Abstract
Background
The entry point is crucial to an accurate reduction in femoral nailing. Fluoroscopy-based navigation was developed to aid in reducing femur fractures and selecting entry points.
Questions/purposes
We asked: (1) Can the piriformis fossa (PF) and tip of the greater trochanter (TT) be identified with high reproducibility? (2) What is the range of nonneutral images clinically acceptable for entry point selection? (3) Does navigation improve accuracy and precision of landmarking the TT and PF? And (4) does off-angle fluoroscopy within the acceptable range affect landmark accuracy?
Methods
Three orthopaedic surgeons digitized the PF and TT under direct visualization on 10 cadaveric femurs, quantifying the reproducibility of the targeted PF and TT landmarks. Arcs of acceptable AP and lateral images of each femur were acquired in increments of 5° with a C-arm. An experienced orthopaedic surgeon rejected or accepted images for entry point selection by qualitatively assessing the relative positions and sizes of the greater trochanter, lesser trochanter, and femoral neck. Entry points were identified on each image using fluoroscopy and navigation. Hierarchical linear modeling was used to compare accuracy and precision between navigation and fluoroscopy and the effects of image angle.
Results
A 29° average arc of acceptable images was found. Reproducibility of the target landmarks for the PF and TT under direct visualization was excellent. Navigation had similar accuracy to fluoroscopy for PF localization but less for TT. Navigation increased precision compared to fluoroscopy for both PF and TT. Image angle affected accuracy of the PF and TT under fluoroscopy and navigation.
Conclusions
Nonorthogonal images reduce accuracy of PF and TT identification with both navigation and fluoroscopy. Navigation increased precision but decreased accuracy and cannot overcome inaccuracies induced by nonorthogonal images.
Introduction
Closed intramedullary (IM) nailing of the femur has become the standard of care for treating closed femoral shaft fractures in adults and has been shown to have high union rates [4, 20, 23]. Despite the popularity and high union rates of this approach, IM nailing is associated with complications due to malreduction and iatrogenic fractures [1, 8, 12]. One factor that may influence the incidence of such complications is the point of entry for the nail [9, 15].
In antegrade nailing, the nail is driven into the IM canal through an entry point located at either the piriformis fossa (PF) or the tip of the greater trochanter (TT) [20, 21]. This is done under fluoroscopic guidance and using tactile feedback to locate the entry point for the nail. The importance of accuracy in the location of the entry point has been established for both the PF and TT [6, 9, 12, 13, 15–17, 23]. Incorrect location of the entry point may lead to an increased risk of malalignment. In the case of both the PF and TT, it has been shown medial and lateral inaccuracies in the entry point can raise hoop stresses, leading to an increased risk of iatrogenic fracture in the proximal segment and the femoral neck [6, 13, 16, 17, 23]. Additionally, for the PF, anterior translation of the entry point has been shown to increase hoop stresses and risk of femoral neck fractures [9, 15]. Currently, there is little documentation on the tolerance for the entry point selection, with the majority of the aforementioned studies describing the qualitative variations used to examine the effect of the displaced entry point. A single study quantified the variation used to examine the position of the TT and the effects of a 2- to 3-mm shift in the mediolateral (ML) direction on the alignment of the reduction using a variety of IM nails [17]. Other than this, there is currently little documentation regarding the tolerance or acceptable deviations in the PF entry point (in either the AP or ML directions) or the TT in the AP direction, and although the effects of offsetting the entry point in the two planes have been documented, the threshold at which these effects become unacceptable has not been documented. Also, uncertainty in the description and identification of both the PF and TT has been indicated in the literature, presenting an even greater challenge for their intraoperative identification under fluoroscopic guidance [10].
Navigation systems have been developed, enabling the surgeon to simultaneously view the position of the tip of a tool in biplanar fluoroscopic images [5, 7, 11]. However, to date, there is no documentation on whether these systems can improve overall accuracy and precision of the localization of entry points for IM nailing by overcoming inaccuracies induced by nonorthogonal images.
We asked four questions: (1) Is the localization of the PF and TT landmarks, under direct visualization, reproducible? (2) Is there a range of nonneutral AP and lateral images that would be deemed clinically acceptable for entry point selection? (3) Does fluoroscopy-based computer navigation increase the accuracy and precision with which the PF and TT entry points are selected when compared to conventional fluoroscopic guidance? And (4) does off-angle acquisition within the acceptable range of AP or lateral images affect the accuracy of landmark selection?
Materials and Methods
We obtained 10 fresh-frozen cadaveric right femurs (two male, eight female; age range, 43–93 years) with approval from the institutional research ethics board. All specimens were stripped of soft tissue. A right angle was used to mark the direction of the mediolateral ML and AP axes on the distal portion of the diaphysis. A 3-mm drill was used to form three holes denoting a coordinate system used as reference points for the measurement of the entry points.
