Abstract
Objective
Rapid Eye Movement (REM) sleep behavior disorder is often co-morbid with Parkinson's disease (PD). The current study aimed to provide a detailed understanding of the impact of having REM sleep behavior disorder on multiple NMS in patients with PD.
Methods
86 participants were evaluated for REM-sleep behavior disorder and assessed for multiple non-motor symptoms of PD. Principal component analysis was utilized to model multiple measures of non-motor symptoms in PD and a multivariate analysis of variance was used to assess the relationship between REM-sleep behavior disorder and the multiple non-motor symptoms measures. Seven non-motor symptoms measures were assessed: cognition, quality of life, fatigue, sleepiness, overall sleep, mood, and overall non-motor symptoms of PD.
Results
36 PD patients were classified as having REM-sleep behavior disorder (objective polysomnography and subjective findings), 26 as not having REM-sleep behavior disorder (neither objective nor subjective findings), and 24 as probable REM-sleep behavior disorder (either subjective or objective findings). REM-sleep behavior disorder was a significant predictor of increased non-motor symptoms in PD while controlling for dopaminergic therapy and age (p=0.01). The REM-sleep behavior disorder group reported more non-motor symptoms of depression (p=0.012), fatigue (p=0.036), overall sleep (p=0.018), and overall non-motor symptoms (p=0.002).
Conclusion
In PD, REM-sleep behavior disorder is associated with more non-motor symptoms, particularly increased depressive symptoms, sleep disturbances, and fatigue. More research is needed to assess whether PD patients with REM-sleep behavior disorder represent a subtype of PD with different disease progression and phenomenological presentation.
Keywords: Parkinson's disease, REM sleep Behavior Disorder, Non-motor Symptoms, Sleep Disorders, Quality of Life, Mood, Fatigue, Daytime Sleepiness
Introduction
Parkinson's disease (PD) is strongly associated with non-motor symptoms (NMS) including sleep disturbances, daytime sleepiness, dementia, fatigue, and depression [1]. In a large multicenter study, 99% of 1072 PD patients reported NMS [2]. NMS have a major negative impact on the lives of patients and on their families, contribute to the severe disability these patients experience, impair quality of life (QOL), and even shorten life expectancy [3]. Some studies have suggested that NMS are more significant than motor symptoms when assessing caregiver distress, institutionalization rates, QOL, and overall economics of PD [1, 3, 4].
Rapid-eye-movement (REM) sleep behavior disorder (RBD) is a parasomnia characterized by REM sleep without atonia (RSWA), i.e., abnormal increase of muscle activity during REM sleep that is evident by phasic and/or tonic muscle activity on the electromyogram (EMG) channel on the overnight polysomnograph (PSG) recording. This loss of muscle atonia is typically described by patients as “acting out” their dreams. A high percentage of idiopathic RBD patients eventually develop neurodegenerative disease [5], and RBD has been recognized as a strong predictor of the development of synucleinopathies, including PD [6, 7].
RBD has been reported to affect up to 58% of PD patients [8], yet few studies have systematically assessed the relationship between RBD and NMS in PD. In a recent study we reported that having PD and with multiple sleep disorders, was associated with increased NMS [9]. Those results showed that RBD is a significant predictor of increased NMS while controlling for other sleep disorders. The current study aimed to further assess and provide a more detailed understanding of the impact of having RBD on multiple NMS in patients with PD.
Methods
Participants
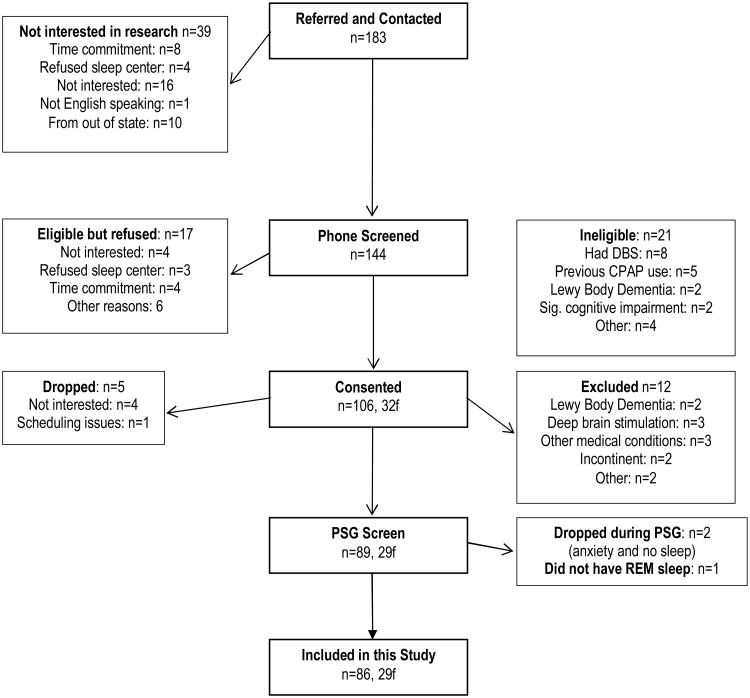
183 PD patients were referred to the study by neurologists at the University of California, San Diego (UCSD) or by San Diego County community neurologists, volunteered after hearing a talk at support group meetings or responded to flyers and advertisements (Figure 1). 106 patients met inclusion/exclusion criteria (Table 1), agreed to participate, and consented for this study. However, 7 patients dropped out prior to completing the overnight PSG, 12 were excluded (did not meet inclusion criteria after medical records were reviewed), and 1 patient did not have REM sleep. The remaining 86 patients, all of whom completed the overnight PSG evaluation, participated in this study. The study was approved by UCSD Human Research Protection Program and San Diego Veterans Administration Healthcare System. All patients met UK brain bank criteria for the diagnosis of PD.
