Abstract
Objective
The APOE4 allele is the strongest genetic risk factor for sporadic Alzheimer’s disease (AD). Case-control studies suggest the APOE4 link to AD is stronger in women. We examined the APOE4-by-sex interaction in conversion risk (from healthy aging to mild cognitive impairment (MCI)/AD or from MCI to AD) and cerebrospinal fluid (CSF) biomarker levels.
Methods
Cox proportional hazards analysis was used to compute hazards ratios (HR) for an APOE-by-sex interaction on conversion in controls (N=5,496) and MCI patients (N=2,588). The interaction was also tested in CSF biomarker levels of 980 subjects from the AD Neuroimaging Initiative.
Results
Among controls, male and female carriers were more likely to convert to MCI/AD, but the effect was stronger in women (HR=1.81 women; HR=1.27 men; interaction P=0.0106). The interaction remained significant in a pre-defined sub-analysis restricted to APOE3/3 and APOE3/4 genotypes. Among MCI patients, male and female carriers were more likely to convert to AD (HR=2.16 women; HR=1.64 men). The effect was nominally stronger in women, but the interaction was not significant (P=0.136). In the sub-analysis restricted to APOE3/3 and APOE 3/4 genotypes, the interaction was significant (P= 0.022; HR=2.17 women; HR=1.51 men). The APOE4-by-sex interaction on biomarker levels was significant for MCI patients in total-tau and the tau-to-Abeta-ratio (P=0.0088 and P=0.020, respectively; more AD-like in women).
Interpretation
APOE4 confers greater AD risk in women. Biomarker results suggest that increased APOE-related risk in women may be associated with tau pathology. These findings have important clinical implications and suggest novel research approaches into AD pathogenesis.
Introduction
Alzheimer’s disease (AD) is an increasingly prevalent, fatal neurodegenerative disease that has proven resistant thus far to all attempts to prevent it, forestall it, or slow its progression. The ε4 allele of the Apolipoprotein E gene (APOE4) is a potent genetic risk factor for sporadic and late-onset familial AD1. The ε3 allele (APOE3) is the most common APOE polymorphism in the general population and considered risk-neutral, while the ε2 allele (APOE2) is the least common and is thought to reduce AD risk. While estimates vary across studies and ethnic backgrounds, the APOE4 allele is typically present in more than 50% of AD patients but is found only in about 15% of healthy older controls2. Basic science research has suggested several roles that the ε4 isoform of apolipoprotein E (ApoE4) may play in augmenting the development of AD. Cell culture and animal models have identified potential pathogenic mechanisms related to beta-amyloid (Abeta) clearance, tau hyperphosphorylation, and synaptic function, among others3
In human studies, some, but not all, imaging biomarker studies have shown early AD-like findings in healthy older APOE4 carriers4–6. Cerebrospinal fluid (CSF) biomarker studies are more consistent and tend to show reduced (more AD-like) Abeta levels, but normal tau levels, in healthy older APOE4 carriers. Longitudinal studies of clinical decline from mild cognitive impairment (MCI) to AD are mixed with some, but not all, suggesting that the APOE4 allele increases the risk of conversion from MCI to AD7. The data on clinical conversion from healthy aging to MCI or AD are similarly mixed. To date, there have been six longitudinal studies examining the role of APOE4 in the risk of converting from healthy aging to MCI or AD 8–13. Of these studies, four found a significant effect of APOE4 and two did not, even when combining APOE4 heterozygotes and homozygotes. Thus, while the link between APOE4 and AD is strong, many expected effects, like increasing the risk of conversion from healthy aging to MCI or from MCI to AD, have not been widely replicable.
A critical, and commonly overlooked, feature of the APOE4 link to AD is that several case-control studies suggest it is far more pronounced in women. Shortly after the initial linkage studies, a prominent interaction between APOE and sex was reported14. The first large meta-analysis of APOE4 studies confirmed the interaction and found that the effect was most prominent among subjects with one copy of the APOE4 allele and one copy of the risk-neutral APOE3 allele. Women with one APOE4 allele had up to a four-fold increased risk when compared to women homozygous for the APOE3 allele. By contrast, men with one APOE4 allele had little to no bump in risk15. This finding has been replicated and yet is rarely considered in clinical AD research where male and female APOE4 carriers are generally viewed as having equal risk16, 17.
Although case-control studies of AD support an interaction between APOE4 and sex, such studies are less conclusive than prospective cohort studies, particularly in diseases like AD with a long preclinical phase18, 19. The interaction between APOE4 and sex has not been established either in prospective cohorts of healthy older controls converting to MCI or AD or in prospective cohorts of MCI patients converting to AD. Most prospective studies examining the main effect of APOE4 on incident MCI or AD have included sex as a covariate, but not explicitly tested for an APOE4-by-sex interaction. To our knowledge, only one prospective study has examined the effect of this interaction on clinical conversion, in this case from healthy aging to AD. Beydoun and colleagues reported a main effect of APOE4 but no significant interaction with sex8. As acknowledged by the authors, this study, with 113 incident cases of all-cause dementia, may not have been adequately powered to detect a sex interaction.
