Abstract
Recent studies suggest that memory formation in the hippocampus is modulated by the motivational significance of events, allowing past experience to adaptively guide behavior. The effects of motivation on memory are thought to depend on interactions between the hippocampus, the ventral tegmental area (VTA), and the nucleus accumbens (NAcc). Indeed, animal studies reveal anatomical pathways for circuit-level interaction between these regions. However, a homologue circuit connectivity in humans remains to be shown. We characterized this circuitry in humans by exploiting spontaneous low-frequency modulations in the fMRI signal (termed resting-state functional connectivity) which are thought to reflect functionally related regions and their organization into functional networks in the brain. We examined connectivity in this network across two datasets (hi-resolution, n=100; standard resolution, n=894). Results reveal convergent connectivity between the hippocampus and both the NAcc and the VTA centered on ventral regions in the body of the hippocampus. Additionally, we found individual differences in the strength of connectivity within this network. Together, these results provide a novel task-independent characterization of circuitry underlying interactions between the hippocampus, NAcc and VTA and provide a framework with which to understand how connectivity might reflect, and constrain, the effects of motivation on memory.
Keywords: Reward, VTA, Dopamine, Memory, Humans, Functional Imaging
Memory is central to behavior, allowing past experience to guide later choices. For memory to be adaptive it must be modulated by the motivational significance of events. Indeed, rewards, rewarding contexts and reward-driven motivation all enhance long-term memory (e.g. Adcock et al., 2006; Tse et al., 2007; Wittmann et al., 2005; Wolosin et al.; for review see Shohamy and Adcock, 2010). The effects of reward on memory are thought to depend on interactions between two specialized neural circuits: a memory circuit in the medial temporal lobe (MTL) that includes the hippocampus and surrounding MTL cortices, and a reward-circuit in the midbrain that includes the ventral tegmental area (VTA) and the nucleus accumbens (NAcc) (Lisman et al., 2011; Shohamy and Adcock, 2010).
Support for the existence of such a network comes primarily from animal studies. Both anatomical and pharmacological studies demonstrate important modulatory inputs on the hippocampus coming from VTA and NAcc. Dopamine neurons in the VTA project directly to the hippocampus (Gasbarri et al., 1994; Samson et al., 1990) and modulate plasticity of hippocampal neurons (e.g. Huang and Kandel, 1995; for review see Lisman et al., 2011 ). VTA neurons also project to the NAcc, which itself has strong anatomical links to the MTL (Groenewegen et al., 1987). Together, these connections provide a putative mechanism by which motivationally important events, signaled in the VTA and NAcc, could impact memory formation in the hippocampus.
The hippocampus, in turn, also modulates activity in the VTA, providing opportunities for bidirectional influences of memory and context on motivational responses. In rodents at least two distinct polysynaptic pathways between the hippocampus and VTA have been described: one originating in the ventral hippocampus (Floresco et al., 2001; Valenti et al., 2011; for review see Lisman and Grace, 2005) and one in the dorsal hippocampus (Luo et al., 2011; Rossato et al., 2009).
Neuroimaging studies in humans have also begun to reveal functional interactions between midbrain dopamine regions and the hippocampus during memory formation (Adcock et al., 2006; Shohamy and Wagner, 2008; for review see Shohamy and Adcock, 2010). However, many open questions remain about functional connectivity between subregions of the hippocampus, the VTA and the NAcc, and the characterization of this circuit in the human brain.
Here, we sought to examine intrinsic functional connectivity between the hippocampus, VTA and the NAcc in humans using resting state functional MRI. Resting-state fMRI detects large-amplitude spontaneous low-frequency (< 0.1Hz) modulations in the fMRI signal observed during rest and reveals correlations across functionally related regions. Previous studies have separately characterized memory networks centered on the MTL (Kahn et al., 2008; Libby et al., 2012) and on subregions of the striatum (Di Martino et al., 2008). These studies reveal important characterization of each of these networks, but leave open the question of whether there exists intrinsic cross-talk between them. In particular, it remains unknown (1) to what extent the hippocampus, VTA and NAcc are interconnected at rest; (2) the specific subregions within the hippocampus that are involved in this network; (3) whether expression of this network varies across individuals, and thus might reflect, and constrain, variability in experience and behavior.
To address these questions, we defined regions of interest anatomically and compared these to reported activated voxels in previously published fMRI studies, focusing on the VTA, NAcc and hippocampus (subdivided along the posterior–anterior axis). We computed the correlation between these regions of interest and whole-brain correlation maps.