Each femur was clamped to a table with the posterior condyles parallel to its surface. A three-dimensional (3-D) digital scribe (MicroScribe™ 3Dx; GoMeasure3D, Amherst, VA, USA), accurate to 0.23 mm, was clamped to the same table and its reference coordinate system set based on the coordinate system created in the femoral diaphysis. Three fellowship-trained orthopaedic surgeons (AK, BC, MRE) separately digitized the location of the PF and TT with the scribe using direct visualization and tactile feedback to locate the points. This provided a 3-D set of coordinates for the location of both the PF and TT. The digital scribe did not create any visible or palpable indentations or alterations in the surface anatomy of the specimen and, as such, the other observers were not able to use cues from previous observers’ point selection to guide their point selection. These measurements were repeated after a minimum of 2 weeks to assess the reproducibility (defined as the closeness of agreement between the selected points for each of the entry points on the same specimen but selected by different observers or the same observer at a different time) of point selection. This yielded a data set of 40 data points for each observer (120 data points in total).
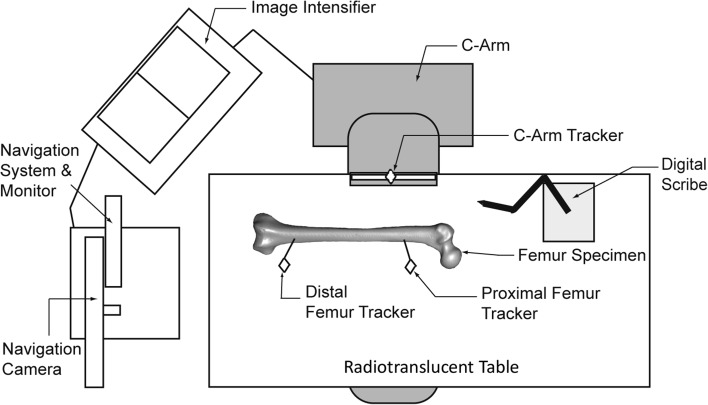
Specimens were then clamped to a radiolucent table and again positioned so that the posterior condyles were parallel to the plane of the table. A single fellowship-trained surgeon (MRE) digitized the location of the PF and TT using direct visualization and tactile feedback (providing the gold standard for both entry points for that specimen). A fluoroscopy-based navigation system (Stryker Corp, Kalamazoo, MI, USA) was connected to a C-arm and image intensifier (Philips Healthcare, Andover, MA, USA) and its infrared trackers were mounted onto the C-arm and two bicortical screws in the diaphysis of the femur (Fig. 1). Neutral AP and lateral images were acquired based on the position of the C-arm relative to the table (0° = neutral AP and 90° = neutral lateral).
Fig. 1.
A schematic diagram illustrates the experimental setup. The navigation unit is positioned at the distal end of the table with the C-arm in a neutral lateral alignment. The digital scribe is clamped to the table by the proximal femur.
From the 0° position, the C-arm was rotated in 5° increments and images were acquired until the surgeon identified an image that would no longer be an acceptable intraoperative AP image. The acceptability of an image for entry point selection was determined based on the opinion of an experienced orthopaedic surgeon, as was done in previous studies on the tibial entry points [22]. The surgeon qualitatively inspected the relative sizes and positions of the femoral neck, greater trochanter, and lesser trochanter, as would be done intraoperatively, to estimate whether an image was excessively rotated (internally or externally) to be used for entry point selection (the same method was used for the lateral image range). As such, images were acquired at predetermined positions of 5°, 10°, and 15° of internal and external rotation, sequentially, until they were found to appear to be too rotated to be used to determine the entry point. This was repeated from the 90° position, yielding two arcs of acceptable AP and lateral images. A fourth tracker was mounted on the scribe and the tip of the scribe was calibrated for the navigation system. The femur was draped, and holding the lateral image at 90°, the AP image was varied, sequentially, through the previously identified AP arc. Under the guidance of the image intensifier and tactile feedback, the PF and TT entry points were digitized. The AP and lateral images were uploaded into the navigation software. Using tactile feedback and the ability to track the real-time position of the scribe in the AP and lateral fluoroscopic images, simultaneously, both entry points were redigitized. The complete navigation workflow (such as the registration of the femoral landmarks required for fracture reduction) was not performed, as these steps do not provide any additional quantitative or visual data or guidance for the portion of the workflow designed for entry point selection in this system. This was repeated for the range of AP and lateral images for all specimens. The observer was given no feedback as to the accuracy or relative positioning of any of their selected entry points to the gold standard entry points of the specimen. This yielded two measurements for each entry point per angle for each femur: a fluoroscopy-guided point (with tactile feedback) and a navigation-guided point (with tactile feedback). The number of data points for each entry point for a given specimen was dependent on the size of the arc of AP and lateral images and ranged from 13 to 14 measurements for each method of guidance (fluoroscopy and navigation) and a total of 26 to 28 measurements for each entry point per femur.