In the current study we hypothesized that the APOE-by-sex interaction would be evident in the risk of converting from healthy aging to MCI/AD and from MCI to AD and specifically that a single APOE4 allele would confer greater risk of conversion in women than in men. To test this we took advantage of a large, multisite, longitudinal aging and dementia database available through the National Alzheimer’s Coordinating Center (NACC). In addition, to explore potential biochemical changes underlying these hypothesized effects, we also examined the APOE-by-sex interaction in cerebrospinal fluid (CSF) data from healthy older controls and MCI patients available in a second, multisite aging and dementia dataset, the Alzheimer’s Disease Neuroimaging Initiative (ADNI). In this case, we hypothesized that a single copy of the APOE4 allele would result in more AD-like changes in women than in men (i.e., lower beta-amyloid levels, higher tau levels, higher phospho-tau levels, and higher tau-to-Abeta ratio).
Methods
Assessing Conversion Risk in the NACC Dataset
The NACC curates data collected at 34 past and present Alzheimer’s Disease Centers (ADCs) across the United States. For this study we used data from 11,654 non-demented subjects collected in the longitudinal Uniform Data Set (UDS)20–22 with visits spanning from 09/2005 to 05/2013 (date of database access: 06/12/2013).
We restricted our analysis to subjects who were rated as healthy control (HC) or MCI at their initial assessment at study entry, who had an APOE genotype available, and who had a minimum of one follow-up visit at 12 months or later. These filter criteria led to a cohort of 8,084 subjects (HC N=5,496; MCI N=2,588; Table 1).
Table 1. Population statistics for healthy controls and MCI subjects in the NACC data.
Columns show total subjects, subjects in each genotype, the p-value for the variable being different between ε2/ε3 and ε3 homozygotes, and the p-value for the characteristic being different between ε3 homozygotes and ε3/ε4 heterozygotes. P-values were computed using a logistic regression. Age refers to age at entry into the cohort, number of visits to the number of visits recorded in the database. For continuous values median and interquartile range in brackets are provided, for categorical data, the raw counts as well as percentage are given.
| Total | ε2/ε2 | ε2/ε3 | ε2/ε4 | ε3/ε3 | ε3/ε4 | ε4/ε4 | P-value APOE | P-value sex | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HC | N | 5,496 | 30 | 661 | 139 | 3,210 | 1,320 | 136 | NA | NA |
| Female (%) | 3,652 (66.4) | 19 (63.3) | 456 (69.0) | 96 (69.1) | 2,117 (66.0) | 872 (66.1) | 92 (67.6) | 0.71 | NA | |
| Age | 73.0 [66.6–79.8] | 74.1 [68.1–79.8] | 74.6 [67.1–81.8] | 70.8 [65.3–77.8] | 73.9 [67.4–80.4] | 71.1 [65.5–78.1] | 67.6 [62.1–73.3] | 0.57 | 0.006 | |
| No. of visits | 4 [3–6] | 3.5 [3–4] | 4 [3–6] | 4 [3–5.5] | 4 [3–6] | 4 [3–6] | 4 [2.8–6] | 0.06 | 0.06 | |
| Years of follow-up | 3.9 [2.2–5.5] | 3.6 [2.2–4.3] | 4.0 [2.3–5.7] | 4.0 [2.6–5.2] | 4.0 [2.2–5.5] | 3.9 [2.1–5.4] | 3.3 [2.1–5.3] | 0.27 | 0.40 | |
| Converters (%female) | 964 (60.5) | 2 (0) | 111 (70.3) | 29 (65.5) | 521 (56.6) | 268 (63.1) | 33 (66.7) | 0.08 | 0.000019 | |
| Years of education | 16 [13–18] | 16 [14–18] | 16 [13–18] | 16 [13.5–18] | 16 [14–18] | 16 [14–18] | 16 [14–18] | 0.63 | <2.2e-16 | |
| Non-Hispanic White (%) | 4,458 (81.1) | 20 (66.7) | 524 (79.3) | 101 (72.7) | 2,654 (82.7) | 1,047 (79.3) | 112 (82.4) | 0.06 | 1e-15 | |
| MMSE | 29 [28–30] | 29 [28–30] | 29 [28–30] | 29 [28–30] | 29 [28–30] | 29 [28–30] | 29 [28–30] | 0.68 | 4.4e-11 | |
| MCI | N | 2,588 | 5 | 226 | 70 | 1237 | 858 | 192 | NA | NA |
| Female (%) | 1,275 (49.3) | 3 (60.0) | 117 (51.8) | 35 (50.0) | 602 (48.7) | 425 (49.5) | 93 (48.4) | 0.63 | NA | |
| Age | 74.5 [68.5–80.2] | 82.7 [78.2–84.2] | 76.5 [70.0–83.2] | 74.1 [67.3–82.3] | 75.7 [68.8–81.6] | 73.8 [68.6–79.0] | 70.5 [65.9–74.5] | 0.43 | 1.0 | |
| No. of visits | 4 [2–5] | 3 [2–3] | 3 [3–5] | 3 [2–5] | 4 [3–5] | 3 [2–5] | 4 [3–5] | 0.21 | 0.11 | |
| Years of follow- up | 3.0 [2.0–4.7] | 2.1 [2.1–3.5] | 3.05 [2.03–4.68] | 2.4 [1.6–4.4] | 3.1 [2.0–4.8] | 3.0 [1.8–4.4] | 3.2 [2.1–4.9] | 0.53 | 0.9 | |
| Converters (%female) | 867 (48.8) | 0 (0) | 50 (56.0) | 23 (43.5) | 324 (45.1) | 364 (49.5) | 106 (45.3) | 0.04 | 0.73 | |
| Years of education | 16 [12–18] | 16 [12–16] | 16 [12–18] | 16 [12–18] | 16 [12–18] | 16 [12–18] | 16 [14–18] | 0.84 | <2.2e-16 | |
| Non-Hispanic White (%) | 2,071 (80.0) | 2 (40) | 176 (77.9) | 58 (82.9) | 967 (78.2) | 704 (82.1) | 164 (85.4) | 0.05 | <2.2e-16 | |
| MMSE | 28 [26–29] | 28 [28–29] | 28 [26–29] | 28 [26–29] | 28 [26–29] | 28 [26–29] | 27 [25–29] | 0.31 | 0.19 |