Resting state fMRI scans from a total of 994 participants were included in the present study. All fMRI data were collected during a continuous passive session in which participants were instructed to stay awake and remain still. Two datasets were included. A high-resolution dataset was used to identify the nature of functional connectivity between the hippocampus, ventral tegmental area and nucleus accumbens. An additional more expanded dataset was used to confirm the results in standard acquisition resolution, and test whether individual variability resulting from age, gender and acquisition protocol is correlated with connectivity patterns.
Dataset 1 (high-resolution; n=100) was acquired on a 3 T TimTrio system at Massachusetts General Hospital using a gradient-echo echo-planar pulse sequence and a 12-channel phased-array whole-head coil (TR = 5000 ms, TE = 30 ms, FA = 90°, 55 axial slices, 2 × 2 × 2 mm voxels, no interslice gap). These data have been used in previous analyses of spontaneous BOLD correlations (Kahn et al., 2008; Vincent et al., 2008; Vincent et al., 2010). Informed consent was obtained in accordance with guidelines set forth by the ethics committee of the Massachusetts General Hospital.
Dataset 2 (n = 894) was derived from the 1000 Functional Connectomes Project (http://fcon_1000.projects.nitrc.org/). Resting-state fMRI scans were aggregated from 21 community-based datasets (mean age 29.7 ± 13.6 years; only participants over age 18; 1–5 scans per participant; duration: 216–560 s; n = 860 at 3 T, n = 34 at 1.5 T; voxel size, in-plane 1.5–5 mm; slice thickness, 3–8 mm). Each contributor’s respective ethics committee approved submission of deidentified data. The institutional review boards of NYU Langone Medical Center and New Jersey Medical School approved the receipt and dissemination of the data.
Data preprocessing and analyses were identical to previous published work (Kahn et al., 2008; Vincent et al., 2008; Vincent et al., 2010). Both datasets were normalized to a 2 mm3 voxel target atlas. Seed-based Fisher’s r-to-z transformed (Zar et al. 1996) correlation maps and seed-to-seed correlation analyses were computed for regions of interest (ROIs) identified previously (Figure 1A). Midbrain and striatum ROIs were based on Adcock et al. (2006) and eight hippocampal ROIs along the long axis of the hippocampus covering its full extent were based on Kahn et al. (2008). We computed the correlation values from NAcc (Montreal Neurological Institute [MNI] coordinates; [−11 4 0]; 8 mm diameter sphere; 28 voxels × 2 mm3 = 224 mm3 volume) and VTA ([−4 −15 −9]; 8 mm diameter sphere; 36 voxels × 2 mm3 = 288 mm3 volume) with each of the anatomically defined seed regions in the hippocampus (3 mm diameter spheres; 7 voxels × 2 mm3 = 56 mm3 volume; for coordinates of all hippocampal seeds see Table 1 in Kahn et al. [2008]). Note that variation in the volumes of NAcc and VTA seeds resulted from the transformation between previously reported MNI coordinates and the normalized target volume (sampled at 2 mm3). To characterize the pattern of connectivity between ROIs such that we are able to distinguish between the contributions of sub-networks independently, we computed partial correlations between each pair of regions while controlling for the third region. The partial correlation between X and Y given a set of n controlling variables Z = {Z1, Z2, …, Zn}, written ρXY·Z, is the correlation between the residuals RX and RY resulting from the linear regression of X with Z and of Y with Z, respectively.
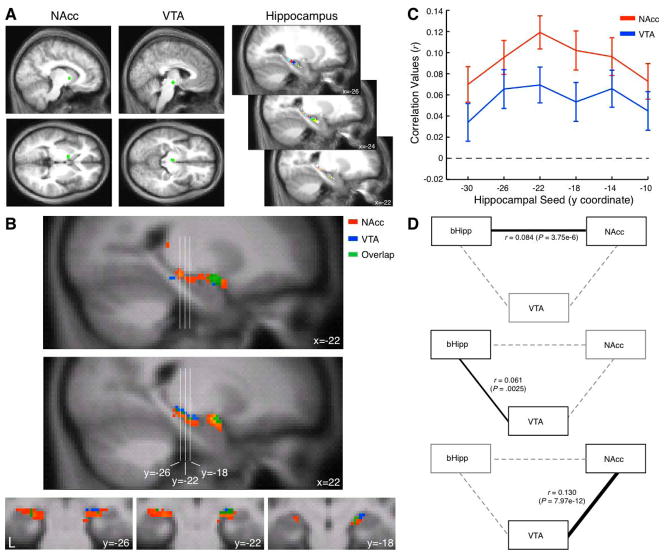
Figure 1. Functional connectivity of the hippocampus to ventral striatum and midbrain regions.