Entry point digitizations produced a set of 3-D coordinates. The fluoroscopy- and navigation-based landmark coordinates were transformed into the distance from the gold standard, enabling a 3-D AP and ML comparison of the accuracy (the proximity of a selected point to the gold standard point for each of the two entry points on each specimen) and precision (the agreement between the multiple points selected under each method of guidance for each of the two entry points on each specimen) of the two techniques.
Finally, to investigate the effects of the off-neutral images, the transformed landmarks from the entry point digitizations (as described above) were grouped across all specimens according to the selected entry point (PF or TT), guidance method (navigation or fluoroscopy), and imaging angle (0 to 30°). This enabled a comparison of the accuracy of the entry point selection at each of the imaging angles for each of the two entry points and each guidance method for all specimens.
We used a two-way, random-effects intraclass correlation coefficient (ICC) to examine the inter- and intraobserver repeatability in the selection of the AP and ML location of both entry points under direct visualization (SPSS® Version 13; SPSS Inc, Chicago, IL, USA) [2]. To investigate the accuracy and precision for the selected method for each entry point, a hierarchical linear model was developed (SAS®; SAS Institute, Inc, Cary, NC, USA). This model was also used to determine the effects of the angle of the fluoroscopic image on the accuracy of each method.
Results
The reproducibility of both the PF and TT entry points was high for both the repeated measurements of one observer, as well as between observers. Based on the values of the ICC, entry point selection using direct visualization was highly reproducible for both the AP and ML positions for the PF entry point (0.99 and 0.96, respectively) and TT entry point (0.93 and 0.98, respectively). The lowest value for reproducibility of the entry point selection for one observer (intraobserver) was 0.89 (PF in the ML direction). All other reproducibility values for each observer (intraobserver) and between observers (interobserver) were greater than 0.9 for the AP and ML positions for the PF and TT, thus yielding a suitable gold standard for image-based comparisons and indicating the PF and TT landmarks can be localized with high reproducibility (Table 1).
Table 1.
ICC for the reproducibility of the gold standard method for selecting the PF and TT in the ML and AP directions
| Observer | ICC | |||
|---|---|---|---|---|
| PF | TT | |||
| ML | AP | ML | AP | |
| Observer 1 | 0.894 | 0.909 | 0.992 | 0.995 |
| Observer 2 | 0.933 | 0.955 | 0.924 | 0.975 |
| Observer 3 | 0.910 | 0.972 | 0.904 | 0.978 |
| Interobserver | 0.960 | 0.994 | 0.938 | 0.986 |
The values in the rows labeled Observers 1, 2, and 3 indicate the reproducibility of the selection of each landmark for each observer (intraobserver); the values in the row labeled Interobserver are the reproducibility of the selection of each landmark across the three observers; ICC = intraclass correlation coefficient; PF = piriformis fossa; TT = tip of the greater trochanter; ML = mediolateral.
A range of acceptable AP and lateral images was observed for all specimens. The average range of angles through which AP and lateral images were deemed to be clinically acceptable was 29° (range, 25°–30°). Images taken beyond these angulations appeared distorted and were deemed to be unusable, based on the opinion of an experienced orthopaedic surgeon, for landmark identification.