The clinical conversion risk was modeled using a Cox proportional hazards model. We performed the Cox regression analysis in two subgroups of the cohort: (i) HC only and (ii) MCI only. For controls, clinical conversion was defined as the first detection of MCI or AD (using primary diagnoses of possible or probable AD based on the NINCDS-ADRDA criteria23) and the Cox model was used to model the hazard of developing MCI or AD among controls, whichever occurred first. For MCI subjects, conversion was defined as the first detection of AD (using primary diagnoses of possible or probable AD) and the Cox model was used to model the hazard of developing AD among MCI subjects. Any additional outcomes (such as reversions from MCI to HC or development of non-AD dementia) were treated as non-conversions. The subject age at the visit where clinical progression was detected served as time of event; subjects who had not progressed at their last recorded visit were right-censored. In addition, to account for the period prior to inclusion in the cohort, a left-truncated design was used. Furthermore, we corrected the dependence between truncation and failure time (detected using Tsai’s test24) by estimating time-dependent effects of the age at study entry in the Cox regression. This method is a generalization of the one proposed by Gail et al.25. More precisely, we included age as a covariate in the Cox regression model and allowed the coefficient of age to be different within follow-up periods starting before and after the median age of the study sample (73.0 for controls and 74.5 for MCI; Table 1). The data were analyzed across sexes with covariates for APOE2 carrier status, APOE4 carrier status, APOE2 homozygosity, APOE4 homozygosity, sex, APOE2-by-sex interaction, and APOE4-by-sex interaction. Due to the small sample size (see Table 1), APOE2 homozygosity was not modeled in MCI subjects. Further, the model was adjusted for years of education, Mini Mental State Examination (MMSE) score at study entry, and stratified by race and Hispanic origin by grouping all non-Hispanic white subjects in one group and everyone else in a second group. In addition to the full regression model including the APOE2-by-sex and APOE4-by-sex variables, we assessed the risk of clinical conversion attributable to carrying the APOE2 or APOE4 allele for each sex separately in sex-stratified models. Hazard ratios along with their 95% interval are reported. In order to visualize conversion rates with left-truncated and right-censored data, we used the Breslow method to estimate the survival function of the conversion time for subjects entering the study at 55.
Given our hypothesis, based on the Farrer et al. meta-analysis15, that the APOE4-by-sex interaction would be strongest in ε3/ε4 heterozygotes versus ε3 homozygotes, we also performed the Cox regression on a restricted subset of individuals having either the ε3 homozygote (ε3/ε3) or the ε3/ε4 heterozygote genotype.
Effects on Spinal Fluid Biomarkers in the ADNI Database
We studied whether sex modulates the APOE4 carrier effect on the most commonly used CSF biomarkers of AD. This analysis used data from the ADNI database (www.loni.usc.edu/ADNI; date accessed: 11/04/2013). See Weiner et al.26 for an overview of the ADNI cohort. It should be noted that some ADNI subjects are likely also included in the NACC dataset though it is not currently possible to identify which subjects are in both datasets.
Cerebrospinal fluid (CSF) Biomarkers
In ADNI, a particular emphasis was placed on four established CSF biomarkers: Abeta, total tau, phosphorylated tau181p (p-tau) and the ratio of total tau to Abeta. Biomarkers were assessed at study entry and at follow-up visits for a subset of subjects (see Shaw et al.5 for details on biomarker acquisition).
CSF biomarkers were available for N=1,094 subjects at study entry. Again, the analysis was restricted to the HC (N=272) and MCI (N=618) subgroups. Table 2 lists sample sizes for each genotype and clinical category along with population demographics.
Table 2. Population statistics for healthy controls and MCI subjects in the ADNI database for whom CSF biomarker data were available.
Columns show total subjects and subjects for each APOE genotype, Age refers to age at entry into the cohort. For continuous values median and interquartile range in brackets are provided, for categorical data, the raw counts as well as percentage are given. The last two columns contain the P-value for the variables being different between APOE genotypes and sex, respectively. P-values were computed using logistic regression.
