Abstract
Background
Osseous defects reconstructed with cryopreserved structural allografts are poorly revascularized and therefore are prone to nonunion, infection, deterioration of mechanical properties, and fracture. Whether this can be mitigated by specific interventions such as intramedullary surgical revascularization has been incompletely evaluated.
Questions/purposes
We aimed to study surgical revascularization as a means to improve bone remodeling in cryopreserved allograft. Second, we questioned whether spatial histomorphometric differences occur in cortical bone areas after intramedullary surgical revascularization. Third, biomechanical properties of the graft-recipient construct in surgically revascularized allograft were compared with those of conventional allografts.
Methods
Allografts were harvested from 10 Brown Norway rats, cryopreserved, and transplanted orthotopically in a 10-mm defect in two groups of 10 Lewis rats each (major histocompatibility mismatch). In the control group, no surgical revascularization was performed, whereas in the experimental group, a saphenous arteriovenous bundle was transposed in the bone marrow cavity. Bone remodeling was measured with histomorphometry, histology, and microcomputed tomography at 16 weeks. Spatial differences were analyzed with histomorphometry. To determine biomechanical properties, load at failure and structural stiffness in bending were evaluated by the three-point bend testing. In both groups, normal values of the contralateral femur also were analyzed.
Results
Surgically revascularized allografts had increased bone remodeling (bone formation rate to bone surface ratio: 130 ± 47 µm3/µm2/year versus 44 ± 43 µm3/µm2/year, p = 0.006) and higher cortical osteocyte counts (18.6% ± 12.7% versus 3.1% ± 2.8%, p = 0.002) than nonrevascularized grafts. In nonrevascularized grafts, the bone formation rate to bone surface ratio was 35% of the contralateral normal values, whereas in surgically revascularized grafts, the bone formation rate to bone surface ratio in the grafts exceeded the contralateral values (110%). Microcomputed tomography did not show differences in bone volume between groups, however in both groups, bone volume was less in grafts compared with the contralateral femurs. Inner cortical bone formation rate to bone surface ratio was greater in surgically revascularized grafts (65 ± 30 µm3/µm2/year versus 13 ± 16 µm3/µm2/year in the control group, p = 0.012). Outer cortical bone formation rate to bone surface ratio also increased in surgically revascularized grafts (49 ± 31 µm3/µm2/year versus 19 ± 21 µm3/µm2/year, p = 0.032). No differences were found in load at failure and structural stiffness between both groups. In the control group, load at failure and structural stiffness were lower in grafts than in the contralateral femurs (p = 0.004 and p = 0.02, respectively). In the experimental group, surgically revascularized grafts also had lower load at failure and structural stiffness than the contralateral femurs (p = 0.008 and p = 0.02, respectively).
Conclusions
Surgical revascularization of large segmental allografts improved bone remodeling and viability without an adverse effect on total bone volume or bending strength and stiffness in this short-term analysis.
Clinical Relevance
Cryopreserved allografts remain largely necrotic and are associated with a high rate of complications. Surgical revascularization increases graft healing which could contribute to graft survival with time.
Introduction
In structural defects in the extremities, autologous bone transplantation is not always feasible because of the inability to match missing bone size and shape from the few expendable donor sites and the potential for donor site morbidity. The use of cryopreserved allogeneic bone has been a widely used and appropriate alternative to reconstruct segmental defects [2]. However, a serious shortcoming of bone allograft is the high frequency of complications such as infection, fracture, and nonunion [4, 12, 14, 26]. Study of retrieved postmortem specimens has shown limited remodeling and minimal revitalization with time [10, 11, 24]. Wheeler and Enneking [26] found a gradual diminution in bone mineral density and increase in microfractures with time, resulting in 50% loss of strength after 10 years in vivo. These findings show the essential necrotic nature of allograft structural bone segments, which are frozen before transplantation to decrease the immunogenic response in the recipient. After transplantation, revascularization in frozen allografts is restricted and insufficient to revitalize and incorporate the graft biologically [10, 11, 23]. Furthermore, vascularization of the graft directly relates to the biomechanical competence of the reconstructed bone, including the intrinsic properties of the graft and the graft-recipient interface [6].
Successful biomechanical incorporation of allograft is dependent on stable fixation and adequate bone-to-bone contact. However, because no patent vascular bed exists in cryopreserved allografts, neoangiogenesis purely originates from recipient bone and surrounding soft tissue. As soon as the graft is revascularized, remodeling occurs as described by the activation-resorption-formation sequence of bone remodeling; an osteoclastic cutter head is followed by a layer of osteoblasts [13]. One could theorize that incompletely revascularized allograft will weaken with time as a result of limited bone remodeling, having lost the ability to recover from microfractures. Conversely, rapid revascularization could incite abundant bone remodeling in which osteoclastic activity dominates osteoblastic activity, which could lead to an initial net bone loss and fracture [3]. Allograft bone remodeling and incorporation after revascularization therefore seems to be a delicately balanced process. We previously presented an orthotopic rat model to study surgical revascularization in frozen bone allografts [27]. A strong angiogenic response with increase of cortical bone blood flow was observed after intramedullary arteriovenous bundle implantation. However, whether increased bone blood flow in the graft would alter bone remodeling, morphologic features of the bone, or biomechanical properties remained unanswered.
