Abstract
Background
After a decade of war in Iraq and Afghanistan, we have observed an increase in combat-related injury survival and a paradoxical increase in injury severity, mainly because of the effects of blasts. These severe injuries have a devastating effect on each patient’s immune system resulting in massive upregulation of the systemic inflammatory response. By examining inflammatory mediators, preliminary data suggest that it may be possible to correlate complications such as wound failure and heterotopic ossification (HO) with distinct systemic and local inflammatory profiles, but this is a relatively new topic.
Questions/purposes
We asked whether systemic or local markers of inflammation could be used as an objective means, independent of demographic and subjective factors, to estimate the likelihood of (1) HO and/or (2) wound failure (defined as wounds requiring surgical débridement after definitive closure, or wounds that were not closed or covered within 21 days of injury) in patients sustaining combat wounds.
Methods
Two hundred combat wounded active-duty service members who sustained high-energy extremity injuries were prospectively enrolled between 2008 and 2012. Of these 200 patients, 189 had adequate followups to determine the presence or absence of HO, and 191 had adequate followups to determine the presence or absence of wound failure. In addition to injury-specific and demographic data, we quantified 24 cytokines and chemokines during each débridement. Patients were followed clinically for 6 weeks, and radiographs were obtained 3 months after definitive wound closure. Associations were investigated between these markers and wound failure or HO, while controlling for known confounders.
Results
The presence of an amputation (p < 0.001; odds ratio [OR], 6.1; 95% CI. 1.63–27.2), Injury Severity Score (p = 0.002; OR, 33.2; 95% CI, 4.2–413), wound surface area (p = 0.001; OR, 1.01; 95% CI, 1.002–1.009), serum interleukin (IL)-3 (p = 0.002; OR, 2.41; 95% CI, 1.5–4.5), serum IL-12p70 (p = 0.01; OR, 0.49; 95% CI, 0.27–0.81), effluent IL-3 (p = 0.02; OR, 1.75; 95% CI, 1.2–2.9), and effluent IL-13 (p = 0.006; OR, 0.67; 95% CI, 0.50–0.87) were independently associated with HO formation. Injury Severity Score (p = 0.05; OR, 18; 95% CI, 5.1–87), wound surface area (p = 0.05; OR, 28.7; 95% CI, 1.5–1250), serum procalcitonin ([ProCT] (p = 0.03; OR, 1596; 95% CI, 5.1–1,758,613) and effluent IL-6 (p = 0.02; OR, 83; 95% CI, 2.5–5820) were independently associated with wound failure.
Conclusions
We identified associations between patients’ systemic and local inflammatory responses and wound-specific complications such as HO and wound failure. However, future efforts to model these data must account for their complex, time dependent, and nonlinear nature.
Level of Evidence
Level II, prognostic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Injuries sustained during combat operations are usually the result of high-energy mechanisms including high-velocity gunshot wounds, but even more commonly, they are the result of blasts [2]. Blast wounds are devastating. They often produce severe open fractures and/or traumatic amputations and often are contaminated with foreign material. Zones of injury are massive and evolve slowly with time. Treating surgeons rely on negative-pressure wound therapy and serial débridements to remove devitalized tissue, deliver local antibiotics, and determine whether the wound is ready for closure or flap coverage.
The manner in which surgeons assess combat wounds has not changed substantively during the last century [1, 13, 18, 20]. We are taught, when débriding muscle, to look for color, consistency, contractility when stimulated, and the capacity to bleed when incised. Wounds that meet all criteria of these “four Cs”, and lack signs of infection, are deemed ready for closure or coverage. Although subjective and somewhat controversial [1], this technique is successful in the majority of cases, and our rate of wound failure during the last decade of war has been reported to be between 17% and 26% [6, 7, 11, 25]. Although some would argue that an “80% solution” is acceptable given the severe nature of these injuries, the cost of wound failure, whether pursuing limb salvage or attempting to salvage a functional amputation level, is unquestionably high [10, 22, 23].
The timing of closure is tied to another frequent complication that emerged in this patient population. Heterotopic ossification (HO) complicates the majority of combat wounds [9, 16] but is more common in wounds that fail after attempted closure [6, 7], particularly in the residual limbs of amputees [15, 22]. During the débridement process, wounds become progressively less compliant with time as fibrosis and ectopic mineralization progress. This effect hinders efforts to fashion and mobilize local soft tissue flaps necessary for most functional amputation levels or for adequate coverage of open fractures or exposed neurovascular structures [21, 24]. Surgeons strive to close wounds as soon as possible, but often struggle with the timing, lest it be too early or too late. As such, there is considerable interest among surgeons who treat blast wounds in developing more objective ways of assessing not only the wounds, but each patient’s physiologic response to injury.
The massive physiologic insult caused by blast wounds produces a severe and poorly regulated systemic inflammatory response [8, 11]. By measuring mediators of systemic and local inflammation, preliminary data suggest it may be possible to risk-stratify patients for HO and wound failure during the débridement process [6, 8, 11, 25]. This would allow surgeons to base decisions regarding whether to give HO prophylaxis and the timing of wound closure on objective rather than subjective data if the relationships between these mediators and outcomes of interest could be modeled.