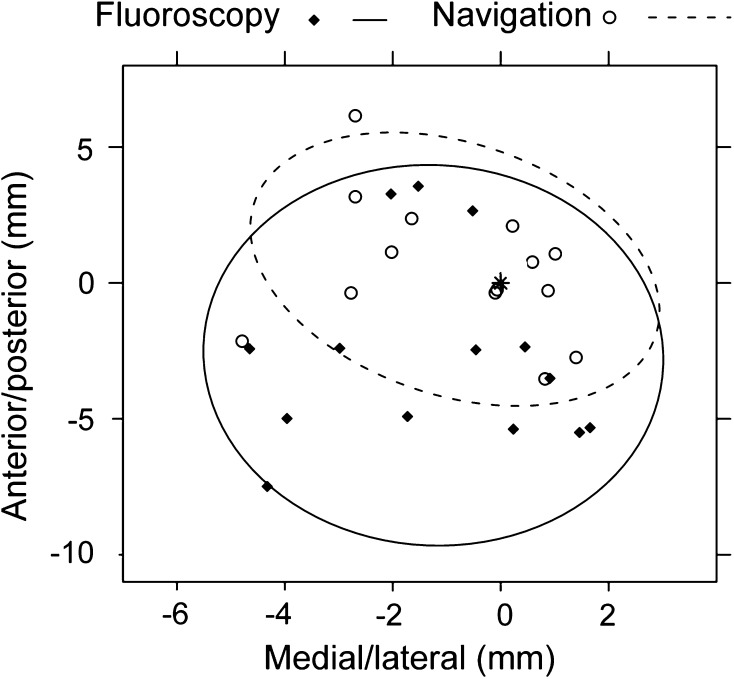
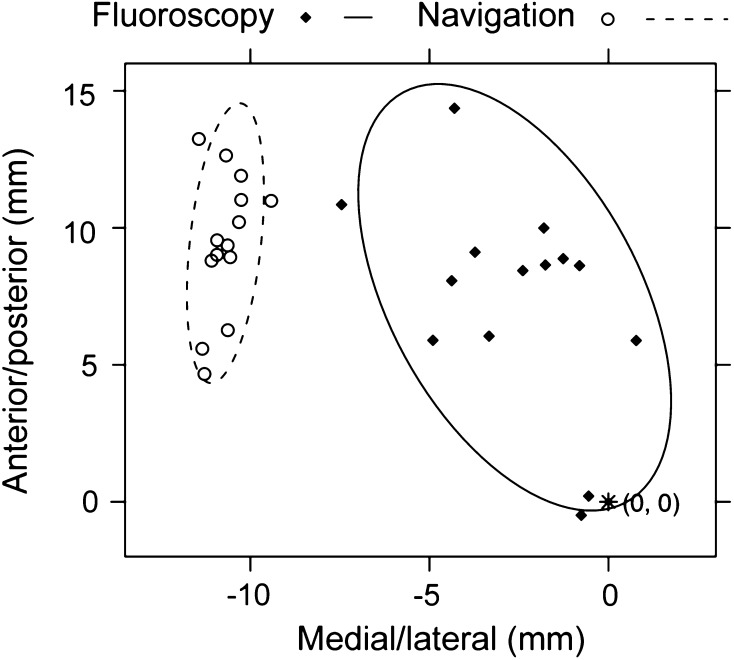
In general, the use of navigation did not result in improved accuracy in entry point selection. Based on the identified acceptable AP and lateral images, similar accuracy (p = 0.26; mean difference, 2.9 mm; range, 0.1–10.0 mm) was found in the 3-D localization of the PF under navigation (mean distance, 5.0 mm; range, 0.2–13.4 mm) and fluoroscopy (mean distance, 4.1 mm; range, 0.5–15.7 mm). In two-dimensional (2-D) space, the location of the PF in the ML direction demonstrated no difference (p = 0.93; mean difference, 2.0 mm; range, 0.0–10.3 mm) in accuracy between navigation (mean distance, 2.4 mm; range, 0.3–7.1 mm) and fluoroscopy (mean distance, 2.4 mm; range, 0.1–9.0 mm). In contrast, navigation (mean distance, 4.0 mm; range, 0.1–12.0 mm) improved the accuracy of the PF landmark in the AP direction (p = 0.003; mean difference, 4.0 mm; range, 0.1–15.3 mm), compared to fluoroscopy (mean distance, 2.9 mm; range, 0.0–14.0 mm). For the PF, the navigated point tended to lie anteriorly and medially to the points selected under fluoroscopic guidance (p < 0.001 and p = 0.082, respectively) (Fig. 2). Improved 3-D accuracy (p < 0.001; mean difference, 6.4 mm; range, 0.1–20.5 mm) was found for TT using fluoroscopy (mean distance, 6.8 mm; range, 0.6–24.7 mm) as compared to navigation (mean distance, 11.6 mm; range, 0.8–21.9 mm). In 2-D space, the accuracy under fluoroscopy (mean distance, 3.1 mm; range, 0.1–10.4 mm) in the ML direction was better (p < 0.001; mean difference, 7.7 mm; range, 0.1–18.0 mm) than navigation (mean distance, 9.2 mm; range, 0.1–17.4 mm). In the AP direction, the points selected using fluoroscopy (mean distance, 5.5 mm; range, 0.0–24.0 mm) demonstrated a trend toward higher (p = 0.058; mean difference, 4.3 mm; range, 0.220.8 mm) accuracy than those selected using navigation (mean distance, 6.2 mm; range, 0.1–18.3 mm). The navigated points tended to lie anteriorly and laterally to those selected under fluoroscopic guidance (p = 0.002 and p < 0.001, respectively) (Fig. 3). The percentage of the selected points within 3 mm of the optimal entry points (a threshold for the accuracy of the TT based on the work of Ostrum et al. [17]) was 22.6% for the navigated PF points, 40.1% for the fluoroscopic PF points, 2.9% for the navigated TT points, and 19.7% for the fluoroscopic TT points. Navigation was more precise than fluoroscopy for selection of the PF and TT entry points (p = 0.001 and p = 0.024, respectively).
Fig. 2.
