Abstract
Purpose of the review
This article provides an overview of the latest advances on in vitro modeling of inherited cardiomyopathies using human induced pluripotent stem cells (iPSCs).
Recent findings
Inherited cardiomyopathies have been recently modeled by generating iPSCs from patients harboring mutations in genes associated with the pathogenesis of HCM, DCM, and ARVC/D.
Summary
Patient-specific iPSCs and their differentiated cardiomyocytes (iPSC-CMs) now provide a novel model to study the underlying molecular mechanism of the pathogenesis of familial cardiomyopathies as well as for in vitro drug screening and drug discovery.
Keywords: induced pluripotent stem cells, inherited cardiomyopathy, hypertrophic cardiomyopathy, dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy
Introduction
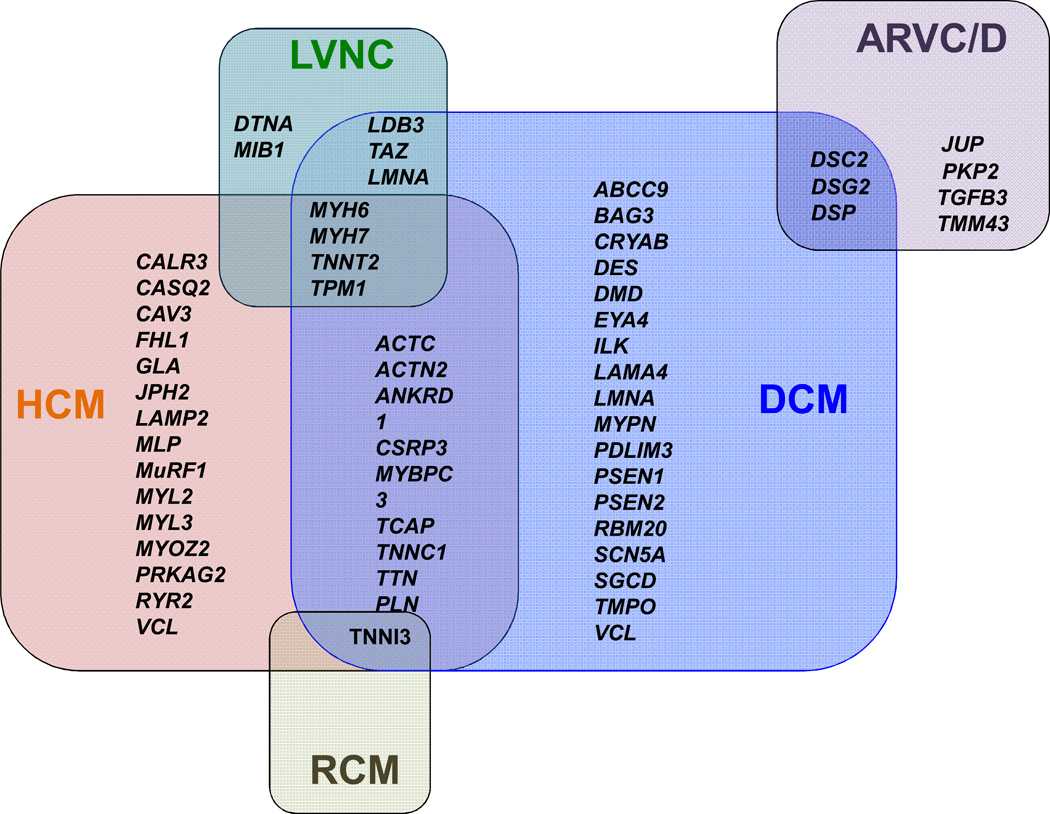
Inherited cardiomyopathies are a major cause of morbidity and mortality. In recent years, exciting progress has been made in defining the etiology of inherited cardiomyopathies, including hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), left ventricular non-compaction cardiomyopathy (LVNC), and arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) [1]. To date, numerous mutations in more than 50 genes that are implicated in the pathogenesis of inherited cardiomyopathies have been discovered [2] (Figure 1). The clinical course of these disease-causing mutations is remarkably variable, ranging from early severe phenotype and sudden cardiac death in adolescents to lifelong, asymptomatic, mutation-carrier status. Although the molecular analysis efforts have revealed important insights regarding the role of genetics in cardiomyopathies, the underlying mechanisms of HCM, DCM, LVNC, and ARVC/D remain unclear. An improved understanding of the pathological mechanisms involved in inherited cardiomyopathies could potentially lead to novel therapies.
