Abstract
Background
Cluster analysis can be used to identify individuals similar in profile based on response to multiple pain sensitivity measures. There are limited investigations into how empirically derived pain sensitivity subgroups influence clinical outcomes for individuals with spine pain.
Objective
The purposes of this study were: (1) to investigate empirically derived subgroups based on pressure and thermal pain sensitivity in individuals with spine pain and (2) to examine subgroup influence on 2-week clinical pain intensity and disability outcomes.
Design
A secondary analysis of data from 2 randomized trials was conducted.
Methods
Baseline and 2-week outcome data from 157 participants with low back pain (n=110) and neck pain (n=47) were examined. Participants completed demographic, psychological, and clinical information and were assessed using pain sensitivity protocols, including pressure (suprathreshold pressure pain) and thermal pain sensitivity (thermal heat threshold and tolerance, suprathreshold heat pain, temporal summation). A hierarchical agglomerative cluster analysis was used to create subgroups based on pain sensitivity responses. Differences in data for baseline variables, clinical pain intensity, and disability were examined.
Results
Three pain sensitivity cluster groups were derived: low pain sensitivity, high thermal static sensitivity, and high pressure and thermal dynamic sensitivity. There were differences in the proportion of individuals meeting a 30% change in pain intensity, where fewer individuals within the high pressure and thermal dynamic sensitivity group (adjusted odds ratio=0.3; 95% confidence interval=0.1, 0.8) achieved successful outcomes.
Limitations
Only 2-week outcomes are reported.
Conclusions
Distinct pain sensitivity cluster groups for individuals with spine pain were identified, with the high pressure and thermal dynamic sensitivity group showing worse clinical outcome for pain intensity. Future studies should aim to confirm these findings.
Response to the management of musculoskeletal pain is highly variable, and individual factors such as pain sensitivity (eg, response to standard experimental stimuli) are being considered as important components in explaining this variation. Pain sensitivity has received considerable attention within physical therapist research and practice, especially in relation to diagnosis and intervention.1–4 Studies have been conducted to examine whether pain sensitivity differentiates individuals with and without a musculoskeletal pain condition, gives indication to the underlying pain mechanisms, or is related to treatment response. For example, recent evidence has shown enhanced pain sensitivity at areas remote to the primary area of complaint in select groups of individuals with common pain complaints in the spine,5–9 upper extremity,10–13 and lower extremity,14–18 implicating alterations in central nervous system (CNS) processing (eg, central sensitization) as a component of the musculoskeletal pain condition.
Although enhanced localized pain sensitivity would be expected in a peripherally sensitized state, the spreading of enhanced pain sensitivity to areas beyond the local site (eg, remote anatomical regions) would suggest central sensitization, which more closely reflects conditions such as fibromyalgia and may indicate a higher likelihood of unresponsiveness to traditional treatment strategies.19–21 Some authors22 have conceptualized that certain pain conditions, such as fibromyalgia, temporomandibular disorders, and headache, may be appropriately described as “central sensitivity syndromes.”22 Other authors23,24 have suggested that pain conditions progress along a continuum, where central sensitization is the final common pathway for the development and maintenance of chronic symptoms. These considerations are relevant for clinical decision making, especially if treatment modalities are shown to be impactful in reducing pain sensitivity or halting the progression of central sensitization. Studies on the effects of spinal manipulation have shown a favorable, immediate response to pain sensitivity, highlight a potential pain modulation mechanism for the clinical benefits following manual therapy application, and may be an early indication of the utility of spinal manipulation in settings where reducing pain sensitivity is a goal.25–29
Although evidence is emerging on pain sensitivity, the clinical relevance of best measurement for pain sensitivity in individuals with common musculoskeletal pain conditions remains unclear.19,30 Heightened local and remote pain sensitivity have been observed in groups of individuals with musculoskeletal pain conditions, but the associations between individual pain sensitivity responses and clinical reports of pain and disability are not strong.31,32 Furthermore, there is limited evidence that pain sensitivity can be used to predict treatment outcome.33 There are potential reasons for these limitations. First, inter-individual variability in pain sensitivity has been observed among patients with similar musculoskeletal pain presentation. Among a sample of patients with shoulder pain, we observed heightened generalized pain sensitivity in some patients, but not all, compared with healthy controls.11 We concluded that there is heterogeneity in pain sensitivity responses within a clinical condition, but the extent to which these individual differences in pain sensitivity affect clinical outcome remains undetermined.
A second reason is related to pain sensitivity measurement. Pain sensitivity can be measured through a variety of methods where aspects of the measure such as stimulus modality (ie, pressure, heat), location of stimulation (ie, local to pain complaint, remote), and desired response (ie, threshold, tolerance) can be modified. There is not a strong association between the different pain sensitivity modalities.34 Furthermore, there is no standardized form of pain sensitivity assessment, so results are not easily comparable.30 One potentially relevant measurement distinction worth noting is between static and dynamic measures.35 Static pain sensitivity measures, including pain threshold or tolerance, are considered reflective of the basal state of pain perception and often involve application of a single standard stimulus to determine sensory function. An example of this measure is pain threshold, where a single pressure stimulus is applied until an individual reports the first onset of pain.
Conversely, dynamic pain sensitivity measures are reflective of the modulation or processing of pain and involve paradigms where repetitive stimuli are applied and the degree of pain facilitation or inhibition is inferred dependent on the dynamic paradigm.24,36,37 An example of dynamic pain sensitivity is temporal summation, where a repetitive stimulus is applied to the individual and successive pain responses are obtained. There is evidence to suggest that dynamic pain sensitivity measures have an association with clinical pain and can be more informative than static measures in assessing the pain experience.32,35,38–42 The apparent upside of dynamic pain sensitivity, however, must be tempered by limitations that include poor stability over time and uncertainty how direct pain ratings infer alterations in spinal nociceptive processing.43,44
Few previous studies have investigated subgrouping based on pain sensitivity. Cluster analysis offers the advantage of examining responses across multiple pain sensitivity measures of different parameters and modalities and may overcome limitations of using single pain sensitivity measures. Moreover, a cluster analysis approach is in conformity with recommendations encouraging use of multimodal pain sensitivity assessments.34 The resultant pain sensitivity subgroups can be assessed for differences in demographic and clinical measures. Previous studies using cluster analysis on pain sensitivity measures have focused on groups of healthy individuals or individuals with chronic pain conditions such as fibromyalgia.45–48 For example, Hastie et al47 performed a cluster analysis using both static and dynamic measures in individuals who were asymptomatic and found that a dynamic measure of temporal summation differentiated subgroups. Kindler et al48 identified a subgroup of healthy individuals with differing response patterns to opioids, which resulted in a unique effect on temporal summation. Collectively, these studies provide preliminary evidence that differing pain sensitivity profiles within groups of individuals exist, with dynamic measures being a potential distinguishing subgroup factor. We sought to advance this line of study by examining pain sensitivity profiles in a clinical population of individuals with musculoskeletal pain.
Thus, the primary aims of this study were: (1) to identify pain sensitivity subgroups using cluster analysis within a sample of individuals with neck or low back pain and (2) to validate these empirically derived pain sensitivity subgroups on demographic, psychological, and 2-week clinical outcome measures. We included static and dynamic pain sensitivity measures as clustering variables and hypothesized that a subgroup of individuals would be differentiated based on responses to static or dynamic measures, which is consistent with prior studies.47,48 Furthermore, we hypothesized that the pain sensitivity subgroups would be differentiated by clinical outcome but that these subgroups would demonstrate similar psychological distress.11 Specifically, we expected a high pain sensitivity subgroup to not differ on pain-associated psychological distress measures but to have worse clinical outcomes compared with a subgroup with lower pain sensitivity.