With this in mind, we asked whether systemic or local markers of inflammation could be used during the débridement process to estimate the likelihood of (1) HO and/or (2) wound failure in patients sustaining combat-related injuries.
Patients and Methods
This study was approved by the institutional review boards at the Walter Reed National Military Medical Center and the Naval Medical Research Center. Only patients who sustained high-energy penetrating extremity wounds larger than 75 cm2 treated with negative-pressure wound therapy were eligible for enrollment. Third-party consent was obtained in some cases, from the patient’s legally authorized representative. Patients enrolled by this method were given the opportunity to withdraw from or continue study participation once he or she regained consciousness and competency to make his or her own medical decisions.
Between March 31, 2008 and October 27, 2012, we screened 435 patients with combat-wounds who were evacuated to our center. Of these, 200 met inclusion criteria. Informed consent was obtained from each. None of the potential enrollees declined to participate. This resulted in a series of 200 patients, of whom 189 (95%) had complete followup data to determine HO formation at 3 months after definitive wound closure and 191 (96%) had complete followup data to determine wound healing at 6 weeks after definitive wound closure. Because informed consent was obtained before surgical intervention, the exact wound dimensions under the negative-pressure wound therapy device could not be determined. On examination of wounds during the first débridement, eight wounds (4%) were found to be less than 75 cm2 and the participants were withdrawn from the study. In addition, one patient (< 1%) requested withdrawal from the study after the first débridement procedure. Two additional patients (1%) were lost to followup after being discharged from the hospital and lacked radiographic and other data.
Demographic and injury-specific data were gathered from several sources, including the Armed Forces Health Longitudinal Technology Application, which is the electronic medical record and coding system for the Military Health System; the Joint Theater Trauma Registry that contains medical treatment information for soldiers injured in combat operations abroad, and our institution’s Surgical Scheduling System. The following demographic data were collected: age, sex, mechanism