Abstract
Purpose
The risk of febrile neutropenia (FN) in cancer patients receiving chemotherapy is mainly due to the type of chemotherapy regimen and the presence of specific risk factors in patients. The recent trend of using a dose-dense treatment schedule has enhanced the risk of FN. In the present prospective study, we evaluated the feasibility of a reduction of duration of therapy with colony-stimulating factor (G-CSF) in a dose-dense regimen.
Methods
Between June 2002 and December 2011, 107 patients with a new diagnosis of non-Hodgkin lymphoma (NHL) receiving dose-dense chemotherapy, every 14 days, were included in the study. The primary endpoint was defined as the completion of planned chemotherapy cycles as scheduled. Secondary endpoints were median number of administered G-CSF doses (vials), incidence of FN, hospitalization and toxicity.
Results
The planned chemotherapy cycles (primary endpoint) were completed by 84.1 % of patients. The median number of G-CSF (lenograstim) doses administered for each patient was 24 (range 10–35), which corresponds to a median of five vials (range 0–10) for each cycle. Grades 3–4 toxicities, related to G-CSF administration, included neutropenia and thrombocytopenia (14.0 and 1.9 %, respectively). No grades 3–4 bone pain was detected. The incidence of FN and hospitalization was 9.3 % (10/107) and 4.5 % (5/107), respectively.
Conclusions
Reduced dosage of G-CSF allows dose-dense chemotherapy scheduling, limits exposure to G-CSF and also represents an opportunity for cost savings.
Keywords: G-CSF, Lenograstim, Nadir, Neutropenia, NHL, Pegfilgrastim
Introduction
Chemotherapy-induced febrile neutropenia (FN) is one of the most common adverse events in cancer patients receiving myelosuppressive drugs [1]. The risk of FN is mainly due to the type of chemotherapy regimen and the presence of specific risk factors (age, gender, comorbidities) [2]. The risk of FN is increased by the recent trend of using a dose-dense treatment schedule compared to a standard-dose chemotherapy regimen [2].
Prophylactic administration of granulocyte colony-stimulating factor (G-CSF) provides protection for patients at risk of FN in reducing hospital admissions, antibiotic usage and the need for dose reductions or delays during chemotherapy administration, which are associated with poorer oncological outcome [1, 2].
The intensity (frequency and total dose) of chemotherapy is a major factor to be taken into account when assessing the risk of FN and the possible administration of G-CSF prophylaxis. As suggested by the European Organisation for Research and Treatment of Cancer (EORTC) guidelines, G-CSF should be administered as primary prophylaxis for FN in cancer patients receiving dose-dense chemotherapy [2].
According to international guidelines [2, 3] and registrational clinical trials [4–6], prophylaxis with daily subcutaneous (s.c.) administration of G-CSF (filgrastim or lenograstim) should start from 24 to 72 h after chemotherapy and continued until sufficient/stable post-nadir absolute neutrophil count (ANC) recovery [1]. Alternatively, a single s.c. administration of pegfilgrastim (6 mg) is considered equally effective and comparable to 11 injections of daily G-CSF [4–6]. The return to normal range of the absolute ANC requires approximately 9–14 daily G-CSF injections per chemotherapy cycle [1]. A large survey has challenged these indications and highlighted that the mean G-CSF administration is 5.5 days: only 9.3 % of patients exceed 7 days, and 6 % exceed 10 days, confirming that the timing and doses of adequate daily G-CSF prophylaxis are still debatable [7].