This study was a translational effort to bridge a gap between laboratory-based efforts and clinical application. Our overall goal was to identify pain sensitivity subgroups that could serve as prognostic factors or treatment monitoring tools in future clinical studies.
Method
Design Overview
We conducted a secondary analysis of data from 2 randomized controlled trials involving participants with low back pain (NCT01168999) and neck pain (NCT01168986). Both randomized controlled trials investigated the effects of manual therapy intervention over a 2-week period. Data from these trials had not been published at the time of manuscript submission.
Setting and Participants
Participants were recruited from the campus of the University of Florida and the surrounding communities with the use of posted flyers and electronic advertisement from November 2009 until December 2012. For the low back pain trial, participants were considered eligible if they were: (1) between the ages of 18 and 60 years, (2) English-speaking, (3) currently experiencing low back pain that did not extend below the knees and of a clinical pain intensity of 4/10 or higher during the previous 24 hours, and (4) deemed appropriate for conservative treatment. Participants were excluded from enrollment into the low back pain trial if they met any of the following: (1) previous surgery for low back pain within previous 6 months, (2) systemic disease known to cause peripheral neuropathy, (3) current or history of chronic pain condition unrelated to low back pain, and (4) fracture as a cause of low back pain.
For the neck pain trial, participants were considered eligible if they were: (1) between the ages of 18 and 60 years, (2) English-speaking, (3) currently experiencing neck pain with or without associated arm pain extending below the elbow and of a clinical pain intensity of 4/10 or higher during the previous 24 hours, and (4) deemed appropriate for conservative treatment. Participants were excluded from enrollment into the neck pain trial if they met any of the following: (1) previous surgery for neck pain within the previous 6 months, (2) systemic disease known to cause peripheral neuropathy, (3) current or history of chronic pain condition unrelated to neck pain, (4) fracture as a cause of neck pain, and (5) history of whiplash injury or trauma as a cause of the current neck pain.
Demographic and Clinical Characteristics
Participants completed a standard demographic questionnaire (eg, age, sex, pain duration) and psychological measures. The psychological measures included the Pain Catastrophizing Scale (PCS), Fear-Avoidance Beliefs Questionnaire (physical activity subscale [FABQ-PA] and work subscale [FABQ-W]), and 11-item Tampa Scale of Kinesiophobia (TSK-11). These measures have shown good psychometric properties in participants with clinical pain.49 The PCS is a 13-item self-report questionnaire (score=0–52) that measures thoughts on various pain experiences, where higher scores indicate higher levels of pain catastrophizing.50 The FABQ-PA is a 4-item subscale (score=0–24) and the FABQ-W is a 7-item subscale (score=0–42) where higher levels on both subscales indicate higher fear-avoidance beliefs.51 The TSK-11 is a shortened version of the 17-item questionnaire and measures fear of movement (total score: 11–44).52 Higher scores on the TSK-11 indicate higher levels of fear of movement.
Clinical pain intensity was measured using the Patient-Centered Outcome Questionnaire (PCOQ).53,54 The PCOQ includes a numeric rating scale of 0 (“none”) to 100 (“worst imaginable”) for usual levels of pain over the previous week. Clinical disability also was measured using the Oswestry Disability Questionnaire (ODQ, for the low back pain trial) or Neck Disability Index (NDI, for the neck pain trial).55,56 The ODQ and NDI are 10-item questionnaires where each item is scored on a 6-point scale from 0 to 5. Both of these measures were computed to a score from 0% to 100%, where higher scores indicate greater perceived disability.
Pain Sensitivity
Pain sensitivity assessment included pressure and thermal pain sensitivity.34 The anatomical location of assessment varied based on trial enrollment. For the low back pain trial, pain sensitivity assessment was obtained at the lower extremity (foot), and for the neck pain trial, the upper extremity (forearm or hand) was used. These extremity locations were selected as standard measurement locations for several reasons. These extremity locations are within the general area of tissue innervated by regions of pain complaint and in which intervention was applied.25,29,57 Furthermore, extremity locations are not as sensitive as the trunk, and individuals have reported difficulty in distinguishing between thermal testing parameters (eg, initial and delayed pain) in the trunk region.29,58 Finally, changes in pain sensitivity in regions of dermatome innervation following intervention have been observed previously.25,29 All pain sensitivity assessments were conducted on the dominant-side extremity.
Static pressure pain sensitivity.
Measurements of pressure pain sensitivity were collected using a handheld algometer (Pain Diagnostics & Treatment, Great Neck, New York) with a 1-cm2-diameter probe. For participants with low back pain, pressure pain sensitivity was measured at the webspace between the first and second toes, and for participants with neck pain, pressure pain sensitivity was measured at the webspace between the first and second fingers. A standard force of 6 kg was applied to all participants at a rate of 1 kg/s. Participants were instructed to report a pain rating for the 6-kg stimulus using a 10-cm mechanical visual analog scale anchored with 0 meaning “no pain” and 100 meaning “most intense pain sensation imaginable.” Because this was a non–threshold-based measurement, we termed this measurement suprathreshold pressure pain.
Static thermal pain sensitivity: threshold and tolerance.
Thermal heat pain sensitivity was assessed using a computer-controlled NeuroSensory Analyzer (TSA-2001, Medoc Ltd, Ramat Yishai, Israel) with a Peltier-element-based stimulator. Two thermal static procedures (threshold and tolerance) and one dynamic procedure (temporal summation) were performed. The baseline temperature for thermal static procedures was approximately 35°C. For the static procedures, temperature increases occurred at a rate of 0.5°C/s, and the site of stimulus application was at the dorsum of the foot (low back pain) or anterior forearm (neck pain). For thermal heat threshold, participants were instructed to notify the assessor when the temperature sensation changed from a warm sensation to pain. For thermal heat tolerance, participants were instructed to notify the assessor when the temperature sensation became so painful they could no longer tolerate it. A total of 2 trials were conducted for each static procedure, and the average temperatures for thermal heat threshold and tolerance were calculated.
Dynamic thermal pain sensitivity: temporal summation.
Thermal temporal summation was assessed at the plantar surface of the foot (low back pain) or palm of the hand (neck pain) with the same experimental setup as for static thermal pain sensitivity. We measured thermal temporal summation using an established protocol where each participant experienced 10 consecutive heat pulses that rose rapidly (approximately 10°C/s) from a baseline of 35°C to a maximum of 51°C at an interstimulus frequency of 0.33 Hz (eg, approximately 3-second interval between stimulus peaks).59,60 For each heat pulse, participants were instructed to rate the pain intensity on a 101-point numeric rating scale where 0 represented “no pain” and 100 represented “worst pain imaginable.” Furthermore, participants were encouraged to focus on the “second pain,” which was a delayed pain intensity following the initial onset of heat. Pain ratings associated with this temporal summation protocol are moderately reliable within a single testing session but have lower reliability across sessions.43 Two dynamic pain sensitivity measurements were obtained using this protocol. Temporal summation was computed as the highest pain rating after the first pulse minus the pain rating of the first pulse.61 Additionally, the fifth pulse pain rating, termed suprathreshold heat pain, also was used as a measure of dynamic pain sensitivity, as this measure has been shown to be associated with clinical pain ratings.39
Procedure
After providing informed consent, all participants commenced with standardized questionnaires and pain sensitivity testing. Randomization occurred after baseline testing was completed. All intervention procedures were provided by a licensed physical therapist. Participants in the low back pain study (NCT01168999) were randomly assigned to receive a spinal manipulation to the lumbar spine, a placebo spinal manipulation, or no treatment. Participants in the neck pain study (NCT01168986) were randomly assigned to receive a mechanical manual therapy intervention to the cervical spine, a cervical spine exercise (supine chin retraction), or no treatment. Participants in each study who received manual therapy, exercise, or placebo were seen for 6 sessions over 2 weeks, and participants who received no treatment were seen for 2 sessions over 2 weeks. Follow-up measurements of clinical pain intensity and disability were obtained on all participants by the intervention provider at the completion of the 2-week period.