of injury, Injury Severity Score, number of surgical débridements, wound location, amputation level (if applicable), wound size, associated vascular injury, and wound closure method. None of the patients received primary prophylaxis for HO, which was in keeping with our institution’s practice regarding the treatment of acute combat-related extremity injuries.
Sample Collection
Because not all patients arrived with negative-pressure wound therapy, wound effluent was collected 2 hours after the first débridement performed at our institution and 12 hours before each subsequent débridement procedure using a gel-free negative-pressure wound canister (Kinetic Concepts Inc [KCI®], San Antonio, TX, USA). Wound effluent was processed in a manner previously described [6]. During each débridement procedure, serum was collected from a peripheral vein, placed in a red-topped serum BD Vacutainer® (BD Corp, Franklin Lakes, NJ, USA), and processed in a manner previously described [6]. All samples were flash-frozen in liquid nitrogen and stored at −80° F until analysis. Photographs were taken of each wound for documentation and surface area measurements using PictZar® planimetry software planimetry software (BioVisual Technologies LLC, Elmwood Park, NJ, USA). During each débridement procedure, the surgeon’s subjective assessment of the wound was obtained, categorized as not ready for closure indicating that the wound lacked one or more of the four Cs or showed frank purulence, or ready for closure, indicating that all four Cs were present.
Cytokine and Chemokine Analysis
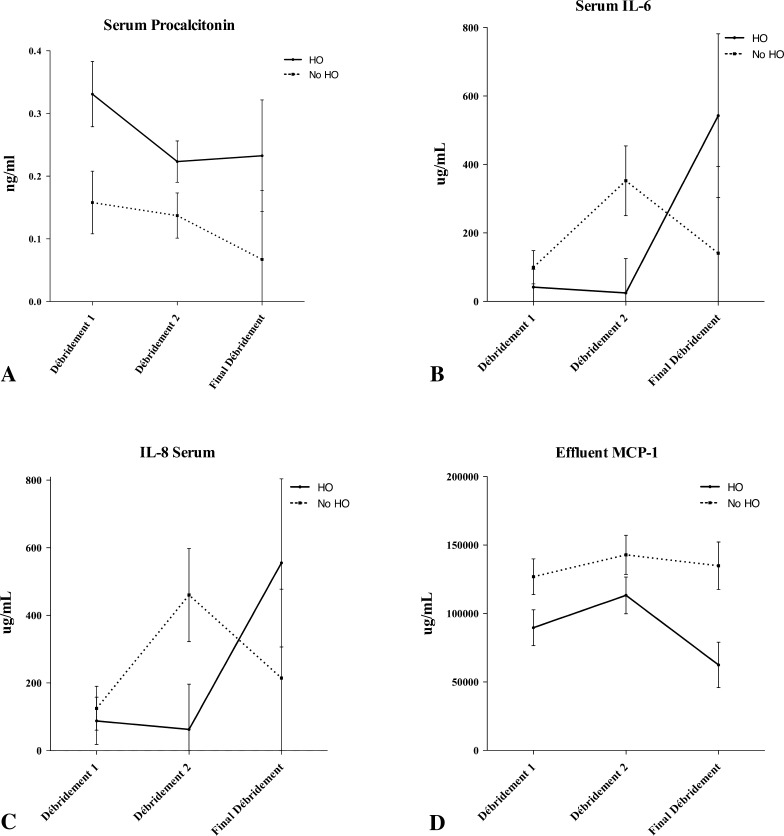
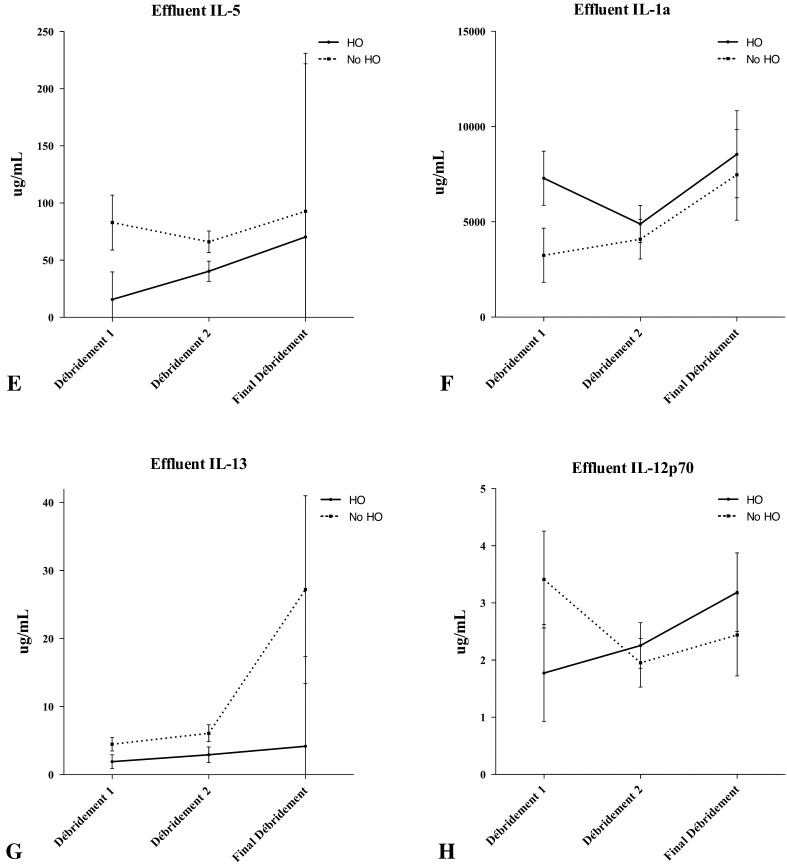
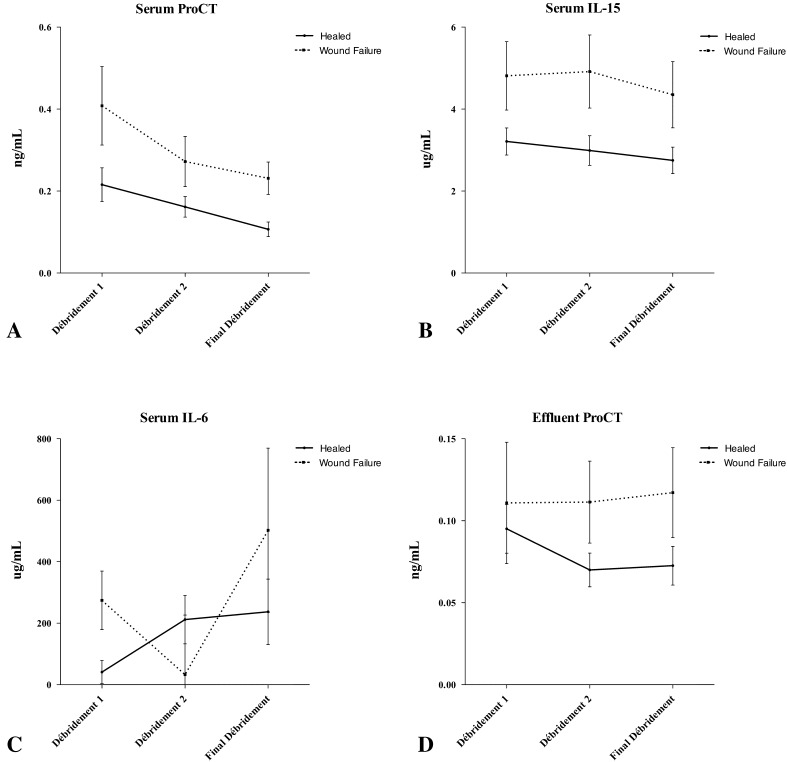
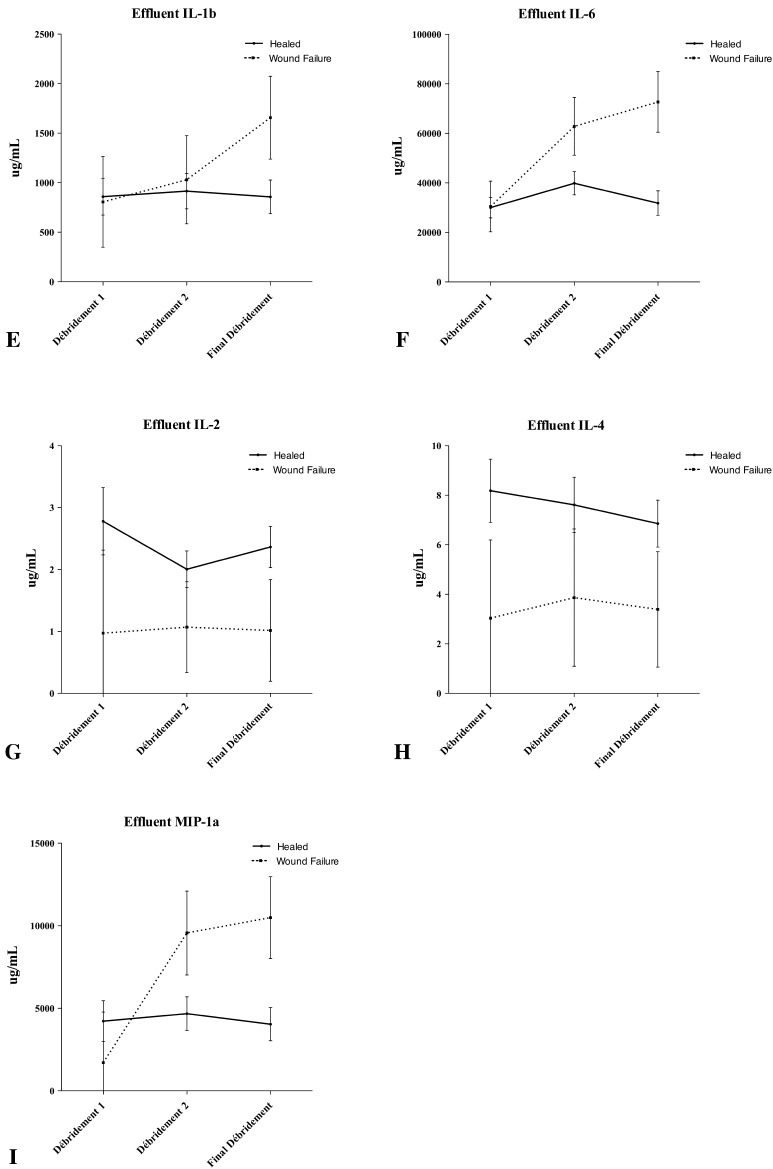
Serum and effluent were analyzed for 24 inflammatory cytokines and chemokines at the Naval Medical Research Center. We quantified the concentrations of procalcitonin (ProCT); interleukin (IL)-1a, IL-1b, IL-2 through 8, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, and IL-17; interferon (IFN)-α2, IFN-g; eotaxin; tumor necrosis factor-α; monocyte chemotactic protein-1; granulocyte-macrophage colony-stimulating factor; monocyte inflammatory protein-1α; and IP-10 using a Beadlyte® Human 22-Plex Multi-Cytokine Detection System (Upstate/Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. All proteins were measurable in serum and wound effluent in concentrations amenable to the assay techniques used for this study. Each of these inflammatory proteins was observed to vary with time. Subjectively, unique patterns were observed between wounds that formed HO (Fig. 1) and wounds that failed (Fig. 2).