A graph shows the scatter of the PF entry points selected under navigation guidance and fluoroscopic guidance for a single specimen. The 95% CI is indicated with the ellipses for each set and the gold standard is denoted by the point (*) located at (0, 0).
Fig. 3.
A graph shows the scatter of the TT entry points selected under navigation guidance and fluoroscopic guidance for a single specimen. The 95% CI is indicated with the ellipses for each set and the gold standard is denoted by the point (*) located at (0, 0).
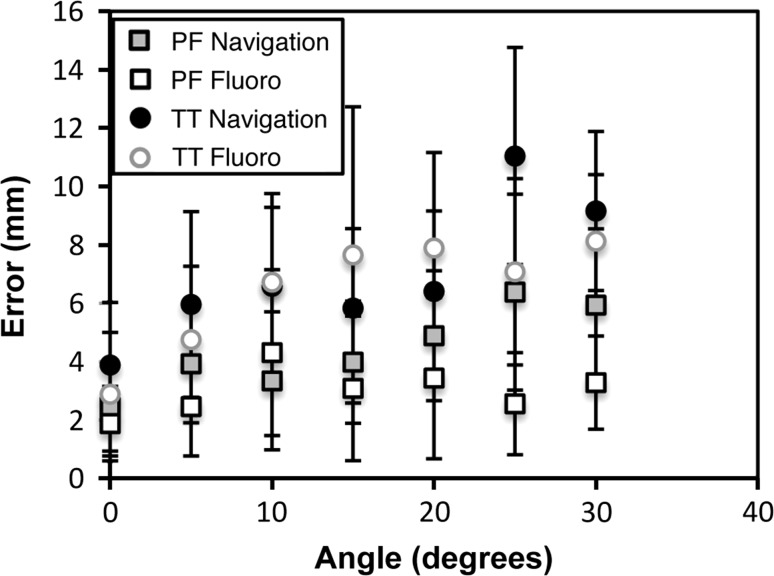
Off-neutral images were found to affect the accuracy of entry point selection of both the PF and TT entry points under the fluoroscopic and navigated guidance. As the angle of the images was increased (ie, the more the images deviated from the true AP or lateral views), the accuracy of entry point selection decreased for both entry points when using fluoroscopy or navigation to guide the selection (Fig. 4). From the hierarchical linear model, altering the angle of the image affected the PF and TT entry points for both fluoroscopy (p = 0.009 and p < 0.001, respectively) and navigation (p = 0.003 and p < 0.001). The effect of the angle of the image was greater for the TT and was also greater for the navigated point selection. The percentage of selected entry points within 3 mm of the optimal entry points was highest for the neutral images for both entry points and both methods of guidance. Even with neutral images, only 61.5% of the navigated PF points, 75.0% of the fluoroscopic PF points, 42.8% of the navigated TT points, and 59.0% of the fluoroscopic TT points were found to be within 3 mm of the optimal entry points.
Fig. 4.
A graph shows the mean distance of the selected points from the target point with respect to image angle for the PF and TT guided by navigation and fluoroscopy.
Discussion
Successful fracture reduction in closed IM nailing requires accurate location of the point through which the nail is introduced into the canal. For both the PF and TT entry points, the importance of accuracy in entry point selection has been well documented [9, 13, 15, 17, 19, 21]. Fluoroscopy-based navigation systems have been developed for IM nailing in the treatment of femoral shaft fractures to assist with entry point selection and fracture reduction. However, there has been little documentation on whether fluoroscopy-based navigation can improve the accuracy and precision of entry point selection and overcome some of the limitations of fluoroscopically-guided landmarking. Accordingly, we evaluated the following questions: (1) Is the localization of the PF and TT landmarks, under direct visualization, reproducible? (2) Is there a range of nonneutral AP and lateral images that would be deemed clinically acceptable for entry point selection? (3) Does fluoroscopy-based computer navigation increase the accuracy and precision with which the PF and TT entry points are selected when compared to conventional fluoroscopic guidance? And (4) does off-angle acquisition within the acceptable range of AP or lateral images affect the accuracy of landmark selection?
This study had several limitations. First, femur specimens were stripped of their soft tissue. This represents a “best-case scenario” that is not representative of clinical practice. The lack of soft tissue enabled direct visualization of the proximal femur and likely increased reproducibility during the investigation of the reproducibility of the PF and TT landmarks. This was necessary to determine whether these landmarks were suitable to act as targets for the investigation of the accuracy and precision of navigation compared to fluoroscopy. This work has shown, even in an idealized scenario, there is a range of AP and lateral images that may appear to be acceptable for entry point selection and, based on this range of images, current fluoroscopy-based navigation does not provide increased accuracy in entry point selection compared to conventional fluoroscopy.
