Summary
Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, Boyd Barr D, Schrader SM, Kim S, Chen Z, Sundaram R, on behalf of the LIFE Study. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development – the LIFE Study. Paediatric and Perinatal Epidemiology 2011; 25: 413–424.
The relationship between the environment and human fecundity and fertility remains virtually unstudied from a couple-based perspective in which longitudinal exposure data and biospecimens are captured across sensitive windows. In response, we completed the LIFE Study with methodology that intended to empirically evaluate a priori purported methodological challenges:
implementation of population-based sampling frameworks suitable for recruiting couples planning pregnancy;
obtaining environmental data across sensitive windows of reproduction and development;
home-based biospecimen collection; and
development of a data management system for hierarchical exposome data.
We used two sampling frameworks (i.e. fish/wildlife licence registry and a direct marketing database) for 16 targeted counties with presumed environmental exposures to persistent organochlorine chemicals to recruit 501 couples planning pregnancies for prospective longitudinal follow-up while trying to conceive and throughout pregnancy. Enrolment rates varied from <1% of the targeted population (n = 424 423) to 42% of eligible couples who were successfully screened; 84% of the targeted population could not be reached, while 36% refused screening. Among enrolled couples, ~85% completed daily journals while trying; 82% of pregnant women completed daily early pregnancy journals, and 80% completed monthly pregnancy journals. All couples provided baseline blood/urine samples; 94% of men provided one or more semen samples and 98% of women provided one or more saliva samples. Women successfully used urinary fertility monitors for identifying ovulation and home pregnancy test kits.
Couples can be recruited for preconception cohorts and will comply with intensive data collection across sensitive windows. However, appropriately sized sampling frameworks are critical, given the small percentage of couples contacted found eligible and reportedly planning pregnancy at any point in time.
Keywords: cohort, environment, fecundity, fertility, LIFE Study, preconception, pregnancy, study design, enrolment
Introduction
Successful human reproduction and development requires completion of a series of highly timed and interrelated processes involving both partners, underscoring the importance of a couple-based approach when assessing couple-dependent outcomes such as conception or pregnancy.1 A number of sensitive windows underlie these processes and capture key outcomes ranging from spermatogenesis and folliculogenesis to ovulation followed by fertilisation, implantation and in utero development.2 Of late, considerable interest has arisen regarding the relationship between early or in utero development and health across the lifespan, globally referred to as the developmental origins of health and disease (DOHaD) paradigm.3 This paradigm encompasses both the testicular dysgenesis syndrome (TDS) and ovarian dysgenesis syndrome (ODS) hypotheses, which posit that fecundity impairments in men and women may have an in utero origin with implications for both urological and gynaecological health and later onset diseases.4,5 The underlying mechanisms for the observed relationships are largely unknown, given the absence of exposure data during sensitive windows or so-called exposome data.6
A number of methodological challenges confront investigators in the design of epidemiological research aimed at assessing environmental influences on reproduction and development and questions pertaining to the DOHaD hypothesis. First, prospective cohort designs are best suited for this avenue of research and require the recruitment of couples discontinuing contraception prior to attempting pregnancy. Couples are followed throughout pregnancy if pregnancy is achieved. This allows the measurement of both baseline and time-sensitive exposures for both partners of the couple, given growing evidence suggesting that parental exposures are important for fecundity and fertility. For example, partners’ preconception cigarette smoking, caffeine and alcohol consumption and body mass indices are reported to affect couple fecundity7,8 including among couples undergoing assisted reproductive technologies.9,10
The absence of readily available population-based sampling frameworks suitable for delineating couples of reproductive age with explicit pregnancy intentions is perhaps one of the most pressing challenges to overcome.11 Another notable challenge is the intensive data collection protocol required for capturing prospective longitudinal data on an hourly, daily or monthly basis consistent with reproductive events such as hormonal profiles, menstruation or ovulation along with bio-specimen collection timed to sensitive windows for the study's outcome(s). As couples attempting pregnancy are typically healthy, do not necessarily ‘report’ their pregnancy intentions and reside in disparate locations, the use of the home for collecting exposure and outcome data and biospecimens is particularly attractive and helps to minimise participant burden. Another important challenge is the development of a web-based data management infrastructure that offers field staff support in communicating with study participants, managing data collection, tracking biospeci-mens, and technological capabilities for managing the complex hierarchical exposome data structure such research produces.
We designed and successfully completed the Longitudinal Investigation of Fertility and the Environment (LIFE) Study to achieve two goals: (i) to empirically evaluate the methodology for designing a population-based prospective cohort design with longitudinal collection of data and biospecimens during sensitive windows of human reproduction and development; and (ii) to assess the effects of environmental chemicals in the context of couples’ life styles on five sensitive outcomes, viz., time-to-pregnancy, infertility, pregnancy loss, gestation at delivery and birth size. Thus, the LIFE Study developed a methodology relevant for couples residing in areas with known environmental chemical exposures. This paper addresses the LIFE Study's first goal.