Fig. 1A–H.
Concentrations of (A) serum procalcitonin, (B) serum IL-6, and (C) serum IL-8, and effluents (D) monocyte chemotactic protein (MCP)-1, (E) IL-5, (F) IL-1a, (G) IL-13, and (H) IL-12p70 are shown throughout the débridement process as a function of HO formation. The error bars show the mean and standard error of the mean. Wound closure or flap coverage was performed at the final débridement. Although not statistically significant, general trends are evident in most proteins.
Fig. 2A–I.
Concentrations of (A) serum procalcitonin (ProCT), (B) serum Il-15, and (C) serum IL-6, and effluents (D) ProCT, (E) IL-1b, (F) IL-6, (G) IL-2, (H) IL-4, and (I) monocyte inflammatory protein (MIP)-1a are shown throughout the débridement process as a function of wound failure. The error bars show the mean and standard error of the mean. Wound closure or flap coverage was performed at the final débridement. Although not statistically significant, general trends are evident in most proteins.
Clinical and Radiographic Followup
The formation of HO was the primary outcome. To evaluate it, we confirmed the radiographic presence or absence of HO at least 3 months from the time of wound closure on good quality orthogonal plain radiographs. We chose this duration of radiographic followup based on our experience in treating combat-related HO, which is reliably evident by this time [6, 7, 9, 15]. Two of the authors (JAF, EMP) reviewed radiographs after patient enrollment and data collection were complete. HO developed in 92 (48%) of the 189 wounds with at least 3 months of radiographic followup, with complete agreement between the two reviewers.
Wound failure was the secondary outcome, therefore study team members followed patients clinically for 6 weeks. In this time, wounds that required additional débridement procedures (eg, for deep infection or dehiscence) after attempted definitive closure or that were closed more than 21 days after injury were considered failures. Twenty-one days was chosen because it is 2 SDs longer than our institution’s historical mean time to closure (10 days) for combat wounds [25]. Using these criteria, 24 (13%) of the 191 wounds failed, and 168 (88%) healed uneventfully.