A, Nucleus accumbens (NAcc; [−11 4 0]) and ventral tegmental area (VTA; [−4 −15 −9]) seeds (8 mm diameter spheres) were used to derive a correlation map in the hippocampus. Hippocampal seeds (3 mm diameter spheres) were used to identify the locus of maximal correlation to NAcc and VTA seeds. B, Sagittal (top) and coronal (bottom) cross sections in the hippocampus depict correlation to NAcc (red), VTA (blue) or both (green) in the hippocampus and amygdala. Reliable correlation is observed in the body of the hippocampus and amygdala. C, Correlation along the long axis of the hippocampus to the NAcc and VTA seeds demonstrates reliable correlation values with peak response at y = −22. D, Partial correlation between the three regions of interest bHipp (at the peak correlation value region, y = −22), NAcc and VTA are depicted. Each graph depicts the partial correlation between a pair of regions (black edge) while controlling for the third region (dashed edges).
Midbrain and striatal ROIs identified from prior functional studies provided a conservative approach to delineating functional connectivity of these networks. We found reliable correlation between VTA and NAcc to the hippocampus (Figure 1B). Correlation was maximal in the body of the hippocampus at y = −22 (Figure 1C). Partial correlation analyses focused on NAcc, VTA and the body of the hippocampus (seed ROI [−26 −22 −14]; bHipp) revealed that these patterns of correlation are independent for each pair of ROIs (Figure 1D). Specifically, NAcc and VTA were maximally correlated with each other when controlling for bHipp (r = 0.13; P = 7.97e-12). Next, NAcc and bHipp demonstrated reliable correlation when controlling for VTA (r = 0.084; P = 3.75e-6), suggesting that these regions are correlated independently of the correlation between NAcc and VTA. Similarly, VTA and bHipp were reliably correlated when controlling for NAcc (r = 0.061; P =.0025).
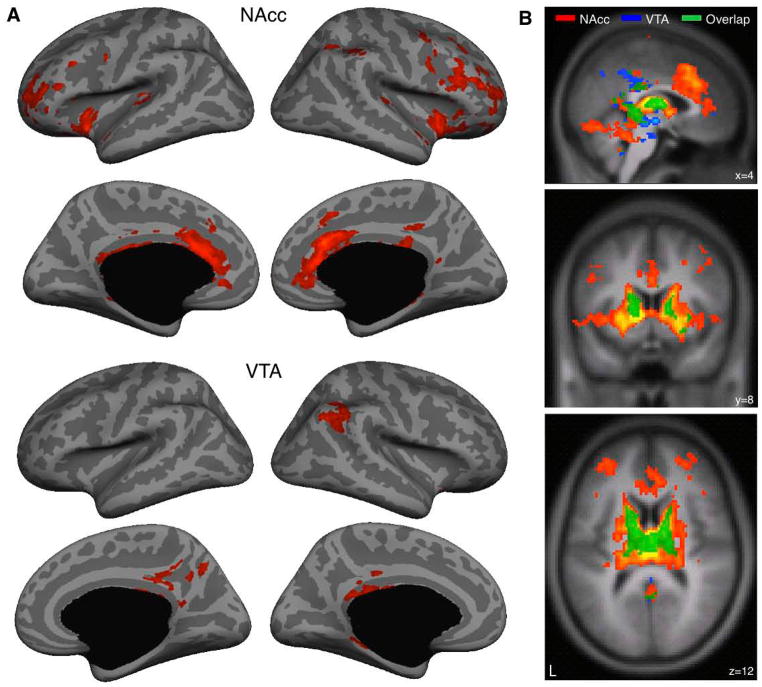
Next, we sought to examine the selectivity of these results by comparing connectivity across the brain for each of these networks – hippocampus with NAcc and hippocampus with VTA (Figure 2). Specifically, we aimed to characterize the extent to which these two networks overlap. This analysis revealed overlap in the body of the hippocampus. Outside of the hippocampus the patterns of connectivity were more distinct and displayed only partial overlap. In particular, spontaneous fluctuations in the NAcc seed correlated with a broader network of regions, including the full extent of the caudate and putamen, as well as the hippocampus, thalamus, dorsolateral prefrontal cortex and cingulate cortex. VTA by contrast showed a more selective pattern of connectivity with regions within the midbrain, the body of the caudate, dorsal putamen, and the hippocampus. Together, then, overlapping connectivity for both the NAcc and the VTA was found primarily in the body of the hippocampus, the body of the caudate, dorsal putamen and the thalamus, suggesting that these regions comprise a functional unit..
Figure 2. Functional connectivity of ventral striatum and midbrain reveals limited connectivity to the cerebral cortex and extensive connectivity in the basal ganglia and thalamus.