Methods
Study design and population
The LIFE Study used a prospective cohort design suitable for following couples across sensitive windows of human reproduction and development. Specifically, couples interested in becoming pregnant in the next 2 months were recruited and followed until pregnant or up to 12 months of attempting pregnancy between 2005 and 2009. In addition, pregnant women were followed to delivery or through a pregnancy loss. The target population comprised individuals residing in four Michigan counties with reported exposure to persistent organochlorine chemicals (i.e. Berrien, Calhoun, Ingham, Kalamazoo) and 12 counties in Texas (i.e. Aransas, Brazoria, Calhoun, Chambers, Fort Bend, Galveston, Harris, Jefferson, Matagorda, Montgomery, Nueces and Orange) with presumed exposure to persistent environmental chemicals. By design, the targeted population comprising potentially exposed individuals and their partners was intended to be inclusive of couples irrespective of gynaecological and/or urological history who were interested in becoming pregnant apart from sterilised couples or those who were told by a physician that they could not achieve pregnancy without medical assistance. The inclusion criteria were: (a) married or in a committed relationship; (b) females aged 18–40 and males aged 18+ years; (c) able to communicate in English or Spanish; (d) self-reported menstrual cycles ranging from 21 to 42 days consistent with the fertility monitor's requirement; and (e) no hormonal birth control injections in the past 12 months, given the uncertain return of ovulation. Our a priori sample size was 500 couples powered to be able to detect a reduction in fecundity in relation to differences in environmentally relevant concentration of organochlorine chemicals. We used data from the New York State Angler Cohort Study for the range of power assumptions, as it is the only prospective time-to-pregnancy study that had individual serum organochlorine concentrations for participating women.12
A different sampling framework was utilised in each geographical location to recruit individuals, given the absence of a uniformly available approach in each state for identifying and recruiting reproductive aged couples planning pregnancies. This provided a unique opportunity to assess the efficacy of sampling frameworks on recruitment. Specifically, the Texas site used the Texas Parks and Wildlife Department's angler database for recruitment, while the Michigan site used a commercially available marketing database – InfoUSA® – that utilised recruitment filters to identify individuals with fishing interests (e.g. fishing magazine subscriptions). The former sampling framework comprises all commercial and recreational fishing licences in Texas, and was stratified by licence type and census track to achieve diversity. As race and ethnicity are not reported in this registry, the North American Association of Central Cancer Registries Hispanic Identification Algorithm was used to help oversample on presumed Hispanic ethnicity. The InfoUSA® sampling framework was assessed in two Michigan counties and found to provide complete coverage of households based upon data from the 2000 Census assuming some level of migration. Additional efforts were undertaken to oversample under-represented minorities and individuals living in census tracts with low median household incomes (≥$40 000). Prior to mailing letters, contact information was updated with commercially available software (e.g. Telematch and Metronet).
Recruitment began with an introductory letter and study brochure mailed to targeted individuals followed by a telephone call within 2 weeks, at which time contacted individuals were screened for eligibility. Up to 10 follow-up telephone calls were placed at varying times and days to reach targeted individuals by two call centres (http://ppri.tamu.edu; http://www.rti.org) consistent with their established survey methods. Each partner of the couple was individually screened for enrolment even if that meant additional telephone calls. The contact information of eligible and preliminarily consenting couples was given to the research coordinators at each site, who then assigned interviewers to couples. In-home interviews and training sessions were scheduled at the couple's convenience. The LIFE Study was conducted between 2005 and 2009.
Data and biospecimen collection
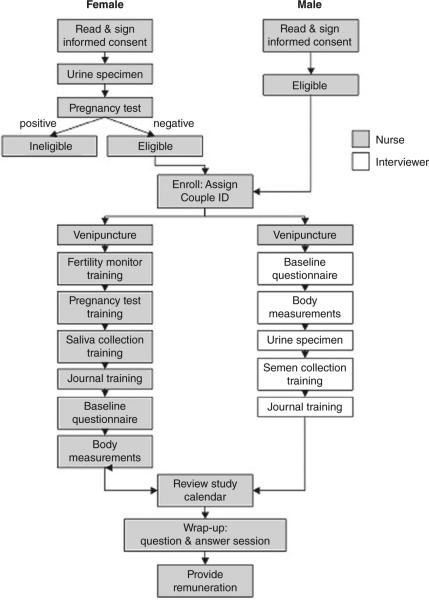
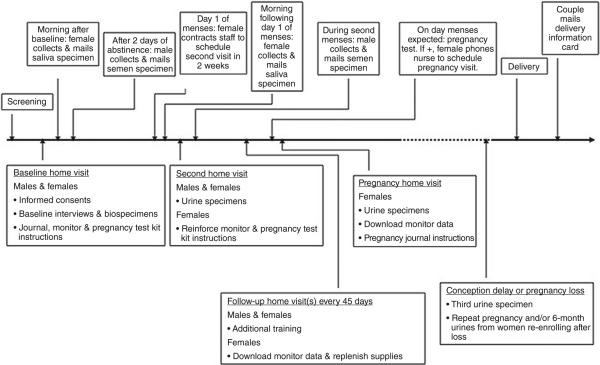
Figure 1 illustrates the process for consenting, enrolling and interviewing couples including biospecimen collection while providing instruction for using daily journals, fertility monitors, pregnancy test kits and collection of future biospecimens. A research nurse and assistant visited the home to facilitate simultaneous interviewing and training of the couple, while minimising participant burden. The timeline for time-varying data and biospecimen collection beginning with a baseline interview followed by completion of daily journals by each partner while attempting pregnancy is illustrated in Figure 2. Home-based follow-up visits to ensure compliance with the study and to deliver additional supplies and download data from the monitor were planned every 45 days, while the couple was attempting to conceive. Women achieving pregnancy continued daily journal data collection throughout 8 weeks post-conception using the estimated day of ovulation determined by fertility monitors, at which time pregnancy data collection was captured in monthly journals targeted to events occurring during specific weeks of gestation. A home pregnancy visit was conducted to train women in the use of pregnancy journals and to collect early pregnancy biospecimens. Couples returned standardised birth announcements following delivery allowing for the capture of delivery date, infant gender and birth size (birthweight, length and head circumference, delivery mode). Couples experiencing a pregnancy loss completed a brief pregnancy loss card that collected information regarding the temporal ordering of signs and symptoms associated with the loss. Also, couples were encouraged to continue in the study with another attempt to conceive.