Descriptive statistics regarding patient’s mechanism of injury, overall severity of injury, and fracture-specific variables were collected on the study subjects. Continuous variables are presented as median (interquartile range) and categorical variables as number (%) (Table 1). The distribution of each continuous variable was compared with the normal distribution using the Shapiro-Wilk test. Equality of variance for continuous variables was determined using the Brown-Forsythe and Levene test. Statistical differences between continuous variables versus the bivariate outcome variables (wound status and HO formation) were evaluated using the Mann-Whitney U test and the post hoc Tukey-Kramer assessment. The levels of significance for the demographic and wound-specific data were adjusted using the Bonferroni method and for the proteomic data using the false discovery rate method [14]. Associations between categorical variables were compared using Fisher’s exact test or chi-square analysis, depending on the number of expected values in the contingency matrix. After univariate screening of the variables using the methods described, those with p values less than 0.25 were included for the multivariable analysis. Two linear models were developed. The first identified variables associated with HO formation using information from the first débridement while controlling for known confounders. The second identified variables associated with wound dehiscence using information from the final débridement while controlling for known confounders. We used JMP® Version 9.0.2 (SAS Institute, Inc, Cary, NC, USA) and R© Version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria) for all statistical estimations.
Table 1.
Patient demographics (n = 191)
| Demographic | Number (%) or median (interquartile range) |
|---|---|
| Age (years) | 23 (21, 25) |
| Sex | |
| Male | 189 (99) |
| Female | 2 (1) |
| Mechanism of Injury | |
| Blast | 162 (85) |
| Gunshot wound | 28 (14) |
| Blunt | 1 (1) |
| Injury Severity Score | 17 (12, 26) |
| Wound surface area (cm2) | 210 (120, 375) |
| Wound location | |
| Upper extremity | 28 (15) |
| Lower extremity | 163 (85) |
| Wound type | |
| Amputation | 95 (50) |
| Transfemoral | 33 |
| Knee disarticulation | 6 |
| Transtibial | 50 |
| Shoulder disarticulation | 1 |
| Transhumeral | 2 |
| Transradial | 2 |
| Open fracture | 57 (29) |
| Femur | 10 |
| Tibia | 33 |
| Foot | 5 |
| Humerus/shoulder | 7 |
| Radius/ulna | 3 |
| Hand | 1 |
| Soft tissue injury (without fracture) | |
| Hip/gluteal | 39 (21) |
| Thigh | 6 |
| Leg | 19 |
| Arm | 7 |
| Forearm | 4 |
| 2 | |
| Arterial injury, relevant to wound | 90 (47) |
| Traumatic brain injury | 100 (52) |
| Heterotopic ossification | 92 (49) |
| Wound failure | 24 (13) |
Results
Using data from the first débridement performed when the patient was in the continental United States, multivariate analysis, observed serum IL-3 (p = 0.002; odds ratio [OR], 2.41; 95% CI, 1.5–4.5), serum IL-12p70 (p = 0.0013; OR, 0.49; 95% CI, 0.27–0.81), effluent IL-3 (p = 0.02; OR, 1.75; 95% CI, 1.2–2.9) and effluent IL-13 (p = 0.006; OR, 0.67; 95% CI, 0.50–0.87) were associated with HO formation while controlling for known confounders, including mechanism of injury (blast), type of injury (amputation), Injury Severity Score, traumatic brain injury, and wound surface area (Table 2). Serum IL-12p70 and effluent IL-13 showed inverse relationships (OR, < 1), in which higher concentrations were associated with less likelihood of HO developing. Using data from the final débridement, the relationships surrounding wound failure were less clear. This is likely attributable to the small number of wounds that failed. Multivariate analysis showed that serum ProCT (p = 0.03; OR, 1596; 95% CI, 5.1–1,758,613) and effluent IL-6 (p = 0.02; OR, 83; 95% CI, 2.5–5820) associated with wound failure while controlling for wound surface area, Injury Severity Score, arterial injury, and the surgeon’s assessment (Table 3). As expected, the surgeon’s subjective assessment followed a general trend from not ready for closure to ready for closure throughout the débridement process. However, a determination of ready for closure was inversely associated with wound outcome (p = 0.04; OR, 7.6; 95% CI, 1.1–176). Two of 27 (7%) wounds that lacked at least one of the four Cs dehisced compared with 22 of 164 (13%) that exhibited acceptable color and consistency, contractility when stimulated, and the capacity to bleed when sharply incised.
Table 2.