Figure 1. The genetic basis of inherited cardiomyopathies.
Mutations in more than 50 genes have been associated with inherited cardiomyopathies, characterized by significant overlap. HCM, hypertrophic cardiomyopathy; DCM, dilated cardiomyopathy; LVNC, left ventricular non-compaction cardiomyopathy; ARVC/D, arrhythmogenic right ventricular cardiomyopathy/dysplasia.
The emergence of human pluripotent stem cells (PSCs), which include both human embryonic stem cells (ESCs) [3] and human induced pluripotent stem cells (iPSCs) [4], and the rapidly advancing technology and differentiation protocols associated with them, have made it possible to quickly interrogate the effects of genetic modifications. The ability to generate patient-specific iPSCs provides new opportunities to further understand disease pathogenesis and to develop new therapies. In this review, we will summarize recent advances in iPSC technology to study the molecular mechanisms underlying the pathogenesis of inherited cardiomyopathies.
Familial Hypertrophic Cardiomyopathy
Familial hypertrophic cardiomyopathy (HCM) is the most common monogenically inherited form of heart disease, predominantly caused by mutations in sarcomere protein genes [5]. Interestingly, most of these genes encode sarcomeric proteins, such as cardiac muscle β-myosin heavy chain (MYH7), cardiac muscle troponin T (TNNT2), and cardiac myosin-binding protein C (MYBPC3). However, the molecular events that link the sarcomere mutation to the development of the clinical phenotype of HCM are still unclear. In a recent study by Lan et. al. [6]*, human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) were generated from a family carrying an autosomal dominant missense mutation on exon 18 of the MYH7 gene that encodes for an Arginine-to-Histidine substitution at amino acid position 663 (Arg663His) [6]. The study showed for the first time that iPSC-CMs can recapitulate the HCM disease phenotype at the single-cell level, including cellular hypertrophy, calcineurin/nuclear factor of activated T-cells (NFAT) activation, upregulation of hypertrophic transcription factors, and arrhythmia. The study also demonstrated that elevation in intracellular calcium ([Ca2+]i) preceded the presentation of other phenotypic abnormalities, suggesting that dysregulation of Ca2+ cycling is a central mechanism for pathogenesis of the disease. Importantly, cellular hypertrophy and Ca2+ cycling abnormalities can be prevented with calcium channel blockers such as verapamil, diltiazem, and nifedipine. These findings validated iPSC technology as a novel method to understand how sarcomeric mutations can cause the development of cardiac hypertrophy and arrhythmia, and to potentially identify new therapeutic targets for the disease. Furthermore, while iPSCs can be used to complement existing zebrafish [7] and mouse [8] models of hypertrophic cardiomyopathy, the main advantage here is that beating human heart cells are being studied directly.
Familial Dilated Cardiomyopathy (DCM)
Dilated cardiomyopathy (DCM) is a clinical disease entity defined by left ventricular dilatation and impaired systolic function. DCM is composed of a heterogeneous group of diseases with different etiologies, with familial disease being responsible for one third to half of the cases [9]. Familial DCM is a leading cause of heart failure and is caused by mutations in at least 40 different individual genes of diverse ontologies. DCM was first modeled by Sun et al. using iPSCs from patients carrying a heterozygous mutation in the cardiac troponin T (TNNT2), namely p.R173W [10]*. In this model, an increased heterogeneous sarcomeric organization and a pronounced punctate distribution of sarcomeric α-actinin were observed. Individual DCM iPSC-CMs also exhibited altered Ca2+ handling compared to iPSC-CMs from control individuals. In addition, β-adrenergic stimulation (with norepinephrine) increased the number of DCM iPSC–CMs with abnormal sarcomeric α-actinin distribution, compromised cellular contractility, and induced failure of spontaneous contraction.