In clinical practice, alternative G-CSF schedules have been tested, suggesting that reduction of the number of G-CSF administrations without altering the outcome is feasible [8–10]. A shorter G-CSF schedule may reduce the risk and severity of short-term side effects and is also more cost-effective [11–15]. For patients receiving dose-dense chemotherapy regimens, the days of G-CSF administration should be planned accurately since it should be given before nadir onset and far from chemotherapy administration, avoiding the toxic priming effect of G-CSF [1]. The choice of timing of G-CSF administration, compared to chemotherapy, is chosen by the physician considering the risks, benefits and costs. Based on these considerations, we evaluated the feasibility of administering fewer doses of G-CSF (lenograstim) during treatment of non-Hodgkin lymphoma (NHL) with a dose-dense rituximab-cyclophosphamide, vincristine, doxorubicin, prednisone (R-CHOP)-14 regimen.
Methods
This was a single-centre, prospective, non-comparative study conducted in the Haematology Unit of Careggi Hospital (Florence, Italy) according to Good Clinical Practice guidelines and the Declaration of Helsinki and was approved by the local ethics committee. Patients with histologically or cytologically confirmed NHL, at any stage, and eligible for a first-line R-CHOP regimen every 14 days were enrolled in the study. Patients were required to be >18 years of age, with an Eastern Cooperative Oncology Group performance status of zero to one and normal hepatic and renal functions. Normal cardiac (left ventricular ejection fraction), neutrophil count of ≥1,500 × 109/l, haemoglobin of ≥9 g/dl and platelet count of >100,000 were required before the first cycle. Patient candidates for multiple-day/high-dose chemotherapy or bone marrow/peripheral blood stem cell transplantation and patients who were pregnant or breastfeeding were excluded.
Informed consent was obtained from all patients at study entry. The R-CHOP regimen was rituximab the day before chemotherapy (day 0) and cyclophosphamide of 750 mg/m2, doxorubicin of 50 mg/m2 and vincristine of 1.4 mg/m2 (max. 2 mg) on day 1 plus prednisone of 100 mg orally for 5 days. The R-CHOP regimen was administered every 14 days for six cycles. Lenograstim was administered subcutaneously at a dose of 263 mcg from day 5 to day 11 (seven vials). Blood cell counts were obtained the day of rituximab administration (on day 0 of each cycle), and if patients reached a number of leucocytes over 20,000/mm3, the lenograstim dose was reduced by one vial (Fig. 1). This reduction was applied in a sequential order.
Fig. 1.
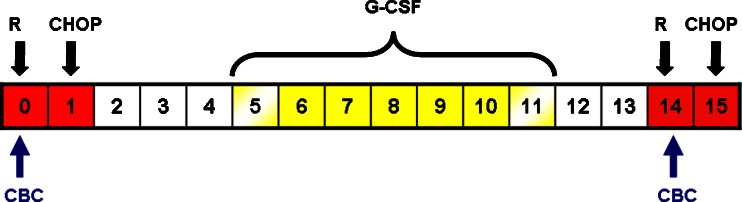
Treatment schedule of the first chemotherapy administration and study evaluations. The red boxes indicate days of chemotherapy administration; yellow boxes indicate days of G-CSF administration, and yellow-shaded boxes (day 5 and day 11) indicate the potential days of G-CSF reduction according CBC results; CBC, complete blood count; R, rituximab; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone
The primary endpoint of the study was completion of planned chemotherapy cycles, defined as the percentage of patients experiencing dose reduction or treatment delays due to haematological toxicity. Secondary endpoints included the proportion of patients needing hospitalization and those experiencing febrile episodes, defined as a single temperature of >38.3 °C (101 °F) or a sustained temperature of ≥38 °C (100.4 °F) for more than 1 h.
Secondary endpoints included evaluation of complete remission rate, progression-free survival (PFS) and overall survival (OS). Disease status was assessed between the third and fourth R-CHOP cycles and at the end of the therapy. Response to therapy was evaluated with standard response criteria for lymphoma [16]. PFS was defined as the time between the first chemotherapy administration and the occurrence of disease relapse, disease progression, death from any cause or last follow-up. OS was defined as the time from first chemotherapy administration until death or the date of last follow-up when the patient was known to be alive.