Analysis of variables associated with HO using information from the first débridement
| Variable | Coefficient | Standard error | OR (95% CI) | p value |
|---|---|---|---|---|
| Mechanism of injury (blast) | 0.74 | 0.44 | 2.54 (0.47–15.7) | 0.29 |
| Type of injury (amputation) | 2.1 | 0.73 | 6.1 (1.63–27.2) | < 0.001 |
| Injury Severity Score | 0.07 | 0.02 | 33.2 (4.2–413) | 0.002 |
| Traumatic brain injury | 0.15 | 0.27 | 2.2 (0.80–7.0) | 0.56 |
| Wound surface area | 0.006 | 0.002 | 1.005 (1.002–1.009) | 0.001 |
| Serum IL-3 | 0.48 | 0.29 | 2.41 (1.5–4.5) | 0.002 |
| Serum IL-12p70 | −0.41 | 0.28 | 0.49 (0.27–0.81) | 0.013 |
| Effluent IL-3 | 0.56 | 0.24 | 1.75 (1.2–2.9) | 0.02 |
| Effluent IL-13 | −0.40 | 0.14 | 0.67 (0.50–0.87) | 0.006 |
HO = heterotopic ossification; OR = odds ratio; IL = interleukin.
Table 3.
Analysis of variables associated with wound failure using information from the final débridement
| Variable | Coefficient | Standard error | OR (95% CI) | p value |
|---|---|---|---|---|
| Surgeon’s assessment (ready for closure) | 1.0 | 0.61 | 7.6 (1.1–176) | 0.04 |
| Arterial injury | 0.02 | 0.61 | 1.0 (0.3–3.5) | 0.95 |
| Injury Severity Score | 0.09 | 0.05 | 18 (5.1–87) | 0.05 |
| Wound surface area | 0.004 | 0.002 | 28.7 (1.5–1250) | 0.05 |
| Serum ProCT | −4.6 | 2.1 | 1596 (5.1–1758613) | 0.03 |
| Effluent IL-6 | 8,804,510 | 3,919,800 | 83 (2.5–5820) | 0.02 |
OR = odds ratio; Pro-CT = procalcitonin; IL = interleukin.
Discussion
Blast victims are subject to an exaggerated and prolonged systemic inflammatory response that influences the local wound environment. Because each patient’s physiologic response to injury varies, it is difficult for surgeons to assess objectively which wounds may be subject to untoward complications like HO or wound failure. We defined the systemic and local inflammatory responses in 191 combat-injured patients in this prospective study. In doing so, we identified patterns of unique inflammatory profiles independently associated with wound dehiscence and the formation of HO at times useful for surgical decision-making.
The results of our study must be interpreted in the context of its limitations. Despite the heterogeneous nature of combat-related blast wounds, our patient population is a homogeneous one, consisting of young, previously healthy service members who had sustained high-energy penetrating extremity wounds in combat. In addition, the majority (85%) of these patients sustained blast injuries, which may confound the results. Nevertheless, all patients sustained high-energy trauma requiring intercontinental aeromedical evacuation, multiple débridement surgeries, and negative-pressure wound therapy. The results of this study still may not be applicable to less severe injury mechanisms more commonly seen after other types of blunt trauma commonly encountered in the civilian setting. Nevertheless, we believe critically injured patients, whether civilian or military, may exhibit similar inflammatory profiles during the débridement process. To explore this, prospective validation of these results is underway in civilian patients with trauma. Although 35 surgeons were involved in the care of the patients in the current study, two of the authors (JAF, EMP), who reviewed the radiographs, were involved in approximately ½ of the cases. This may be a source of potential bias; however, we sought to minimize this by analyzing radiographs at the completion of data collection. It is possible that other parameters not collected by the research team, such as wound bioburden [3, 12, 17] may be associated with both outcomes. In addition, the inflammatory patterns observed in patients who have HO form may be evident sooner than was measured in this study, or that primary HO prophylaxis might not be effective at the time at which these samples were collected. Although we obtained samples from the first surgical débridement performed with the patient in the continental United States out of necessity, ongoing studies allow for collection of specimens sooner after injury, before intercontinental aeromedical evacuation. The current study was not designed to assess the clinical or financial implications of wound failure or HO, therefore assigning accurate measures of cost, either to the individual or the institution, is not directly possible using these data. However, the clinical implications of using these or similar data, in real time, to objectively guide surgical decision-making may be considerable when one considers clinical morbidity and cost-savings potential of models developed to estimate the likelihood of untoward complications such as wound failure.
Finally, the relationships between inflammatory mediators are highly complex, and vary with time. Although the linear regression methods we used identified independent associates with HO and wound failure, less than ½ of the variance was explained in either case. Regarding the outcome of wound failure, the CIs of serum ProCT and effluent IL-6 were quite large, owing to instability of the model, which may be attributable to an inadequate sample size. As such, nonlinear methods may be necessary to codify these time-dependent data into coherent models if the goal is to estimate likelihoods of these outcomes in the future.
We observed an association between HO and the patient’s inflammatory response to injury. This may be related to the time at which we chose to sample these wounds—the first débridement performed with the patient in the continental United States. However, it is then that we would realistically risk-stratify patients to receive primary prophylaxis for HO. Serum IL-3 is produced by activated T-lymphocytes early during the wound-healing process and stimulates differentiation of pluripotent hematopoietic stem cells down a myeloid lineage and also stimulates mast cell differentiation. Although myeloid hyperplasia has been described in combat related HO [4], IL-3 is a potent inhibitor of osteoblastic differentiation [5]. Similarly, IL-13, in addition to inhibiting macrophage activity, has been shown to increase osteoprotegerin expression that serves to inhibit osteoclastogenesis [19]. Its role during the early stages of HO is unknown. We believe the initial signaling process for HO development begins shortly after, if not simultaneously with, the initial injury once the host begins to respond to the insult. As such, our analysis of downstream mediators of inflammation was done to characterize the inflammatory state of the host rather than to identify potential therapeutic targets.
