Abstract
Vertebral discitis usually arises from haematogenous spread of pathogens to the discs and bones. Vertebral discitis can rarely occur as a complication after laparoscopic operations with fixating sutures on the promontory. We report the case of an 81-year-old woman who underwent a laparoscopic resection rectopexy because of rectal prolapse. Weeks after the operation, the patient developed lower back pain with radiation to both legs not responding to symptomatic therapy. Two months later, a magnetic resonance imaging of the lumbar spine showed vertebral osteomyelitis and discitis. A fixation on the promontory may be sufficiently traumatic to the spine to pave the way for subsequent infection. A high index of suspicion should be raised in patients with persistent, severe back pain. Anamnesis, imageing and an adequate specimen from the affected area for microbiological analysis are crucial for timely diagnosis and appropriate management involving targeted and prolonged antimicrobial therapy.
INTRODUCTION
The most common way of vertebral infection is haematogenous. Sometimes, pathogens may reach the bone directly during a neurosurgical operation, e.g. lumbar discectomy [1].
Rarely, vertebral discitis is reported as a complication of laparoscopic operations with fixating sutures on the promontory.
CASE REPORT
An 81-year-old woman underwent laparoscopic resection rectopexy because of rectal prolapse. The initial hospitalization was uneventful and during the operation no epidural analgesy was applied. Three weeks later, she presented with increasing back pain without neurological signs and symptoms or fever. Due to elevated C-reactive protein (CRP), a deep-seated surgical site infection was suspected. Computed tomography (CT) scan and magnetic resonance imaging (MRI) of the pelvis were normal besides presacral seroma. However, one of four blood culture bottles was positive with Pseudomonas aeruginosa and the patient was treated with Piperacillin/Tazobactam for 14 days. Pain improved transiently but returned shortly after cessation of antibiotics. Subsequent symptomatic therapy was without benefit.