| total | ε2/ε2 | ε2/ε3 | ε2/ε4 | ε3/ε3 | ε3/ε4 | ε4/ε4 | P- value APOE | P- value sex | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HC | N | 272 | 0 | 39 | 2 | 162 | 61 | 8 | NA | NA |
| Age | 73.7 [70.8–78.1] | NA | 72.4 [70.6–76.3] | 72.3 [71.3–73.2] | 74.0 [71.0–78.5] | 73.7 [68.2–77.5] | 76.8 [69.0–83.3] | 0.42 | 0.11 | |
| Female (%) | 135 (49.8) | NA | 22 (56.4) | 1 (50) | 79 (48.8) | 30 (49.2) | 3 (38) | 0.37 | NA | |
| Years of education | 16 [14–18] | NA | 16 [13.5–18] | 14.5 [13.3–17.8] | 16 [14–18] | 16 [14–18] | 17 [16–18.5] | 0.29 | 1.e–06 | |
| MMSE | 29 [29–30] | NA | 29 [28–30] | 27.5 [26.3– 28.8] | 29 [29–30] | 28 [29–30] | 29.5 [29–30] | 0.31 | 0.038 | |
| MCI | N | 618 | 1 | 36 | 10 | 274 | 230 | 67 | NA | NA |
| Age | 72.8 [67.3–77.6] | 77.6 | 73.2 [69.8–78.3] | 70.0 [64.3– 74.6] 74.6] | 74.0 [67.9– 79.7] 79.7] | 72.7 [67.5– 76.7] 76.7] | 70.0 [64.7– 73.9] 73.9] | 0.48 | 0.0002 | |
| Female (%) | 249 (40.5) | 1 (100) | 13 (36.1) | 5 (50) | 110 (40.3) | 94 (41.0) | 26 (39.4) | 0.18 | NA | |
| Years of education | 16 [14–18] | 17 | 16 [13.8–18] | 17.5 [14.3– 18] | 16 [14–18] | 16 [14–18] | 16 [14–18] | 0.73 | 3.6e- 06 | |
| MMSE | 28 [26–29] | 29 | 28 [27–29] | 28 [26.3– 29.8] | 28 [27–29] | 28 [26–29] | 28 [26–30] | 0.43 | 0.14 |
The APOE4-by-sex interaction on CSF biomarker levels at study entry was examined separately in HC and MCI subjects using an ANCOVA adjusting for APOE2 and APOE4 carrier status, APOE4 homozygosity, APOE2-by-sex interaction, sex, age, age-squared, years of education, and ADNI study phase (ANDI1, ANDIGO or ADNI 2). Due to the small sample sizes (see Table 2), APOE2 homozygosity was modeled neither in the HC nor in the MCI analyses. P-values for the eight tests (i.e., four CSF biomarkers for each HC and MCI) for an APOE4-by-sex interaction were corrected for multiple testing using the Holm-Bonferroni method. In an effort to examine potential Abeta-independent effects of APOE4, we report the total tau and p-tau analyses before and after adjusting for Abeta levels.
Again, given our hypothesis of a stronger APOE4-by-sex interaction in ε3/ε4 heterozygotes versus ε3 homozygotes, we performed the ANCOVA on a restricted subset of individuals having either the ε3 homozygote (ε3/ε3) or the ε3/ε4 heterozygote.
Results
Clinical Conversion Risks
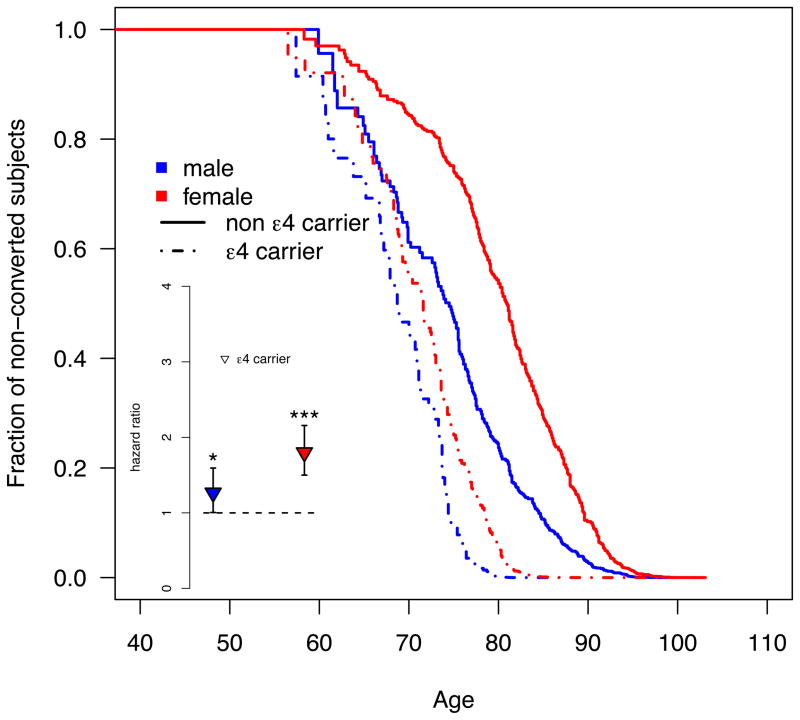
During the observation period 959 healthy subjects (17.5%) converted to MCI or AD. The APOE4-by-sex interaction is significant (P=0.0106) and driven by an increased APOE4 effect on conversion in women. Among healthy older men, there is a marginally significant increase in conversion risk for APOE4 carriers compared to non-carriers (HR=1.27; CI: 1.01–1.59; P=0.045). Among healthy older women, APOE4 carriers show a highly significant 1.8-fold increase in risk (CI: 1.5–2.16; P=2.5e–10). The survival function plot (Figure 1) shows that non-carrier females are the group with the least risk in progressing from healthy control to MCI or AD (median conversion age [95% CI]: 80.9 [79.7–83.0]). APOE4 carriers, regardless of sex, show the highest risk for clinical progression (68.7 [67.6–70.7] and 71.6 [69.7–72.9] for males and females, respectively), while non-carrier males are at intermediate risk (74.5 [70.2–77.6]).
Figure 1. The APOE4 carrier status increases the risk of clinical decline in healthy older women, but not men.