Data Analysis
Data were analyzed with IBM SPSS Statistics for Windows, version 20 (IBM Corp, Armonk, New York). Descriptive statistics were computed for the entire sample for demographic characteristics, psychological measures, pain sensitivity, and clinical outcome. For all analyses, alpha was set at the .05 level for statistical significance.
Prior to conducting cluster analysis, raw scores for the pain sensitivity measures were transformed to z scores for standardization. Because the pain sensitivity measures were tested at different body regions for each clinical cohort (eg, lower extremity for low back pain, upper extremity for neck pain), z scores were computed within each group separately prior to combining into one z score measure. This procedure was performed to avoid any potential differences in responses based on testing region alone. Any observed differences in pain sensitivity among clinical cohorts could then be attributed to clinical condition, not testing location. Thus, pain sensitivity responses in the lower extremity were standardized to other responses in the lower extremity, and those in the upper extremity were standardized to other responses in the upper extremity. Additionally, 2 pain sensitivity measures (thermal heat threshold and tolerance) were reflected prior to z score transformation for ease of interpretation of cluster groupings. Thus, when interpreting the cluster graph, higher scores would indicate higher pain sensitivity for all measures.
We conducted an exploratory hierarchical agglomerative cluster analysis using Ward's clustering method and squared Euclidean distances to identify unique subgroups based on pain sensitivity measures. The optimal cluster solution was identified based on statistical and theoretical criteria. We examined the resultant dendrogram and schedule of agglomeration coefficients as well as considering pain sensitivity cluster groupings from previous publications.45,47,48 To validate our cluster groupings, we performed 2 follow-up analyses. First, discriminant function analysis with cross-validated classification was performed to determine accuracy of cluster group classification from the cluster analysis. Second, a one-way analysis of variance (ANOVA) with Bonferonni post hoc correction was used for descriptive purposes to determine the composition of the cluster groups from the pain sensitivity responses.
Cluster group differences in baseline demographic, psychological, and clinical factors also were examined with separate one-way ANOVAs with Bonferonni post hoc correction for continuous variables and chi-square test for categorical variables. To determine differences in clinical outcome, we examined an estimate of percent change. We were primarily interested in whether groups differed in meeting an a priori cutoff score for clinical outcome. Thus, we used a 30% change in clinical pain intensity and disability as a measure of successful outcome. A 30% change in clinical outcome has been used as a clinically meaningful criterion in previous studies.62,63 Frequencies of individuals within each cluster group meeting a 30% change in outcome were examined along with crude and adjusted odds ratios as a measure of effect size. Odds ratios were adjusted for other potential prognostic factors such as age, sex, pain duration, and baseline pain intensity and disability.
Role of the Funding Source
This study was supported by the University of Florida Research Opportunity Fund. Mr Coronado was supported by NIH T32 Interdisciplinary Training in Rehabilitation and Neuromuscular Plasticity grant 5T32HD043730. Dr Bialosky received support from the Rehabilitation Research Career Development Program (5K12HD055929-02).
Results
Descriptive
Data from 157 participants were included in these analyses. Descriptive results for demographic characteristics, psychological measures, pain sensitivity, and clinical outcome are presented in Table 1. The entire sample consisted of 110 participants with low back pain and 47 with neck pain.
Table 1.
Demographic Characteristics, Psychological Measures, Pain Sensitivity, and Clinical Outcome for Individuals With Spine Pain (N=157)a
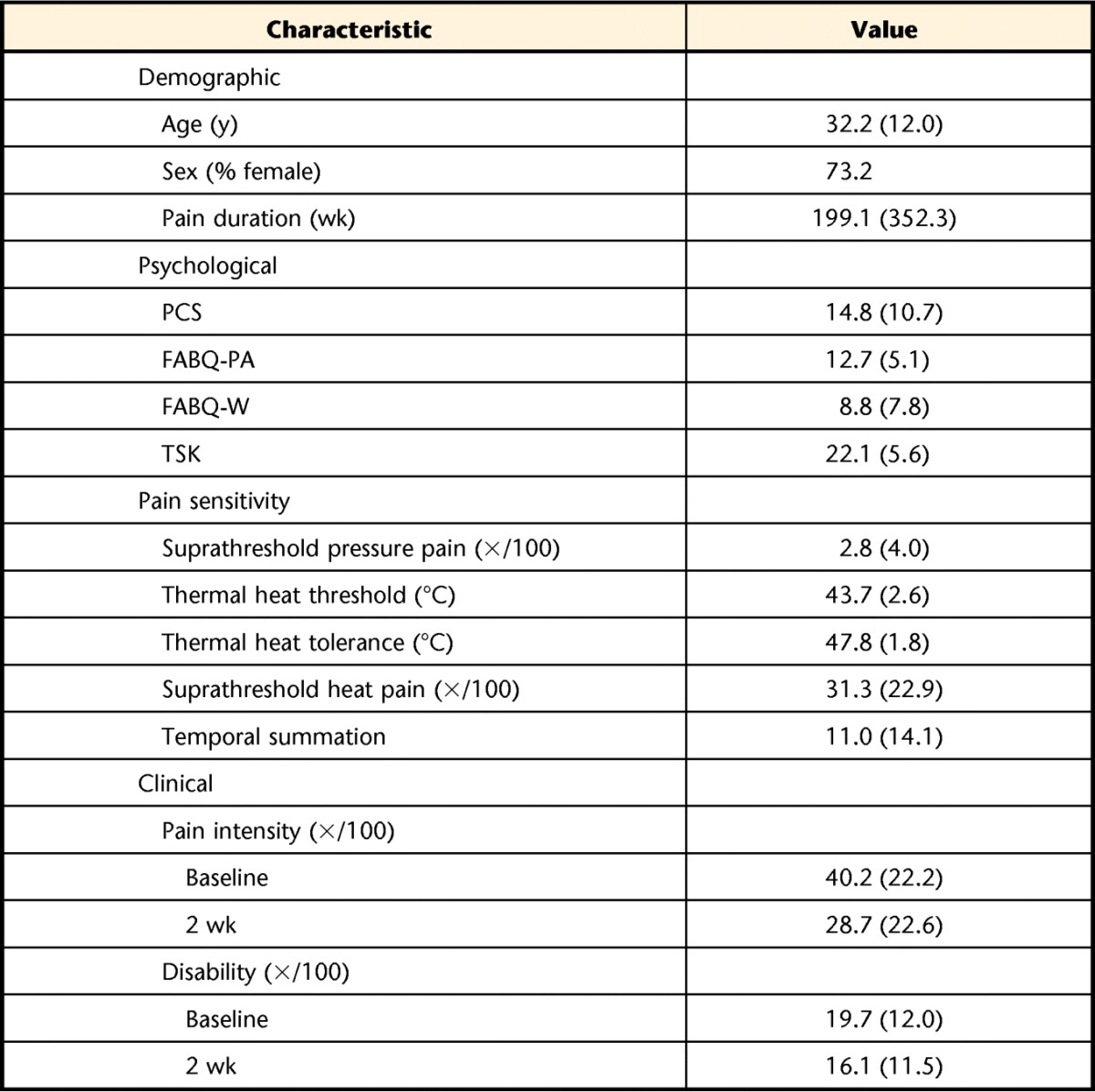
Values are X̅ (SD) unless otherwise indicated. PCS=Pain Catastrophizing Scale, FABQ-PA=Fear-Avoidance Beliefs Questionnaire physical activity subscale, FABQ-W=Fear-Avoidance Beliefs Questionnaire work subscale, TSK=Tampa Scale of Kinesiophobia.