Patient-specific DCM iPSC lines have also been generated from a patient with a novel heterozygous mutation of p.A285V codon conversion on exon 4 of the desmin (DES) [11], as well as from an autosomal dominant nonsense mutation (p.R225X) in exon 4 of the lamin A/C (LMNA) gene found in a Chinese family with familial DCM spanning 3 generations [12]. The DES mutation was associated with diffuse abnormal DES aggregations in DCM iPSC-CMs [11], whereas the LMNA mutation was shown to accelerate nuclear senescence and apoptosis of iPSC-CMs under electrical stimulation, which was rescued by therapeutic blockade of stress-related ERK1/2 pathway [12]. Taken together, these studies demonstrated that iPSC-CM recapitulated certain aspects of the DCM phenotype in vitro, providing a proof-of-concept that iPSCs can used as a model to study DCM pathogenesis.
Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D)
ARVC/D is a predominantly genetically determined and heritable form of cardiomyopathy that is characterized pathologically by the replacement of cardiomyocytes by fibrous or fibro-fatty tissue mostly in the right ventricle, and clinically by elevated risks of arrhythmia and sudden cardiac death [13]. Several mutations in several genes encoding components of the cardiac desmosome genes have been linked to ARVC/D, including junction plakoglobin (JUP), desmoplakin (DSP), plakophilin-2 (PKP-2), and desmoglein-2 (DSG-2). Three separate studies have examined the effect of mutations in PKP2 gene in a patient-specific iPSC model of ARVC/D. Kim et al. [14] generated iPSCs from two patients with clinical ARVC/D carrying either a homozygous (c.2484C>T) or a heterozygous (c.2013delC) mutation in the PKP2 gene. Although the investigators observed an abnormal nuclear translocation of junction plakoglobin proteins and very low β-catenin activity in mutant iPSC-CMs from both patients, the disease phenotype was not fully recapitulated in standard culture conditions. In subsequent experiments, the authors discovered that the induction of an adult-like metabolism using an adipogenic cocktail that co-activates the normal PPAR-alpha-dependent metabolism and abnormal PPAR-γ pathway was imperative to induce the classical signs of ARVC/D, such as intracellular lipid accumulation in iPSC-CMs. These findings suggested that metabolic abnormalities could play a central role in the pathogenesis of ARVC/D. Importantly, this study demonstrated that induction of adult-like metabolism is essential in establishing an adult-onset disease model using patient-specific iPSC-CMs.
The ARVD/C was also modeled by generating iPSCs from patients harboring two novel mutation in the PKP2 gene, namely c.1841T>C [15]* and c.972InsT/N [16], as well as a known mutation on the same gene (c.148_151delACAG/N) [16]. All three models showed the mutant iPSC-CMs were prone to lipid accumulation following treatment with various adipogenic stimuli, displaying a functional pro-adipogenic state that is considered to be one of the hallmarks of ARVC/D. In the study by Gepstein and colleagues [16], the authors observed that intracellular lipid droplet accumulation in mutant iPSC-CMs was also shown to be correlated with the degree of the desmosomal abnormalities within the same cell, suggesting a causal link between desmosomal malfunction and lipid accumulation in ARVC/D. Interestingly, the small molecule BIO, a specific inhibitor of GSK-3b, could prevent the effect of the adipogenic stimuli on the mutant cardiomyocytes, implying a possible role of the canonical Wnt pathway in the ARVC/D pathogenesis. Collectively, these studies underscore the unique potential of the iPSC technology for modeling ARVC/D in vitro. The iPSCs-CMs that were derived from ARVC/D patients recapitulated, to some extent, the ARVC/D phenotype in the dish, and provided mechanistic insights into early disease pathogenesis.