Accordingly, in the current article, we studied bone remodeling and incorporation after surgical revascularization of bone allograft in the orthotopic model. We hypothesized that bone remodeling as measured with histology, histomorphometry, and micro-CT will be improved in surgically revascularized allografts as compared with conventional allografts. Second, we compared histomorphometric parameters at the inner cortex to outer cortical parameters to determine the spatial differences in bone remodeling after intramedullary surgical revascularization. Third, we hypothesized that surgical revascularization of allografts improves biomechanical properties of the graft-recipient construct.
Materials and Methods
Orthotopic Allotransplantation
This study was approved by the Institutional Animal Care and Use Committee.
Surgical revascularization of the graft was performed in our previously described orthotopic rat model [27]. In brief, a 10-mm segment of diaphyseal femurs from 10 female Brown Norway (RT1n) rats (weight, 200–250 g) were harvested bilaterally, reamed with a 2-mm hand drill to allow intramedullary implantation of the arteriovenous bundle, and frozen at −80° C for at least 1 month before transplantation. At transplantation surgery, male Lewis (RT1l) rats (20 in total; weight, 250–300 g), representing a major histocompatibility mismatch, were anesthetized with ketamine (90 mg/kg intramuscularly and xylazine (10 mg/kg intramuscularly). A single dose of Fragmin® (Eisai Inc, Woodcliff Lake, NJ, USA) (10 IU subcutaneously) was given preoperatively and daily postoperatively during 5 days. The frozen graft was thawed to room temperature. A 10-mm segment of bone was removed in the recipient rat at either the left or right femur, matching the side at which the graft was harvested from the donor. The defect was created with a minisaw and standardized by using a custom-made 10-mm steel mold. This created a large segmental bone defect which was approximately 22% of the complete femoral length. The graft then was transplanted in the defect. Next, the ipsilateral saphenous arteriovenous bundle was dissected from its femoral origin distally to the ankle (Fig. 1A). It remained attached proximally to its femoral origin and the bundle was sutured distally with a nylon monofilament suture (Fig. 1B). A small hole (1.2 mm) was drilled in the distal end of the remaining recipient femur and the arteriovenous bundle was pulled through the vascular entry hole and transposed in the intramedullary canal of the graft in a retrograde fashion (Fig. 1C). The graft was fixed with a custom-made stainless steel plate from Mayo Clinic Division of Engineering, and 1.2 mm × 8 mm screws; additional nylon monofilament sutures were used to achieve a stable construct. The graft-host junctions were created with a 15° oblique osteotomy to increase rotational stability (Fig. 1C).
Fig. 1A–C.
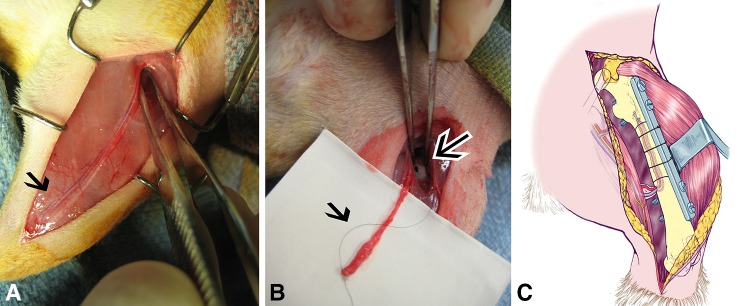
(A) On the medial side of the lower extremity, the saphenous arteriovenous bundle is marked before dissection (black arrow). (B) Dissection of the arteriovenous bundle (black arrow), which is transposed in the femur through a small cortical hole (black arrow with white border) is shown. (C) The schematic drawing shows the orthotopic model. The arteriovenous bundle is placed in the intramedullary canal and the graft is fixed with a custom-made plate and screws with additional nylon sutures.