Figure 1. Consort Table.
Table 1. Inclusion/Exclusion Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Study design
Consented patients received a brief cognitive assessment using the Montreal Cognitive Assessment (MoCA) [10]. Patients were admitted to the General Clinical Research Center (now Clinical and Translational Research Institute) Gillin Laboratory for Sleep and Chronobiology for an overnight PSG recording. During their visit, a neurologist (JCB) evaluated the patients with the Hoehn and Yahr [11] and the Unified Parkinson's Disease Rating Scale (UPDRS) [12], a physician trained in sleep medicine (JSL or JEM) conducted a history and physical including detailed sleep history, review of overall medical conditions, medication use, and completion of the REM Behavior Disorder Sleep Questionnaire (RBDSQ) [13]. All patients also completed a self-administered NMS questionnaire packet including: 1. Non-motor Symptoms Questionnaire (NMSQuest) [14] assessing non-motor features of PD (neuropsychiatric, autonomic, gastrointestinal, sensory and other disturbances); 2. Beck Depression Inventory- 2nd edition (BDI-II) [15] assessing depressive symptoms; 3. Epworth Sleepiness Scale (ESS) [16] assessing daytime sleepiness; 4. Short Form of the Multidimensional Fatigue Symptom Inventory (MFSI-SF) [17] evaluating fatigue on five subscales: General, Physical, Emotional, Mental, and Vigor; 5. Parkinson's Disease Sleep Scale (PDSS) [18, 19] assessing sleep complaints and nocturnal difficulties specific to PD; 6. Parkinson's Disease Questionnaire (PDQ-39) [20] evaluating QOL.
Polysomnographic evaluation
The first four participants had PSG recorded with Embla (Planegg, Gernamy). The remaining participants had PSG recorded with Compumedics Somté (Charlotte, NCUSA). Video-enabled PSG was available for 47% of the sample. Additionally, detailed clinical notes of nighttime behaviors (e.g., talking, yelling, excessive movement, going to bathroom) were maintained by the technician and recorded on the PSG record at the time of occurrence. Electroencephalography (F4, C4, O1 or O2), electrooculography (left and right outer canthus), submental EMG, respiratory effort (thoracic and abdominal piezoelectric bands), airflow (nasal pressure transducer), electocardiogram, oximetry and tibialis EMG were recorded.
Sleep recordings were scored by a scorer blinded to the clinical assessment, treatment condition, and questionnaire data. All PSG records were staged and scored according to accepted criteria [21] while allowing for RSWA in REM-sleep.
RBD scoring
Subjective
RBDSQ was used to assess clinical history of dream enactment behavior. This screening tool for RBD is based on the clinical criteria of the International Classification of Sleep Disorders, second edition (ICSD-II) [22] and is composed of 10 yes/no items with maximum score of 13. The RBDSQ was previously validated with a cutoff score of 5 exhibiting 96% sensitivity and 56% specificity. [13, 23]
Objective
Submental EMG was assessed for RSWA. RSWA scoring method was first developed by Lapierre and Montplaisir [24] and validated by Consens et al, [25] including scoring of tonic and phasic epochs. The American Academy of Sleep Medicine (AASM) guidelines also provide the scoring method of tonic and phasic activity [21]. This study followed the AASM guidelines, but integrated Lapierre and Montplaisir's and Consens et al.'s ideas [24, 25] and also accommodated to PSG software, i.e., in order to score tonic and phasic epochs, the mini-epoch length and reference activity amplitude were adjusted.
Tonic epoch was defined as a REM-sleep epoch with >50% of the duration exhibiting an increase in chin EMG amplitude that was at least 2× the non-REM baseline. On the other hand, the AASM guidelines define a tonic epoch as a REM-sleep epoch with >50% of the epoch's duration showing chin EMG amplitude greater than the minimum amplitude present in non-REM-sleep.
Phasic epoch was defined as at least 50% of the mini-epochs scored as phasic activity. Phasic activity was defined as transient bursts of muscle activity 0.1-5.0 seconds in duration with at least 4× the amplitude of background EMG-activity.
An EMGscore was calculated as the percent of tonic and phasic REM-sleep epochs over the total number of REM-sleep epochs. The EMGscore is similar to the RBD measure (called PSG-score) previously proposed by Consens et al. which established a cut-off score of 10% with a sensitivity of 89% and specificity of 57% [25]. A cut-off of 10% EMGscore was also used in this study.
Finally, patients were diagnosed with RBD as suggested by the ICSD-II.[22] Current criteria of confirmed-RBD according to the ICSD-II involves subjective clinical history with objective documentation of either RSWA or dream enactment behavior during an overnight PSG. Therefore, patients were classified into groups based on subjective (RBDSQ) and objective (EMGscore) measures: Yes-RBD group (yRBD; n=36; RBDSQ≥5 and EMGscore≥10% or having video- and/or technician-recorded evidence of motor behaviors during REM sleep) or No-RBD group (nRBD; n=26; RBDSQ<5 and EMGscore<10). Patients not falling into either the yRBD or nRBD groups were not included in the primary analyses as they may be fundamentally different from the yRBD and nRBD groups; however, these patients (called probable RBD, pRBD; n=24; RBDSQ ≥5 or EMGscore≥10%) were included in secondary analyses for exploratory purposes.