Left Ventricular Non-Compaction (LVNC) and Restrictive Cardiomyopathy (RCM)
To date, iPSC models of LVNC and RCM have not been reported, although several laboratories are also working actively in these diseases
Using Patient Specific Stem Cell–Derived Cardiomyocytes to Study Proarrhythmia
Cardiac toxicity is a leading cause of regulatory delay in approval and market withdrawal of new pharmaceuticals [17]. Drug-induced blocking of human Ether-à-go-go Related Gene (hERG) potassium channel, which conducts the rapid component of the delayed rectifier potassium current (IKr), can induce ventricular tachyarrhythmia in the heart known as Torsade de Pointes (TdP) [18]. To minimize cardiac risks associated with drug development, both the pharmaceutical industry and U.S. Food and Drug Administration (FDA) have mandated in vitro cardiotoxicity screening early in new chemical entities (NCE) development. Given that the majority of adverse cardiac drug reactions occur in patients with hereditary cardiac disease who are vulnerable to fatal arrhythmias, the recent advances in efficient generation of iPSC-CMs [19] have important implications for drug toxicity screening. Human iPSC technology holds the potential to serve as a human-based model that reflects a variety of genetic backgrounds for both drug development and cardiotoxicity screening. Liang et al. recently assessed the susceptibility of patients from different genetic backgrounds to drug-induced cardiotoxicity in a library of human iPSC-CMs derived from patients with common hereditary cardiac disorders such as familial HCM, familial DCM, and long QT syndrome (LQTS; KCNQ1 mutation) [20]*. Not surprisingly, healthy and diseased individuals exhibited different susceptibilities to cardiotoxic drugs (see Table 1 for the complete list the drugs studied), which were more accurately modeled in iPSC-CMs than the standard cardiotoxicity testing currently performed using Chinese hamster ovary (CHO) or human embryonic kidney (HEK) cells overexpressing hERG. In agreement with these findings, another study suggested that LQTS2 patients (HERG mutation) are at an increased risk of developing excessive QT prolongation and potentially TdP when exposed to blocking drugs, as indicated by an iPSC-CM based assay [21]. Finally, iPSC-CMs have been used in conjunction with the higher throughput multielectrode array (MEA) platform for screening a panel of cardiovascular drugs such as quinidine, sotalol, verapamil, nifedipine, etc. [22].
Table 1.
Recent publications on iPSC disease models of inherited cardiomyopathies
| Disease | Gene mutation | Proband’s phenotype | iPSC-CM phenotype | Drug testing | Ref |
|---|---|---|---|---|---|
| DCM |
TNNT2 p. R173W |
Ventricular dilatation, systolic dysfunction | Altered Ca2+ regulation, reduced contractility, myofibril disarray | Metoprolol, SERCA2a overexpression, norepinephrine Cisapride, Nicorandil, Alfuzosin, Verapamil |
[10, 20] |
|
DES p. A285V |
Ventricular dilatation, impaired left ventricular ejection fraction (LVEF), ventricular tachycardia | Diffuse abnormal DES aggregations | N/A | [11] | |
|
LMNA p. R225X |
Dilated right and left ventricles, impaired LVEF, atrial fibrillation, atrioventricular block | Nuclear senescence and cellular apoptosis | MEK1/2 inhibitors | [12] | |
| HCM |
MYH7 p. R663H |
Concentric left ventricular hypertrophy (LVH) with prominent thickening of the inferior septum and inferior wall | Cellular hypertrophy, disorganization of sarcomeres, calcineurin activation, nuclear translocation of NFAT, contractile arrhythmia and DADs, Ca2+ cycling dysfunction | Quinidine, Procainamide, Lidocaine, Mexiletine, Ranolazine, Flecainide, Propafenone, Propranolol, Metoprolol, Amiodarone, Sotalol, Dofetilide, Verapamil, Diltiazem, Nifedipine, Cisapride, Nicorandil, Alfuzosin | [6, 20] |
| ARVC/D |
PKP2 c.2484C>T c.2013delC |
Severe right ventricular (RV) dilatation and reduced RV ejection fraction with no left ventricular impairment | Abnormal plakoglobin nuclear translocation and decreased β-catenin activity, exaggerated lipogenesis and apoptosis upon adipogenic stimulus, Ca2+ handling deficits | Overexpression of WT PKP2, PPAR-γ antagonists | [14] |
|
PKP2 c.1841T>C |
Dilated right ventricle with regional akinesia and reduced ejection fraction, normal left ventricular size and function | Decrease expression of PKP2 protein, increased lipid accumulation upon adipogenic stimulus | N/A | [15] | |
|
PKP2 c.972InsT/N c.148_151delACAG/N |
Dilated and trabeculated RV with reduced global RV function, ventricular tachycardia | Reduction in the gene expression levels of PKP2, desmosomal abnormalities, accumulation of lipid-droplets, upregulation of PPAR-γ | GSK-3β inhibitor | [16] | |
| LVNC | N/A | N/A | N/A | N/A | N/A |
| RCM | N/A | N/A | N/A | N/A | N/A |
Anticipated Advances in the Next Decade
The results from recent studies reviewed here provide proof-of-principle that iPSC-based disease models can generally be expected to reproduce the disease phenotype. These novel iPSC models of inherited cardiomyopathies are being rapidly implemented for disease research and drug toxicity screening, offering new opportunities for therapeutic intervention. However, obtaining cells in sufficient quantities with consistent quality at high purity is one of the hurdles that need to be addressed in order to broaden the applicability of these models. We anticipate that robust protocols will be developed in the next few years that reproducibly yield human iPSC-CMs with more adult phenotype, making them more amendable to large-scale production [19]. In addition, further refinement of differentiation protocols could lead to the generation of ESC- or iPSC-derived cardiac subtypes such as atrial, nodal, or ventricular cells [23].