The main figure shows the survival function plot for conversions from healthy control to either MCI or possible/probable AD based on left-truncated and right-censored data. In contrast to the Cox regression no stratification or covariate adjustment was applied. Subjects younger than 55 years were excluded (N=211; 3.8%) from the plot for visualization purposes only; the Cox model featured all available subjects. The inset depicts the hazards ratio for converting from HC to MCI or AD computed separately for each sex using a Cox regression model (* and *** signify significant effects with P≤0.5 and P<0.001, respectively). Blue and red refer to males and females, respectively.
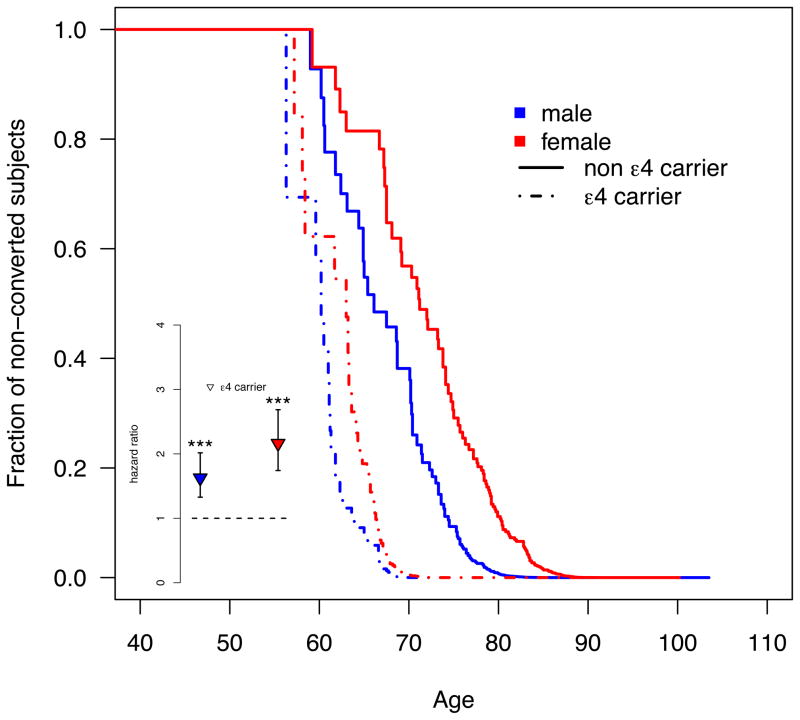
Among MCI subjects, the APOE4 effect was significant in men and women. While the effect was nominally stronger in women, the interaction was not significant (P=0.136; Table 3). Male APOE4 carriers show a HR of 1.64 (CI: 1.33–2.02; P=3.8e-6) while female APOE4 carriers exhibit a HR of 2.16 (CI: 1.74–2.69; P=2.9e-12; Figure 2).
Table 3. Cox proportional hazards ratios.
Rows correspond to the covariates in the Cox regression models (Edu=years of education; APOE2=APOE2 carrier status; APOE4=APOE4 carrier status;APOE2hom=APOE2 homozygosity; APOE4hom=APOE4 homozygosity). Columns correspond to the Cox proportional hazards ratio, its 95% confidence interval, and the corresponding P-value for the analysis including all subjects for HC and MCI subjects.
| HC | MCI | |||||
|---|---|---|---|---|---|---|
| Covariate | Cox HR | 95% CI | P-value | Cox HR | 95% CI | P-value |
| Edu | 0.98 | 0.96–1.00 | 0.12 | 1.04 | 1.01–1.06 | 0.00115 |
| MMSE | 0.85 | 0.82–0.89 | 2.2e–13 | 0.83 | 0.81–0.85 | <2.e–16 |
| Sex | 0.69 | 0.58–0.82 | 3.2e-05 | 0.98 | 0.79–1.22 | 0.86 |
| APOE2 | 0.75 | 0.55–1.02 | 0.069 | 0.83 | 0.59–1.19 | 0.31 |
| APOE2hom | 0.48 | 0.12–1.94 | 0.30 | NA | NA | NA |
| APOE4 | 1.25 | 1.00–1.57 | 0.048 | 1.70 | 1.39–2.07 | 1.7e-07 |
| APOE4hom | 1.59 | 1.11–2.29 | 0.012 | 1.39 | 1.11–1.74 | 0.00396 |
| APOE2xsex | 1.48 | 1.01–2.17 | 0.045 | 0.99 | 0.61–1.62 | 0.97 |
| APOE4xsex | 1.44 | 1.09–1.91 | 0.0106 | 1.23 | 0.94–1.63 | 0.136 |
Figure 2. The APOE4 carrier status increases the risk of clinical decline in women with MCI more than in men.
The main figure shows the survival function plot for conversions from MCI to possible/probable AD based on left-truncated and right-censored data. In contrast to the Cox regression no stratification or covariate adjustment was applied. Subjects younger than 55 years were excluded (N=71; 2.7%) from the plot for visualization purposes only; the Cox model featured all available subjects. The inset depicts the hazards ratio for converting from MCI to AD computed separately for each sex using a Cox regression model (*** signifies a significant effect with P<0.001). Blue and red refer to males and females, respectively.
The APOE2-by-sex interaction is significant only in healthy controls (P=0.045). Here, men carrying the APOE2 allele showed a non-significant decrease in risk (HR=0.74; CI: 0.53–1.01; P=0.061) while female APOE2 carriers exhibit a non-significant increase in risk (HR=1.12; CI: 0.90–1.40; P=0.32).