The cytokine profile of wounds that failed is less clear. Although we observed an association between wound failure and elevated serum ProCT and effluent IL-6 at the final débridement, the results should be interpreted with caution. Nevertheless, these results are similar to those of a previous study in which we observed that ProCT, measured in the serum and the effluent, and IL-13 were associated with wound failure in combat injuries [8]. These data suggest wound failure is associated with a persistent inflammatory state. This was suggested previously [11] and is evidenced by higher concentrations of serum ProCT, a relatively late-stage inflammatory mediator, and effluent IL-6, an acute phase mediator, that were persistent until the final stages of the débridement process.
This study showed unique local and systemic inflammatory profiles associated with untoward wound outcomes in combat wounds. Local and systemic inflammatory data are attractive as promising future tools for the clinician, as they can be assessed intraoperatively, and the analyses performed in the current study could be reliably available the same day or by the time of the patient’s next return to the operating room, 24 to 72 hours later. There is of considerable interest in developing models that will codify these data while combining them with injury-specific and demographic information to provide usable information for surgeons. However, future efforts geared toward modeling these data must account for their complex, time-dependent, and nonlinear nature.
Acknowledgments
We thank Trevor Brown PhD, from the Regenerative Medicine Department, Naval Medical Research Center, for data assembly; Stacia Moreno, Xochitl Ceniceros, Fred Gage, Felipe Lisboa MD, Tala Ghadimi, Andrew Greenhalgh, and Aileen Mooney MS, from the Regenerative Medicine Department, who enrolled patients, collected, and processed the samples needed to perform this study, Matthew Wagner PhD, also from the Regenerative Medicine Department, and Meng Shi MS, from the Department of Biostatistics at the Naval Medical Research Center for statistical coding advice.
Footnotes
The institution of one or more of the authors (JAF) has received, during the study period, funding from a grant from the Orthopaedic Trauma Research Program (OTRP) and the US Navy Bureau of Medicine and Surgery Advance Medical Development Program.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Naval Medical Research Center, Silver Spring, MD, USA, and the National Military Medical Center, Bethesda, MD, USA.
The views expressed this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
This study protocol was approved by the Institutional Review Boards at both the National Military Medical Center and Naval Medical Research Center in compliance with all applicable Federal regulations governing the protection of human subjects.
One or more of the authors is a military service member (or employee of the U.S. Government). This work was prepared as part of his or her official duties. Title 17 U.S.C §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
References
- 1.Bartlett CS. Clinical update: gunshot wound ballistics. Clin Orthop Relat Res. 2003;408:28–57. doi: 10.1097/00003086-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Belmont PJ, Jr, McCriskin BJ, Hsiao MS, Burks R, Nelson KJ, Schoenfeld AJ. The nature and incidence of musculoskeletal combat wounds in Iraq and Afghanistan (2005–2009) J Orthop Trauma. 2013;27:e107–e113. doi: 10.1097/BOT.0b013e3182703188. [DOI] [PubMed] [Google Scholar]
- 3.Brown TS, Hawksworth JS, Sheppard FR, Tadaki DK, Elster E. Inflammatory response is associated with critical colonization in combat wounds. Surg Infect (Larchmt). 2011;12:351–357. doi: 10.1089/sur.2010.110. [DOI] [PubMed] [Google Scholar]
- 4.Davis TA, Lazdun Y, Potter BK, Forsberg JA. Ectopic bone formation in severely combat-injured orthopedic patients: a hematopoietic niche. Bone. 2013;56:119–126. doi: 10.1016/j.bone.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich LA, Chung HY, Ghobrial I, Choi SJ, Morandi F, Colla S, Rizzoli V, Roodman GD, Giuliani N. IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood. 2005;106:1407–1414. doi: 10.1182/blood-2005-03-1080. [DOI] [PubMed] [Google Scholar]
- 6.Evans KN, Forsberg JA, Potter BK, Hawksworth JS, Brown TS, Andersen R, Dunne JR, Tadaki D, Elster EA. Inflammatory cytokine and chemokine expression is associated with heterotopic ossification in high-energy penetrating war injuries. J Orthop Trauma. 2012;26:e204–e213. doi: 10.1097/BOT.0b013e31825d60a5. [DOI] [PubMed] [Google Scholar]
