Abstract
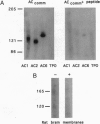
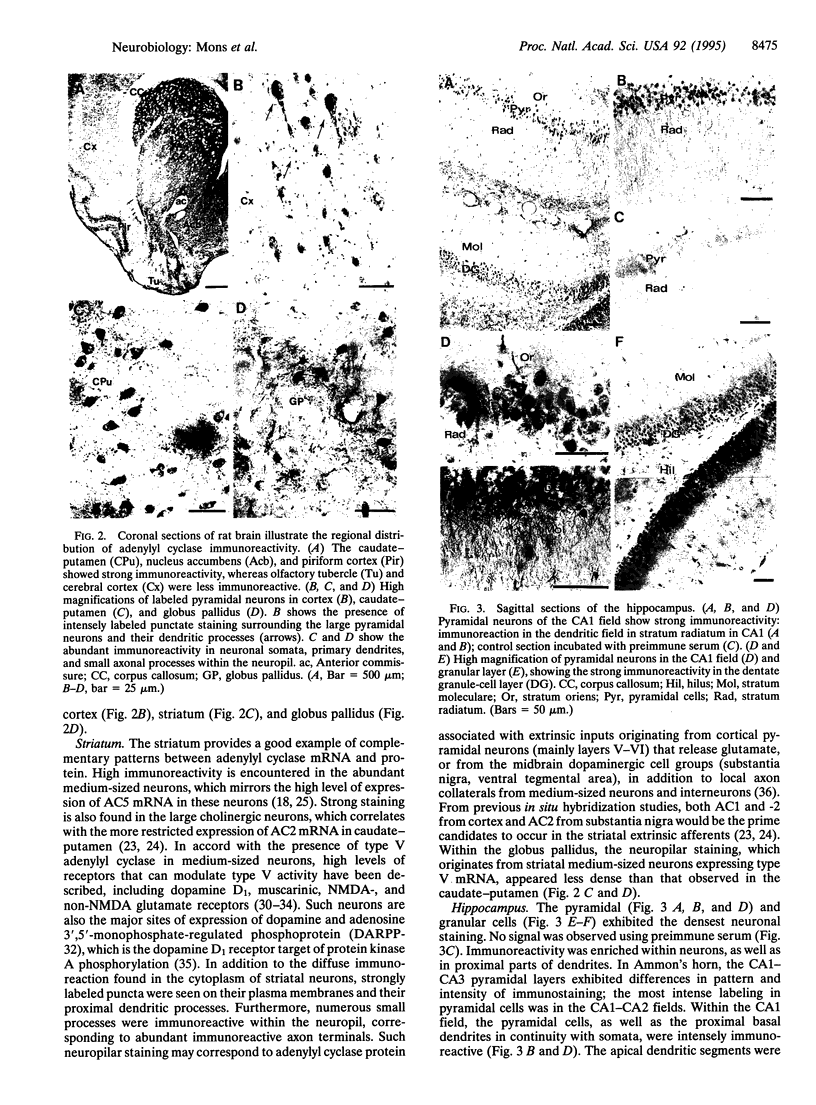
Only three isoforms of adenylyl cyclase (EC 4.6.1.1) mRNAs (AC1, -2, and -5) are expressed at high levels in rat brain. AC1 occurs predominantly in hippocampus and cerebellum, AC5 is restricted to the basal ganglia, whereas AC2 is more widely expressed, but at much lower levels. The distribution and abundance of adenylyl cyclase protein were examined by immunohistochemistry with an antiserum that recognizes a peptide sequence shared by all known mammalian adenylyl cyclase isoforms. The immunoreactivity in striatum and hippocampus could be readily interpreted within the context of previous in situ hybridization studies. However, extending the information that could be gathered by comparisons with in situ hybridization analysis, it was apparent that staining was confined to the neuropil--corresponding to immunoreactive dendrites and axon terminals. Electron microscopy indicated a remarkably selective subcellular distribution of adenylyl cyclase protein. In the CA1 area of the hippocampus, the densest immunoreactivity was seen in postsynaptic densities in dendritic spine heads. Labeled presynaptic axon terminals were also observed, indicating the participation of adenylyl cyclase in the regulation of neurotransmitter release. The selective concentration of adenylyl cyclases at synaptic sites provides morphological data for understanding the pre- and postsynaptic roles of adenylyl cyclase in discrete neuronal circuits in rat brain. The apparent clustering of adenylyl cyclases, coupled with other data that suggest higher-order associations of regulatory elements including G proteins, N-methyl-D-aspartate receptors, and cAMP-dependent protein kinases, suggests not only that the primary structural information has been encoded to render the cAMP system responsive to the Ca(2+)-signaling system but also that higher-order strictures are in place to ensure that Ca2+ signals are economically delivered and propagated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakalyar H. A., Reed R. R. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990 Dec 7;250(4986):1403–1406. doi: 10.1126/science.2255909. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Nicoll R. Molecular machines integrate coincident synaptic signals. Cell. 1993 Jan;72 (Suppl):65–75. doi: 10.1016/s0092-8674(05)80029-7. [DOI] [PubMed] [Google Scholar]
- Cali J. J., Zwaagstra J. C., Mons N., Cooper D. M., Krupinski J. Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem. 1994 Apr 22;269(16):12190–12195. [PubMed] [Google Scholar]
- Chetkovich D. M., Gray R., Johnston D., Sweatt J. D. N-methyl-D-aspartate receptor activation increases cAMP levels and voltage-gated Ca2+ channel activity in area CA1 of hippocampus. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6467–6471. doi: 10.1073/pnas.88.15.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. M., Mons N., Karpen J. W. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature. 1995 Mar 30;374(6521):421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- Defer N., Marinx O., Stengel D., Danisova A., Iourgenko V., Matsuoka I., Caput D., Hanoune J. Molecular cloning of the human type VIII adenylyl cyclase. FEBS Lett. 1994 Aug 29;351(1):109–113. doi: 10.1016/0014-5793(94)00836-1. [DOI] [PubMed] [Google Scholar]