Medications
All patients were assessed for medication use (type, dose, frequency, time, reason, and duration of use). As dopaminergic therapy regimen highly differs between patients, and to allow comparisons among patients on different dopaminergic regimens, drug dosages were converted to Levodopa Dosage Equivalents (LDE) according to the formula provided by Tomlinson et al. [26]
Data analysis
Given that seven NMS variables (e.g., mood, sleepiness, sleep dysfunction, fatigue, cognition, overall NMS, and QOL) were under consideration and to avoid multiple comparisons, principal component analysis (PCA) was utilized to derive component/factor scores for the NMS of PD. This was accomplished by factoring the relationships among the multiple observed variables designed to assess the seven different NMS [27]. The PCA was conducted utilizing the correlation matrix with a varimax rotation. The first principal component was derived, which by construction captured the maximum variability in the NMS variables. An analysis of variance (ANOVA) was used to assess the relationship between the NMS factor (i.e., first principal component) and the presence of RBD while controlling for age and dopaminergic therapy (LDE). A significant model showing RBD status as a significant predictor (p<0.05) would serve as an omnibus test of any association between RBD status and NMS variables and would suggest that post-hoc analysis of this relationship would be appropriate.
To extract a subset of NMS variables for further analysis, a multivariate ANOVA (MANOVA) was used to assess the relationship between RBD groups (yRBD vs. nRBD) and the seven NMS measures while controlling for age and dopaminergic therapy. A significant overall model would further indicate a relationship between RBD and NMS. Furthermore, a significant relationship (p<0.05) between RBD status and the individual measures was used to guide post-hoc analyses of individual variables. Post-hoc analyses were conducted using independent-sample t-tests. No Type I error protection was utilized as these tests were exploratory.
Finally, MANOVA was used for exploratory analysis, which included the pRBD group to examine possible differences between the pRBD vs. yRBD or nRBD. These exploratory analyses were intended to examine the clinical utility of this classification.
All analyses were executed using SPSS (version 17.0, SPSS, Chicago, IL).
Results
A total of 86 PD patients (mean age=67.2±9.4 years; Range: 47-89, 29f) participated in the study and were evaluated for RBD (Figure 1). The majority of participants were Caucasian (90%), married (73%), and had at minimum an undergraduate degree (70%). There were no significant differences between yRBD and nRBD in any of the demographic variables except UPDRS total score (p=0.017), UPDRS part 1 (p<0.001) and part 2 (p=0.02), and antidepressant use (p=0.05) (Table 2).
Table 2. Demographics.
| Variable | Total | nRBD | yRBD |
|---|---|---|---|
|
| |||
| N | 86 | 26 | 36 |
|
| |||
| Age: mean years (SD) | 67.4 (8.8) | 68.38 (10.0) | 67.25 (7.3) |
|
| |||
| Gender: n (%) | |||
| Male | 57 (66.3) | 18 (69.2) | 25 (69.4) |
| Female | 29 (33.7) | 8 (30.8) | 11 (30.6) |
|
| |||
| UPDRS: mean (SD) | |||
| Part 1 | 3.1 (2.3) | 1.5 (1.3) | 4.1 (2.7) |
| Part 2 | 11.0 (4.9) | 9 (3.5) | 11.8 (5.0) |
| Part 3 | 18.5 (7.5) | 16.9 (6.7) | 19.4 (7.5) |
| Part 4 | 3.5 (2.3) | 3.3 (2.2) | 3.5 (2.4) |
| Total | 36.1 (13.2) | 30.7 (10.5) | 38.7 (13.3) |
| Missing: n | 6 | 2 | 2 |
|
| |||
| Hoehn & Yahr: n (%) | |||
| Stage I | 24 (27.9) | 7 (26.9) | 10 (27.8) |
| Stage II | 44 (51.1) | 13 (50.0) | 19 (52.8) |
| Stage III | 12 (14.0) | 4 (15.4) | 5 (13.9) |
| Missing | 6 (7.0) | 2 (7.7) | 2 (5.6) |
|
| |||
| PD duration: mean years (SD) | 6.3 (5.3) | 5.8 (4.0) | 6.9 (6.4) |
|
| |||
| Medications: | |||
| LDE: mean (SD) | 853.0 (592.1) | 845.2 (572.1) | 828.9 (618.9) |
| Benzodiazepine: n (%) | 9 (10.5) | 0 | 6 (16.7) |
| Antidepressants: n (%) | 24 (27.9) | 4 (15.4) | 9 (25.0) |
|
| |||
| AHI: mean (SD) | 14.1 (13.8) | 13.4 (10.7) | 10.25 (10.9) |
|
| |||
| PLMI: mean (SD) | 21.0 (25.4) | 13.67 (20.2) | 25.2 (27.1) |
|
| |||
| TST: mean min (SD) | 349.2 (73.3) | 336.39 (87.1) | 358.82 (63.8) |
|
| |||
| REM-sleep percent: mean % (SD) | 11.7 (7.1) | 11.76 (5.9) | 13.05 (7.9) |
|
| |||
| Total REM Time: mean min (SD) | 43.1 (29.5) | 42.44 (26.2) | 48.43 (31.4) |
|
| |||
| EMGsocre: mean % (SD) | 12.8 (12.2) | 4.33 (2.1) | 19.62 (11.1) |
|
| |||
| RBDSQ: mean (SD) | 5.9 (3.5) | 2.35 (1.1) | 8.08 (2.7) |
RBD, REM-sleep behavior disorder; nRBD, no RBD; yRBD, yes RBD; AHI, Apnea hypopnea index; PLMI, periodic limb movement index; TST, total sleep time; REM, rapid eye movement; RBDSQ, REM behavior disorder screening questionnaire. RBD, REM-sleep behavior disorder; UPDRS, Unified Parkinson's Disease Rating Scale;
Omnibus testing of RBD and NMS
The PCA resulted in a single component that explained 54% of the variance. The remaining components explained 14% or less of the variability in the set of NMS variables. A regression model with NMS factor (i.e. the first principle component) as the dependent variable and LDE, age, and RBD status as independent variables was significant (R2=0.29, F3,61=7.9, p<0.001). All variables included in this model were significant predictors of the NMS factor score [Age (β=-0.26, p=0.021), LDE (β=0.34, p=0.003), and RBD status (β=0.31, p=0.008)].