Figure 1.
Illustration of the enrolment and baseline interview process, LIFE Study 2005–2009.
Figure 2.
Illustration of time-varying data collection, LIFE Study, 2005–2009.
Upon entering the home for the baseline visit, the female participant was asked to take a home pregnancy test to verify the absence of a pre-existing pregnancy. If positive, the couple was thanked and the research team departed; if negative, the team completed couple enrolment and began the baseline (~20 min) interview with each partner of the couple questioned separately prior to attempting pregnancy to ascertain medical and reproductive history and behaviours in the past 12 months that are purported to affect fecundity and fertility (e.g. exercise, use of tobacco products, alcohol and caffeine). Included in the baseline was a four-item version of Cohen's Perceived Stress Scale13 shown to be a valid measure of perceived stress. All data were entered into databases preloaded onto laptop computers and synchronised with the web-based data management system (AdvantageEDCSM; EMMES Corp. http://www.emmes.com) prior to the baseline visit. This system allowed for remote offline enrolment and baseline data collection followed by immediate back-up onto flash drives (memory sticks) and subsequent uploading to the web-based data management structure. This online and offline data entry system used a customised computer-assisted personal interview programme to minimise burden while ensuring quality control. All participants were weighed using standardised Tanita scales and measured for height using a portable Shorr Board stadiometer. Hip and waist circumferences were measured using a standardised measuring tape and anatomical markings consistent with the anthropometric protocol adapted from the third National Health and Nutrition Examination Survey.14
Women were given and instructed in the use of the Clearblue® Easy fertility monitor and the digital Clearblue® Easy pregnancy tests. The Clearblue® Easy monitor tracks daily levels of oestrone-3-glucuronide (E3G), a metabolite of oestradiol, and luteinising hormone, using urinary test sticks and stores summary information for up to six test cycles, which is used in optimising the prediction of fertility status according to previous test results. Detailed daily monitor data are retained by the monitor memory for up to two cycles allowing for the transfer of such data into the study's web-based data management system. The monitor is reported to be highly accurate (99%) in detecting the luteinising hormone surge and in predicting peak fertility (91%) in comparison with ultrasonography, which is considered to be the gold standard,15 and for timing biospecimen collection to the menstrual cycle.16 The pregnancy test kit was selected because the results display ‘pregnant’ or ‘not pregnant’ in lieu of more subjective lines and symbols and its demonstrated sensitivity and reliability for detecting 25 mIU/mL of human chorionic gonadotropin.17
From both partners of the couple, the nurse interviewer obtained non-fasting blood (~20 mL) for quantification of persistent organic pollutants (i.e. organochlorine pesticides, perfluorinated chemicals, polybrominated diphenyl ethers, polychlorinated biphenyls), metals, cotinine and serum lipids along with non-fasting urine (~120 mL) samples for the quantification of phytoestrogens and creatinine. Blood was collected into a 3-mL EDTA purple top tube and either one 15-mL or two 10-mL red top tubes. All remaining biospecimens were timed to sensitive windows as illustrated in Figure 2:
first-morning saliva samples were obtained the morning following the interview and at the time the menses button was pressed on the fertility monitor;
semen samples were obtained following 2 days of abstinence after the baseline interview and 1 month later;
urine samples were obtained from both partners at the second home visit (or second trying cycle);
additional urine samples were obtained at the first pregnancy visit or after 6 months without conception.
We purposefully tried to time baseline specimens as early as possible after enrolment to minimise missed samples among couples conceiving quickly. The nurse returned the blood and urine to the laboratory for processing, while couples processed and returned semen and saliva samples via an overnight delivery service using the provided collection devices and shipping supplies. All biospecimens were bar-coded for tracking through all phases, from collection to receipt by the local study site to receipt by the National Institute for Occupational Safety and Health's andrology laboratory, a commercial saliva analysis laboratory (http://www.salimetrics.com), or the laboratories at the Centers for Disease Control and Prevention and, ultimately, in the National Institute of Child Health and Human Development repository.
Couples were remunerated up to $75 as follows: $25 for the blood, $5 for each of the two urine specimens and $20 for each semen or saliva sample. Additional non-cash incentives (e.g. books, candles, newsletters, photo albums) during follow-up were also provided. Full institutional review board approval at all collaborating institutions was granted for this work; written informed consent was obtained from all participating couples. The web-based data system enabled ongoing monitoring of protocol violations and health alerts, which included blood lead levels ≥ 25 μg/dL, azoospermia in both semen samples or three consecutive cycles without ovulation detection. These alerts were intended to identify couples that might benefit from medical care. A Certificate of Confidentiality was granted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development in August 2004 as an added confidentiality and privacy measure, and the Office of Management and Budget approval for the conduct of the study was obtained in March 2005.