Pain Sensitivity Clusters
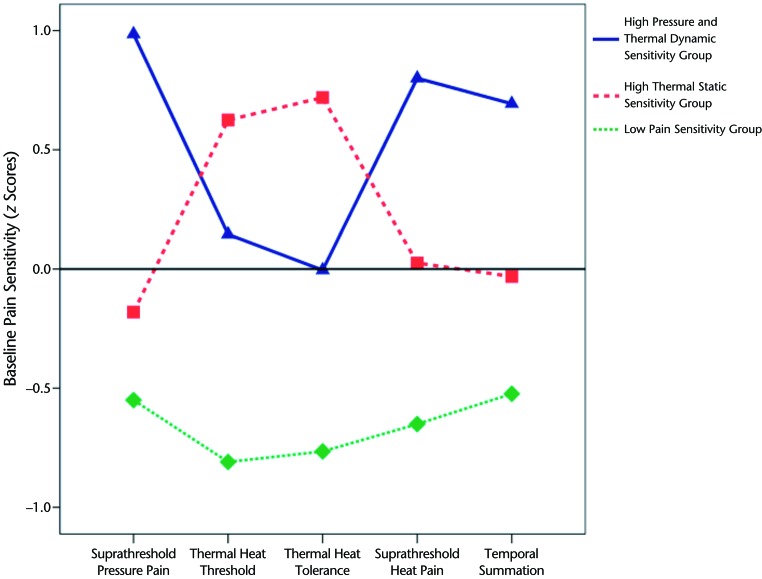
Transformation of pain sensitivity raw scores to z scores was done prior to cluster analysis. Based on inspection of the dendrogram and plotted agglomeration coefficients, a 3-cluster solution was deemed appropriate (Figure). Cluster 1 was labeled “high pressure and thermal dynamic sensitivity” (n=40) and included individuals with higher sensitivity for suprathreshold responses (pressure and heat) and thermal temporal summation. Cluster 2 was labeled “high thermal static sensitivity” (n=59) and included individuals with higher sensitivity for thermal heat threshold and tolerance. Cluster 3 was labeled “low pain sensitivity” (n=50) and included individuals with low sensitivity for all pain sensitivity measures.
Figure.
Cluster subgroups based on pain sensitivity measures. Data for thermal head threshold and tolerance are reflected. Higher z scores denote higher pain sensitivity for all measures.
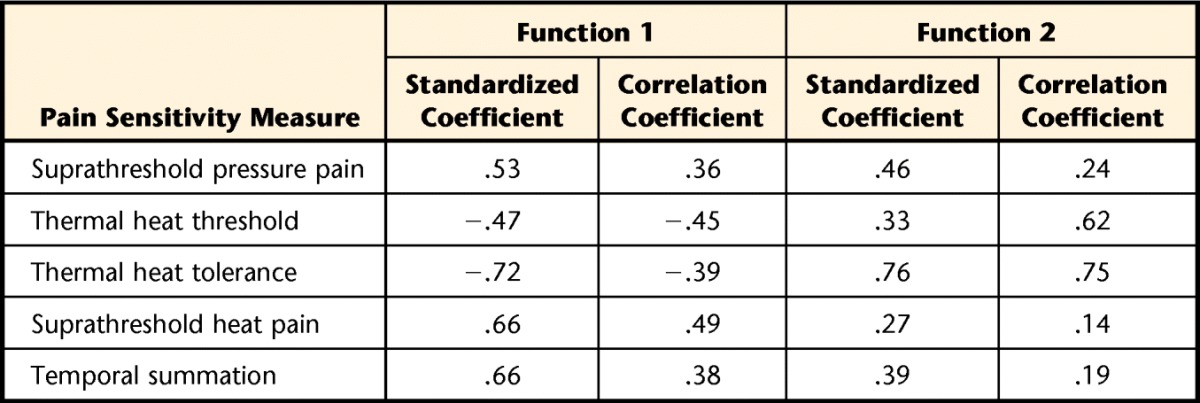
Validation of the cluster solution was performed first using discriminant function analysis with 5 predictors simultaneously entered into analysis: suprathreshold pressure pain (Wilks λ=.78, P<.001), thermal heat threshold (Wilks λ=.61, P<.001), thermal heat tolerance (Wilks λ=.59, P<.001), suprathreshold heat pain (Wilks λ=.78, P<.001), and temporal summation (Wilks λ=.70, P<.001). Two discriminant functions were extracted, with significant results for the overall test for functions 1 and 2 (χ102=226.42, P<.001, Wilks λ=.21) and for the test for function 2 (χ42=79.49, P<.001, Wilks λ=.58). Collectively, these findings indicate that both functions discriminate among the cluster groups. Functions 1 and 2 accounted for 64% (canonical correlation=.80) and 42% (canonical correlation=.65), respectively, of the total relationship between predictors and cluster groups. Table 2 presents the discriminant function coefficients for each of the predictors within each function and illustrates the relative and absolute relationships between the predictors and discriminant function. For function 1, the strongest relationship was observed with thermal heat tolerance followed by the 2 thermal dynamic measures, and the weakest relationship was observed with thermal heat threshold. Classification using cross-validation demonstrated that the functions were able to correctly classify 86.6% of the 3 cluster groups (98.0% of the low pain sensitivity group, 89.8% of the high thermal static sensitivity group, 67.5% of the high pressure and thermal dynamic sensitivity group).
Table 2.
Standardized Canonical Coefficients and Correlation (Pooled Within-Group) Coefficients of the Pain Sensitivity Variables of the Discriminant Function
Subgroup Differences in Pain Sensitivity
One-way ANOVA results for differences between pain sensitivity measures among the 3 cluster groups as a second step for validation are presented in Table 3 along with differences in other baseline factors. Significant differences among groups were noted for all pain sensitivity measures (P<.05). Compared with the other 2 cluster groups, the low pain sensitivity group had higher scores for thermal heat threshold and tolerance and lower scores for suprathreshold heat pain and temporal summation (P<.05), suggesting less sensitivity to these stimuli. Lower scores for suprathreshold pressure pain were observed in the low pain sensitivity group in comparison with the high pressure and thermal dynamic sensitivity group (P<.05) but not when compared with the high thermal static sensitivity group (P>.05). The high pressure and thermal dynamic group had higher scores on all 5 pain sensitivity measures compared with the high thermal static sensitivity group (P<.05), which suggests higher relative sensitivity to pressure and thermal dynamic stimuli but not to thermal static stimuli in the high pressure and thermal dynamic sensitivity group.
Table 3.