Toxicity was graded using the Common Terminology Criteria for Adverse Events (CTCAE) v.4.03. All adverse events attributed to G-CSF administration that occurred during the study, as observed by the investigator or reported by the subject, were recorded.
If a cycle was delayed for seven or more days because of myelosuppression caused by the prior cycle, doses of chemotherapy agents were reduced by 25 %. In case of intolerance or in the case of two delays, treatment was switched to R-CHOP-21. Endpoints were evaluated during all planned R-CHOP-14 cycles. Patients were followed up for clinical response to chemotherapy and survival until progression or death.
Descriptive statistics refer to all included patients. For continuous variables, the median, minimum and maximum values were calculated. For each discrete variable, the number of cases in each category, in relation to all cases with non-missing values of that variable, was calculated.
Sample size was calibrated with the aim to obtain primary endpoint estimate with adequate precision. A total sample size of 110 patients ensures a precision quantified in the order of 15 % assuming that a primary endpoint estimate equals to 80 %. Precision is measured by the width of the two-sided 95 % confidence interval of the primary endpoint rate. The Score-Wilson Confidence Interval Formula implemented in PASS 2008 software was employed for sample size computations [17].
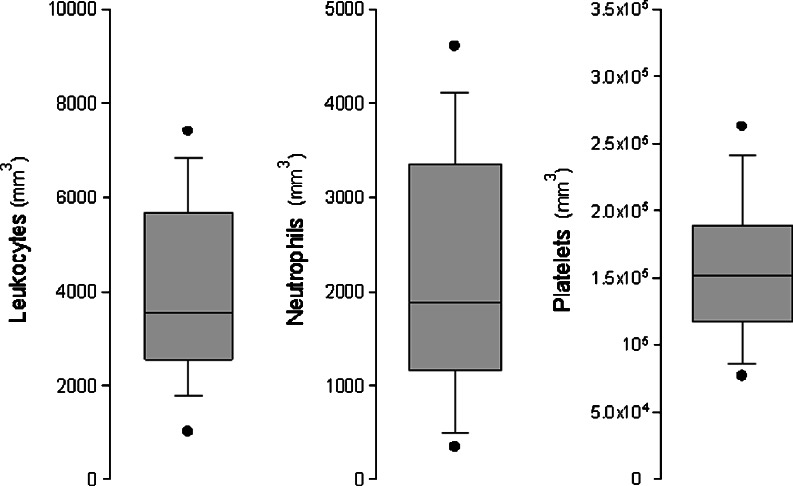
The distribution of leukocytes, neutrophils and platelet data were descriptively analysed using box-plot graphs. The boundary of the box closest to zero indicates the 25th percentile, a line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers above and below the box indicate the 90th and 10th percentiles. In addition, the 95th and 5th percentiles were graphed as points.
Results
Between June 2002 and December 2011, 107 patients with a new diagnosis of NHL (92.5 % DLBCL, 7.5 % follicular lymphoma grade IIIb) were included in the study. The median age was 61 years (range 20–75); 29 % of patients had a high-intermediate or high international prognostic index (IPI).
Bulky disease, elevated LDH and symptoms were present in 34.6 % (37/107), 31.8 % (34/107) and 42.1 % (45/107) of patients, respectively. Twenty-two patients (20.6 %) had bone marrow involvement. The median number of administered R-CHOP cycles was 6 (range 3–6). The main patient characteristics are reported in Table 1.
Table 1.
Patient characteristics
| Variable | % (N) |
|---|---|
| Non-Hodgkin lymphoma | 100 (107) |
| Age (years) | |
| Median (range) | 61 (20–75) |
| Gender | |
| Male | 61.7 (66) |
| Female | 38.3 (41) |
| Histology | |
| DLBCL | 92.5 (99) |
| FL-3b | 7.5 (8) |
| Stage | |
| I | 19.7 (21) |
| II | 33.6 (36) |
| III | 16.8 (18) |
| IV | 29.9 (32) |
| IPI score | |
| High | 10.3 (11) |
| Intermediate-high | 18.7 (20) |
| Intermediate-low | 23.3 (25) |
| Low | 47.7 (51) |
DLBCL diffuse large B cell lymphoma, FL-3b follicular lymphoma at stage 3b, IPI international prognostic index
All patients received a daily administration of G-CSF according to protocol (Fig. 1). The median number of lenograstim doses (vials) administered for each patient was 24 (range 10–35), which corresponds to a median of five vials (range 0–10) for each cycle.