- Feinstein P. G., Schrader K. A., Bakalyar H. A., Tang W. J., Krupinski J., Gilman A. G., Reed R. R. Molecular cloning and characterization of a Ca2+/calmodulin-insensitive adenylyl cyclase from rat brain. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10173–10177. doi: 10.1073/pnas.88.22.10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T., Inagaki S., Takagi H. Distribution of type II adenylyl cyclase mRNA in the rat brain. Brain Res Mol Brain Res. 1993 Jul;19(1-2):165–170. doi: 10.1016/0169-328x(93)90163-j. [DOI] [PubMed] [Google Scholar]
- Gao B. N., Gilman A. G. Cloning and expression of a widely distributed (type IV) adenylyl cyclase. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10178–10182. doi: 10.1073/pnas.88.22.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers D. L., Lowe D. G. Guanylyl cyclase receptors. J Biol Chem. 1994 Dec 9;269(49):30741–30744. [PubMed] [Google Scholar]
- Glatt C. E., Snyder S. H. Cloning and expression of an adenylyl cyclase localized to the corpus striatum. Nature. 1993 Feb 11;361(6412):536–538. doi: 10.1038/361536a0. [DOI] [PubMed] [Google Scholar]
- Hellevuo K., Yoshimura M., Kao M., Hoffman P. L., Cooper D. M., Tabakoff B. A novel adenylyl cyclase sequence cloned from the human erythroleukemia cell line. Biochem Biophys Res Commun. 1993 Apr 15;192(1):311–318. doi: 10.1006/bbrc.1993.1415. [DOI] [PubMed] [Google Scholar]
- Hersch S. M., Gutekunst C. A., Rees H. D., Heilman C. J., Levey A. I. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994 May;14(5 Pt 2):3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y., Katsushika S., Chen L., Halnon N. J., Kawabe J., Homcy C. J. Isolation and characterization of a novel cardiac adenylylcyclase cDNA. J Biol Chem. 1992 Jul 5;267(19):13553–13557. [PubMed] [Google Scholar]
- Iyengar R. Molecular and functional diversity of mammalian Gs-stimulated adenylyl cyclases. FASEB J. 1993 Jun;7(9):768–775. doi: 10.1096/fasebj.7.9.8330684. [DOI] [PubMed] [Google Scholar]
- Jacobowitz O., Iyengar R. Phorbol ester-induced stimulation and phosphorylation of adenylyl cyclase 2. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10630–10634. doi: 10.1073/pnas.91.22.10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangeer S., Rodbell M. The disaggregation theory of signal transduction revisited: further evidence that G proteins are multimeric and disaggregate to monomers when activated. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8782–8786. doi: 10.1073/pnas.90.19.8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsushika S., Chen L., Kawabe J., Nilakantan R., Halnon N. J., Homcy C. J., Ishikawa Y. Cloning and characterization of a sixth adenylyl cyclase isoform: types V and VI constitute a subgroup within the mammalian adenylyl cyclase family. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8774–8778. doi: 10.1073/pnas.89.18.