Twenty rats were randomly allocated to two groups. In the control group, no surgical revascularization was performed (conventional allograft). In the experimental group, grafts were surgically revascularized with the saphenous arteriovenous bundle in the medullary canal. Postoperatively and for 2 days thereafter buprenorphine (0.05–0.1 mg/kg subcutaneously) was given once daily and rats were allowed to move freely in their cage. At 14 days and 4 days before euthanasia, respectively, calcein green and tetracycline hydrochloride fluorescent labels (20 mg/kg) were administered to enable later histomorphometric studies (Fig. 2). After a survival period of 16 weeks, the rats were euthanized with pentobarbital (200 mg/kg intravenously). The aorta and vena cava were cannulated to allow irrigation of the lower extremity vasculature with 50 mL of heparinized saline followed by Microfil® contrast agent (Microfil®; Flow Tech Inc, Carver, MA, USA) to determine arteriovenous bundle patency. No complications occurred during the 16 weeks survival and fixation material remained intact. All arteriovenous bundles were patent at 16 weeks (Fig. 3). The grafted femur and the contralateral femur were removed for analysis.
Fig. 2.
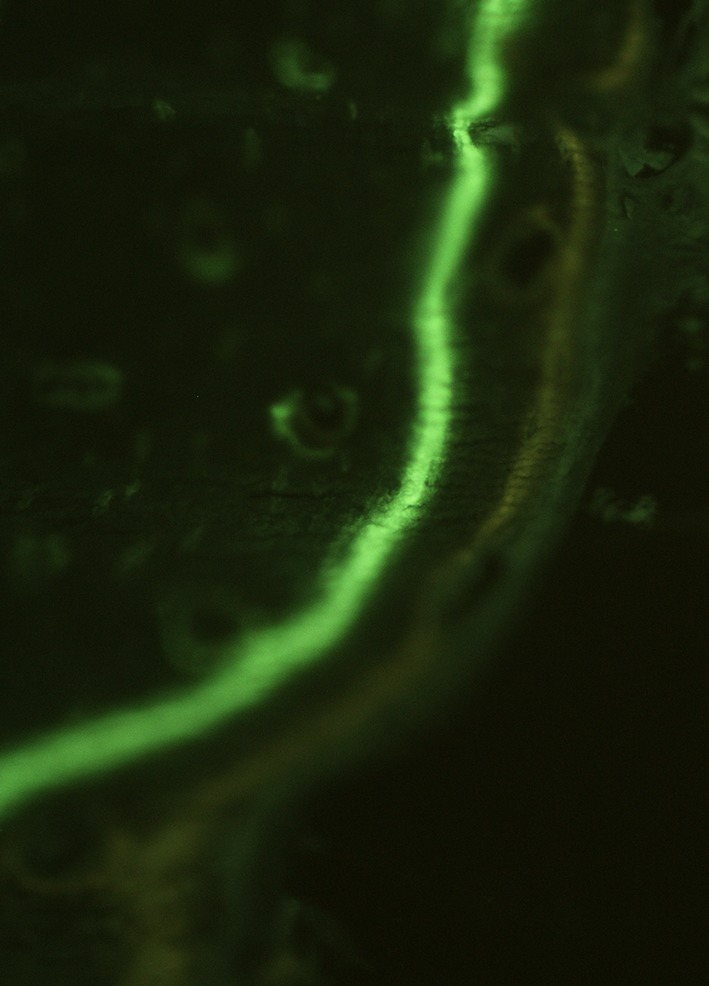
Calcein green and tetracycline hydrochloride fluorescent labels (20 mg/kg) were administered 14 days and 4 days before euthanasia, respectively, to enable histomorphometric studies.
Fig. 3.

At 16 weeks, femurs were removed and patency was confirmed by filling the arteriovenous bundle with Microfil® (Flow Tech Inc) in all specimens.
Micro-CT
Immediately after sacrifice, plate, screws, and soft tissue were carefully removed from the femurs. Grafts and contralateral femurs were scanned using a micro-CT system (MicroCT40; Scanco Medical, Basserdorf, Switzerland). The femur was placed in a polyethylenimine holder with saline. The graft was scanned at a voltage of 55 KvP and a current of 144 µA at 10-µm thickness resulting in 1000 axial-cut slices. After scanning and reconstruction, a region of interest was determined encircling the outer perimeter of the bone. The ratio of the bone volume to total volume, bone surface to bone volume, and bone mineral density were measured with MicroCT40 software (Scanco Medical).
Histology: Cortical Osteocyte Viability
After biomechanical three-point bend testing (described below), all grafts and contralateral femurs were fixed in 10% formalin for 24 hours. For histologic analysis, a transverse section was harvested consistently from the proximal 1/3 of each graft and decalcified in 14% ethylenediaminetetraacetic acid for 7 hours in a calibrated laboratory microwave at 750 W (PELCO BioWave® 3450 Laboratory Microwave, Ted Pella, Inc, Redding, CA, USA). At the site where the transverse sections were harvested, biomechanical testing had not disturbed cortical integrity. Staining was performed with hematoxylin and eosin for histologic analysis. Bone cellular repopulation was quantified by determining the percentage of lacunae filled with viable osteocytes to the total number of lacunae (empty + osteocyte-containing) in 10 random fields at × 400 magnification.