Baseline Demographic Characteristics, Psychological Measures, and Pain Sensitivity Factors Based on Pain Sensitivity Subgroupsa
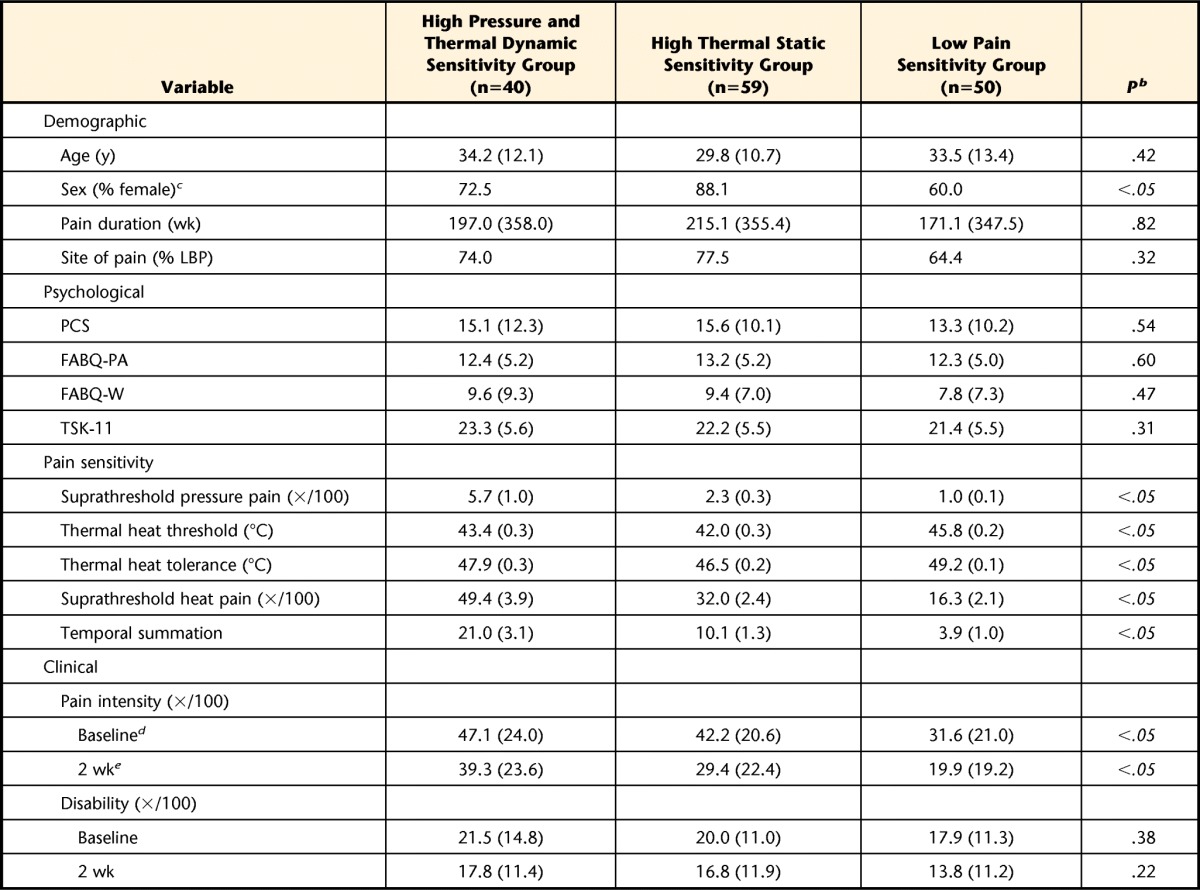
Values are X̅ (SD) unless otherwise indicated. LBP=low back pain, PCS=Pain Catastrophizing Scale, FABQ-PA=Fear-Avoidance Beliefs Questionnaire physical activity subscale, FABQ-W=Fear-Avoidance Beliefs Questionnaire work subscale, TSK=Tampa Scale of Kinesiophobia.
b Italicized P values denote significance.
c Difference in sex between high thermal static sensitivity group and low pain sensitivity group (P<.05).
d Difference in baseline pain intensity between high pressure and thermal dynamic sensitivity group and low pain sensitivity group (P<.05) and between high thermal static sensitivity group and low pain sensitivity group (P<.05).
e Difference in 2-wk pain intensity between high pressure and thermal dynamic sensitivity group and low pain sensitivity group (P<.05).
Subgroup Differences in Demographics and Psychological Status
No baseline demographic or psychological differences were noted among the cluster groups, except for a difference in sex between the high thermal static sensitivity group and the low pain sensitivity group, with a relatively higher proportion of female participants seen in the former group (P<.05) (Tab. 3).
Subgroup Differences in Clinical Outcome
Lower baseline pain intensity was reported in the low pain sensitivity group (P<.05), but a similar difference in 2-week pain intensity was noted only when compared with the high pressure and thermal dynamic pain sensitivity group (P<.05) (Tab. 3). We observed differences in the proportion of individuals within each group meeting a 30% clinical pain intensity change (χ2=6.90, P<.05), with a higher proportion of individuals in the low pain sensitivity group (63%) meeting this criterion compared with the high pressure and thermal dynamic sensitivity group (34%) but not when compared with the high thermal static sensitivity group (51%). No differences were observed in the proportion of individuals in each group meeting a 30% change in disability (χ2=1.90, P>.05). The proportions of individuals meeting this criterion for disability were 40% for the low pain sensitivity group, 35% for the high thermal static sensitivity group, and 26% for the high pressure and thermal dynamic sensitivity group. Crude and adjusted odds ratios for pain sensitivity status on pain intensity and disability outcome are presented in Tables 4 and 5, respectively. After controlling for potential confounding factors, lower odds for achieving a successful pain outcome were still associated with the high pressure and thermal dynamic sensitivity group.
Table 4.
Influence of Pain Sensitivity Subgroup on Pain Intensity Outcome (Proportion of Individuals Meeting 30% Change)a
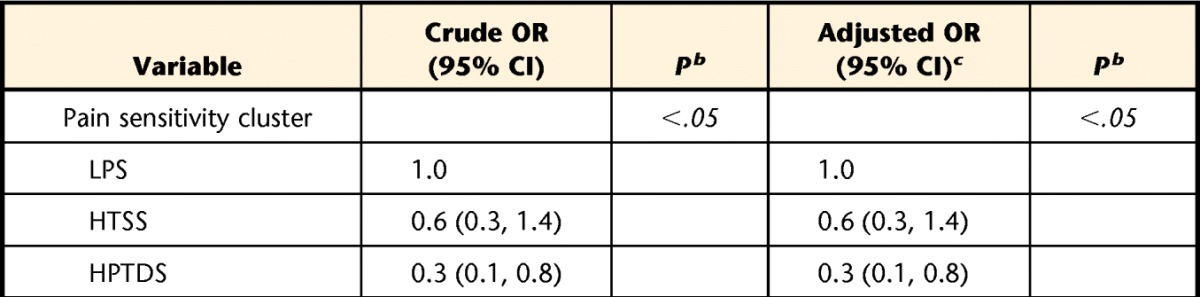
HPTDS=high pressure and thermal dynamic sensitivity, HTSS=high thermal static sensitivity, LPS=low pain sensitivity, OR=odds ratio, 95% CI=95% confidence interval.
b Italicized P values denote significance.
c Adjusted for age, sex, pain duration, and baseline pain intensity.
Table 5.
