubMed] [Google Scholar]