Quantitative Histomorphometry
Complete transverse sections were harvested from the proximal 1/3 of the graft and from the contralateral femur at the same anatomic location after 24 hours of fixation in 10% formalin. Undecalcified, unstained sections were embedded with the glycol methylmethacrylate procedure and the block was cut on a Leica Microtome 2065® (Leica Microsystems Inc, Buffalo Grove, IL, USA). A Nikon® Eclipse 50i microscope (Nikon Instruments Inc, Melville, NY, USA) and Nikon® Intensilight C-HGFI fluorescent system was used with a DS-Fi1 high-definition color camera head (Nikon Instruments Inc) for image capturing. A blue-violet filter set was used with an excitation of 425/40 (405–445), a dichroic reflector at 455, and a long pass filter of 475.
Complete sections were viewed at ×200 for fluorochrome labeling uptake and histomorphometric parameters were determined using imaging analysis software (Osteomeasure®; Osteometrics, Atlanta, GA, USA). The mineralizing bone surface to total bone surface ratio was determined, which is the ratio of the surface of fluorescent labeled mineralizing bone to total bone surface. This value represents the percentage of bone surface involved in bone remodeling. Mineral apposition rate is calculated by measuring the distance between the two labels (calcein green and tetracycline hydrochloride) and relating this to the 10 day period in between fluorescent label delivery. Mineral apposition rate is a measure of the rate at which new bone is deposited. The bone formation rate to bone surface ratio is the product of mineral apposition rate and mineralizing bone to total bone surface ratio and represents the annual fractional volume of bone formed per unit bone surface area. This was measured from the complete section in each graft and at the inner and the outer perimeter of the cortex in each graft to determine if any spatial differences existed in the bone remodeling process.
Biomechanics
The strength of the complete construct of graft and recipient bone was measured in three-point bending to determine the overall competence of the femur to sustain loading at 16 weeks. In each group, grafted and contralateral femurs were analyzed after micro-CT scanning and before histologic analysis. Three-point bending was chosen to extract mechanical properties of the graft-recipient construct and contralateral femurs [6]. Bones were kept moist in saline before testing, which was conducted at room temperature. Each bone was oriented alike with the posterior side placed on two supporting bars separated by 18 mm with the graft placed centrally. A rounded bar loaded the construct centrally at a rate of 5 mm/minute in the AP direction. Load at failure (N) and deflection displacement (mm) were measured. The deflection was read directly by the actuator of the servohydraulic testing system (MTS systems Corporation, Eden Prairie, MN, USA). Structural stiffness was calculated as load divided by displacement (N/mm). The data for bones at which failure occurred at the graft-recipient interface and the bones with failure through the graft were combined and analyzed together, since the main objective of biomechanical testing was to extract some measure of mechanical properties of the complete graft-recipient construct.
Statistical Analysis
Histomorphometric, micro-CT, and biomechanical measurements also were performed on the contralateral femurs to compare graft properties with those of the normal femoral properties in each group. Analysis between groups was performed with absolute graft values and with normalized values (graft values as a percentage of the normal contralateral values), which adjusts for potential biologic variability between animals. Therefore, data are presented as absolute and normalized values (expressed as a percentage). The Kolmogorov test was applied for each data set to determine distribution. Data derived from bone remodeling analysis (micro-CT, histology, and histomorphometry) were analyzed with the Mann-Whitney U test to detect differences between groups (conventional grafts versus revascularized grafts) and with the paired Wilcoxon signed-rank test to detect differences in each group (grafts versus contralateral femurs). Analysis of histomorphometric spatial data was performed with the Mann-Whitney U test to detect differences in inner cortical and outer cortical bone remodeling between conventional grafts and revascularized grafts. Biomechanical property differences between experimental groups were analyzed with the Mann-Whitney U test and the paired Wilcoxon signed-rank test was used to detect differences between graft-recipient constructs and contralateral femurs. GraphPadPrism™ Version 5.0 (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis.