Web-based data management
The database was a priori designed for this longitudinal observational study to address key elements for successfully managing the study in disparate locations. The system was designed to: (i) manage all aspects of the study including multiple remote users using standard web browsers that might be found in the home; (ii) assign site-preferred participant identifiers defined by enrolment site and a unique code randomly generated; (iii) provide flexibility for either offline or online data collection by site staff, and for mail-in or web-based participant journals; (iv) permit tracking of bio-specimens from collection through shipment and archiving; (v) provide 24/7 access for all investigators and research staff while maintaining a public website for study participants (http://www.lifestudy.us); (vi) monitor adherence and timely data submission; (vii) provide timely notification of protocol violations and health alerts; (viii) implement ongoing quality control procedures; and (ix) accommodate the hierarchical data structure.
Results
The research sites completed weekly production reports to monitor the study from the time the study samples were loaded into computerised telephone calling centres until completion of the study. Irrespective of sampling framework, the majority (n = 354 371, 65%) of households could not be contacted or screened, despite 10 attempts on varying days and times (Table 1). The screening refusal rate was 36% (n =126 459). Among the 51 715 (12%) households successfully screened, only 2% were eligible for enrolment. As Table 2 reflects, the leading reasons for couples screening ineligible were (in descending order): age (27%), not in a committed relationship (19%) and not interested in becoming pregnant (19%). Thus, 2% of the target population were successfully contacted, recruited and enrolled in the study between June 2005 and February 2009 including 104 couples from Michigan counties and 397 from Texas counties; 69% of the enrolled cohort successfully completed the study protocol.
Table 1.
Recruitment yield from targeted reference population by research site, LIFE Study, 2005–09
| Michigan site n (%) | Texas site n (%) | Total n (%) | |
|---|---|---|---|
| Telephone sample loaded | 69 336 (100) | 355 087 (100)a | 424 423 (100)a |
| Unable to complete screening | 49 643 (72) | 304 728 (86) | 354 371 (84) |
| Non-working telephone number | 4884 (10) | 138 627 (45) | 143 511 (41) |
| Refusals | 20 149 (41) | 106 310 (35) | 126 459 (36) |
| Unable to reach, deceased, language barrier | 24 610 (49) | 59 791 (20) | 84 401 (24) |
| Completed screening | 19 693 (28) | 32 022 (9) | 51 715 (12) |
| Screened eligible | 207 (1) | 981 (3) | 1188 (2) |
| Screened ineligible | 19 486 (99) | 31 041 (97) | 50 527 (98) |
| Recruited | 203 (1) | 981 (3) | 1184 (2) |
| Enrolled (each partner signed consent) | 104 (51) | 397 (40) | 501 (42) |
| Completed studyb | 73 (70) | 275 (69) | 348 (69) |
LIFE Study recruitment was completed in Michigan on 31 December 2006 and in Texas on 15 February 2009. Percentages are rounded.
Approximately 5% (n = 18 337 individuals) of the sample targeted by the call-in centre had not been contacted at the time recruitment was completed.
Includes couples with a birth, pregnancy loss (with or without continuation in the study) or who completed 12 months of trying without a pregnancy.
Table 2.
Reasons for ineligibility by site, LIFE Study, 2005–09
| Reason – screened ineligible | Michigan site (19 486) n (%) | Texas site (31 041) n (%) | Total (50 527) n (%) |
|---|---|---|---|
| Age | 5840 (30) | 7611 (25) | 13 451 (27) |
| Not interested in becoming pregnant | 1683 (9) | 8131 (26) | 9814 (19) |
| Not in committed relationship | 1760 (9) | 7684 (25) | 9444 (19) |
| Moved or is moving outside study area | 7031 (36) | 1256 (4) | 8287 (16) |
| Surgically sterile/unable | 1467 (8) | 1823 (6) | 3290 (7) |
| Off contraception >2 months | 801 (4) | 1835 (6) | 2636 (5) |
| Currently pregnant | 257 (1) | 1694 (5) | 1951 (4) |
| Menstrual cycles outside range | 328 (2) | 727 (2) | 1055 (2) |
| Birth control shota in past 12 months | 46 (<1) | 30 (<1) | 76 (<1) |
| Not sexually active | 71 (<1) | – | 71 (<1) |
| Other | 202 (1) | 250 (<1) | 452 (<1) |
Injection.
Withdrawals from the cohort occurred at varying times as noted in Table 3 with the majority occurring before, at or shortly after enrolment, largely because of a change in interest. Reasons for withdrawing shortly before/after recruitment were varied but did not differ notably by sampling framework, with the most common reasons being no longer interested (27%) or the inability to locate the couple (20%). Reasons for withdrawing before or after pregnancy were similar across sites, and largely attributed to non-compliance with journals (46%) or no longer interested in the study (20%).
Table 3.