Correlation maps were obtained after within-subject transformation using Fisher’s r-to-z and submitted to a second level analysis using a random effects model (threshold P < 0.05 corrected for multiple comparisons using family-wise error). A, Two distinct networks were observed in the cerebral cortex. The NAcc correlated with dorsolateral prefrontal, frontopolar, anterior cingulate and insular cortices. The VTA correlated with lateral inferior parietal and posterior cingulate cortices. B, Sagittal, coronal and axial cross sections depict overlap primarily in the body of the hippocampus, body of the caudate, dorsal putamen and the thalamus.
Next we sought to confirm the results obtained in Dataset 1 by asking whether the same pattern would be observed in Dataset 2, across differing scanning parameters, age groups and genders. In Dataset 2 we computed the correlation values for the NAcc and VTA to the hippocampal seeds. Confirming the results from Dataset 1, we found that the maximal correlation value was observed at y = −22 for both seeds (NAcc: r = .044; VTA: r = 0.037; P values < .001 corrected for multiple comparisons using Bonferroni correction).
The large sample size in Dataset 2 allowed us additionally to ask whether there is individual variability in the extent of connectivity within these networks. This question is particularly important given that the behaviors thought to depend on this circuit have been shown to vary substantially across individuals (e.g. Adcock et al., 2006; Shohamy and Wagner, 2008; Wimmer and Shohamy, 2012). Thus, individual variability in network connectivity might suggest that behavioral differences may be driven, in part, by differences in intrinsic connectivity.
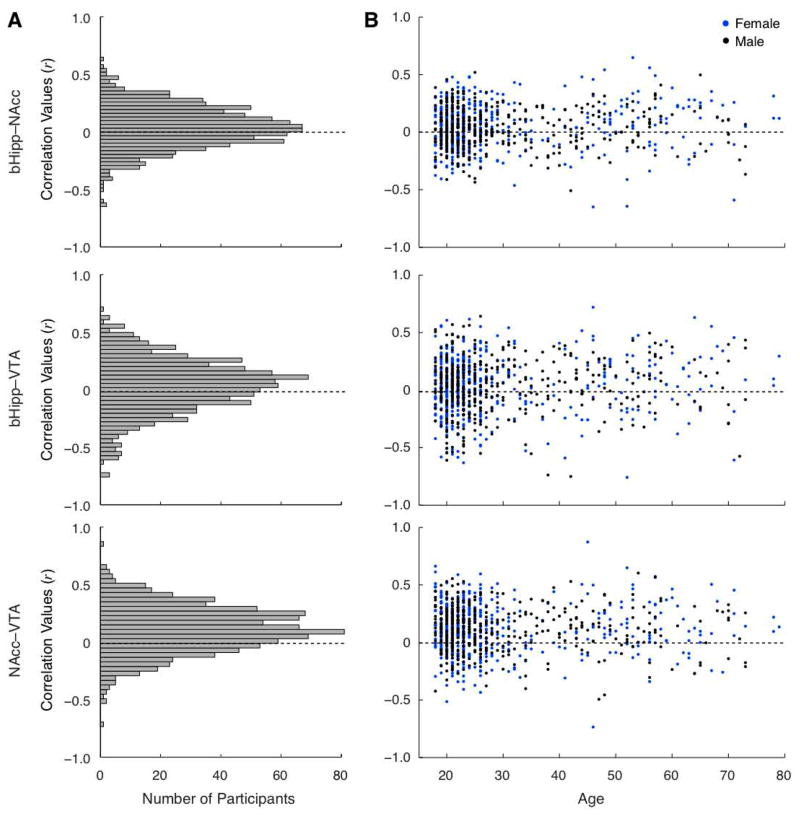
To address this question, we explored variability in functional connectivity between the bHipp and NAcc, bHipp and VTA and NAcc and VTA. As shown in Figure 3A, there was indeed variability across participants in intrinsic connectivity between these regions. This variability was not found to be significantly related to gender (unpaired two-tail t-test NAcc–VTA: t892=.21, P = .84; bHipp–NAcc: t892=1.245, P = .21; bHipp–VTA: t892=.07, P = .94) (Figure 3B). Linear regression demonstrated no reliable correlation with age for NAcc–VTA (F=.10, P > .1) or for bHipp–NAcc (F=2.61, P > .1); connectivity between bHipp–VTA did show a trend for correlating with age (F=5.46, P = .06) (all analyses corrected for multiple comparisons using Bonferroni method).
Figure 3. Individual variability in functional connectivity of the hippocampus to ventral striatum and midbrain.