Influence of Pain Sensitivity Subgroup on Disability Outcome (Proportion of Individuals Meeting 30% Change)a
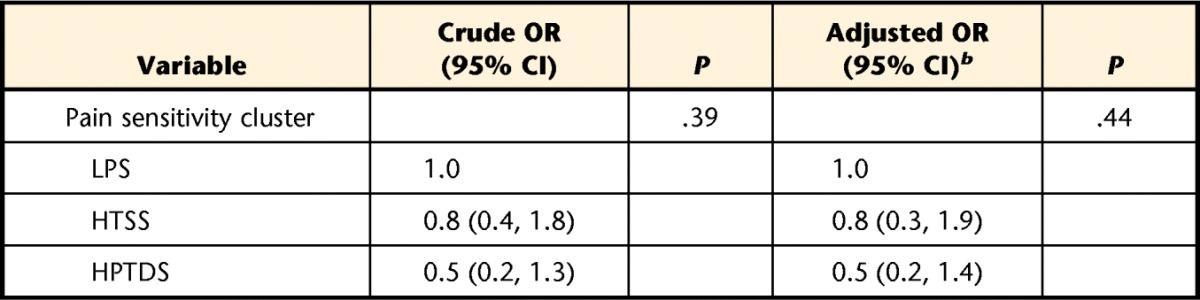
HPTDS=high pressure and thermal dynamic sensitivity, HTSS=high thermal static sensitivity, LPS=low pain sensitivity, OR=odds ratio, 95% CI=95% confidence interval.
b Adjusted for age, sex, pain duration, and baseline disability.
Discussion
We performed a cluster analysis on pain sensitivity responses in a group of individuals with spine pain and found 3 subgroups of individuals who differed in response to pressure and thermal stimuli. We confirmed our initial hypothesis and noted that these groups included a low pain sensitivity group, a high thermal static sensitivity group, and a high pressure and thermal dynamic sensitivity group. These groups were distinguished by stimulus modality, namely whether measures were static or dynamic, a similar finding found in participants who were pain-free.47 Furthermore, we found support for our second hypothesis through observation of a differential subgroup association with clinical pain intensity outcome but not disability or psychological factors. The specificity of this finding (ie, pain sensitivity specific to pain intensity) is noteworthy and in need of further consideration in studies.
Few investigations have examined patient subgroups based on pain sensitivity measures, particularly in musculoskeletal pain conditions commonly seen in physical therapy. In a recent study, Borsbo et al45 examined pain sensitivity subgroups using static pain sensitivity measures in a group of women with chronic whiplash-associated disorder. They found 2 pain sensitivity subgroups within the clinical sample, where the higher pain sensitive subgroup demonstrated a worse clinical picture (ie, higher pain intensity) than the less sensitive subgroup. Furthermore, when compared with a pain-free cohort, only the higher pain sensitive group had significantly greater pain sensitivity. In our current study, we had a similar general finding of differing pain sensitivity among individuals with spine pain (eg, less sensitive and more sensitive); however, we also observed subgroup differences in the higher pain sensitive subgroups dependent on static and dynamic pain sensitivity measures.
Only 2 previous studies, to our knowledge, have incorporated static and dynamic pain sensitivity procedures via cluster analysis.47,48 These studies examined responses within a sample of healthy individuals and showed that a dynamic measure of temporal summation was a distinguishing variable among subgroups. We also found comparable findings where dynamic pain sensitivity measures can differentiate among subgroups. Given our current results, we suggest incorporating both static and dynamic pain sensitivity measures when determining pain profiles, as dynamic measures appear to distinguish among individuals with a similar clinical presentation. Moreover, our group with enhanced dynamic pain sensitivity showed poorer clinical pain intensity outcome than our group with low pain sensitivity. This finding adds support to the potential clinical relevance of dynamic pain sensitivity measures as opposed to static pain sensitivity measures alone.
Our results further highlight the potential uniqueness of elevated pain sensitivity within a clinical population. We did not observe subgroup differences in psychological status or area of chief pain complaint (eg, neck or low back pain). We previously observed a similar finding in our study with individuals with shoulder pain who exhibited differing pain sensitivity profiles but did not differ on psychology.11 These findings suggest that pain sensitivity may reflect distinct aspects of the pain experience not redundant with psychology or reflective of the subtype of pain condition. This finding, however, is not definitive. Some investigators46,47,64 have observed differences in psychological factors between pain sensitivity subgroups, in contrast to our current observation.
There is evidence suggesting pain sensitivity is predictive of clinical outcome and a potential prognostic factor of interest.42,65 The influence of pain sensitivity corresponds most highly to pain-related outcomes such as clinical pain intensity and is supported in the current study where individuals in the high pressure and thermal dynamic sensitivity group showed higher pain intensity ratings and a lower proportion of individuals achieving a clinically meaningful pain outcome. The association between subgroup status and pain outcome did not change after controlling for other prognostic variables and seems robust enough for further investigation. The relationship between pain sensitivity and disability is not as clear. Given the lack of association between pain sensitivity subgroup and disability outcome, we regard other factors as more influential in driving disability-related outcomes (ie, psychological status). For example, Beneciuk et al66 subgrouped individuals with low back pain based on fear-avoidance measures and showed an influence of psychological subgroup on both pain and disability outcome. Findings such as these are consistent with the fear-avoidance model of pain and support the need to incorporate both psychology and pain sensitivity in the assessment of musculoskeletal pain.67,68
This study has limitations to consider. We included data from 2 separate randomized trials with short-term outcome (eg, trial conclusion at 2 weeks) and used an analytical analysis strategy appropriate for prognostic studies. This study was an initial attempt to determine whether pain sensitivity subgroups can be identified and the extent to which they are related to clinical outcome as justification for their use as a prognostic factor in future studies. Further investigation of these pain sensitivity clusters as an additional prognostic factor within stronger study designs is warranted. For example, quality features of a prognostic study in the future that investigate these subgroups would need to include long-term follow-up, incorporation of an inception cohort, and blinding of examiners. We did not examine a specific response to a particular intervention through interaction effects due to lack of power. Thus, our results of differing subgroup influence on outcome are best interpreted as a potential prognostic influence with no direct effect from specific treatment (eg, manual therapy). Although not reported in this study, preliminary analyses from the randomized trials suggest no significant differences in clinical outcome among the multiple arms of the randomized trials. It also is important to note that the proportion of those individuals with neck or low back pain did not differ between subgroups; thus, subgroup differences cannot be attributed to pain complaint location.
In addition, because we used 2 different spine pain conditions, we standardized assessment and analyses of the data to include only pain sensitivity locations within a dermatome-related region for each of the clinical conditions. We did not have data related to certain clinical characteristics such as referral pattern of low back pain (eg, pain distribution), other coexisting areas of pain complaint, or alternative measures of pain intensity (ie, resting pain, pain with specific movements). These data may provide indications of severity of condition or potential neurologic involvement. Additionally, we did not perform assessments to identify neuropathic injury or disease, which could be associated with the aberrant sensory findings in this study. We did exclude participants who reported pain complaints extending past the elbow (neck pain) or knee (low back pain) and other current chronic pain conditions. Although we cannot confirm individuals did not have neuropathic symptoms, the subjective criteria indicate that individuals were without neuropathic injury or disease. Our current study did not include a control, nonpatient sample, so we are unable to discuss the relevance of the differences in pain sensitivity compared with a healthy population or to determine the presence of altered sensitization.
Our study attempted to examine whether pain sensitivity subgroups can be distinguished in individuals with similar pain complaints and whether there is a differential impact on clinical outcome based on subgroup status. These results suggest that a subgroup of individuals with heightened dynamic pain sensitivity have a worse pain intensity clinical outcome. Efforts are needed to develop clinically feasible pain sensitivity protocols that include multiple stimulus modalities and are able to deliver static and dynamic parameters. Additionally, future research should focus on how subgroups with differing responses to experimental stimuli could direct physical therapy treatment or interact with specific physical therapy treatments to improve clinical outcome rates. These efforts have been initiated with clinical factors69–71 as well as psychological factors.71–74 Future research will determine whether subgroup-based intervention strategies based on pain sensitivity can be used to better direct clinical management. For example, previous studies have indicated manual therapy reduces static and dynamic measures of pain sensitivity,26 but these therapies have not been targeted to a subgroup of patients with heightened dynamic pain sensitivity profiles to determine whether larger treatment effects are observed.