Three months after the initial operation, the patient presented with severe back pain radiating to both legs. The lower lumbar spine was highly tender on percussion, but the neurologic examination revealed only a slight loss of muscle strength in the big right toe. Vascular diseases of the lower extremities were excluded. The patient did not have fever at any time. Leucocytes were normal and CRP was moderately elevated to 42 mg/l. The MRI of the spine now revealed vertebral discitis without epidural abscess formation (Fig. 1). A barium enema excluded fistulas to the rectum (Fig. 2). Culture of CT-guided needle biopsy was positive with P. aeruginosa and antibiotic therapy with 6× 1 g Ceftazidime i.v. per day was started. The pain slowly improved and CRP values declined. After 20 days, the patient developed β-lactam-associated neutropenia. Therefore, treatment was changed to oral ciprofloxacin and continued for another 9 weeks. Pain was managed with fentanyl skin patch, paracetamol, metamizol and pregabalin. The patient fully recovered from the lower back pain. The loss of muscle strength in the big right toe persisted and was interpreted as stenosis of right S5 foramina due to infective destruction which did not require neurosurgical decompression.
Figure 1:
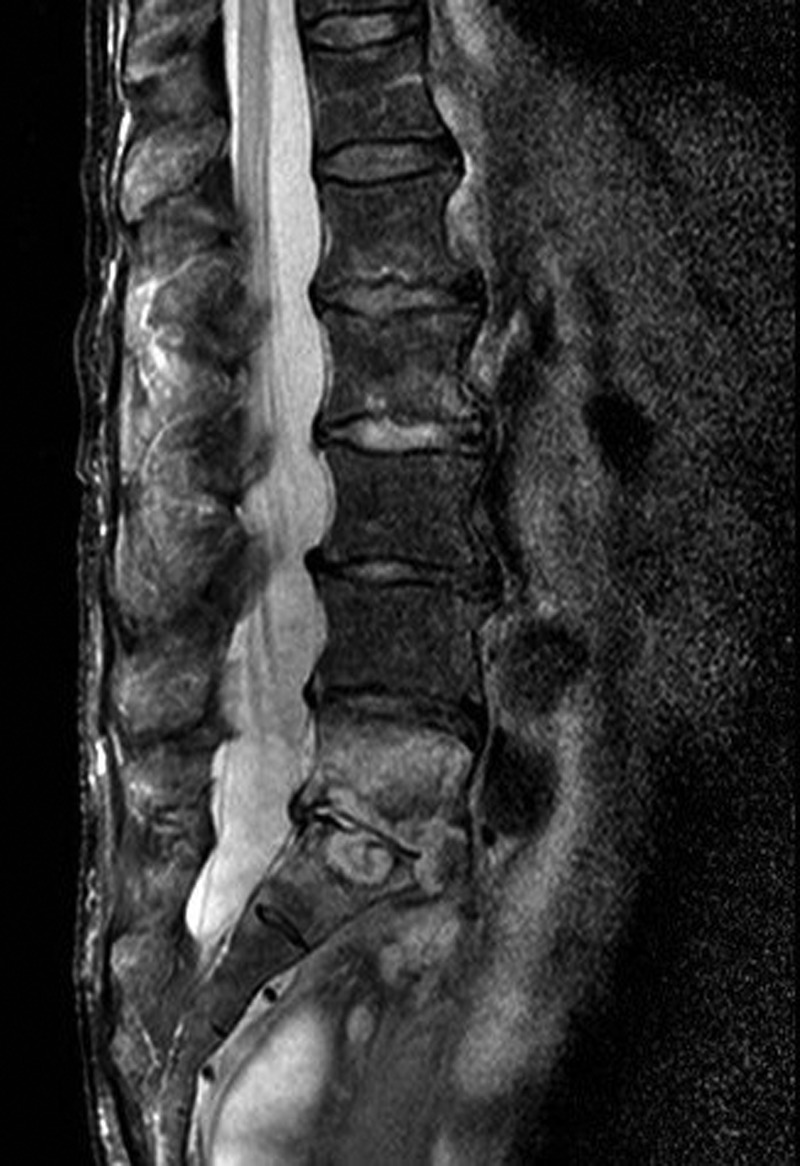
MRI of the spine with vertebral discitis L5/S1.
Figure 2:
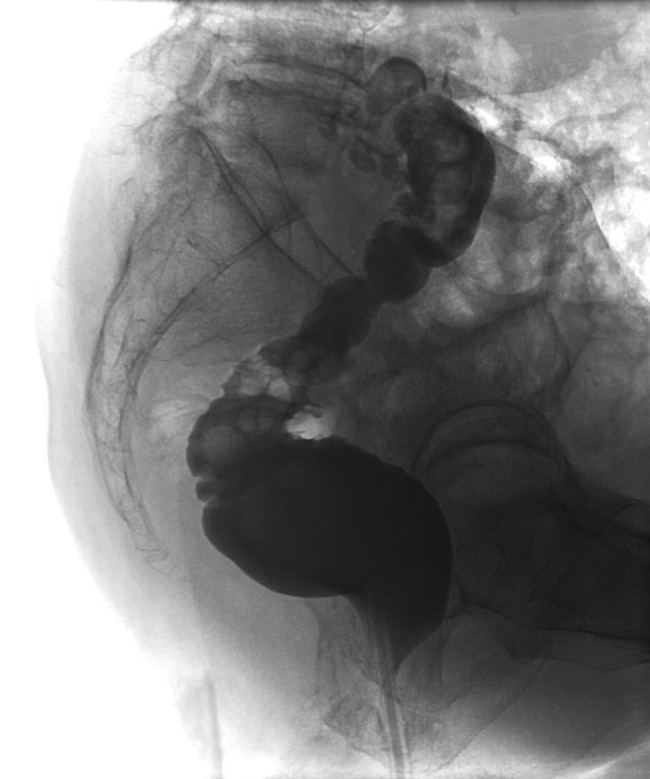
Barium enema without fistula at infection site.
DISCUSSION
In most cases vertebral discitis is resulting from haematogenous spread of pathogens. More rarely they develop through direct inoculation after vertebral surgery. In our case it is also possible that after the operation the presacral seroma was not the primary infectious site but got infected during the Pseudomonas sepsis. Staphylococcus is by far the most common pathogen [2]. Spinal tuberculosis and brucellosis mainly occur in endemic areas, but should also be suspected in migrants.
The signs and symptoms of a vertebral discitis are not specific. Most patients present with back pain, but motor weakness and sensory changes are only found in a minority. Symptoms often worsen over weeks and months. Less than half of the patients with bacterial spondylodiscitis have fever [3]. Physical examination is often similar to common back pain. However, tenderness to percussion of the spine may exist locally. A high level of suspicion is required in patients with opioid-dependent back pain and an unexplained inflammatory response. Leucocyte counts may be elevated or normal [4], whereas CRP levels are increased in 58 [3] to 82% of patients [5].