Results
Bone Remodeling
Micro-CT analysis showed no difference in bone volume to total volume between conventional and revascularized allografts (respectively, 0.56 ± 0.15 mm3/mm3 versus 0.54 ± 0.14 mm3/mm3, p = 0.7; normalized for contralateral femurs, p = 0.76). Revascularized grafts had lower bone volume to total volume than their own contralateral femurs (0.64 ± 0.02 mm3/mm3; p = 0.008). In the control group, no difference was observed between conventional grafts and contralateral femurs (0.64 ± 0.02 mm3/mm3; p = 0.13). Bone surface to bone volume showed no differences (conventional graft, 3.75 ± 2.7 mm2/mm3; revascularized graft, 4.24 ± 2.1 mm2/mm3, p = 0.52; normalized to contralateral femurs, p = 0.27). The bone surface to bone volume ratio was greater in conventional and revascularized allografts when compared with contralateral values (p = 0.002 and p = 0.008, respectively). Bone mineral density did not differ between conventional and revascularized grafts (1143 ± 42 mg/cm3 versus 1122 ± 39 mg/cm3, respectively, p = 0.35). In conventional grafts, bone mineral density was lower compared with that of the contralateral femurs (1213 ± 17 mg/cm3; p = 0.0002). Similarly, bone mineral density was lower in revascularized grafts compared with that of the contralateral femurs (1225 ± 12 mg/cm3; p = 0.008). The histologic osteocyte viability score was greater in revascularized grafts (18.6 ± 12.7%) compared with conventional grafts (3.1 ± 2.8%; p = 0.002). The range of osteocyte repopulation was 0% to 8% in conventional grafts and 4% to 40% in revascularized grafts. Quantitative histomorphometric analysis showed that in conventional grafts, the mineralizing bone surface to total bone surface was 30%, mineral apposition rate was 105%, and bone formation rate to bone surface rate was 35% of the normal contralateral values (Table 1). Revascularized grafts had greater absolute and normalized remodeling parameters: 78% mineralizing bone surface to total bone surface (p = 0.002), 137% mineral apposition rate (p = 0.16), and 110% bone formation rate to bone surface (p = 0.005).
Table 1.
Quantitative histomorphometry
| Histomorphometry | Absolute /normalized | Conventional allograft | Revascularized allograft | p value |
|---|---|---|---|---|
| Mineralizing bone surface to total bone surface ratio (%) | Graft values (%), | 9 ± 9 | 19 ± 5 | 0.01 |
| normalized values (%)*, | 30 ± 24 | 78 ± 26 | 0.002 | |
| p value graft versus contralateral | 0.008 | 0.039 | ||
| Mineral apposition rate (µm/day) |
Graft values (µm/day), | 1.3 ± 0.5 | 1.8 ± 0.4 | 0.07 |
| normalized values (%)*, | 105 ± 43 | 137 ± 39 | 0.16 | |
| p value graft versus contralateral | 0.38 | 0.05 | ||
| Bone formation rate to bone surface ratio (µm3/µm2/year) |
Graft values (µm3/µm2/year), | 44 ± 43 | 130 ± 47 | 0.006 |
| normalized values (%)*, | 35 ± 28 | 110 ± 53 | 0.005 | |
| p value graft versus contralateral | 0.008 | 0.95 |
Values are mean ± SD; *normalized values represent the percentage of graft values to contralateral (normal) values, therefore, a value higher than 100% indicates remodeling of a revascularized bone exceeds that of the contralateral normal femur.
Histomorphometric Analysis of Spatial Differences
Inner cortical and outer cortical bone formation rate to bone surface ratio were greater in revascularized grafts compared with conventional grafts (Table 2). After vascular bundle implantation, inner cortical bone formation rate to bone surface ratio exceeded that of contralateral values at 509%, whereas outer cortical bone formation rate to bone surface ratio was 45% of contralateral values. In conventional grafts, inner cortical bone formation rate to bone surface ratio was approximately equal to that of the contralateral inner cortical bone formation rate to bone surface ratio (105%) and the outer cortical bone formation rate to bone surface ratio was only 18% of the contralateral values.
Table 2.
Spatial results of bone formation rate to bone surface
| Bone formation rate to bone surface ratio (µm3/µm2/year) | Conventional allograft |
Revascularized allograft |
p value |
|---|---|---|---|
| Endosteal bone formation rate to bone surface ratio | 13 ± 16 (105%)* |
65 ± 30 (509%)* |
0.01 |
| Periosteal bone formation rate to bone surface ratio | 19 ± 21 (18%)* |
49 ± 31 (45%)* |
0.03 |
Values are mean ± SD; *graft bone formation rate to bone surface ratio as a percentage of contralateral femur normal values.
Biomechanics
Under three-point bending, failure occurred at the graft-recipient interface in three conventional grafts and four revascularized grafts. The rest of the grafted femurs and all contralateral femurs failed midway, at the central loading bar. In conventional grafts, load at failure was lower than in the contralateral femurs: 39.3 ± 15.6 N versus 134.2 ± 34.6 N (p < 0.001). In revascularized grafts, load at failure also was lower than that of their contralateral femurs: 23.2 ± 19.6 N versus 133.4 ± 9.6 N (p = 0.008). The structural stiffness in conventional grafts was lower compared with the contralateral side (20.8 ± 18.4 N/mm and 120.4 ± 44.4 N/mm, respectively, p < 0.001). In revascularized grafts, the structural stiffness also was lower than the contralateral values (16.2 ± 17.8 N/mm versus 110.3 ± 24.9 N/mm, p < 0.001). No differences in load at failure or structural stiffness were found between revascularized and conventional grafts when comparing absolute or normalized values (Table 3).