The MANOVA which modeled the relationship between the multiple NMS measures and RBD status (yRBD vs. nRBD) showed that RBD was a significant predictor of NMS (p=0.05) while controlling for age (p=0.001) and LDE (p=0.03). This analysis further revealed that in this model controlling for age and LDE, having RBD was a significant predictor for PDSS (R2=0.27, F3,61=5.74, p=0.02), BDI-II (R2=0.22, F3,61=8.48, p=0.005), NMSQuest (R2=0.25, F3,61=11.1, p=0.002), and MFSI-SF (R2=0.23, F3,61=4.58, p=0.037). RBD was not a significant predictor for PDQ-39 (p=0.25), MoCA (p=0.67), or ESS (p=0.66).
NMS Assessment
yRBD, compared to nRBD, endorsed significantly more NMS on the NMSQuest (yRBD=13.1±5.0 vs. nRBD=9.2±4.4, t=-3.21, p=0.002) (Figure 2). The NMSQuest includes 2 questions regarding RBD symptoms. Omitting these questions from the analyses did not significantly change the results (yRBD=11.9±4.7 vs. nRBD=9.3±4.4, t=-2.14, p=0.037). Frequency of positive answers on the NMSQuest is provided in Table 3. Exploratory post-hoc analyses revealed that, as expected, the yRBD patients more commonly endorsed the questions associated with RBD (both p<0.001). Additionally, yRBD patients reported more changes in taste and smell (p=0.039), unexplained changes in weight (p=0.03), and feeling light headed, dizzy or weak when standing from a sitting or lying position (p=0.004).
Figure 2.
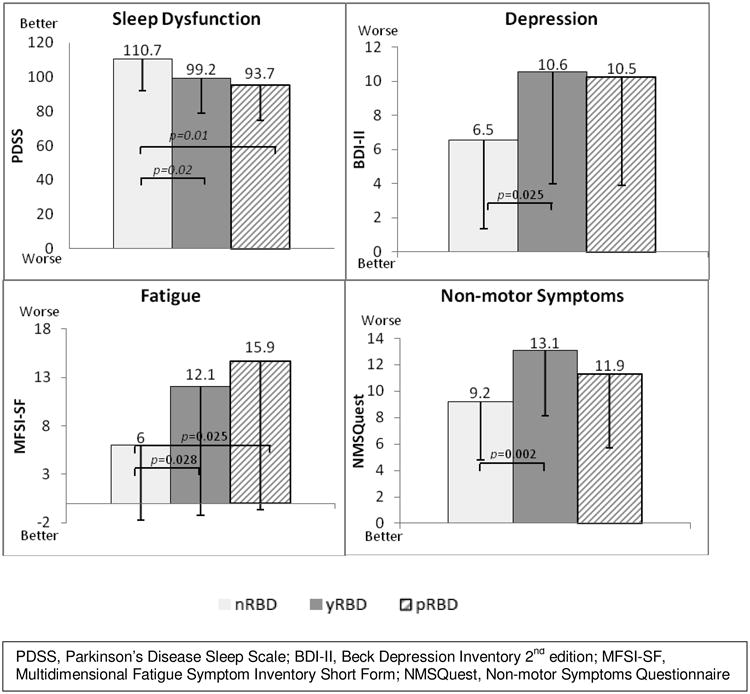
Mood, sleep, fatigue and overall NMS Differences between PD patients without RBD (nRBD), with RBD (yRBD), or probable RBD (pRBD).
Table 3. Frequency of positive answers on the NMSQuest (30-items) in our total Parkinson's disease sample and in Parkinson's disease patients with RBD (yRBD) and with no RBD (nRBD).
| Question | Total (N=86) |
nRBD (n=26) |
yRBD (n=36) |
p | |
|---|---|---|---|---|---|
| 1. | Dribbling | 51 (59.3) | 16 (61.5) | 22 (61.1) | 0.97 |
| 2. | Taste/smell | 36 (41.9) | 7 (26.9) | 19 (52.8) | 0.04 |
| 3. | Swallowing | 28 (32.6) | 8 (30.8) | 12 (33.3) | 0.83 |
| 4. | Vomiting | 16 (18.6) | 1 (3.8) | 6 (16.7) | 0.12 |
| 5. | Constipation | 50 (58.1) | 13 (50.0) | 26 (72.2) | 0.07 |
| 6. | Bowel incontinence | 8 (9.3) | 2 (7.7) | 4 (11.1) | 0.65 |
| 7. | Bowel emptying incomplete | 33 (38.4) | 8 (30.8) | 15 (41.7) | 0.38 |
| 8. | Urgency to pass urine | 61 (70.1) | 18 (69.2) | 28 (77.8) | 0.45 |
| 9. | Nocturia | 64 (74.4) | 20 (76.9) | 29 (80.6) | 0.73 |
| 10. | Pains | 37 (43.0) | 10 (38.5) | 17 (47.2) | 0.49 |
| 11. | Weight | 14 (16.3) | 1 (3.8) | 9 (25.0) | 0.03 |
| 12. | Forgetfulness, memory | 51 (59.3) | 16 (61.5) | 24 (66.7) | 0.68 |
| 13. | Loss of interest | 29 (33.7) | 7 (26.9) | 14 (38.9) | 0.33 |
| 14. | Hallucinations | 11 (12.8) | 1 (3.8) | 7 (19.4) | 0.07 |
| 15. | Concentrating | 45 (42.3) | 11 (42.3) | 22 (61.1) | 0.14 |
| 16. | Sad, blues | 38 (44.2) | 10 (38.5) | 16 (44.4) | 0.64 |
| 17. | Anxiety | 31 (36.0) | 6 (23.1) | 14 (38.9) | 0.19 |
| 18. | Sex drive | 35 (40.7) | 9 (34.6) | 16 (44.4) | 0.44 |
| 19. | Sex difficulty | 38 (44.2) | 9 (34.6) | 19 (52.8) | 0.2 |
| 20. | Dizzy | 35 (40.7) | 5 (19.2) | 20 (55.6) | 0.004 |
| 21. | Falling | 15 (17.4) | 6 (23.1) | 5 (13.9) | 0.35 |
| 22. | Daytime sleepiness | : 29 (33.7) | 8 (30.8) | 12 (33.3) | 0.83 |
| 23. | Insomnia | 47 (54.7) | 13 (50.0) | 20 (55.6) | 0.67 |
| 24. | Intense, vivid dreams | 34 (39.5) | 1 (3.8) | 19 (52.8) | <0.001 |
| 25. | Acting out during dreams | 36 (41.9) | 0 | 26 (72.2) | <0.001 |
| 26. | Restless legs | 43 (50.0) | 11 (42.3) | 19 (52.8) | 0.42 |
| 27. | Swelling | 28 (32.6) | 8 (30.8) | 12 (33.3) | 0.83 |
| 28. | Sweating | 18 (20.9) | 6 (23.1) | 7 (19.4) | 0.73 |
| 29. | Double vision | 22 (25.6) | 8 (30.8) | 11 (30.6) | 0.99 |