If a vertebral discitis is suspected, an MRI of the affected area should be performed. This is the gold standard with sensitivity, specificity and accuracy of 96, 92 and 94%, respectively. Typically, MRI shows decreased signal intensity of vertebral bodies in T1-weighted images and increased disc signal intensity in T2-weighted images. However, MRI may be negative early in the disease course and should be repeated after 7–14 days if the diagnosis is still likely. In the absence of positive blood cultures with a typical microorganism CT-guided biopsy for microbiology and histology should be performed before initiation of empiric antimicrobial therapy. The sensitivity of cultures from biopsy is described from 36% up to 100%. Blood cultures alone are positive in 72% [4]. If microbiological culture of a representative biopsy remains negative for >5 days, broad spectrum bacterial DNA amplification may help to identify a causative microorganism, particularly if antibiotics had been administered before biopsy [6].
If empirical therapy is needed before the availability of first microbiologic results, the choice of antimicrobials is dependent on likely pathogens, individual patient history and local resistance patterns. However, intravenous therapy with high doses of antimicrobials sufficiently covering staphylococci and other Gram-positive bacteria are the first priority to rapidly reach bactericidal drug levels in bone, disc and epidural space. In regions and/or situations with a low pre-test probability for MRSA, a penicillinase-resistant penicillin may be sufficient. Empirical treatment for Gram-negative bacteria including Pseudomonas is usually reserved for patients with additional risk factors. Intravenous therapy should be continued for a minimum of 2 weeks. The switch to oral therapy depends on oral bioavailability, drug susceptibility, individual patient parameters and on a good clinical response. The total duration of treatment for bacterial spondylodicitis is typically 6–12 weeks. Response to treatment should be monitored clinically and with CRP, if initially elevated. Blood counts, liver and kidney function tests should be used to guide optimal dosage and monitor adverse events of prolonged and high-dose antimicrobial therapy. Routine follow-up imaging is not recommended unless disease progression or treatment failure is suspected based on clinical and laboratory assessment [7]. Surgical debridement is usually needed in the case of epidural abscess formation or disease progression despite adequate antimicrobial therapy [2].
A review of the literature in Medline revealed only a few case reports of vertebral discitis after fixation on the promontory. Two cases of lumbar discitis after laparoscopic rectopexy were recently reported from the Netherlands [8]. Few other cases have been described in gynaecological journals after sacrocolpopexy.
A fixation on the promontory can be a trauma of the spine leading to a locus of minor resistance to subsequent infection. The clinical signs and symptoms of spondylodiscitis are not specific. A follow-up examination should include the explicit question for back pain. Only straight forward diagnostic followed by a consequent therapy can avoid unnecessary postoperative morbidity by persistent neurological deficits because of this rare complication.
REFERENCES
- 1.Rohde V, Meyer B, Schaller C, Hassler WE. Spondylodiscitis after lumbar discectomy. Incidence and a proposal for prophylaxis. Spine. 1998;23:615–20. doi: 10.1097/00007632-199803010-00016. [DOI] [PubMed] [Google Scholar]
- 2.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 3.Kim CJ, Song KH, Jeon JH, Park WB, Park SW, Kim HB, et al. A comparative study of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 1976). 2010;35:E1096–100. doi: 10.1097/BRS.0b013e3181e04dd3. [DOI] [PubMed] [Google Scholar]
- 4.Enoch DA, Cargill JS, Laing R, Herbert S, Corrah TW, Brown NM. Value of CT-guided biopsy in the diagnosis of septic discitis. J Clin Pathol. 2008;61:750–3. doi: 10.1136/jcp.2007.054296. [DOI] [PubMed] [Google Scholar]
- 5.Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990–95. Scand J Infect Dis. 2001;33:527. doi: 10.1080/00365540110026566. [DOI] [PubMed] [Google Scholar]
- 6.Lecouvet F, Irenge L, Vandercam B, Nzeusseu A, Hamels S, Gala JL. The etiologic diagnosis of infectious discitis is improved by amplification-based DNA analysis. Arthritis Rheum. 2004;50:2985. doi: 10.1002/art.20462. [DOI] [PubMed] [Google Scholar]
- 7.Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Osmon DR. Do follow-up imaging examinations provide useful prognostic information in patients with spine infection? Clin Infect Dis. 2006;43:172. doi: 10.1086/505118. [DOI] [PubMed] [Google Scholar]
- 8.Draaisma WA, van Eijck MM, Vos J, Consten EC. Lumbar discitis after laparoscopic ventral rectopexy for rectal prolapse. Int J Colorectal Dis. 2011;26:255–6. doi: 10.1007/s00384-010-0971-0. [DOI] [PubMed] [Google Scholar]