In conclusion, subgroups of individuals with differing response to experimental stimuli exist in patients with spine pain, and subgroup status is associated with differential improvement in pain intensity but not disability. Future research should investigate whether screening procedures can identify pain sensitivity subgroups and whether management can be modified based on pain sensitivity status.
Footnotes
All authors provided concept/idea/research design and writing. Dr Bialosky provided data collection. Dr Coronado, Dr Bialosky, and Dr George provided data analysis. Dr Coronado provided project management and clerical support. Dr Robinson provided facilities/equipment. Dr Robinson and Dr George provided consultation (including review of manuscript before submission). The authors acknowledge Maggie Horn, PT, PhD, for her assistance with data collection and Jason Beneciuk, PT, PhD, FAAOMPT, and Mark D. Bishop, PT, PhD, for assistance with statistical analyses.
The study protocols for these analyses were approved by the University of Florida Institutional Review Board.
This research was presented at the International Association for the Study of Pain World Congress on Pain; August 27–31, 2012; Milan, Italy, and the Combined Sections Meeting of the American Physical Therapy Association; February 3–6, 2014; Las Vegas, Nevada.
This study was supported by the University of Florida Research Opportunity Fund. Dr Coronado was supported by NIH T32 Interdisciplinary Training in Rehabilitation and Neuromuscular Plasticity grant 5T32HD043730. Dr Bialosky received support from the Rehabilitation Research Career Development Program (5K12HD055929-02).
References
- 1. Courtney CA, Clark JD, Duncombe AM, O'Hearn MA. Clinical presentation and manual therapy for lower quadrant musculoskeletal conditions. J Man Manip Ther. 2011;19:212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Courtney CA, Kavchak AE, Lowry CD, O'Hearn MA. Interpreting joint pain: quantitative sensory testing in musculoskeletal management. J Orthop Sports Phys Ther. 2010;40:818–825 [DOI] [PubMed] [Google Scholar]
- 3. de-la-Llave-Rincon IA, Puentedura EJ, Fernandez-de-Las-Penas C. Clinical presentation and manual therapy for upper quadrant musculoskeletal conditions. J Man Manip Ther. 2011;19:201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nijs J, Van Houdenhove B. From acute musculoskeletal pain to chronic widespread pain and fibromyalgia: application of pain neurophysiology in manual therapy practice. Man Ther. 2009;14:3–12 [DOI] [PubMed] [Google Scholar]
- 5. O'Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain. 2007;11:415–420 [DOI] [PubMed] [Google Scholar]
- 6. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623 [DOI] [PubMed] [Google Scholar]
- 7. Sterling M, Jull G, Vicenzino B, Kenardy J. Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain. 2003;104:509–517 [DOI] [PubMed] [Google Scholar]
- 8. Puta C, Schulz B, Schoeler S, et al. Enhanced sensitivity to punctate painful stimuli in female patients with chronic low back pain. BMC Neurol. 2012;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott D, Jull G, Sterling M. Widespread sensory hypersensitivity is a feature of chronic whiplash-associated disorder but not chronic idiopathic neck pain. Clin J Pain. 2005;21:175–181 [DOI] [PubMed] [Google Scholar]
- 10. Fernandez-Carnero J, Fernandez-de-Las-Penas C, de la Llave-Rincon AI, et al. Widespread mechanical pain hypersensitivity as sign of central sensitization in unilateral epicondylalgia: a blinded, controlled study. Clin J Pain. 2009;25:555–561 [DOI] [PubMed] [Google Scholar]
- 11. Coronado RA, Simon CB, Valencia C, George SZ. Experimental pain responses support peripheral and central sensitization in patients with unilateral shoulder pain. Clin J Pain. 2014;30:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gwilym SE, Oag HC, Tracey I, Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. J Bone Joint Surg Br. 2011;93:498–502 [DOI] [PubMed] [Google Scholar]
- 13. Chiarotto A, Fernandez-de-Las-Penas C, Castaldo M, Villafane JH. Bilateral pressure pain hypersensitivity over the hand as potential sign of sensitization mechanisms in individuals with thumb carpometacarpal osteoarthritis. Pain Med. 2013;14:1585–1592 [DOI] [PubMed] [Google Scholar]
- 14. Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581 [DOI] [PubMed] [Google Scholar]
- 15. Jensen R, Hystad T, Kvale A, Baerheim A. Quantitative sensory testing of patients with long lasting patellofemoral pain syndrome. Eur J Pain. 2007;11:665–676 [DOI] [PubMed] [Google Scholar]
- 16. Wylde V, Palmer S, Learmonth ID, Dieppe P. Somatosensory abnormalities in knee OA. Rheumatology (Oxford). 2012;51:535–543 [DOI] [PubMed] [Google Scholar]
- 17. Gwilym SE, Keltner JR, Warnaby CE, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–1234 [DOI] [PubMed] [Google Scholar]
- 18. Lee YC, Lu B, Bathon JM, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken). 2011;63:320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curatolo M. Diagnosis of altered central pain processing. Spine (Phila Pa 1976). 2011;36(25 suppl):S200–S204 [DOI] [PubMed] [Google Scholar]
- 20. Staud R. Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia. Curr Rheumatol Rep. 2011;13:513–520 [DOI] [PubMed] [Google Scholar]
- 21. Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum. 2008;37:339–352 [DOI] [PubMed] [Google Scholar]
- 23. Arendt-Nielsen L, Graven-Nielsen T. Translational musculoskeletal pain research. Best Pract Res Clin Rheumatol. 2011;25:209–226 [DOI] [PubMed] [Google Scholar]
- 24. Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol. 2010;6:599–606 [DOI] [PubMed] [Google Scholar]
- 25. Bialosky JE, Bishop MD, Robinson ME, et al. Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Phys Ther. 2009;89:1292–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coronado RA, Gay CW, Bialosky JE, et al. Changes in pain sensitivity following spinal manipulation: a systematic review and meta-analysis. J Electromyogr Kinesiol. 2012;22:752–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fernandez-Carnero J, Fernandez-de-las-Penas C, Cleland JA. Immediate hypoalgesic and motor effects after a single cervical spine manipulation in subjects with lateral epicondylalgia. J Manipulative Physiol Ther. 2008;31:675–681 [DOI] [PubMed] [Google Scholar]
- 28. Fernandez-de-las-Penas C, Perez-de-Heredia M, Brea-Rivero M, Miangolarra-Page JC. Immediate effects on pressure pain threshold following a single cervical spine manipulation in healthy subjects. J Orthop Sports Phys Ther. 2007;37:325–329 [DOI] [PubMed] [Google Scholar]
- 29. George SZ, Bishop MD, Bialosky JE, et al. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord. 2006;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Backonja MM, Attal N, Baron R, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154:1807–1819 [DOI] [PubMed] [Google Scholar]
- 31. Kamper SJ, Maher CG, Hush JM, et al. Relationship between pressure pain thresholds and pain ratings in patients with whiplash-associated disorders. Clin J Pain. 2011;27:495–501 [DOI] [PubMed] [Google Scholar]
- 32. Hubscher M, Moloney N, Leaver A, et al. Relationship between quantitative sensory testing and pain or disability in people with spinal pain—a systematic review and meta-analysis. Pain. 2013;154:1497–1504 [DOI] [PubMed] [Google Scholar]
- 33. Grosen K, Fischer IW, Olesen AE, Drewes AM. Can quantitative sensory testing predict responses to analgesic treatment? Eur J Pain. 2013;17:1267–1280 [DOI] [PubMed] [Google Scholar]