Withdrawal reasons by timing of study protocol, LIFE Study, 2005–09
| Timing & reason | Michigan n (%) | Texas n (%) | Total n (%) |
|---|---|---|---|
| Before or upon recruitment | 93 (100) | 581 (100) | 674 (100) |
| No longer interested | 25 (27) | 155 (27) | 180 (27) |
| Cannot contact/schedule couple | 21 (23) | 112 (19) | 133 (20) |
| Self-reported pregnancy | 14 (15) | 62 (11) | 76 (11) |
| Change in pregnancy intentions | 17 (18) | 43 (7) | 60 (9) |
| Othera | – | 57 (10) | 57 (8) |
| Moved/will be moving | 3 (3) | 44 (8) | 47 (7) |
| Off birth control >2 months | – | 38 (7) | 38 (6) |
| Refused biospecimens | 5 (5) | 29 (5) | 34 (5) |
| Too busy | 3 (3) | 16 (3) | 19 (3) |
| Wishes to pursue infertility treatment | – | 17 (3) | 17 (3) |
| No longer with partner | 3 (3) | 2 (<1) | 5 (<1) |
| Health reasons | 2 (2) | 3 (<1) | 5 (<1) |
| Unknown | – | 3 (<1) | 3 (<1) |
| Withdrew before pregnancy (reason) | 24 (100) | 90 (100) | 114 (100) |
| Non-compliant/insufficient journals returned | 12 (50) | 41 (46) | 53 (46) |
| No longer interested | 6 (25) | 17 (19) | 23 (20) |
| Pursuing infertility treatment | 2 (8) | 10 (11) | 12 (11) |
| No longer with partner | 3 (13) | 6 (7) | 9 (8) |
| Family illness/health reasons | – | 4 (4) | 4 (4) |
| Male partner found to have azoospermia | – | 5 (6) | 5 (4) |
| Otherb | – | 4 (4) | 4 (4) |
| Changes in pregnancy intention | 1 (4) | 1 (1) | 2 (2) |
| Moved | – | 2 (2) | 2 (2) |
| Does not wish to give biospecimens | – | – | – |
| Withdrew after pregnancy (reason) | 8 (100) | 32 (100) | 40 (100) |
| Non-compliant/insufficient journals returned | 3 (38) | 17 (53) | 20 (50) |
| No longer wishes/eligible after pregnancy loss | 5 (63) | 14 (44) | 19 (48) |
| Family illness/health reason | – | 1 (3) | 1 (3) |
Recruitment denotes willingness to schedule baseline interview among couples screened eligible.
Stated reasons included decided to postpone trying, study requires too much, and found to be ineligible.
Stated reasons included no longer wishes to use monitor, entering menopause and disruption from hurricanes.
Table 4 demonstrates the high degree of compliance observed among enrolled couples irrespective of partner. Virtually all couples completed the baseline data and biospecimens collection with ~85% completing daily journals. An overwhelming majority of couples chose hardcopy journals to the online option, including couples who attempted but later abandoned online entry. Good response was obtained for collecting two challenging time-specific biospecimens – the second semen sample (77% completion) and a urine sample from women not achieving pregnancy within six cycles (77% completion).
Table 4.
Data and biospecimen completeness by site, LIFE Study, 2005–09
| Data or biospecimen collection | Michigan (n = 104) % | Texas (n = 397) % | Total (n = 501) % |
|---|---|---|---|
| Data collectiona | |||
| Male baseline interview | 100 | 100 | 100 |
| Female baseline interview | 100 | 100 | 100 |
| Male daily journal | 82 | 85 | 84 |
| Female daily journal | 84 | 88 | 87 |
| Daily early pregnancy journal | 80 | 82 | 82 |
| Monthly pregnancy journal | 76 | 81 | 80 |
| Biospecimen collection | |||
| Male baseline blood | 100 | 99.2 | 99.4 |
| Female baseline blood | 100 | 99.7 | 99.8 |
| Male baseline urine | 100 | 100 | 100 |
| Female baseline urine | 100 | 100 | 100 |
| Baseline saliva – female | 98 | 98 | 98 |
| Second saliva – female | 81 | 89 | 87 |
| Baseline semen – male | 94 | 95 | 94 |
| Second semen – male | 69 | 79 | 77 |
| Male second urine | 85 | 96 | 94 |
| Female second urine | 91 | 95 | 94 |
| Six-month urine | 79 | 76 | 77 |
| Pregnancy urine | 92 | 95 | 95 |
Baseline bloods were not obtained for one couple in Texas, given the nurse's inability to successfully complete venipuncture after two attempts.
Denotes the average per cent of journals/cards submitted divided by the expected number per participant.
Despite different sampling frameworks used by the two sites, few significant differences were observed with regard to completion status or across research locations (Table 5). Similar to research in general even when population-based, couples who completed the study were more likely to have higher educational attainments, household incomes and health insurance than couples who withdrew. We did not have the ability to a priori oversample by under-represented groups as our sampling frameworks did not have the necessary information for the stratification needed. Modest differences in race were observed by completion status, but interpretation is limited given individuals’ ability to self-identify with more than one race consistent with the US Census methodology. Of particular note is the lack of difference in gravidity or parity, characteristics that are particularly relevant for preconception studies.
Table 5.