| 30. | Delusions | 5 (5.8) | 0 | 2 (5.6) | 0.22 |
Depression
yRBD, compared to nRBD, rated themselves as more depressed, scoring significantly higher on the BDI-II (yRBD=10.56±6.6 vs. nRBD=6.54±5.2, t=-2.59, p=0.025) (Figure 2). The BDI-II includes a question regarding changes in sleep; excluding this question from the analysis did not significantly change the results (yRBD=9.6±6.2 vs. nRBD=5.9±5.0, t=-2.51, p=0.015). Exploratory post-hoc analyses revealed that yRBD patients reported more symptoms of loss of pleasure (p=0.001), crying (p=0.018), indecisiveness (p=0.043), and fatigue (p=0.02). Differences in depressive symptoms were also noted on part 1 of the UPDRS with yRBD patients scored significantly higher compared to nRBD (p=0.001).
Fatigue
yRBD, compared to nRBD patients, rated themselves as more fatigued, scoring significantly higher on the MFSI-SF (yRBD=12.06±13.3 vs. nRBD=6.0±7.7, t=-2.26, p=0.028) (Figure 2). Exploratory analyses revealed no significant differences between the groups in the individual subscales.
Sleep Dysfunction
yRBD, compared to nRBD patients, reported more sleep dysfunction on the PDSS (yRBD=99.2±20.1 vs. nRBD=110.7±18.6, t=2.3, p=0.025) (Figure 2). However, when omitting the two questions assessing RBD, the analyses resulted in no significant differences between the group on this measure (yRBD=84.9±17.2 vs. nRBD=92.1±17.8, t=1.62, p=0.11).
Probable RBD group
The exploratory MANOVA that modeled the relationship between the three RBD groups (yRBD, nRBD, and pRBD) and the multiple NMS measures while controlling for age and LDE showed that RBD (p=0.021), age (p<0.001) and LDE (p=0.003) were all significant predictors in this multivariate model. This analysis further revealed that in a model controlling for age and LDE, RBD classification was a significant predictor for PDSS (R2=0.21, F4,83=3.96, p=0.023), BDI-II (R2=0.20, F4,83=4.45, p=0.015), NMSQuest (R2=0.23, F4,83=5.21, p=0.007), and MFSI-SF (R2=0.24, F4,83=3.13, p=0.049). RBD classification was not a significant predictor for PDQ-39 (p=0.29), MoCA (p=0.18), and ESS (p=0.66).
As shown in Figure 2, post-hoc analyses revealed that pRBD scored significantly lower on PDSS compared to nRBD (p=0.012) indicating poorer sleep, with no differences between yRBD and pRBD. Excluding the two questions related to RBD symptoms did not significantly change these results (p=0.028). Additionally, pRBD scored significantly higher on MFSI-SF compared to nRBD group (p=0.025) indicating more fatigue, again with no differences between yRBD and pRBD. Assessing the MFSI-SF subscales showed that pRBD, compared to nRBD scored higher on the Physical (p=0.011) and Emotional (p=0.001) subscales. On the BDI-II, pRBD did not score significantly different from yRBD (p=0.637) or nRBD (p=0.06). Similarly, pRBD did not differ on the NMSQuest compared to either the yRBD (p=0.096) or nRBD (p=0.205).
Discussion
This observational study assessed the complex relationship between RBD and NMS in PD. The results suggest that, after controlling for age and dopaminergic therapy, PD patients with RBD experienced more NMS than PD patients without RBD. Our results showed that PD patients with RBD reported more NMS on measures of depression, fatigue, sleep, and overall NMS. Our multivariate model of NMS in PD revealed a moderate effect size (R2=0.29) and moderate effect sizes were observed in the models of the individual measures of depression, fatigue, sleep, and overall NMS. These data provide further support for an association between RBD and different NMS in PD and raises the possibility that RBD could be a useful biomarker for a subgroup of PD with more NMS.