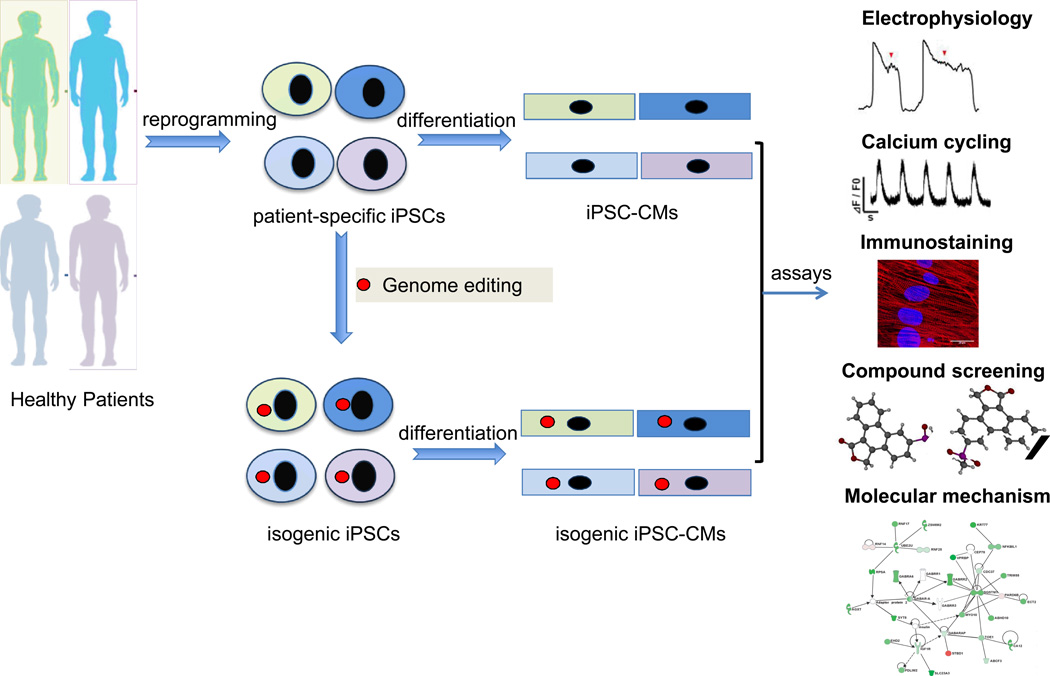
Furthermore, the recent emergence of genome editing technologies in combination with iPSC technology could make it feasible to rapidly interrogate the effects of genetic modification in isogenic human model systems [24]. Genome editing by engineered nucleases is a promising approach that could be readily applied to patient-specific iPSC lines to correct or induce mutation-specific phenotypes in iPSC models. The ideal study design will probably involve the insertion of an inherited cardiomyopathy-related mutation into wild-type iPSC lines from diverse genetic backgrounds, which will allow investigators to test for causal effects of different mutations without the need for patient recruitment (Figure 2). In the long run, the creation of engineered human iPSC disease-in–a-dish models will enhance our understanding of underlying mechanisms related to inherited cardiomyopathies and provide a novel platform for patient-specific therapeutics.
Figure 2. Studying disease-causing gene variants by combining nuclease-mediated gene engineering and iPSC technologies.