Baseline description of study participants by site, LIFE Study, 2005–09
| Characteristic | Michigan completed n (%) 77 (74.0) | Michigan withdrew n (%) 27 (26.0) | Texas completed n (%) 289 (72.8) | Texas withdrew n (%) 108 (27.2) |
|---|---|---|---|---|
| Females | ||||
| Age (years) | ||||
| ≤24 | 7 (9.1) | 2 (7.4) | 18 (6.2) | 12 (11.1) |
| 25–29 | 30 (39.0) | 12 (44.4) | 132 (45.7) | 43 (39.8) |
| 30–34 | 26 (33.8) | 8 (29.6) | 101 (34.9) | 31 (28.7) |
| ≥35 | 14 (18.2) | 5 (18.5) | 38 (13.1) | 21 (19.4) |
| Mean (±SD) | 30.4 (4.3) | 30.1 (4.4) | 29.9 (3.9) | 30.0 (4.6) |
| Racea | ||||
| American Indian/Alaska Native* | 0 (0.0) | 0 (0.0) | 10 (3.5) | 6 (5.6) |
| Black or African American | 2 (2.6) | 5 (18.5) | 9 (3.1) | 9 (8.3) |
| Native Hawaiian/Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| White | 70 (90.9) | 22 (81.5) | 250 (86.5) | 84 (77.8) |
| Asian | 2 (2.6) | 0 (0.0) | 9 (3.1) | 2 (1.9) |
| Other** | 0 (0.0) | 0 (0.0) | 23 (8.0) | 7 (6.5) |
| Education | ||||
| <High school | 1 (1.3) | 0 (0.0) | 0 (0.0) | 2 (1.9) |
| High school graduate/GED | 4 (5.2) | 2 (7.4) | 12 (4.2) | 6 (5.6) |
| Some college/technical school | 12 (15.6) | 12 (44.4) | 41 (14.2) | 29 (26.9) |
| College graduate or higher | 60 (77.9) | 13 (48.1) | 236 (81.7) | 70 (64.8) |
| Household income ($)*** | ||||
| <10 000 | 0 (0.0) | 1 (3.7) | 2 (0.7) | 2 (1.9) |
| 10 000–29 999 | 5 (6.5) | 4 (14.8) | 5 (1.7) | 7 (6.5) |
| 30 000–49 999 | 17 (22.1) | 8 (29.6) | 29 (10.0) | 10 (9.3) |
| 50 000–69 999 | 18 (23.4) | 4 (14.8) | 29 (10.0) | 18 (16.7) |
| 70 000–89 999 | 12 (15.6) | 8 (9.6) | 67 (23.2) | 25 (23.1) |
| ≥90 000 | 23 (29.9) | 1 (3.7) | 153 (52.9) | 46 (42.6) |
| Health insurance | ||||
| No | 1 (1.3) | 6 (22.2) | 16 (5.5) | 17 (15.7) |
| Yes | 76 (98.7) | 21 (77.8) | 273 (94.5) | 91 (84.3) |
| Gravidity (no. prior pregnancies) | ||||
| Nulligravida | 28 (36.4) | 11 (40.7) | 127 (43.9) | 44 (40.7) |
| 1 | 24 (31.2) | 7 (25.9) | 89 (30.8) | 30 (27.8) |
| 2+ | 25 (32.5) | 9 (33.3) | 71 (24.6) | 34 (31.5) |
| Mean (± SD) | 1.3 (1.6) | 1.3 (1.6) | 1.0 (1.2) | 1.2 (1.4) |
| Parity (no. prior livebirths) | ||||
| 0 | 37 (48.1) | 15 (55.6) | 152 (52.6) | 59 (54.6) |
| 1 | 24 (31.2) | 7 (25.9) | 103 (35.6) | 28 (25.9) |
| 2+ | 16 (20.8) | 5 (18.5) | 31 (10.7) | 21 (19.4) |
| Mean (±SD) | 0.8 (1.0) | 0.8 (1.2) | 0.6 (0.7) | 0.7 (0.8) |
| Males | ||||
| Age (years) | ||||
| ≤24 | 2 (2.6) | 3 (11.1) | 8 (2.8) | 6 (5.6) |
| 25–29 | 24 (31.2) | 8 (29.6) | 94 (32.5) | 31 (28.7) |
| 30–34 | 26 (33.8) | 9 (33.3) | 113 (39.1) | 38 (35.2) |
| ≥35 | 25 (32.5) | 7 (25.9) | 74 (25.6) | 33 (30.6) |
| Mean (±SD) | 32.2 (4.6) | 30.9 (4.8) | 31.7 (4.8) | 31.8 (5.6) |
| Racea | ||||
| American Indian/Alaska Native | 0 (0.0) | 1 (3.7) | 13 (4.5) | 9 (8.3) |
| Black or African American | 2 (2.6) | 4 (14.8) | 13 (4.5) | 10 (9.3) |
| Native Hawaiian/Pacific Islander | 0 (0.0) | 0 (0.0) | 2 (0.7) | 1 (0.9) |
| White | 71 (92.2) | 20 (74.1) | 252 (87.2) | 91 (84.3) |
| Asian | 2 (2.6) | 0 (0.0) | 6 (2.1) | 1 (0.9) |
| Other* | 2 (2.6) | 1 (3.7) | 30 (10.4) | 14 (13.0) |
| Education | ||||
| <High school | 1 (1.3) | 0 (0.0) | 1 (0.3) | 1 (0.9) |
| High school graduate/GED | 3 (3.9) | 6 (22.2) | 12 (4.2) | 20 (18.5) |
| Some college/technical school | 26 (33.8) | 11 (40.7) | 74 (25.6) | 32 (29.6) |
| College graduate or higher | 47 (61.0) | 9 (33.3) | 201 (69.6) | 54 (50.0) |
| Health insurance | ||||
| Yes | 5 (6.5) | 5 (18.5) | 21 (7.3) | |
| No | 72 (93.5) | 22 (81.5) | 268 (92.7) | 96 (88.9) |
Self-identified race based upon the US Census classification.