Restriction of the analysis to ε3/ε3 and ε3/ε4 subjects confirms the APOE4-by-sex interaction in healthy controls (P=0.019) and reveals a significant APOE4-by-sex interaction among MCI subjects (P=0.022; Table S1; Figures S1–S2). Among controls, a single copy of the APOE4 allele significantly increases the conversion risk in women (HR=1.8; CI:1.48–2.19; P=3.5e-9) but not in men (HR=1.23; CI:0.96–1.57; P=0.09). Among MCI patients, a single copy of the APOE4 allele significantly increases conversion risk in men (HR=1.51; CI:1.22–1.88; P=0.0002) and women (HR=2.17; CI:1.72–2.72; P=3.6e-11) but, as indicated by the significant interaction, to a significantly greater degree in women.
CSF Biomarkers
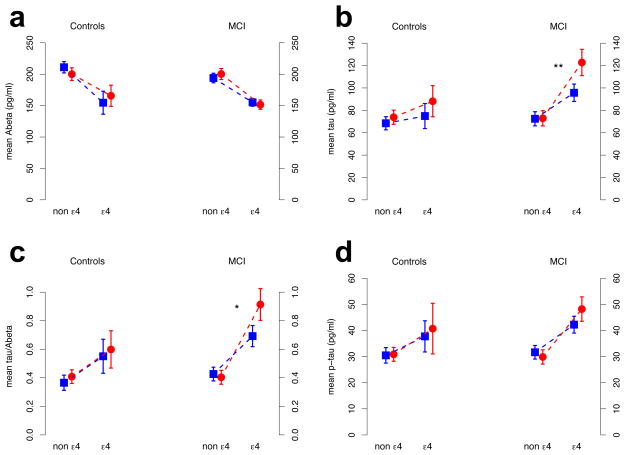
The APOE4-by-sex interaction showed only a nominal significant effect (P=0.0197; Pcor = 0.12) for CSF Abeta levels in healthy controls (more AD-like in men; Figure 3a); there was no evidence for an interaction by sex on the other three biomarkers among controls (Table 4). Among MCI subjects, we detected a significant APOE4-by-sex interaction on CSF tau levels (Pcor= 0.0088) and on the tau-Abeta-ratio (Pcor= 0.0199) as well as a nominal significant effect on CSF p-tau (P=0.0253; Pcor= 0.13) with a more AD-like pattern in female APOE4 carriers (Figure 3c-d). Results for tau and p-tau remained qualitatively unchanged after adjusting for Abeta (Table 4; Figure S3).
Figure 3. Sex modifies the APOE4 effect on spinal fluid biomarker levels.
CSF biomarker levels in HCs and MCI subjects are shown with 95% CI. Depicted CSF Biomarker levels were adjusted for age, age-squared, years of education, MMSE score and ADNI study phase. Blue squares and red circles correspond to men and women, respectively. Dashed lines highlight the change in CSF levels between ε3 homozygotes and ε4 heterozygotes. P-values for the APOE by sex effect were computed using an ANCOVA. *: P<0.05 (corrected for multiple comparisons) **: P<0.01 (corrected). Subfigures a, b, c, and d correspond to Abeta, total tau, tau-to-Abeta-ratio, and p-tau, respectively.
Table 4. P-values from the ANOVA analysis on CSF biomarker levels.
Columns correspond to the main covariates of interest: sex, APOE2 carrier status, APOE2-by-sex interaction, APOE4 carrier status, APOE4 homozygosity, and APOE-by-sex interaction. The last column reports the corrected P-value for the APOE4-by-sex interaction for the eight main tests. Rows correspond to different CSF biomarkers: Abeta, tau, p-tau, and the tau-Abeta-ratio. Results for tau* and p-tau* were corrected for Abeta levels.
| sex | APOE2 | APOE2x sex | APOE4 | APOE4hom | APOE4x sex | Pcor | ||
|---|---|---|---|---|---|---|---|---|
| HC | Abeta | 0.070 | 0.0023 | 0.23 | 0.0499 | 0.00765 | 0.01968 | 0.12 |
| tau | 0.24 | 0.52 | 0.97 | 0.018 | 0.072 | 0.62 | 1.0 | |
| p-tau | 0.90 | 0.44 | 0.54 | 0.00137 | 0.30 | 0.99 | 1.0 | |
| tau/Abeta | 0.41 | 0.41 | 0.86 | 0.00247 | 0.88 | 0.83 | 1.0 | |
| tau* | 0.38 | 0.94 | 0.86 | 0.051 | 0.021 | 0.37 | NA | |
| p-tau* | 0.76 | 0.13 | 0.36 | 0.00543 | 0.085 | 0.58 | NA | |
| MCI | Abeta | 0.71 | 0.33 | 0.13 | 2.3e-13 | 1.1e-07 | 0.21 | 0.84 |
| tau | 0.70 | 0.28 | 0.45 | 4.6e-13 | 0.34 | 0.0011 | 0.0088 | |
| p-tau | 0.52 | 0.54 | 0.94 | 2.2e-10 | 0.24 | 0.02529 | 0.13 | |
| tau/Abeta | 0.71 | 0.27 | 0.38 | <2e-14 | 0.00521 | 0.002836 | 0.0199 | |
| tau* | 0.56 | 0.39 | 0.74 | 3.7e-07 | 0.49 | 0.00264 | NA | |
| p-tau* | 0.58 | 0.80 | 0.48 | 0.00027 | 0.37 | 0.0596 | NA |
The restricted analysis on ε3 homozygous and ε3/ε4 heterozygous subjects, confirmed the same APOE4-by-sex interactions (Table S2; Figures S4-S5).