The genetic variability among human iPSCs can influence the outcome in cardiomyopathy modeling experiments. Nuclease-mediated gene targeting could in principle eliminate the variation arising from the iPSC line derivation and the genetic background. Isogenic iPSC lines carrying specific mutations can be generated using well-characterized parental iPSC lines derived from healthy patients of diverse genetic backgrounds. Generation of isogenic iPSC-CMs can be used to establish whether the observed phenotype is the direct result of the disease-causing variant independently of the genetic background noise. Functional assays can be performed to identify disease-related mechanisms. Similarly, this approach can be utilized in compound screening assays in order to determine the susceptibility of the genetic variant in question to drug-induced cardiac toxicity and arrhythmias. iPSCs, induced pluripotent stem cells; iPSC-CMs, induced pluripotent stem cell-derived cardiomyocytes.
Conclusion
The literature published in recent years demonstrates the growing importance of patient-specific iPSCs in disease modeling of inherited cardiomyopathies (Table 1). The identification of mutations in patients with inherited cardiomyopathies has revealed substantial molecular complexity in the etiologies of these disorders. Despite their complexity, the recent advancement of iPSC-based disease modeling in vitro has provided a human-based platform to improve our understanding of familial cardiomyopathies diseases. Follow-up research is expected to yield additional insights that will lead to new medical approaches to their treatment, but considerable work remains to be done.
Key points.
HCM, DCM and ARVC/D have been recently modeled by patient-specific iPSC-CMs
iPSC-CMs provide a novel human model to study the underlying molecular mechanism of the pathogenesis of inherited cardiomyopathies
iPSC-CMs offer a human-based platform for in vitro drug screening and drug discovery
Acknowledgement
We gratefully acknowledge funding support from NIH K99 HL104002 (IK), Leducq Foundation, AHA 13EIA14420025, NIH R01 HL113006, and NIH U01 HL099776 (JCW).
Footnotes
Conflict of interest
None
References
- 1.Watkins H, Ashrafian H, Redwood C. Inherited Cardiomyopathies. N Engl J Med. 2011 Apr 28;364(17):1643–1656. doi: 10.1056/NEJMra0902923. [DOI] [PubMed] [Google Scholar]
- 2.Hershberger RE, Cowan J, Morales A, et al. Progress with Genetic Cardiomyopathies: Screening, Counseling, and Testing in Dilated, Hypertrophic, and Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circ Heart Fail. 2009 May;2(3):253–261. doi: 10.1161/CIRCHEARTFAILURE.108.817346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science. 1998 Nov 6;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007 Nov 30;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Frey N, Luedde M, Katus HA. Mechanisms of Disease: Hypertrophic Cardiomyopathy. Nat Rev Cardiol. 2012 Feb;9(2):91–100. doi: 10.1038/nrcardio.2011.159. [DOI] [PubMed] [Google Scholar]
- 6. Lan F, Lee AS, Liang P, et al. Abnormal Calcium Handling Properties Underlie Familial Hypertrophic Cardiomyopathy Pathology in Patient-Specific Induced Pluripotent Stem Cells. Cell Stem Cell. 2013 Jan 3;12(1):101–113. doi: 10.1016/j.stem.2012.10.010. This is the first article to demonstrate that HCM can be modeled in iPSCs
- 7.Becker JR, Deo RC, Werdich AA, et al. Human Cardiomyopathy Mutations Induce Myocyte Hyperplasia and Activate Hypertrophic Pathways During Cardiogenesis in Zebrafish. Dis Model Mech. 2011 May;4(3):400–410. doi: 10.1242/dmm.006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geisterfer-Lowrance AA, Christe M, Conner DA, et al. A Mouse Model of Familial Hypertrophic Cardiomyopathy. Science. 1996 May 3;272(5262):731–734. doi: 10.1126/science.272.5262.731. [DOI] [PubMed] [Google Scholar]
- 9.Hershberger RE, Hedges DJ, Morales A. Dilated Cardiomyopathy: The Complexity of a Diverse Genetic Architecture. Nat Rev Cardiol. 2013 Sep;10(9):531–547. doi: 10.1038/nrcardio.2013.105. [DOI] [PubMed] [Google Scholar]