SD, standard deviation; GED, General Education Diploma.
P < 0.05
P < 0.01
P < 0.001. For comparison by site. Racial categories are not mutually exclusive; hence varying P-values.
Discussion
There have been few population-based prospective pregnancy studies with preconception enrolment ever conducted worldwide as recently summarised,11 and each has used a different sampling framework for recruiting women such as a motor vehicle licence registry,18 health maintenance organisation,19 fish licence registry,12 or a single study recruiting couples from a trade union sampling framework.20 Among the prospective pregnancy studies that had a reported denominator, the recruitment yield ranged from 0.8% to 4.0% of targeted women or couples who were planning to conceive in the next few months and who were willing to participate in data intensive prospective cohort studies.12,19 Our findings that 2% of couples, irrespective of sampling framework, were recruited from target populations is within this range. We have no data for the sizeable (84%) percentage of households not contacted, as we were unable to link with other data sources to assess potential bias. Other population-based data suggest a relatively small percentage of reproductive aged couples are either at risk of or planning pregnancy in the next few months. Specifically, Slama and colleagues selected a random sample of 7699 unlisted and listed telephone numbers throughout France to identify woman aged 18–50 years currently at risk of pregnancy, and reported that 1% of the targeted sample were eligible after 15 telephone follow-up attempts, increasing to 5% when restricting the sample to homes that actually answered the telephone.21 Sweeney and colleagues reported that in a sample of middle-class women, 2% reported they were currently trying or planning to become pregnant in the next 3 months, while 46% reported being sexually active without any form of contraception.22
Our findings offer some lessons learned that might be of use for investigators interested in designing prospective fecundity and pregnancy studies in the US. First, couples planning pregnancy can be recruited from targeted populations based upon different sampling frameworks. However, to do so requires a sufficiently sized target population, given the large percentage of couples who cannot be contacted despite repeated attempts and the small percentage of eligible couples at any point in time even when inclusion criteria are minimal. Ultimately, the decision for population vs. convenience sampling depends upon the research question and other logistics and emerging technologies that may provide cost-efficient opportunities for finding populations with a higher yield (e.g. Facebook). Despite our concerted efforts to recruit diverse couples including a diverse research staff and geographically based targeted recruitment, our study participants were similar to those in other types of research in that they were more educated, had higher household incomes and were covered by health insurance than couples who withdrew. As with most research, the exact reasons for such under-representation are often unknown and may reflect the insensitivity of the study methodology to connect with communities and elicit their participation. Based upon our experience, investigators planning population-based preconception studies may wish to consider the utility of up to 10 telephone attempts, as we have no evidence to support its positive impact on recruitment relative to the resources required. We actively monitored couples reporting they were planning pregnancy in the next 2–12 months and found that ~75% of couples were eventually enrolled in the study. Despite these concerted efforts, the overall recruitment yield is low when considering the target population, but does increase to approximately 42% once couples are screened for recruitment. Understanding the recruitment process helps ground the study cohort in relation to its referent or target population, an important step for the translation of research findings. We are currently unaware of any data regarding the extent to which environmental exposures differ by study completion status. Empirical estimation of potential biases in observation cohort studies utilising preconception enrolment designs is forthcoming in the LIFE Study.
Other important lessons learned from the LIFE Study are offered for investigators considering such designs. Our experience affirms that couples, not just women, can be recruited and both will comply with intensive data and biospecimen protocols timed to sensitive windows of human reproduction and development even in the context of limited remuneration. Sixty-nine per cent of couples completed the study protocol fully, with male partners doing as well as female partners. We were surprised by couples’ preferences for using hard copy rather than online journals in both locations. Unfortunately, we did not query couples about the reasons for their choices. Possible reasons may include the diary's easy-to-complete and portable nature. A second key lesson learned is that couples can be taught to use commercially available kits in the home such as fertility monitors to facilitate conception and the timing of data collection, pregnancy test kits and saliva/semen biospecimen collection kits. Lastly, we found that a web-based data management system can be designed to support population-based research characterised by multiple remote users at research sites or in homes, real-time monitoring of the study protocol and communicating health alerts, and the hierarchical data structure inclusive of biospecimen tracking with two participating laboratories. Practical considerations for supporting bilingual studies include the successful translation of technical terminology (e.g. chemical exposures, reproductive hormones) consistent with regional dialects.
Our findings offer support for the exposome paradigm with regard to feasibility of intensive data collection during sensitive windows of human reproduction.6 Such data are critical not only for the early origins of health and disease paradigm of interest to many scientific disciplines, but also for understanding the environmentally induced epigenetic changes. In conclusion, we agree with Bonde and colleagues that prospective cohort studies with preconception enrolment of couples can be conducted, but they require dedicated time and resources for identifying environmental determinants of fecundity and fertility.20 Thus, investigations focusing on couple-based outcomes, such as pregnancy, should strongly consider the couple as the unit of study if we are to understand paternally, maternally or parentally mediated environmental effects on human development.