A second limitation of this work was the range of acceptable images was determined based on the opinion of an experienced surgeon, using the qualitative observation of the relative sizes and positions of anatomic landmarks such as the greater trochanter, lesser trochanter, and femoral neck. Although this is a subjective method for the acceptance of an image, it is representative of what could commonly occur in the clinical setting, where an image may be accepted, intraoperatively, based on the opinion of an experienced surgeon in the room. Also, the range of acceptable images was fairly consistent for all specimens in this study, which may suggest the qualitative criteria on which the surgeon was forming his opinion were relatively consistent throughout the study. It is also worth noting this method of determining an acceptable range of AP angles was used in a study investigating the effect of the angle of an AP image on the entry point for tibial IM nailing [22], and the range of images we accepted was similar to the range of lateral angles investigated for use in selecting the landmarks on the proximal femur for the calculation of femoral anteversion [3, 22].
A third limitation to this work is the possibility that the surgeon was able to gain further information regarding the 3-D anatomy of the specimen from the tactile feedback and the images during the entry point selection for the investigation of the accuracy and precision of navigation compared to fluoroscopy alone (a learning effect). While this is a possible source of error, care was taken to ensure no feedback regarding the relative positions or accuracy of the selected entry points was given to the surgeon after the selection of an entry point. Without such feedback, it is less likely, despite repeated measures of the same specimen, the surgeon would refine the accuracy of landmark selection, particularly since the angle of the images, and subsequently the information within the images, changed between each entry point selection. This work investigated the use of a single navigation system and other systems may yield different results for the accuracy and precision of entry point selection, although insofar as most navigation systems utilize fluoroscopy, these systems likely would be prone to the same inaccuracies from off-angle fluoroscopic images.
This study indicated there is a range of approximately 30° of both AP and lateral images identified as clinically acceptable for intraoperative entry point selection, which is similar to the arc of AP images that appeared suitable for the determination of the entry point for IM nailing in the tibia in a previous study (average, 30°; range, 25°–40°) [22]. It has also been suggested, occasionally, off-angle images are accepted intraoperatively due to limitations in the line of site [3]. A study investigating whether off-angle lateral images would affect landmark selection for femoral anteversion utilized the lateral images of the proximal femur ranging from a neutral lateral to images rotated 40° from the lateral position [3], which is greater than that observed in this study. As there is a range of AP and lateral images that appear to be acceptable for the determination of the entry point, it is possible a surgeon may unwittingly use off-angle images intraoperatively in the selection of an entry point in both a conventional (fluoroscopy alone) and navigated IM nailing procedure.
The tendency of the navigated PF and TT to lie anterior to the fluoroscopic points indicates an increased risk for malalignment and iatrogenic fractures [9, 15, 19–21]. Additionally, in the case of the PF, navigation tended to direct the point selection medially to that based on fluoroscopy alone, which would be consistent with an increased risk of valgus fracture malalignment and of iatrogenic neck fractures [13]. The opposite was found in the ML location of the TT, with the navigated points tending to lie laterally to those based on fluoroscopy alone, increasing the risk of varus malalignment and iatrogenic comminution fractures, as well as fractures of the trochanter [13, 17]. Therefore, although the navigated points were more precise than those selected under fluoroscopic guidance, it is concerning that these points tended to exhibit similar or reduced accuracy to those selected by fluoroscopy alone and, more so, that the offset tended to be in a direction that increased the risk of complications. In this study, the percentage of entry points within the estimated 3-mm tolerance of the gold standard was low, with the highest percentage (40%) occurring in the fluoroscopic PF points [17]. The percentage of points from the navigated PF and both the navigated and fluoroscopic TT data within the 3-mm tolerance was less than 25%. This indicates, even in an idealized scenario, neither method of entry point selection is capable of reliably achieving the estimated clinical tolerances for entry point accuracy. It is of interest to note the relationship between the relative positioning of the navigated and fluoroscopic points was not consistent between the PF and TT entry points (ie, navigated PF points tended to lie medially to the gold standard and the fluoroscopic points, and navigated TT points tended to lie laterally to the gold standard and the fluoroscopic points). Another interesting outcome of this study is the navigation-based points tended to be either similarly or less accurate than fluoroscopy alone in the selection of both entry points. This seems counterintuitive, as one would anticipate the increased level of visualization and guidance to enable selection of a point with accuracy at least equivalent to the fluoroscopy-based point, if not better. It should be noted the navigated points tended to lie anterior to both the gold standard and the fluoroscopic points for both entry points and, as such, a possible error or bias during the registration and calibration cannot be ruled out entirely as a possible explanation for the general decrease in accuracy of the navigated points. In a survey conducted by Manzey et al. [14], the possibility of overtrust and bias for navigation-based calculations and information was identified as an area of concern in automated and image-guided procedures. This has also been documented in other industries in which computer assistance has been integrated [18]. As such, one possible explanation for our finding may be, despite being given identical images for both the navigated and conventional procedures, the surgeon in this study may have placed more trust in the information being presented in the navigated case and, possibly, relied less on tactile feedback when compared to the conventionally selected points.