- 34. Neziri AY, Curatolo M, Nuesch E, et al. Factor analysis of responses to thermal, electrical, and mechanical painful stimuli supports the importance of multi-modal pain assessment. Pain. 2011;152:1146–1155 [DOI] [PubMed] [Google Scholar]
- 35. Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–572 [DOI] [PubMed] [Google Scholar]
- 36. Staahl C, Drewes AM. Experimental human pain models: a review of standardised methods for preclinical testing of analgesics. Basic Clin Pharmacol Toxicol. 2004;95:97–111 [DOI] [PubMed] [Google Scholar]
- 37. Kong JT, Schnyer RN, Johnson KA, Mackey S. Understanding central mechanisms of acupuncture analgesia using dynamic quantitative sensory testing: a review. Evid Based Comp Alt Med. 2013;2013:187182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weissman-Fogel I, Granovsky Y, Crispel Y, et al. Enhanced presurgical pain temporal summation response predicts post-thoracotomy pain intensity during the acute postoperative phase. J Pain. 2009;10:628–636 [DOI] [PubMed] [Google Scholar]
- 39. Valencia C, Fillingim RB, George SZ. Suprathreshold heat pain response is associated with clinical pain intensity for patients with shoulder pain. J Pain. 2011;12:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Staud R, Cannon RC, Mauderli AP, et al. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95 [DOI] [PubMed] [Google Scholar]
- 41. Staud R, Robinson ME, Vierck CJ, Jr, et al. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–222 [DOI] [PubMed] [Google Scholar]
- 42. Werner MU, Mjobo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anesthesiology. 2010;112:1494–1502 [DOI] [PubMed] [Google Scholar]
- 43. Alappattu MJ, Bishop MD, Bialosky JE, et al. Stability of behavioral estimates of activity-dependent modulation of pain. J Pain Res. 2011;4:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Terry EL, France CR, Bartley EJ, et al. Standardizing procedures to study sensitization of human spinal nociceptive processes: comparing parameters for temporal summation of the nociceptive flexion reflex (TS-NFR). Int J Psychophysiol. 2011;81:263–274 [DOI] [PubMed] [Google Scholar]
- 45. Borsbo B, Liedberg GM, Wallin M, Gerdle B. Subgroups based on thermal and pressure pain thresholds in women with chronic whiplash display differences in clinical presentation: an explorative study. J Pain Res. 2012;5:511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Giesecke T, Williams DA, Harris RE, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003;48:2916–2922 [DOI] [PubMed] [Google Scholar]
- 47. Hastie BA, Riley JL, III, Robinson ME, et al. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–237 [DOI] [PubMed] [Google Scholar]
- 48. Kindler LL, Sibille KT, Glover TL, et al. Drug response profiles to experimental pain are opioid and pain modality specific. J Pain. 2011;12:340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. George SZ, Valencia C, Beneciuk JM. A psychometric investigation of fear-avoidance model measures in patients with chronic low back pain. J Orthop Sports Phys Ther. 2010;40:197–205 [DOI] [PubMed] [Google Scholar]
- 50. Sullivan M, Bishop S, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524–532 [Google Scholar]
- 51. Waddell G, Newton M, Henderson I, et al. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52:157–168 [DOI] [PubMed] [Google Scholar]
- 52. Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117:137–144 [DOI] [PubMed] [Google Scholar]
- 53. Brown JL, Edwards PS, Atchison JW, et al. Defining patient-centered, multidimensional success criteria for treatment of chronic spine pain. Pain Med. 2008;9:851–862 [DOI] [PubMed] [Google Scholar]
- 54. Robinson ME, Brown JL, George SZ, et al. Multidimensional success criteria and expectations for treatment of chronic pain: the patient perspective. Pain Med. 2005;6:336–345 [DOI] [PubMed] [Google Scholar]
- 55. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25:2940–2952; discussion 2952 [DOI] [PubMed] [Google Scholar]
- 56. Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–415 [PubMed] [Google Scholar]
- 57. Bishop MD, Beneciuk JM, George SZ. Immediate reduction in temporal sensory summation after thoracic spinal manipulation. Spine J. 2011;11:440–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Robinson ME, Bialosky JE, Bishop MD, et al. Supra-threshold scaling, temporal summation, and after-sensation: relationships to each other and anxiety/fear. J Pain Res. 2010;3:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Price DD, Staud R, Robinson ME, et al. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59 [DOI] [PubMed] [Google Scholar]
- 60. Staud R, Vierck CJ, Cannon RL, et al. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175 [DOI] [PubMed] [Google Scholar]
- 61. Anderson RJ, Craggs JG, Bialosky JE, et al. Temporal summation of second pain: variability in responses to a fixed protocol. Eur J Pain. 2013;17:67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Salaffi F, Stancati A, Silvestri CA, et al. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8:283–291 [DOI] [PubMed] [Google Scholar]
- 63. Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–414 [DOI] [PubMed] [Google Scholar]
- 64. Park JW, Clark GT, Kim YK, Chung JW. Analysis of thermal pain sensitivity and psychological profiles in different subgroups of TMD patients. Int J Oral Maxillofac Surg. 2010;39:968–974 [DOI] [PubMed] [Google Scholar]
- 65. Valencia C, Kindler LL, Fillingim RB, George SZ. Investigation of central pain processing in shoulder pain: converging results from 2 musculoskeletal pain models. J Pain. 2012;13:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Beneciuk JM, Robinson ME, George SZ. Low back pain subgroups using fear-avoidance model measures: results of a cluster analysis. Clin J Pain. 2012;28:658–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pincus T, Smeets RJ, Simmonds MJ, Sullivan MJ. The fear avoidance model disentangled: improving the clinical utility of the fear avoidance model. Clin J Pain. 2010;26:739–746 [DOI] [PubMed] [Google Scholar]
- 68. Leeuw M, Goossens ME, Linton SJ, et al. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–94 [DOI] [PubMed] [Google Scholar]
- 69. Childs JD, Fritz JM, Flynn TW, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med. 2004;141:920–928 [DOI] [PubMed] [Google Scholar]
- 70. Long A, Donelson R, Fung T. Does it matter which exercise? A randomized control trial of exercise for low back pain. Spine. 2004;29:2593–2602 [DOI] [PubMed] [Google Scholar]
- 71. Werneke MW, Hart DL, George SZ, et al. Clinical outcomes for patients classified by fear-avoidance beliefs and centralization phenomenon. Arch Phys Med Rehabil. 2009;90:768–777 [DOI] [PubMed] [Google Scholar]
- 72. Hill JC, Whitehurst DG, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378:1560–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. George SZ, Fritz JM, Bialosky JE, Donald DA. The effect of a fear-avoidance-based physical therapy intervention for patients with acute low back pain: results of a randomized clinical trial. Spine (Phila Pa 1976). 2003;28:2551–2560 [DOI] [PubMed] [Google Scholar]
- 74. George SZ, Zeppieri G, Jr, Cere AL, et al. A randomized trial of behavioral physical therapy interventions for acute and sub-acute low back pain (NCT00373867). Pain. 2008;140:145–157 [DOI] [PMC free article] [PubMed] [Google Scholar]