Discussion
We have demonstrated, in a large, longitudinal sample, that the risk of clinical conversion conferred by the APOE4 allele is significantly greater in women than in men. The interaction was present in the conversion from healthy aging to MCI/AD and in the conversion from MCI to AD. Among healthy controls, the interaction was detectable both in the full analysis (including all genotypes) and in the pre-defined sub-analysis restricted to the two most common genotypes (ε3 homozygotes versus ε3/ε4 heterozygotes, accounting for 82% of controls). In this sample, healthy older male APOE4 carriers were at a marginally significant increased risk of converting to MCI or AD when compared with men who did not carry the APOE4 allele. By contrast, healthy older female APOE4 carriers were almost twice as likely to develop MCI or AD when compared to female non-carriers. In the sub-analysis, the APOE4 effect remained significant in women but was no longer significant in men. Among all MCI subjects, APOE4 carriers of both sexes had an increased risk of conversion to AD. In the sub-analysis (ε3 homozygotes versus ε3/ε4 heterozygotes, accounting for 81% of MCI subjects) the APOE4 effect was significant in men and women but was significantly stronger in women (APOE4-by-sex interaction P=0.022). These prospective findings on clinical conversion support earlier case-control analyses demonstrating that women with a single APOE4 allele were at increased risk of developing AD compared to women who were homozygous for the APOE3 allele, whereas men with a single APOE4 allele were not at increased risk when compared to men who were APOE3 homozygotes15–17. While the APOE2 findings should be considered preliminary, owing to the smaller sample sizes, here too we detected a significant interaction with sex in which the APOE2 allele trended towards being protective in male, but not female, controls.
The CSF biomarker results reported here suggest that the increased risk of AD in female APOE4 carriers occurs downstream of Abeta pathology. Abeta pathology is believed to occur early during disease pathogenesis, before the appearance of tau-related changes reflective of neuronal injury. The effect of carrying an APOE4 allele on lowering beta-amyloid levels was quite pronounced in both healthy older men and women. Total tau and p-tau levels showed a main effect of APOE4 but did not show any APOE-by-sex interactions in the healthy older controls. Among MCI patients, however, APOE4 increased total tau levels significantly more in women than in men, even after controlling for Abeta levels. Similarly, among MCI patients, APOE4 increased the ratio of total tau to beta-amyloid significantly more in women than in men, despite similar beta-amyloid levels. P-tau levels showed a nominally significant trend in the same direction (more AD-like in female APOE4 carriers), but this did not survive Holm-Bonferonni correction for multiple comparisons.
One possible explanation for the increased effect of APOE4 on the tau biomarker in women is that amyloid changes occur earlier in women than in men. This possibility is less likely given that our analyses adjusted for linear and quadratic effects of age on amyloid, but subsequent longitudinal studies can assess whether there is an earlier start to amyloid pathology in women with the APOE4 allele. An alternative explanation is that for an equivalent amount and duration of amyloid pathology, the APOE4 allele results in more tau-related pathology in women compared to men. APOE4 may initially change Abeta processing in a manner that is roughly equivalent for both sexes but then triggers a more robust acceleration of tau pathology in women. This explanation also appears unlikely, since even after adjusting for the effect of amyloid on tau, the APOE-by-sex interaction is still significant. An Abeta-independent effect of APOE on CSF tau has also previously been shown by Cruchaga et al., where polymorphisms in the APOE locus were strongly associated with CSF tau levels even after an adjustment for Abeta levels27.
Two caveats should be considered when interpreting these results. First, our results may generalize imperfectly, as neither the NACC nor the ADNI data are population-based. For example, a recruitment bias could account for the unexpected finding that the risk of conversion from healthy aging to MCI or AD is less for female than male APOE3 homozygotes. This novel finding, while not predicted by our a priori hypothesis, is nonetheless worth pursuing in a population-based study. Second, the Cox model assumes that drop-out and censoring are unrelated to conversion. Impaired subjects may have been more likely to drop out, and there may have been differential effects by sex.
Despite compelling evidence from a large meta-analysis of case-controls studies, the field of AD research has largely overlooked this potent interaction between APOE and sex15. This may have been due, in part, to the lack of any previous, prospective cohort studies supporting this interaction effect on clinical conversion8. It seems likely that a number of inconsistent findings related to APOE, some of which are outlined in the introduction, are a result of investigators overlooking the APOE-by-sex interaction. We hope that the current findings will alert the field to this interaction and the important clinical and scientific implications it carries. From a clinical perspective these results require careful reexamination of how we should interpret the finding of a single APOE4 allele in men. This bears importantly on encounters with individual patients in terms of diagnostics, prognostics and genetic counseling. In regards to clinical trials, appreciating the APOE-by-sex interaction should allow for more refined genotype stratification when, for example, estimating conversion risk in preventative trials28. Further, several drug trials have suggested that efficacy and side effect profiles may differ between APOE4 carriers and non-carriers though these studies have not assessed the sex interaction29. From a scientific standpoint, these findings should motivate investigations into the potential mechanisms of the APOE-by-sex interaction. Explicitly modeling this interaction, both in human studies and animal model studies, as is now only occasionally done, has the potential to yield new insights into the strongest genetic risk factor for late-onset AD30–32.
Supplementary Material
Acknowledgments
The JNA Foundation. The National Institutes of Health: NS073498.