While images with angular deviations of 30° may appear to be acceptable for intraoperative guidance, these images were found to lead to substantial inaccuracies in entry point identification. A previous study on the effect of off-angle images on the appearance of a guidewire placed in the proximal tibia (in the entry point for a tibial IM nail) found the guidewire appeared to move 3% of the width of the tibia with every 5° rotation of the AP image [22]. Another study investigating the intentional use of off-angle images due to limitations in the intraoperative line of site during navigated IM nailing of the femur found images with rotation in excess of 40° from a neutral image led to increased error in the calculation of femoral anteversion based on landmark selection [3]. Our study has also shown off-angle biplanar images decreased the accuracy with which both entry points were selected and, as such, could result in an increased risk of complications [9, 13, 15, 17, 19–21]. The off-angle images affected the navigation-based point selection even more so than the fluoroscopy-based points. The estimation for the threshold of entry point accuracy in the current literature is 3 mm [17]. When using a neutral image, 75% and 59% of the fluoroscopy-based PF and TT points, respectively, and 62% and 43% of the navigation-based PF and TT points, respectively, were within this threshold, and with the effects of image angle on entry point accuracy, even an image with 5° of rotation will yield a higher percentage of points outside the entry point threshold. As such, acquisition of neutral images is important for the accurate and safe determination of the entry point by both navigation and fluoroscopy.
This study has shown there is a range of approximately 30° of images that would be deemed clinically acceptable for entry point selection in antegrade IM nailing. In the case of images that are not true AP and lateral images, the accuracy of the PF and TT entry points is decreased both on navigation and conventional fluoroscopic guidance, increasing the risk of malalignment and iatrogenic fracture during reduction [9, 13, 15, 17, 19–21]. Thus, despite its increased use, current fluoroscopy-based computer-navigated IM nailing cannot overcome the inaccuracies in entry point selection caused by off-angle images. However, both the increased precision achieved with navigation and the high reproducibility with direct visualization are encouraging. As such, future advances in intraoperative imaging that facilitate improved landmark identification may motivate the use of navigation in the selection of the PF and TT entry points in IM nailing.
Acknowldgments
We thank Dr. Yan Yan Wu and the Applied Health Research Centre of St Michael’s Hospital for statistical consultation. We also thank Dr. Barry Cayen of the Division of Orthopaedic Surgery at the Humber River Regional Hospital (Toronto, ON, Canada) and Dr. Ashesh Kumar of the Division of Orthopaedic Surgery at St Michael’s Hospital for their contribution by performing landmark selection in this study.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed in the Martin Orthopaedic Biomechanics Laboratory at St Michael’s Hospital, Toronto, ON, Canada.
References
- 1.Braten M, Terjesen T, Rossvoll I. Torsional deformity after intramedullary nailing of femoral shaft fractures: measurement of anteversion angles in 110 patients. J Bone Joint Surg Br. 1993;75:799–803. doi: 10.1302/0301-620X.75B5.8376444. [DOI] [PubMed] [Google Scholar]
- 2.Burdock EI, Fleiss JL, Hardesty AS. A new view of inter-observer agreement. Personnel Psychology. 1963;16:373–384. doi: 10.1111/j.1744-6570.1963.tb01283.x. [DOI] [Google Scholar]
- 3.Citak M, Kendoff D, Pearle AD, O’Loughlin PF, Krettek C, Hüfner T, Citak M. Navigated femoral anteversion measurements: general precision and registration options. Arch Orthop Trauma Surg. 2009;129:671–677. doi: 10.1007/s00402-008-0804-6. [DOI] [PubMed] [Google Scholar]
- 4.Christie J, Court-Brown C, Kinninmonth AW, Howie CR. Intramedullary locking nails in the management of femoral shaft fractures. J Bone Joint Surg Br. 1988;70:206–210. doi: 10.1302/0301-620X.70B2.3346289. [DOI] [PubMed] [Google Scholar]
- 5.Gosling T, Oszwald M, Kendoff D, Citak M, Krettek C, Hufner T. Computer-assisted antetorsion control prevents malrotation in femoral nailing: an experimental study and preliminary clinical case series. Arch Orthop Trauma Surg. 2009;129:1521–1526. doi: 10.1007/s00402-009-0871-3. [DOI] [PubMed] [Google Scholar]