- 7.Evans KN, Potter BK, Brown TS, Davis TA, Elster EA, Forsberg JA. Osteogenic gene expression correlates with development of heterotopic ossification in war wounds. Clin Orthop Relat Res. 2014;472:396–404. doi: 10.1007/s11999-013-3325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsberg J, Elster E, Andersen R, Nylen E, Brown T, Rose M, Stojadinovic A, Becker K, McGuigan F. Correlation of procalcitonin and cytokine expression with dehiscence of wartime extremity wounds. J Bone Joint Surg Am. 2008;90:580–588. doi: 10.2106/JBJS.G.00265. [DOI] [PubMed] [Google Scholar]
- 9.Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, Tadaki D, Gage FA, Stojadinovic A, Elster EA. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91:1084–1091. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 10.Gwinn DE, Tintle SM, Kumar AR, Andersen RC, Keeling JJ. Blast-induced lower extremity fractures with arterial injury: prevalence and risk factors for amputation after initial limb-preserving treatment. J Orthop Trauma. 2011;25:543–548. doi: 10.1097/BOT.0b013e3181fc6062. [DOI] [PubMed] [Google Scholar]
- 11.Hawksworth JS, Stojadinovic A, Gage FA, Tadaki DK, Perdue PW, Forsberg J, Davis TA, Dunne JR, Denobile JW, Brown TS, Elster EA. Inflammatory biomarkers in combat wound healing. Ann Surg. 2009;250:1002–1007. doi: 10.1097/SLA.0b013e3181b248d9. [DOI] [PubMed] [Google Scholar]
- 12.Lenarz CJ, Watson JT, Moed BR, Israel H, Mullen JD, Macdonald JB. Timing of wound closure in open fractures based on cultures obtained after debridement. J Bone Joint Surg Am. 2010;92:1921–1926. doi: 10.2106/JBJS.I.00547. [DOI] [PubMed] [Google Scholar]
- 13.Moorhead JJ. Surgical experience at Pearl Harbor. JAMA. 1942;118:712–714. doi: 10.1001/jama.1942.62830090002009. [DOI] [Google Scholar]
- 14.Pawitan Y, Michiels S, Koscielny S, Gusnanto A, Ploner A. False discovery rate, sensitivity and sample size for microarray studies. Bioinformatics. 2005;21:3017–3024. doi: 10.1093/bioinformatics/bti448. [DOI] [PubMed] [Google Scholar]
- 15.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476–486. doi: 10.2106/JBJS.F.00412. [DOI] [PubMed] [Google Scholar]
- 16.Potter BK, Forsberg JA, Davis TA, Evans KN, Hawksworth JS, Tadaki D, Brown TS, Crane NJ, Burns TC, O’Brien FP, Elster EA. Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am. 2010;92(suppl 2):74–89. doi: 10.2106/JBJS.J.00776. [DOI] [PubMed] [Google Scholar]
- 17.Robson MC, Heggers JP. Bacterial quantification of open wounds. Mil Med. 1969;134:19–24. [PubMed] [Google Scholar]
- 18.Selcer P. Standardizing wounds: Alexis Carrel and the scientific management of life in the First World War. Br J History Sci. 2008;41:73–107. doi: 10.1017/S0007087407000295. [DOI] [Google Scholar]
- 19.Stein NC, Kreutzmann C, Zimmermann S-P, Niebergall U, Hellmeyer L, Goettsch C, Schoppet M, Hofbauer LC. Interleukin-4 and interleukin-13 stimulate the osteoclast inhibitor osteoprotegerin by human endothelial cells through the STAT6 pathway. J Bone Miner Res. 2008;23:750–758. doi: 10.1359/jbmr.080203. [DOI] [PubMed] [Google Scholar]
- 20.Stromeyer L, Esmarch F. Gunshot Fractures. Resection in Gunshot Injuries. Translated by S.F. Statham. Philadelphia, PA: J.B. Lippincott & Co; 1862:20–32.
- 21.Tintle SM, Baechler MF, Nanos GP., 3rd Forsberg JA, Potter BK. Traumatic and trauma-related amputations: Part II. Upper extremity and future directions. J Bone Joint Surg Am. 2010;92:2934–2945. doi: 10.2106/JBJS.J.00258. [DOI] [PubMed] [Google Scholar]
- 22.Tintle SM, Baechler MF, Nanos GP, 3rd, Forsberg JA, Potter BK. Reoperations following combat-related upper-extremity amputations. J Bone Joint Surg Am. 2012;94:e1191–e1196. doi: 10.2106/JBJS.K.00197. [DOI] [PubMed] [Google Scholar]
- 23.Tintle SM, Keeling JJ, Forsberg JA, Shawen SB, Andersen RC, Potter BK. Operative complications of combat-related transtibial amputations: a comparison of the modified Burgess and modified Ertl tibiofibular synostosis techniques. J Bone Joint Surg Am. 2011;93:1016–1021. doi: 10.2106/JBJS.J.01038. [DOI] [PubMed] [Google Scholar]
- 24.Tintle SM, Keeling JJ, Shawen SB, Forsberg JA, Potter BK. Traumatic and trauma-related amputations: Part I. general principles and lower-extremity amputations. J Bone Joint Surg Am. 2010;92:2852–2868. doi: 10.2106/JBJS.J.00257. [DOI] [PubMed] [Google Scholar]
- 25.Utz ER, Elster EA, Tadaki DK, Gage F, Perdue PW, Forsberg JA, Stojadinovic A, Hawksworth JS, Brown TS. Metalloproteinase expression is associated with traumatic wound failure. J Surg Res. 2010;159:633–639. doi: 10.1016/j.jss.2009.08.021. [DOI] [PubMed] [Google Scholar]