A, Histograms of correlation values in Dataset 2 (n = 894) demonstrates normally distributed reliable responses with positive peak between bHipp and NAcc, bHipp and VTA, and NAcc and VTA. B, Plotting the distribution of correlation values as a function of age (x axis) and gender (blue: female; black: male) reveals that none of these factors explains functional connectivity strength, such that reliable functional connectivity is equivalent across both age and genders.
In summary, our results indicate that there is intrinsic connectivity between the hippocampus, known for its role in long term memory, and between midbrain dopamine regions and the NAcc, known for their role in motivation and reward processing. The current results further reveal that functional connectivity within this network is localized to a relatively subscribed region within the body of the hippocampus. To the best of our knowledge, this finding in humans is the first to describe the localization within the hippocampus for this network, complementing recent characterization of two distinct multi-synaptic hippocampal-VTA pathways in rodents (Floresco et al., 2001; Lisman and Grace, 2005; Luo et al., 2011; Rossato et al., 2009; Valenti et al., 2011).
In addition to the points of convergence within the hippocampus, our results also point to dissociations between the VTA vs. NAcc. In particular, we found that the VTA is interconnected with a more restricted number of targets, while the NAcc is part of a broader PFC-striatal-MTL network, consistent with known anatomical projections (Alexander et al., 1986; Di Martino et al., 2008; Haber and Knutson, 2010; Lisman and Grace, 2005).
Our findings demonstrate correlated activity in this network during rest, when neither motivation nor memory are being manipulated or explicitly engaged. The fact that there is correlated activity between these regions during rest provides evidence for an intrinsically connected network, which could provide a pathway for enhancing effects of motivation on long-term memory formation.
Interestingly, our current results also point to individual differences in the strength of connectivity between the VTA, NAcc, and hippocampus: while some people show very robust connectivity between these regions, others do not. These individual differences may reflect relatively stable trait-like differences in ability across individuals, or, they could reflect differences in engagement of brain networks specific to the rest activity itself. In general, open questions remain about the relationship between rest activity patterns and functional consequences (Buckner and Vincent 2007; Fox and Raichle, 2007). A critical challenge for future research is to address this question by examining the link between intrinsic connectivity, task-evoked connectivity , and, most critically, behavior. Such studies will help determine whether variability in intrinsic connectivity is indeed related to individual differences in behavior, and, if so, how.
Abnormalities in the striatum, midbrain dopamine system, and the hippocampus are implicated in a wide variety of psychiatric and neurological disorders (e.g. Parkinson’s disease, Huntington’s disease, schizophrenia). Much work over the past decades has focused on characterizing the distinct contributions of each of these regions. But converging evidence indicates that they in fact operate as a dynamic network. Characterizing the intrinsic functional relationship between the hippocampus, midbrain dopamine regions and the ventral striatum in the healthy brain, rather than focusing on each region independently, provides a baseline towards starting to understand how these networks are impacted by, and contribute to, disease.
Acknowledgments
We thank Martinos Center for Advanced Neuroimaging, Harvard Center for Brain Science and Howard Hughes Medical Institute for contribution of human imaging data sets and resting state functional MRI analysis software. This work was supported by the Rappaport Institute (I.K.) and by NIDA R03 DA026957 and an NSF Career Development Award (D.S).
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–17. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Vicent LV. Unrest at rest: Default activity and spontaneous network correlations. NeuroImage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18(12):2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21(13):4915–22. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33(4):445–52. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23(1):103–20. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1995;92(7):2446–50. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(1):129–39. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby LA, Ekstrom AD, Ragland JD, Ranganath C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. Journal of Neuroscience. 2012;32(19):6550–6560. doi: 10.1523/JNEUROSCI.3711-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011;34(10):536–47. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–13. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333(6040):353–7. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325(5943):1017–20. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- Samson Y, Wu JJ, Friedman AH, Davis JN. Catecholaminergic innervation of the hippocampus in the cynomolgus monkey. J Comp Neurol. 1990;298(2):250–63. doi: 10.1002/cne.902980209. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends Cogn Sci. 2010;14(10):464–72. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60(2):378–89. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Schemas and memory consolidation. Science. 2007;316(5821):76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31(11):4280–9. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Van Essen DC, Buckner RL. Functional connectivity of the macaque posterior parahippocampal cortex. J Neurophysiol. 2010;103(2):793–800. doi: 10.1152/jn.00546.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Duzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45(3):459–67. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR. Reward modulation of hippocampal subfield activation during successful associative encoding and retrieval. J Cogn Neurosci. doi: 10.1162/jocn_a_00237. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar J. Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall; 1996. [Google Scholar]