- 6.Harper MC, Henstorf J. Fractures of the femoral neck associated with technical errors in closed intramedullary nailing of the femur: report of two cases. J Bone Joint Surg Am. 1986;68:624–626. [PubMed] [Google Scholar]
- 7.Hazan EJ, Joskowicz L. Computer-assisted image-guided intramedullary nailing of femoral shaft fractures. Tech Orthop. 2003;18:191–200. doi: 10.1097/00013611-200306000-00008. [DOI] [Google Scholar]
- 8.Jaarsma RL, Pakvis DF, Verdonschot N, Biert J, van Kampen A. Rotational malalignment after intramedullary nailing of femoral fractures. J Orthop Trauma. 2004;18:403–409. doi: 10.1097/00005131-200408000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Johnson KD, Tencer AF, Sherman MC. Biomechanical factors affecting fracture stability and femoral bursting in closed intramedullary nailing of femoral shaft fractures, with illustrative case presentations. J Orthop Trauma. 1987;1:1–11. doi: 10.1097/00005131-198701010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kale SP, Patil N, Pilankar S, Karkhanis AR, Bagaria V. Correct anatomical location of entry point for antegrade femoral nailing. Injury. 2006;37:990–993. doi: 10.1016/j.injury.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Kendoff D, Citak M, Gardner MJ, Gösling T, Krettek C, Hüfner T. Navigated femoral nailing using noninvasive registration of the contralateral intact femur to restore anteversion: technique and clinical use. J Orthop Trauma. 2007;21:725–730. doi: 10.1097/BOT.0b013e31812f69a9. [DOI] [PubMed] [Google Scholar]
- 12.Khan FA, Ikram MA, Badr AA, al-Khawashki H. Femoral neck fracture: a complication of femoral nailing. Injury. 1995;26:319–321. doi: 10.1016/0020-1383(95)00049-F. [DOI] [PubMed] [Google Scholar]
- 13.Linke B, Ansari Moein C, Bosl O, Verhofstad MH, van der Werken C, Schwieger K, Ito K. Lateral insertion points in antegrade femoral nailing and their influence on femoral bone strains. J Orthop Trauma. 2008;22:716–722. doi: 10.1097/BOT.0b013e318189369e. [DOI] [PubMed] [Google Scholar]
- 14.Manzey D, Rottger S, Bahner-Heyne JE, Schulze-Kissing D, Dietz A, Meixensberger J, Strauss G. Image-guided navigation: the surgeon’s perspective on performance consequences and human factors issues. Int J Med Robot. 2009;5:297–308. doi: 10.1002/rcs.261. [DOI] [PubMed] [Google Scholar]
- 15.Miller SD, Burkart B, Damson E, Shrive N, Bray RC. The effect of the entry hole for an intramedullary nail on the strength of the proximal femur. J Bone Joint Surg Br. 1993;75:202–206. doi: 10.1302/0301-620X.75B2.8444937. [DOI] [PubMed] [Google Scholar]
- 16.Ostrum RF, Levy MS. Penetration of the distal femoral anterior cortex during intramedullary nailing for subtrochanteric fractures: a report of three cases. J Orthop Trauma. 2005;19:656–660. doi: 10.1097/01.bot.0000154481.46693.69. [DOI] [PubMed] [Google Scholar]
- 17.Ostrum RF, Marcantonio A, Marburger R. A critical analysis of the eccentric starting point for trochanteric intramedullary femoral nailing. J Orthop Trauma. 2005;19:681–686. doi: 10.1097/01.bot.0000184145.75201.1b. [DOI] [PubMed] [Google Scholar]
- 18.Parasuraman R, Riley V. Humans and automation: use, misuse, disuse, abuse. Hum Factors. 1997;39:230–253. doi: 10.1518/001872097778543886. [DOI] [Google Scholar]
- 19.Ricci WM, Devinney S, Haidukewych G, Herscovici D, Sanders R. Trochanteric nail insertion for the treatment of femoral shaft fractures. J Orthop Trauma. 2005;19:511–517. doi: 10.1097/01.bot.0000164594.04348.2b. [DOI] [PubMed] [Google Scholar]
- 20.Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17:296–305. doi: 10.5435/00124635-200905000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ricci WM, Schwappach J, Tucker M, Coupe K, Brandt A, Sanders R, Leighton R. Trochanteric versus piriformis entry portal for the treatment of femoral shaft fractures. J Orthop Trauma. 2006;20:663–667. doi: 10.1097/01.bot.0000248472.53154.14. [DOI] [PubMed] [Google Scholar]
- 22.Walker RM, Zdero R, McKee MD, Waddell JP, Schemitsch EH. Ideal tibial intramedullary nail insertion point varies with tibial rotation. J Orthop Trauma. 2011;25:726–730. doi: 10.1097/BOT.0b013e31821148c7. [DOI] [PubMed] [Google Scholar]
- 23.Winquist RA, Hansen ST, Jr, Clawson DK. Closed intramedullary nailing of femoral fractures: a report of five hundred and twenty cases. J Bone Joint Surg Am. 1984;66:529–539. [PubMed] [Google Scholar]