In sum, we estimate that approximately 2% of couples were planning a pregnancy within 2 months, underscoring the importance of a large sampling framework targeted population-based sampling. Once recruited, couples are compliant with intensive home-based data and biospecimen protocols supporting the ability to measure exposures during sensitive windows of reproduction and development consistent with the TDS and ODS paradigms.
Acknowledgements
This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts #N01-HD-3-3355, N01-HD-3-3356, N01-HD-3-3358). The authors acknowledge the support of the Texas Parks and Wildlife Department in providing the database for study purposes.
Footnotes
The authors have no competing interests.
References
- 1.Louis GMB, Cooney MA, Lynch CD, Handal A. Periconception window: advising the pregnancy planning couple. Fertility and Sterility. 2008;89:e119–e121. doi: 10.1016/j.fertnstert.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck Louis GM. Sensitive windows of human development. In: Buck Louis GM, Platt RW, editors. Reproductive and Perinatal Epidemiology. Oxford University Press; New York: 2011. pp. 16–29. [Google Scholar]
- 3.Gluckman PD, Hanson MA, Buklijas T. A conceptual framework for the developmental origins of health and disease. Journal of Developmental Origins of Health and Disease. 2010;1:6–18. doi: 10.1017/S2040174409990171. [DOI] [PubMed] [Google Scholar]
- 4.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Human Reproduction. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 5.Buck Louis GM, Cooney MA, Peterson CM. The ovarian dysgenesis syndrome. Journal of Developmental Origins of Health and Disease. 2011;2:25–35. [Google Scholar]
- 6.Wild CP. Complementing the genome with an ‘exposome’: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 7.Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertility and Sterility. 2004;81:384–392. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TIA, Olsen J. Subfecundity in overweight and obese couples. Human Reproduction. 2007;22:1634–1637. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- 9.Bellver J, Rossal LP, Bosch E, Zuniga A, Corona JT, Melendez F, et al. Obesity and the risk of spontaneous abortion after oocytes donation. Fertility and Sterility. 2003;79:1136–1140. doi: 10.1016/s0015-0282(03)00176-6. [DOI] [PubMed] [Google Scholar]
- 10.Fedorcsak P, Storeng R, Dale PO, Tanbo T, Abyholm T. Obesity is a risk factor for early pregnancy loss after IVF or ICSI. Acta Obstetricia et Gynecologica Scandinavica. 2000;79:43–48. [PubMed] [Google Scholar]
- 11.Buck GM, Lynch CD, Stanford JB, Sweeney AM, Schieve LA, Rockett JC, et al. Prospective pregnancy study designs for assessing reproductive developmental toxicants. Environmental Health Perspectives. 2004;112:79–86. doi: 10.1289/ehp.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck Louis GM, Dmochowski J, Lynch DC, Kostyniak PJ, McGuinness BM, Vena JE. Polychlorinated biphenyl concentrations, lifestyle and time-to-pregnancy. Human Reproduction. 2009;24:451–458. doi: 10.1093/humrep/den373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 14.Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- 15.Behre HM, Kuhlage J, Gahner C, Sonntag B, Schem C, Schneider HPG, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlana® Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Human Reproduction. 2000;15:2478–2482. doi: 10.1093/humrep/15.12.2478. [DOI] [PubMed] [Google Scholar]
- 16.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. American Journal of Epidemiology. 2009;69:105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole LA, Khantian SA, Sutton JM, Davies S, Rayburn WF. Accuracy of home pregnancy tests at the time of missed menses. American Journal of Obstetrics and Gynecology. 2004;190:100–105. doi: 10.1016/j.ajog.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 18.Ellish NJ, Saboda K, O'Connor J, Nasca PC, Stanek EJ, Boyle C. A prospective study of early pregnancy loss. Human Reproduction. 1996;11:406–412. doi: 10.1093/humrep/11.2.406. [DOI] [PubMed] [Google Scholar]
- 19.Brown JE, Jacobs DRJ, Barosso GM, Potter JD, Hannan PJ, Kopher RA, et al. Recruitment, retention and characteristics of women in a prospective study of preconceptional risks to reproductive outcomes: experience of the Diana Project. Paediatric and Perinatal Epidemiology. 1997;11:345–358. doi: 10.1111/j.1365-3016.1997.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 20.Bonde JP, Hjollund H, Jensen TK, Ernst E, Kolstad H, Henriksen TB, et al. A follow-up study of environmental and biological determinants of fertility among 430 Danish first pregnancy planners: design and methods. Reproductive Toxicology. 1998;12:19–27. doi: 10.1016/s0890-6238(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 21.Slama R, Ducot B, Carstensen L, Lorente C, de La Rochebrochard E, Leridon H, et al. Feasibility of the current-duration approach to studying human fecundity. Epidemiology. 2006;17:440–449. doi: 10.1097/01.ede.0000221781.15114.88. [DOI] [PubMed] [Google Scholar]
- 22.Sweeney AM, Meyer MR, Mills JL, Aarons JH, LaPorte RE. Evaluation of recruitment strategies for prospective studies of spontaneous abortion. Journal of Occupational Medicine. 1989;31:980–985. doi: 10.1097/00043764-198912000-00009. [DOI] [PubMed] [Google Scholar]