To our knowledge, this study is the first to show an association between RBD and depressive symptoms. yRBD experienced more depressive symptoms including loss of pleasure, crying, indecisiveness, and fatigue. A recent study by Suzuki et al.[28] reported that PD patients who met the subjective criteria for RBD (using the Japanese version of the RBDSQ [23]) scored significantly higher on the BDI-II compared to a healthy control group but there were no differences between their RBD groups (including those with an RBDSQ≥5 but with no objective findings) on this measure. Nonetheless, in their study the RBD group reported significantly poorer emotional well-being on the subscale of the PDQ-39. Studies assessing depression in PD suggest that depression primarily results from brain dysfunction rather than situational factors [29] and the presence of RBD may indicate more progressive neuropathological processes which may explain such findings. This contrasts with the results of prior studies in patients with idiopathic RBD [30], and studies of RBD in PD [31-33]. In the study by Postuma et al, depression was assessed with the UPDRS Part 1 [31]. In our study, there was a significant difference between the groups in the BDI-II as well as in Part 1 of the UPDRS (see Table 2). Differences in results may be a function of the smaller sample size used in their study. However, yRBD used antidepressant medications more frequently which may explain the increase in depressive symptom in that group as antidepressants such as serotonin reuptake inhibitors (SSRIs) may result in RSWA, thus falsely lead to a positive RBD diagnosis. In fact, more patients in the yRBD (n=5) group were on SSRIs compared to the nRBD group (n=1). Due to small sample in this study, we did not control for antidepressant medication use. Therefore, future studies employing improved controls are necessary to better establish and understand this possible relationship.
To our knowledge, this is also the first study to show an association between RBD and fatigue in PD. Our findings suggest that PD patients with RBD report more fatigue. Increased fatigue may be a function of worse sleep as is suggested by the higher PDSS scores in yRBD. Additional studies are necessary to explore this association.
Our findings of yRBD reporting feeling light headed, dizzy or weak when standing from a sitting or lying position suggests increased symptoms of orthostatic hypotension, and this is well supported by previous studies [34, 35]. Idiopathic RBD has been demonstrated to be closely related to autonomic dysfunction [36]. Other studies showed evidence for autonomic failure in RBD (with and without PD) [35, 37, 38] as well as abnormal orthostatic blood pressure changes [31, 39].
Our study found that yRBD also reported more olfactory symptoms compared to nRBD. Some previous research supports this relationship [39-41], but Postuma et al. did not [31]. This is likely due to methodological issues including a smaller PD sample in their study. More importantly, many of their participants had significantly impaired smell sensation which made the authors conclude that “a floor effect was probably present.”
Our study did not find a relationship between RBD and cognitive performance in this PD sample, similar to other findings in studies utilizing a single measure of cognitive impairment [42]. However, other studies using comprehensive neurocognitive assessment have shown that PD patients with RBD tend to demonstrate more cognitive impairment, likely better representing overall congnitive functioning [43-47].
We utilized a clinical criteria [22] of RBD that requires both evidence of loss of muscle atonia during REM-sleep with a clinical history of dream enactment, or clear evidence of loss of muscle tone or dream enactment behavior as recorded on video during the PSG recordings. Due to this criteria, a third group, called probable RBD (pRBD), was formed which included those meeting only one of the two criteria. We wanted to explore whether these patients were phenomenologically different from those with clear RBD or clear no-RBD. Our results suggest that pRBD patients are not significantly different from the yRBD group on any of the measures, suggesting similar clinical representation. In addition, pRBD patients experienced more NMS than nRBD patients, specifically, more sleep dysfunction including poorer sleep quality, more distressing dreams, as well as more physical and emotional fatigue. pRBD also seemed to endorse more depressive symptoms compared to nRBD but this result was statistically weak (p=0.06). These findings pose interesting clinical and research questions. Additional studies are necessary to indicate any clinical or research relevance of this group of patients.
While increasing reports suggest that PD patients with versus without RBD are clinically different, the etiology of such differences is not well understood. According to Braak et al.'s six-stage neuropathological model of PD, pathology begins at the dorsal motor nucleus of the vagal nerve and at the olfactory bulb and anterior olfactory nucleus, and progresses to the basal portions of the midbrain and forebrain and encroaches the substantia nigra, all of which leads to the classic clinical manifestation of PD [48]. This pathology may explain some of the NMS of PD, but further research is necessary to assess different pathologies in PD patients with versus without RBD and whether those with RBD represent a subtype of PD with different disease progression and phenomenological presentation.
The major strengths of this study include simultaneous use of subjective and objective measures for RBD diagnosis, multiple validated measures of NMS, and utilization of advanced statistics cohesive assessment of NMS thus avoiding multiple comparisons. Nonetheless, this study had several limitations: 1. Video recordings were available for less than half of the sample. However, detailed clinical notes were maintained by the technician and these notes were available for all patients; 2. The scorer of EMG-activity was not blind to technician's notes as notes were made on the PSG records; 3. Only a single night of PSG was recorded and while this more closely resembles clinical practice, single night recordings may miss RBD occurrences due to high night-to-night variability [49]; 4. Increased use of antidepressants (i.e., SSRIs) in the yRBD group may have influenced the EMGscore thus having an impact on diagnosis. Nevertheless, while these issues may have biased RBD diagnosis in our sample, our RBD occurrence rate is similar to other studies using similar methodology [42, 50]; 5. Our study was also limited to a subjective assessment of NMS and future studies should include objective measures; 6. While the NMSQuest is a widely used method and validated measure, two questions relate specifically to RBD. Our analyses were done with and without these questions. It is possible that removal of the two questions might affect the validity of the total score. However, since removal of the two questions did not change the significant difference between the groups, we feel confident that the results were not biased by the inclusion or removal of these 2 questions and the interpretation of the results is not dependent on the full or limited score; 7. Due to inclusion/exclusion criteria our sample may not be generalizable; 8. We did not have sufficient power to strictly protect error-rate of post-hoc analyses. These were exploratory in nature and post-hoc results should be interpreted with caution. Nonetheless, the use of advanced statistical methods allowed us omnibus testing of the relationship between RBD and multiple NMS.