- 10. Sun N, Yazawa M, Liu J, et al. Patient-Specific Induced Pluripotent Stem Cells as a Model for Familial Dilated Cardiomyopathy. Sci Transl Med. 2012 Apr 18;4(130):130ra47. doi: 10.1126/scitranslmed.3003552. This is the first article to demonstrate that DCM can be modeled in iPSCs
- 11.Tse HF, Ho JC, Choi SW, et al. Patient-Specific Induced-Pluripotent Stem Cells-Derived Cardiomyocytes Recapitulate the Pathogenic Phenotypes of Dilated Cardiomyopathy Due to a Novel Des Mutation Identified by Whole Exome Sequencing. Hum Mol Genet. 2013 Apr 1;22(7):1395–1403. doi: 10.1093/hmg/dds556. [DOI] [PubMed] [Google Scholar]
- 12.Siu CW, Lee YK, Ho JC, et al. Modeling of Lamin a/C Mutation Premature Cardiac Aging Using Patient-Specific Induced Pluripotent Stem Cells. Aging (Albany NY) 2012 Nov;4(11):803–822. doi: 10.18632/aging.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Awad MM, Calkins H, Judge DP. Mechanisms of Disease: Molecular Genetics of Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5(5):258–267. doi: 10.1038/ncpcardio1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim C, Wong J, Wen J, et al. Studying Arrhythmogenic Right Ventricular Dysplasia with Patient-Specific IPSCs. Nature. 2013 Feb 7;494(7435):105–110. doi: 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma D, Wei H, Lu J, et al. Generation of Patient-Specific Induced Pluripotent Stem Cell-Derived Cardiomyocytes as a Cellular Model of Arrhythmogenic Right Ventricular Cardiomyopathy. Eur Heart J. 2013 Apr;34(15):1122–1133. doi: 10.1093/eurheartj/ehs226. This is the first article to demonstrate that ARVC/D can be modeled in iPSCs
- 16.Caspi O, Huber I, Gepstein A, et al. Modeling of Arrhythmogenic Right Ventricular Cardiomyopathy with Human Induced Pluripotent Stem Cells. Circ Cardiovasc Genet. 2013 Nov 7; doi: 10.1161/CIRCGENETICS.113.000188. [DOI] [PubMed] [Google Scholar]
- 17.Mordwinkin NM, Lee AS, Wu JC. Patient-Specific Stem Cells and Cardiovascular Drug Discovery. JAMA. 2013 Nov 20;310(19):2039–2040. doi: 10.1001/jama.2013.282409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sauer AJ, Newton-Cheh C. Clinical and Genetic Determinants of Torsade De Pointes Risk. Circulation. 2012 Apr 3;125(13):1684–1694. doi: 10.1161/CIRCULATIONAHA.111.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burridge PW, Keller G, Gold JD, et al. Production of De Novo Cardiomyocytes: Human Pluripotent Stem Cell Differentiation and Direct Reprogramming. Cell Stem Cell. 2012 Jan 6;10(1):16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liang P, Lan F, Lee AS, et al. Drug Screening Using a Library of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Reveals Disease-Specific Patterns of Cardiotoxicity. Circulation. 2013 Apr 23;127(16):1677–1691. doi: 10.1161/CIRCULATIONAHA.113.001883. This is the first article to demonstrate that susceptibilities to cardiotoxic drugs can be accurately modeled in iPSC-CMs
- 21.Braam SR, Tertoolen L, Casini S, et al. Repolarization Reserve Determines Drug Responses in Human Pluripotent Stem Cell Derived Cardiomyocytes. Stem Cell Res. 2013 Jan;10(1):48–56. doi: 10.1016/j.scr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Navarrete EG, Liang P, Lan F, et al. Screening Drug-Induced Arrhythmia Events Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Low-Impedance Microelectrode Arrays. Circulation. 2013 Sep 10;128(11) Suppl 1:S3–S13. doi: 10.1161/CIRCULATIONAHA.112.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karakikes I, Senyei GD, Hansen J, et al. Small Molecule-Mediated Directed Differentiation of Human Embryonic Stem Cells toward Ventricular Cardiomyocytes. Stem Cells Transl Med. 2013 Dec 9; doi: 10.5966/sctm.2013-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merkle FT, Eggan K. Modeling Human Disease with Pluripotent Stem Cells: From Genome Association to Function. Cell Stem Cell. 2013 Jun 6;12(6):656–668. doi: 10.1016/j.stem.2013.05.016. [DOI] [PubMed] [Google Scholar]