Table 3.
Biomechanical analysis in three-point bending of the graft-recipient construct
| Biomechanical analysis | Allograft group | Grafted femur |
Contralateral femur | p value |
|---|---|---|---|---|
| Load at failure (N) | Conventional allograft | 39.3 ± 15.6 | 134.2 ± 34.6 | < 0.001 |
| Revascularized allograft | 23.2 ± 19.6 | 133.4 ± 9.6 | < 0.001 | |
| Stiffness (load/displacement, N/mm) | Conventional allograft | 20.8 ± 18.4 | 120.4 ± 44.4 | < 0.001 |
| Revascularized allograft | 16.2 ± 17.8 | 110.3 ± 24.9 | < 0.001 |
Values are mean ± SD.
Discussion
Cryopreserved allografts frequently are used to restore defects after tumor resection, bone infection, and trauma [2]. Unfortunately, allografts are associated with a high rate of complications, including infection, nonunion, and fracture [7, 8, 12, 18, 19]. This mostly is attributable to the persistent avascular and nonvital state of allograft bone as observed in long-term clinical studies [6, 10]. Improvement of graft vascularization by vascular bundle implantation has been proposed [15, 27]. However, the consequences of surgical revascularization in orthotopically placed allografts on bone remodeling and biomechanical properties has not been determined. Therefore, we analyzed bone volume, osteocyte population, and bone remodeling in surgically revascularized cryopreserved allografts and compared these findings with those from conventional allografts and contralateral femurs. We found increased remodeling and osteocyte population when surgical revascularization was performed, whereas bone volume was reduced compared with that of contralateral femurs. Second, spatial differences in bone remodeling were analyzed and an increase of bone remodeling was found at the inner and outer cortices after intramedullary arteriovenous bundle implantation. Third, biomechanical properties of the graft-recipient construct in three-point bending were analyzed and proved to be inferior in conventional and revacularized grafts compared with contralateral femurs and no major effect of surgical revascularization could be confirmed.
One limitation of this study is the rodent model. The small size of a rat femur does not translate directly to bone of human dimensions. Rat bone furthermore consists of lamellar bone and bone mineral density and composition are different than that of human bone [1]. However, rats are considered useful models and valuable to long bone research [21]. Another limitation concerns the biomechanical analysis. Grafts failed at either the graft-recipient interface or the graft, which implies that grafts were variably incorporated or variably remodeled. It was our aim in this small animal study to investigate the strength of the complete graft-recipient construct and compare these with contralateral femurs. However, in three-point bending, the complete construct between the two supporting bars is loaded with the maximum bending moment under the central loading bar. Failure occurred at either the graft or at the graft-recipient interface. The grafted femurs in each group were analyzed uniformly to provide data on the complete graft-recipient construct. The data do not represent the strength of the individual graft-recipient interface or the strength of the graft. Whether surgical revascularization influences graft incorporation or graft intrinsic properties separately needs to be analyzed in larger animal models. Moreover, other biomechanical techniques such as torsional testing can be of use to investigate properties of the complete construct. However, we were able to determine some mechanical properties to compare these with the contralateral values. Furthermore, the presence of the hole that was created in the distal recipient femur to introduce the vascular bundle was included in the graft-recipient femoral segment between the two supporting bars. The hole was 1.2 mm in diameter and the diameter of the femur at that level was 4.9 mm on average (ratio hole diameter to outer bone diameter: 0.24). In larger animal models the effect of the size of cortical holes on biomechanical properties has been determined. McBroom et al. [20] determined the biomechanical properties with variable cortical hole sizes in canine femurs. They performed four-point bending and concluded that a ratio of drill hole diameter to bone diameter of 0.2 leads to a 38% strength reduction. However, we did not observe failure at the vascular entry hole, nor at the more distant placed screw holes (1.2 mm, ratio 0.24) in this study. Failure at the hole for vascular entry or the cortical drill holes did not occur likely owing to the biomechanical testing set-up with central placement of the graft construct. The maximal bending moment therefore was not implemented on the femoral segment containing the hole. The effect of hole sizes for vascular bundle entry on biomechanical properties of the complete reconstructed femur are better analyzed with torsional testing as mentioned above. Further biomechanical testing in larger animal models should be done before clinical application of the presented technique. This is the subject of future research in our laboratory.