In the first 10 patients, we used seven vials of G-CSF after each cycle of chemotherapy (from day 5 to day 11). As the treatment was well-tolerated (according to a median ANC count of 6,100, range 3,140–10,600 mm3), we prospectively decided to reduce the number of vials of G-CSF to five (from day 6 to day 10).
The planned chemotherapy cycles of R-CHOP were completed by 84.1 % of patients as scheduled; 15.9 % of cases experienced treatment delay due to neutropenia and thrombocytopenia in 15 (14.0 %) and 2 (1.9 %) patients, respectively. Three patients (2.8 %) switched to R-CHOP-21 scheduling because of poor tolerance to the dose-dense chemotherapy or more than one delay. The incidence of febrile episodes was 9.3 % (10/107), and 4.5 % (5/107) of patients required hospitalization due to febrile neutropenia.
Haematological recovery was regularly assessed in each patient before rituximab administration, and the median values of leucocytes, neutrophils, haemoglobin and thrombocytes were 3,550 mm3 (range 400–13,600), 54 % (range 10–79), 10.7 g/dL (range 5.8–15.5) and 152,000 mm3 (range 43,000–328,000), respectively. The box-plot for leukocytes, neutrophils and platelets is shown in Fig. 2.
Fig. 2.
Box-plots for leukocytes, neutrophils and platelets evaluated at each cycle before rituximab administration of the R-CHOP regimen
Clinical response was observed in 96.2 % (103/107) of patients: the complete response rate was 86.9 % (93/107), while the partial response rate was 9.3 % (10/107). After a median follow-up of 25 months (range 4–90 months), PFS was 77.6 % (83/107) and overall survival was 86.9 % (93/107).
Grades 3–4 toxicities related to chemotherapy have been neutropenia (14.0 %) and thrombocytopenia (1.9 %). No grades 3–4 toxicity has been reported related to G-CSF administration; particularly, no grades 3–4 bone pain was observed.
Discussion
FN is a major cause of morbidity and mortality in patients receiving chemotherapy, leading to a decrease in the dose of cytotoxic agents, delay in the intervals between cycles and limiting the dose intensity of the treatment [1]. Dose-dense chemotherapy is increasingly used in an attempt to improve long-term clinical outcomes [1].
According to EORTC guidelines, prophylaxis with G-CSF is suggested in a dose-dense chemotherapy regimen, but timing and doses of the most appropriate G-CSF administration is still a matter of debate [1]. Generally, the correct G-CSF prophylaxis should consider three factors: (i) day of chemotherapy administration, (ii) day of nadir onset (iii) and FN risk factors [18].
It is possible that the timing of G-CSF application, when optimally timed, might help to alleviate the harsh trough in neutrophil counts caused by chemotherapy [19].
There is clinical evidence for the efficacy of G-CSF in supporting delivery of dose-dense R-CHOP in both young and elderly NHL patients [1]. G-CSF is a fundamental drug during R-CHOP-14, as the short treatment cycle of 14 days would not otherwise allow sufficient time for bone marrow recovery between cycles [1]. Consistent with these data, we assessed the efficacy of lower lenograstim doses as FN prophylaxis in high-risk NHL patients receiving dose-dense R-CHOP regimens. In our study, a median of five (range 0–10) injections of lenograstim per cycle allowed the majority of patients (84.1 %) to complete the planned R-CHOP regimens. The amount of G-CSF also lowered the risk of febrile episodes (9.3 %), hospitalizations (4.7 %) and grade 4 neutropenia (14 %).