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J., Coussen F., Bakalyar H. A., Tang W. J., Feinstein P. G., Orth K., Slaughter C., Reed R. R., Gilman A. G. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science. 1989 Jun 30;244(4912):1558–1564. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- Krupinski J., Lehman T. C., Frankenfield C. D., Zwaagstra J. C., Watson P. A. Molecular diversity in the adenylylcyclase family. Evidence for eight forms of the enzyme and cloning of type VI. J Biol Chem. 1992 Dec 5;267(34):24858–24862. [PubMed] [Google Scholar]
- Kötter R. Postsynaptic integration of glutamatergic and dopaminergic signals in the striatum. Prog Neurobiol. 1994 Oct;44(2):163–196. doi: 10.1016/0301-0082(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Ludvig N., Burmeister V., Jobe P. C., Kincaid R. L. Electron microscopic immunocytochemical evidence that the calmodulin-dependent cyclic nucleotide phosphodiesterase is localized predominantly at postsynaptic sites in the rat brain. Neuroscience. 1991;44(2):491–500. doi: 10.1016/0306-4522(91)90072-v. [DOI] [PubMed] [Google Scholar]
- Ludvig N., Ribak C. E., Scott J. D., Rubin C. S. Immunocytochemical localization of the neural-specific regulatory subunit of the type II cyclic AMP-dependent protein kinase to postsynaptic structures in the rat brain. Brain Res. 1990 Jun 18;520(1-2):90–102. doi: 10.1016/0006-8993(90)91694-c. [DOI] [PubMed] [Google Scholar]
- Martin L. J., Blackstone C. D., Levey A. I., Huganir R. L., Price D. L. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience. 1993 Mar;53(2):327–358. doi: 10.1016/0306-4522(93)90199-p. [DOI] [PubMed] [Google Scholar]
- Matsuoka I., Giuili G., Poyard M., Stengel D., Parma J., Guellaen G., Hanoune J. Localization of adenylyl and guanylyl cyclase in rat brain by in situ hybridization: comparison with calmodulin mRNA distribution. J Neurosci. 1992 Sep;12(9):3350–3360. doi: 10.1523/JNEUROSCI.12-09-03350.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mons N., Cooper D. M. Adenylyl cyclase mRNA expression does not reflect the predominant Ca2+/calmodulin-stimulated activity in the hypothalamus. J Neuroendocrinol. 1994 Dec;6(6):665–671. doi: 10.1111/j.1365-2826.1994.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Mons N., Cooper D. M. Selective expression of one Ca(2+)-inhibitable adenylyl cyclase in dopaminergically innervated rat brain regions. Brain Res Mol Brain Res. 1994 Mar;22(1-4):236–244. doi: 10.1016/0169-328x(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Mons N., Yoshimura M., Cooper D. M. Discrete expression of Ca2+/calmodulin-sensitive and Ca(2+)-insensitive adenylyl cyclases in the rat brain. Synapse. 1993 May;14(1):51–59. doi: 10.1002/syn.890140108. [DOI] [PubMed] [Google Scholar]
- Müller W., Connor J. A. Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature. 1991 Nov 7;354(6348):73–76. doi: 10.1038/354073a0. [DOI] [PubMed] [Google Scholar]
- Neubig R. R. Membrane organization in G-protein mechanisms. FASEB J. 1994 Sep;8(12):939–946. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- Ouimet C. C., Greengard P. Distribution of DARPP-32 in the basal ganglia: an electron microscopic study. J Neurocytol. 1990 Feb;19(1):39–52. doi: 10.1007/BF01188438. [DOI] [PubMed] [Google Scholar]