Another limitation was that analysis was done at one time (16 weeks). Shorter- and longer-term analyses would give us more insight into the dynamic and continuing process of bone remodeling and incorporation after conventional and surgically revascularized bone allografting. Longer-term analysis of the effect of surgical revascularization in a larger animal model would be an advisable next step before any clinical use. Such a study must use orthotopic reconstruction of a long bone segmental resection with physiologic weightbearing, because angiogenesis, bone remodeling, and graft incorporation are dependent on mechanical loading conditions [9, 16, 23, 25].
Surgical revascularization improved bone remodeling and viability as measured with histomorphometry and histology, while bone volume remained unchanged compared with conventional grafts. Other investigators have considered the role of revascularizing structural allografts. Kumta et al. [17] described the effect of surgical revascularization on cellular repopulation in heterotopically (nonloading) transplanted cryopreserved rat allografts. In conventional allografts, the repopulation was scarce with 8% at 12 weeks and 10% at 24 weeks. In surgically revascularized allografts, the repopulation was 20% at 12 and 24 weeks. Carneiro and Malinin [5] implanted an arteriovenous bundle in canine allografts, which were placed heterotopically in a subcutaneous pocket. They described histologic signs (without quantification) of increased bone formation after surgical revascularization. Neither study used orthotopic transplantation and no histomorphometric or biomechanical analyses were performed. We found bone surface-to-bone volume ratio to be greater in grafts in both groups compared with that of the normal contralateral femurs. These findings indicate increased osteoclastic activity resulting in an increase of bone surface and decrease in bone volume. To support this assumption, osteoclastic activity needs to be quantified, which we aim to do in future research with the presented orthotopic model.
We observed remodeling to occur on the inner and outer cortical surfaces of allograft bone after surgical revascularization. Not surprisingly, inner cortical bone formation was greater given the intramedullary location of the implanted arteriovenous pedicle. The rate of remodeling was four times higher at the inner cortex and two times higher at the outer cortex than the values in nonrevascularized allograft controls. These data suggest the ability of surgically implanted vessels in the medullary canal to successfully revascularize the entire cortex of rat femoral diaphyses. To our knowledge, this is the first published experimental study of the effect of surgical revascularization on the structural properties of femoral reconstructions with large segmental allografts. Pelker et al. [22] studied nonrevascularized allograft properties in rat femurs. Segmental bone loss was reconstructed with allograft using intramedullary fixation, which produced axial alignment but no rigid fixation. As in our study, they found femurs reconstructed with cryopreserved allografts to be biomechanically inferior to untreated contralateral femurs. It has been postulated that strong bone angiogenesis can disturb the balance of remodeling and initially weaken bone, possibly resulting in stress fracture or collapse as found in Kienbock’s disease [3]. The rationale for this supposition is the process of creeping substitution of necrotic bone, which begins with revascularization followed by osteoclasis and then new bone formation. The potential for substantial bone loss and resultant loss of strength has been a cause for concern in clinical cases. We have not been able to find any experimental evidence to either support or refute the common opinion that structural allograft revascularization is undesirable. Likely the rate of revascularization must be correct to allow for a balance between osteoclasis and replacement with new, viable bone.
In this experimental study, we observed that implantation of an arteriovenous bundle in the medullary canal of a cryopreserved femoral allograft tended to decrease biomechanical properties. However, no statistically significant changes were determined in our study. Larger groups, larger animal models, and longer-term analysis would contribute to further analysis of biomechanical properties after surgical revascularization.
Despite their widespread use, segmental conventional bone allografts are subject to frequent complications. We aimed to induce remodeling and biomechanical properties by revascularizing nonviable cryopreserved femoral allograft bone. The saphenous arteriovenous bundle was placed in a structural cryopreserved allograft used to reconstruct a missing femoral segment in rats. Surgical revascularization increased measures of bone remodeling compared with nonrevascularized controls. Whether surgical revascularization ultimately could improve incorporation and diminish short- and long-term complications is the subject of future research.