The NACC database is funded by NIA Grant U01 AG016976.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI; National Institutes of Health Grant U01 AG024904). ADNI data are disseminated by the Laboratory for Neuroimaging at the University of Southern California, Los Angeles. Cerebrospinal data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.usc.edu/ADNI). As such, ADNI investigators contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Footnotes
Authors’ Contributions
MG conceived the study. MG and VH conducted literature search. AA collected data and prepared figures. AA and LT analyzed data. MG and AA wrote first draft. All authors contributed in analyzing the data and writing the final manuscript.
References
- 1.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 2.Ward A, Crean S, Mercaldi CJ, et al. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer's disease: a systematic review and meta–analysis. Neuroepidemiology. 2012;38(1):1–17. doi: 10.1159/000334607. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y. Abeta–independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer's disease. Trends in molecular medicine. 2010 Jun;16(6):287–94. doi: 10.1016/j.molmed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Sunderland T, Mirza N, Putnam KT, et al. Cerebrospinal fluid beta-amyloid1–42 and tau in control subjects at risk for Alzheimer's disease: the effect of APOE epsilon4 allele. Biological psychiatry. 2004 Nov 1;56(9):670–6. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Annals of neurology. 2009 Apr;65(4):403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuff N, Woerner N, Boreta L, et al. MRI of hippocampal volume loss in early Alzheimer's disease in relation to ApoE genotype and biomarkers. Brain : a journal of neurology. 2009 Apr;132(Pt 4):1067–77. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elias-Sonnenschein LS, Bertram L, Visser PJ. Relationship between genetic risk factors and markers for Alzheimer's disease pathology. Biomarkers in medicine. 2012 Aug;6(4):477–95. doi: 10.2217/bmm.12.56. [DOI] [PubMed] [Google Scholar]
- 8.Beydoun MA, Boueiz A, Abougergi MS, et al. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiology of aging. 2012 Apr;33(4):720–31. e4. doi: 10.1016/j.neurobiolaging.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brainerd CJ, Reyna VF, Petersen RC, et al. The apolipoprotein E genotype predicts longitudinal transitions to mild cognitive impairment but not to Alzheimer's dementia: findings from a nationally representative study. Neuropsychology. 2013 Jan;27(1):86–94. doi: 10.1037/a0030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Archives of neurology. 2006 Mar;63(3):435–40. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 11.Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Archives of neurology. 2002 Apr;59(4):601–6. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 12.Bunce D, Fratiglioni L, Small BJ, Winblad B, Backman L. APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology. 2004 Sep 14;63(5):816–21. doi: 10.1212/01.wnl.0000137041.86153.42. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Schneider JA, Barnes LL, et al. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Archives of neurology. 2002 Jul;59(7):1154–60. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 14.Payami H, Montee KR, Kaye JA, et al. Alzheimer's disease, apolipoprotein E4, and gender. JAMA : the journal of the American Medical Association. 1994 May 4;271(17):1316–7. [PubMed] [Google Scholar]
- 15.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA : the journal of the American Medical Association. 1997 Oct 22–29;278(16):1349–56. [PubMed] [Google Scholar]
- 16.Bretsky PM, Buckwalter JG, Seeman TE, et al. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer disease and associated disorders. 1999 Oct-Dec;13(4):216–21. doi: 10.1097/00002093-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Payami H, Zareparsi S, Montee KR, et al. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. American journal of human genetics. 1996 Apr;58(4):803–11. [PMC free article] [PubMed] [Google Scholar]
- 18.Manolio TA, Bailey-Wilson JE, Collins FS. Genes, environment and the value of prospective cohort studies. Nature reviews Genetics. 2006 Oct;7(10):812–20. doi: 10.1038/nrg1919. [DOI] [PubMed] [Google Scholar]
- 19.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011 May;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer disease and associated disorders. 2006 Oct-Dec;20(4):210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 21.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer disease and associated disorders. 2007 Jul-Sep;21(3):249–58. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer disease and associated disorders. 2009 Apr-Jun;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.Tsai WY. Testing the Assumption of Independence of Truncation Time and Failure Time. Biometrika. 1990 Mar;77(1):169–77. [Google Scholar]
- 25.Gail MH, Graubard B, Williamson DE, Flegal KM. Comments on 'Choice of time scale and its effect on significance of predictors in longitudinal studies'. Stat Med. 2009 Apr 15;28(8):1315–7. doi: 10.1002/sim.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiner MW, Aisen PS, Jack CR, Jr, et al. The Alzheimer's disease neuroimaging initiative: progress report and future plans. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2010 May;6(3):202–11. e7. doi: 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruchaga C, Kauwe JS, Harari O, et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer's disease. Neuron. 2013 Apr 24;78(2):256–68. doi: 10.1016/j.neuron.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Science translational medicine. 2011 Nov 30;3(111):111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farlow MR. Should the ApoE genotype be a covariate for clinical trials in Alzheimer disease? Alzheimer's research & therapy. 2010;2(3):15. doi: 10.1186/alzrt39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Annals of the New York Academy of Sciences. 2004 Jun;1019:24–8. doi: 10.1196/annals.1297.005. [DOI] [PubMed] [Google Scholar]
- 31.Damoiseaux JS, Seeley WW, Zhou J, et al. Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012 Jun 13;32(24):8254–62. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raber J, Wong D, Buttini M, et al. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proceedings of the National Academy of Sciences of the United States of America. 1998 Sep 1;95(18):10914–9. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.