Recently, in the oncology setting, Badalamenti and colleagues also defined a reduced dosage of G-CSF as FN prophylaxis in soft tissue sarcoma patients [20]. The authors found that 5-day lenograstim treatment is efficient as prophylaxis of FN and allowed the maintenance of chemotherapy dose intensity.
No randomized clinical trials, in either pegylated or daily G-CSG forms (filgrastim and lenograstim), have assessed the lowest fully effective dose of G-CSF needed for FN prophylaxis [4–6]. G-CSF administration has been protracted until post-nadir ANC recovery, but the exact time of nadir onset for the chemotherapy regimen remains unknown [21]. Recently, Ria and colleagues defined the nadir onset of most commonly used chemotherapy regimens in a haematologic setting in order to help physicians tailor the correct timing and duration of G-CSF prophylaxis, attempting to define the optimal timing and duration of G-CSF prophylaxis [21].
We speculate that, in our study, five doses of G-CSF were effective in allowing completion of R-CHOP cycles in NHL because we started before nadir onset (at day 9), as recently reported by Ria and colleagues [21], and far from chemotherapy administration, avoiding the priming effect of G-CSF [1]. The lowest fully effective dose of G-CSF allows limited exposure to G-CSF, which may be associated with potential long-term adverse events [8–10], and also represents an opportunity for cost savings [22, 23].
Vainas and colleagues have recently developed a new method for personalizing combined chemotherapeutic and G-CSF schedules for the most efficacious chemotherapy. Their method could reduce neutropenia by tailoring efficacious cytotoxic and supportive treatments, while minimizing side effects [18].
Future and randomized trials should clarify the exact role of G-CSF, the magnitude of its clinical benefit in terms of survival, the most appropriate dosage (number of vials/pegylated or not pegylated form) and time of administration.
In our study and recent publications [20, 21], the possibility to reduce number of G-CSF seems to be safe, efficacious and cost-saving (number of vials and use of pegylated), although more randomized trials are needed to define the correct timing and dosage of G-CSF to prevent FN, evaluating also quality of life, in patients receiving dose-dense chemotherapy.
Acknowledgments
The authors would like to thank the clinicians, patients, nurses and data managers who participated in the trial.
Conflict of interest
Dr Tania Perrone is an employee of Italfarmaco S.p.A., Italy. None of the other authors have any financial or other conflict to declare.
References
- 1.Aapro MS, Bohilus J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy—induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumors. Eur J Cancer. 2011;47(1):8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Aapro M, Crawford J, Kamioner D. Prophylaxis of chemotherapy-induced febrile neutropenia with granulocyte colony-stimulating factors: where are we now? Support Care Cancer. 2010;18(5):529–541. doi: 10.1007/s00520-010-0816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford J, Caserta C, Roila F. Hematopoietic growth factors: ESMO clinical practice guidelines for the applications. Ann Oncol. 2010;21(S5):v248–v251. doi: 10.1093/annonc/mdq195. [DOI] [PubMed] [Google Scholar]
- 4.Holmes FA, Jones SE, O’Shaughnessy S, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose finding study in women with breast cancer. Ann Oncol. 2002;13:903–909. doi: 10.1093/annonc/mdf130. [DOI] [PubMed] [Google Scholar]
- 5.Holmes FA, O’Shaughnessy S, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage III/IV breast cancer. J Clin Oncol. 2002;20:727–731. doi: 10.1200/JCO.20.3.727. [DOI] [PubMed] [Google Scholar]