Footnotes
This research was funded by the Musculoskeletal Transplant Foundation. (WFW, ATB)
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
References
- 1.Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139:663–670. doi: 10.1210/endo.139.2.5751. [DOI] [PubMed] [Google Scholar]
- 2.Aponte-Tinao L, Farfalli GL, Ritacco LE, Ayerza MA, Muscolo DL. Intercalary femur allografts are an acceptable alternative after tumor resection. Clin Orthop Relat Res. 2012;470:728–734. doi: 10.1007/s11999-011-1952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspenberg P, Wang JS, Jonsson K, Hagert CG. Experimental osteonecrosis of the lunate: revascularization may cause collapse. J Hand Surg Br. 1994;19:565–569. doi: 10.1016/0266-7681(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 4.Bullens PH, Minderhoud NM, de Waal Malefijt MC, Veth RP, Buma P, Schreuder HW. Survival of massive allografts in segmental oncological bone defect reconstructions. Int Orthop. 2009;33:757–760. doi: 10.1007/s00264-008-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carneiro R, Malinin T. Vascularized bone allografts: an experimental study in dogs. J Reconstr Microsurg. 1991;7:101–103. doi: 10.1055/s-2007-1006767. [DOI] [PubMed] [Google Scholar]
- 6.Davy DT. Biomechanical issues in bone transplantation. Orthop Clin North Am. 1999;30:553–563. doi: 10.1016/S0030-5898(05)70108-5. [DOI] [PubMed] [Google Scholar]
- 7.Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: what they can offer and what they cannot. J Bone Joint Surg Br. 2007;89:574–579. doi: 10.1302/0301-620X.89B5.19039. [DOI] [PubMed] [Google Scholar]
- 8.Donati D, Di Bella C, Col Angeli M, Bianchi G, Mercuri M. The use of massive bone allografts in bone tumour surgery of the limb. Curr Orthop. 2005;19:393–399. doi: 10.1016/j.cuor.2005.08.001. [DOI] [Google Scholar]
- 9.Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002;13:688–700. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- 10.Enneking WF, Campanacci DA. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg Am. 2001;83:971–986. [PubMed] [Google Scholar]
- 11.Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73:1123–1142. [PubMed] [Google Scholar]
- 12.Fox EJ, Hau MA, Gebhardt MC, Hornicek FJ, Tomford WW, Mankin HJ. Long-term followup of proximal femoral allografts. Clin Orthop Relat Res. 2002;397:106–113. doi: 10.1097/00003086-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Frost HM. Dynamics of bone remodeling. In: Frost HM, ed. Bone Biodynamics. Boston, MA: Little, Brown and Company; 1964:315–333.
- 14.Gouin F, Passuti N, Verriele V, Delecrin J, Bainvel JV. Histological features of large bone allografts. J Bone Joint Surg Br. 1996;78:38–41. [PubMed] [Google Scholar]
- 15.Hori Y, Tamai S, Okuda H, Sakamoto H, Takita T, Masuhara K. Blood vessel transplantation to bone. J Hand Surg Am. 1979;4:23–33. doi: 10.1016/S0363-5023(79)80101-X. [DOI] [PubMed] [Google Scholar]
- 16.Kasashima T, Minami A, Kato H, Kaneda K. Experimental study of vascularized bone grafts: hypertrophy of the grafted bone. J Reconstr Microsurg. 2000;16:121–128. doi: 10.1055/s-2000-7546. [DOI] [PubMed] [Google Scholar]
- 17.Kumta S, Yip K, Roy N, Lee SK, Leung PC. Revascularisation of bone allografts following vascular bundle implantation: an experimental study in rats. Arch Orthop Trauma Surg. 1996;115:206–210. doi: 10.1007/BF00434555. [DOI] [PubMed] [Google Scholar]
- 18.Mankin HJ, Doppelt S, Tomford W. Clinical experience with allograft implantation: the first ten years. Clin Orthop Relat Res. 1983;174:69–86. [PubMed] [Google Scholar]
- 19.Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005;432:210–216. doi: 10.1097/01.blo.0000150371.77314.52. [DOI] [PubMed] [Google Scholar]
- 20.McBroom RJ, Cheal EJ, Hayes WC. Strength reductions from metastatic cortical defects in long bones. J Orthop Res. 1988;6:369–378. doi: 10.1002/jor.1100060308. [DOI] [PubMed] [Google Scholar]
- 21.Mills LA, Simpson AH. In vivo models of bone repair. J Bone Joint Surg Br. 2012;94:865–874. doi: 10.1302/0301-620X.94B7.27370. [DOI] [PubMed] [Google Scholar]
- 22.Pelker RR, McKay J, Jr, Troiano N, Panjabi MM, Friedlaender GE. Allograft incorporation: a biomechanical evaluation in a rat model. J Orthop Res. 1989;7:585–589. doi: 10.1002/jor.1100070417. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson S, Emery SE, Goldberg VM. Factors affecting bone graft incorporation. Clin Orthop Relat Res. 1996;324:66–74. doi: 10.1097/00003086-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson S, Horowitz M. The response to bone allografts. J Bone Joint Surg Am. 1992;74:939–950. [PubMed] [Google Scholar]
- 25.Tamai S. Experimental vascularized bone transplantations. Microsurgery. 1995;16:179–185. doi: 10.1002/micr.1920160404. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;435:36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 27.Willems WF, Kremer T, Friedrich P, Bishop AT. Surgical revascularization induces angiogenesis in orthotopic bone allograft. Clin Orthop Relat Res. 2012;470:2496–2502. doi: 10.1007/s11999-012-2442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