- Parma J., Stengel D., Gannage M. H., Poyard M., Barouki R., Hanoune J. Sequence of a human brain adenylyl cyclase partial cDNA: evidence for a consensus cyclase specific domain. Biochem Biophys Res Commun. 1991 Aug 30;179(1):455–462. doi: 10.1016/0006-291x(91)91392-p. [DOI] [PubMed] [Google Scholar]
- Petralia R. S., Yokotani N., Wenthold R. J. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994 Feb;14(2):667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont R. T., Chen J., Ma H. W., Ponnapalli M., Iyengar R. Two members of a widely expressed subfamily of hormone-stimulated adenylyl cyclases. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9809–9813. doi: 10.1073/pnas.89.20.9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Multiple forms of phospholipase C isozymes and their activation mechanisms. Adv Second Messenger Phosphoprotein Res. 1992;26:35–61. [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 1992 Jun 25;267(18):12393–12396. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Kempner E. S., Rodbell M. Activation of adenylate cyclase in hepatic membranes involves interactions of the catalytic unit with multimeric complexes of regulatory proteins. J Biol Chem. 1979 Jun 25;254(12):5168–5176. [PubMed] [Google Scholar]
- Shu S. Y., Ju G., Fan L. Z. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988 Feb 29;85(2):169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Siegel S. J., Brose N., Janssen W. G., Gasic G. P., Jahn R., Heinemann S. F., Morrison J. H. Regional, cellular, and ultrastructural distribution of N-methyl-D-aspartate receptor subunit 1 in monkey hippocampus. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):564–568. doi: 10.1073/pnas.91.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig R., Gilman A. G. Mammalian membrane-bound adenylyl cyclases. J Biol Chem. 1995 Jan 6;270(1):1–4. doi: 10.1074/jbc.270.1.1. [DOI] [PubMed] [Google Scholar]
- Testa C. M., Standaert D. G., Young A. B., Penney J. B., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994 May;14(5 Pt 2):3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P. A., Krupinski J., Kempinski A. M., Frankenfield C. D. Molecular cloning and characterization of the type VII isoform of mammalian adenylyl cyclase expressed widely in mouse tissues and in S49 mouse lymphoma cells. J Biol Chem. 1994 Nov 18;269(46):28893–28898. [PubMed] [Google Scholar]
- Weiner D. M., Levey A. I., Brann M. R. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z. G., Refsdal C. D., Merchant K. M., Dorsa D. M., Storm D. R. Distribution of mRNA for the calmodulin-sensitive adenylate cyclase in rat brain: expression in areas associated with learning and memory. Neuron. 1991 Mar;6(3):431–443. doi: 10.1016/0896-6273(91)90251-t. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Cooper D. M. Cloning and expression of a Ca(2+)-inhibitable adenylyl cyclase from NCB-20 cells. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6716–6720. doi: 10.1073/pnas.89.15.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]