- 6.Green MD, Koelbl H, Baselga J, et al. A randomized double blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14:29–35. doi: 10.1093/annonc/mdg019. [DOI] [PubMed] [Google Scholar]
- 7.Falandry C, Campone M, Carton C, et al. Trends in G-CSF use in 990 patients after EORTC and ASCO guidelines. Eur J Cancer. 2010;46:2389–2398. doi: 10.1016/j.ejca.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Cairo MS, Shen V, Krailo MD, et al. Prospective randomized trial between two doses of granulocyte colony-stimulating factor after ifosfamide, carboplatin and etoposide in children with recurrent or refractory solid tumors: a children’s Cancer Group report. J Pediatr Hematol Oncol. 2001;23:30–38. doi: 10.1097/00043426-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Papaldo P, Lopez M, Marolla P, et al. Impact of five prophylactic filgrastim schedules on hematologic toxicity in early breast cancer patients treated with epirubicin and cyclophosphamide. J Clin Oncol. 2005;23:6908–6918. doi: 10.1200/JCO.2005.03.099. [DOI] [PubMed] [Google Scholar]
- 10.Hendler D, Rizel S, Yerushalmi R, et al. Different schedules of granulocyte growth factor support for patients with breast cancer receiving adjuvant dose-dense chemotherapy. A prospective nonrandomized study. Am J Clin Oncol. 2011;34:619–624. doi: 10.1097/COC.0b013e3181f94716. [DOI] [PubMed] [Google Scholar]
- 11.Wolff AC, Jones RJ, Davidson NE, et al. Myeloid toxicity in breast cancer receiving adjuvant chemotherapy with pegfilgrastim support. J Clin Oncol. 2006;24:2392–2394. doi: 10.1200/JCO.2006.05.7174. [DOI] [PubMed] [Google Scholar]
- 12.Le Deley MC, Suzan F, Cutuli B, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007;25(3):292–300. doi: 10.1200/JCO.2006.05.9048. [DOI] [PubMed] [Google Scholar]
- 13.Ferretti G, Lopez M, Terzoli E, et al. Myeloid toxicity in breast cancer patients receiving adjuvant chemotherapy: what is the appropriate use of filgrastim? J Clin Oncol. 2006;24(35):5618–5619. doi: 10.1200/JCO.2006.07.6398. [DOI] [PubMed] [Google Scholar]
- 14.Green V, Bounthavong M, Margileth DA et al (2010) Retrospective evaluation to examine efficacy and safety of half-dose pegfilgrastim in breast cancer patients receiving cytotoxic chemotherapy. J Clin Oncol (suppl; abstr 116)
- 15.Ramaekers RC, Olsen J, Obermiller AM et al (2012) Efficacy and safety of half-dose pegfilgrastim in cancer patients receiving cytotoxic chemotherapy. J Clin Oncol 30 (suppl; abstr 9110)
- 16.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 17.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Vainas O, Ariad S, Amir O, et al. Personalising docetaxel and G-CSF schedules in cancer patients by a clinically validated computational model. Br J Cancer. 2012;107:814–822. doi: 10.1038/bjc.2012.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shochat E, Rom-Kedar V. Novel strategies for granulocyte colonystimulating factor treatment of severe prolonged neutropenia suggested by mathematical modeling. Clin Cancer Res. 2008;14(20):6354–6363. doi: 10.1158/1078-0432.CCR-08-0807. [DOI] [PubMed] [Google Scholar]
- 20.Badalamenti G, Incorvaia L, Provenzano S, et al. Lenograstim in preventing chemotherapy-induced febrile neutropenia in patients with soft tissue sarcoma. Anticancer Res. 2013;33(2):679–684. [PubMed] [Google Scholar]
- 21.Ria R, Reale A, Moschetta M, et al. Neutropenia and G-CSF in lymphoproliferative diseases. Hematology. 2012;18(3):131–137. doi: 10.1179/1607845412Y.0000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potosky AL, Malin JL, Kim B, et al. Use of colony-stimulating factors with chemotherapy: opportunities for cost savings and improved outcomes. J Natl Cancer Inst. 2011;103:979–982. doi: 10.1093/jnci/djr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30(14):1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]