In summary, our study showed that presence of co-morbid condition of RBD with PD is associated (with moderate effect sizes) with increased NMS symptoms compared to PD with no RBD. We have found that RBD in PD is associated with increases in depressive symptoms, sleep disturbances, fatigue, olfactory dysfunction, and orthostatic hypotensive symptoms. This adds further support to a growing body of literature that suggests that RBD is related to increased frequency and severity of non-motor impairment and subsequent poorer QOL. These findings also further support the hypothesis that RBD may be an indicator of a subtype of PD patients with a different clinical presentation and pathophysiological processes. Finally, while further research is necessary on that subset of PD patients that do not fulfill the full RBD criteria but have either REM sleep without atonia or history of dream enactment, our findings suggest that these patients present a different clinical picture than those without RBD, but not different than those with RBD.
Supplementary Material
Acknowledgments
Support: NIA AG08415, NIH UL1RR031980, and Department of Veterans Affairs San Diego Center of Excellence for Stress and Mental Health (CESAMH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Findley L, Eichhorn T, Janca A, Kazenwadel J, Baker M, Currie-Gnjesda D, et al. Factors impacting on quality of life in Parkinson's disease: Results from an international survey. Movement Disorders. 2002;17:60–7. doi: 10.1002/mds.10010. [DOI] [PubMed] [Google Scholar]
- 2.Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The Priamo Study: A Multicenter Assessment of Nonmotor Symptoms and Their Impact on Quality of Life in Parkinson's Disease. Movement Disorders. 2009;24:1641–9. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri KR, Healy DG, Schapira AH National Institute for Clinical E. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 2006;5:235–45. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 4.Findley L, Aujla M, Bain PG, Baker M, Beech C, Bowman C, et al. Direct economic impact of Parkinson's disease: a research survey in the United Kingdom. Mov Disord. 2003;18:1139–45. doi: 10.1002/mds.10507. [DOI] [PubMed] [Google Scholar]
- 5.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. 1996;46:388–93. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 6.Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Movement Disorders. 2001;16:622–30. doi: 10.1002/mds.1120. [DOI] [PubMed] [Google Scholar]
- 7.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 8.Iranzo A, Santamaria J, Tolosa E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med Rev. 2009;13:385–401. doi: 10.1016/j.smrv.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Neikrug AB, Maglione JE, Liu L, Natajaran L, Avanzino JA, Corey-Bloom J, et al. Effects of sleep disorders on the non-motor symptoms of Parkinson's diease. J clin sleep med. 2013;9(11):1119–29. doi: 10.5664/jcsm.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 1998;50:318 and 16 pages following. doi: 10.1212/wnl.50.2.318. [DOI] [PubMed] [Google Scholar]
- 12.Fahn S, Elton R, Committee UD. Unified Parkinson's disease rating scale. Recent developments in Parkinson's disease. 1987;2:153–63. [Google Scholar]
- 13.Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord. 2007;22:2386–93. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov Disord. 2006;21:916–23. doi: 10.1002/mds.20844. [DOI] [PubMed] [Google Scholar]
- 15.Beck A, Steer R, Brown G. Beck Depression Inventory–Second Edition: Manual. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Practice. 1998;6:143–52. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, et al. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73:629–35. doi: 10.1136/jnnp.73.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhuri KR, Martinez-Martin P. Clinical assessment of nocturnal disability in Parkinson's disease: the Parkinson's Disease Sleep Scale. Neurology. 2004;63:S17–20. doi: 10.1212/wnl.63.8_suppl_3.s17. [DOI] [PubMed] [Google Scholar]
- 20.Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson's disease quality of life questionnaire and its associated measures. J Neurol. 1998;245(Suppl 1):S10–4. doi: 10.1007/pl00007730. [DOI] [PubMed] [Google Scholar]
- 21.Iber C, Ancoli Israel S, Chesson AL, Jr, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine. 2007 [Google Scholar]
- 22.Medicine AAoS International classification of sleep disorders: diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 23.Miyamoto T, Miyamoto M, Iwanami M, Kobayashi M, Nakamura M, Inoue Y, et al. The REM sleep behavior disorder screening questionnaire: validation study of a Japanese version. Sleep Med. 2009;10:1151–4. doi: 10.1016/j.sleep.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–4. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 25.Consens FB, Chervin RD, Koeppe RA, Little R, Liu S, Junck L, et al. Validation of a polysomnographic score for REM sleep behavior disorder. Sleep. 2005;28:993–7. doi: 10.1093/sleep/28.8.993. [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Movement Disorders. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 27.Meyers LS, Gamst G, Guarino AJ. Applied multivariate research: Design and interpretation. SAGE Publications Incorporated; 2006. [Google Scholar]
- 28.Suzuki K, Miyamoto T, Miyamoto M, Watanabe Y, Suzuki S, Tatsumoto M, et al. Probable rapid eye movement sleep behavior disorder, nocturnal disturbances and quality of life in patients with Parkinson's disease: a case-controlled study using the rapid eye movement sleep behavior disorder screening questionnaire. BMC neurology. 2013;13:18. doi: 10.1186/1471-2377-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tandberg E, Larsen JP, Aarsland D, Laake K, Cummings JL. Risk factors for depression in Parkinson disease. Arch Neurol. 1997;54:625–30. doi: 10.1001/archneur.1997.00550170097020. [DOI] [PubMed] [Google Scholar]
- 30.Postuma RB, Montplaisir J. Potential early markers of Parkinson's disease in idiopathic rapid-eye-movement sleep behaviour disorder. Lancet Neurol. 2006;5:552–3. doi: 10.1016/S1474-4422(06)70478-1. [DOI] [PubMed] [Google Scholar]
- 31.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Mov Disord. 2008;23:1665–72. doi: 10.1002/mds.22099. [DOI] [PubMed] [Google Scholar]
- 32.De Cock VC, Vidailhet M, Leu S, Texeira A, Apartis E, Elbaz A, et al. Restoration of normal motor control in Parkinson's disease during REM sleep. Brain. 2007;130:450–6. doi: 10.1093/brain/awl363. [DOI] [PubMed] [Google Scholar]
- 33.Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson's disease over time. J Neurol Neurosurg Psychiatry. 2008;79:387–91. doi: 10.1136/jnnp.2007.116830. [DOI] [PubMed] [Google Scholar]
- 34.Romenets SR, Gagnon JF, Latreille V, Panniset M, Chouinard S, Montplaisir J, et al. Rapid eye movement sleep behavior disorder and subtypes of Parkinson's disease. Mov Disord. 2012;27:996–1003. doi: 10.1002/mds.25086. [DOI] [PubMed] [Google Scholar]
- 35.Nomura T, Inoue Y, Högl B, Uemura Y, Kitayama M, Abe T, et al. Relationship between 123I-MIBG scintigrams and REM sleep behavior disorder in Parkinson's disease. Parkinsonism & Related Disorders. 2010;16:683–5. doi: 10.1016/j.parkreldis.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Postuma RB, Lanfranchi PA, Blais H, Gagnon JF, Montplaisir JY. Cardiac autonomic dysfunction in idiopathic REM sleep behavior disorder. Mov Disord. 2010;25:2304–10. doi: 10.1002/mds.23347. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto T, Miyamoto M, Suzuki K, Nishibayashi M, Iwanami M, Hirata K. 123I-MIBG cardiac scintigraphy provides clues to the underlying neurodegenerative disorder in idiopathic REM sleep behavior disorder. Sleep. 2008;31:717–23. doi: 10.1093/sleep/31.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyamoto T, Miyamoto M, Suzuki K, Ikematsu A, Usui Y, Inoue Y, et al. Comparison of severity of obstructive sleep apnea and degree of accumulation of cardiac 123I-MIBG radioactivity as a diagnostic marker for idiopathic REM sleep behavior disorder. Sleep Medicine. 2009;10:577–80. doi: 10.1016/j.sleep.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66:845–51. doi: 10.1212/01.wnl.0000203648.80727.5b. [DOI] [PubMed] [Google Scholar]
- 40.Stiasny-Kolster K, Doerr Y, Möller J, Höffken H, Behr T, Oertel W, et al. Combination of ‘idiopathic’REM sleep behaviour disorder and olfactory dysfunction as possible indicator for α-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128:126–37. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- 41.Postuma RB, Montplaisir J, Lanfranchi P, Blais H, Rompre S, Colombo R, et al. Cardiac Autonomic Denervation in Parkinson's Disease Is Linked to REM Sleep Behavior Disorder. Movement Disorders. 2011;26:1529–33. doi: 10.1002/mds.23677. [DOI] [PubMed] [Google Scholar]
- 42.Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology. 2011;77:1048–54. doi: 10.1212/WNL.0b013e31822e560e. [DOI] [PubMed] [Google Scholar]
- 43.Gagnon JF, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5:424–32. doi: 10.1016/S1474-4422(06)70441-0. [DOI] [PubMed] [Google Scholar]
- 44.Marion MH, Qurashi M, Marshall G, Foster O. Is REM sleep behaviour disorder (RBD) a risk factor of dementia in idiopathic Parkinson's disease? Journal of neurology. 2008;255:192–6. doi: 10.1007/s00415-008-0629-9. [DOI] [PubMed] [Google Scholar]
- 45.Sinforiani E, Zangaglia R, Manni R, Cristina S, Marchioni E, Nappi G, et al. REM sleep behavior disorder, hallucinations, and cognitive impairment in Parkinson's disease. Mov Disord. 2006;21:462–6. doi: 10.1002/mds.20719. [DOI] [PubMed] [Google Scholar]
- 46.Sinforiani E, Pacchetti C, Zangaglia R, Pasotti C, Manni R, Nappi G. REM behavior disorder, hallucinations and cognitive impairment in Parkinson's disease: a two-year follow up. Mov Disord. 2008;23:1441–5. doi: 10.1002/mds.22126. [DOI] [PubMed] [Google Scholar]
- 47.Vendette M, Gagnon JF, Decary A, Massicotte-Marquez J, Postuma RB, Doyon J, et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology. 2007;69:1843–9. doi: 10.1212/01.wnl.0000278114.14096.74. [DOI] [PubMed] [Google Scholar]
- 48.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–34. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 49.Sixel-Döring F, Schweitzer M, Mollenhauer B, Trenkwalder C. Intraindividual variability of REM sleep behavior disorder in Parkinson's disease: a comparative assessment using a new REM sleep behavior disorder severity scale (RBDSS) for clinical routine. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2011;7:75. [PMC free article] [PubMed] [Google Scholar]
- 50.Eisensehr I, Jäger M, Noachtar S. REM sleep behavior disorder in sleep-disordered patients with versus without Parkinson's disease: is there a need for polysomnography? Journal of the neurological sciences. 2001;186:7–11. doi: 10.1016/s0022-510x(01)00480-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.