Abstract
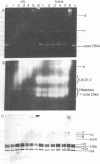
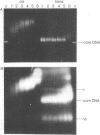
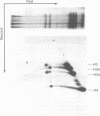
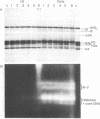
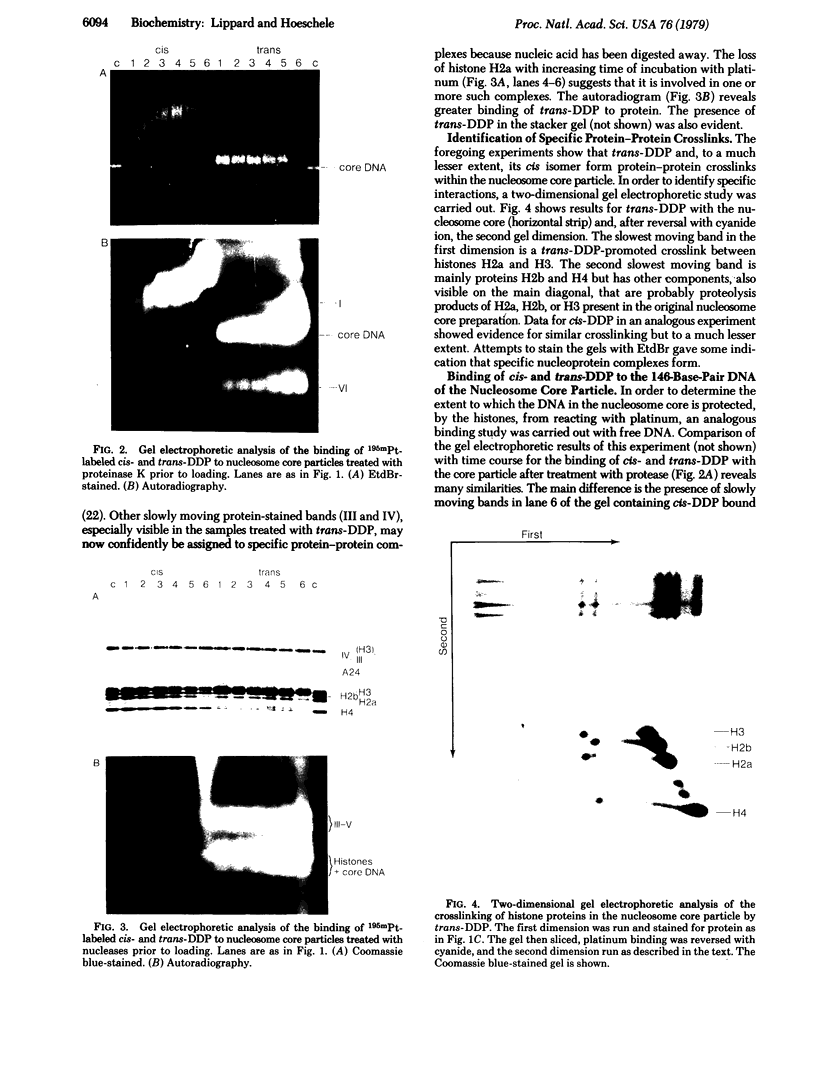
The binding of the antitumor drug cis-dichlorodiammineplatinum(II) and its inactive trans isomer with the nucleosome core particle has been investigated. Kinetic studies show that platinum binding increases with incubation time, from a few bound platinum atoms per nucleosome core in the first 0.5 hr to 40-50 after 40 hr. There is no crosslinking or dissociation of nucleosome cores upon platinum binding, as revealed by sedimentation velocity measurements. Electrophoresis through 0.1% sodium dodecyl sulfate/18% polyacrylamide gels after platinum binding reveals striking differences in the DNA and protein band patterns for the two isomers. cis-Dichlorodiammineplatinum(II) binds first to the DNA, retarding and spreading its migration in the gel. A comparison study with the 146-base-pair nucleosome core DNA showed the binding to be little affected by the presence of the histone octamer. The trans complex, on the other hand, produces DNA—histone and histone—histone crosslinks that only appear for the cis isomer after long incubation times. The protein—protein crosslinks were reversed by soaking the gel in cyanide solution to form [Pt(CN)4]-2. Subsequent two-dimensional gel electrophoresis revealed that trans-dichlorodiammineplatinum(II) forms specific crosslinks between histone protein pairs H3 and H2a and H2b and H4 in the nucleosome core. The occurrence of DNA—protein crosslinks was demonstrated by treating the platinum/nucleosome core reaction mixtures with a protease or with nucleases prior to electrophoresis and observing changes in the gel patterns. Platinum was located in the gels through autoradiography using 195mPt-labeled complexes. This work clearly demonstrates the greater propensity of trans-dichlorodiammineplatinum(II) to form histone—histone and histone—DNA crosslinks compared with the antitumor active cis isomer, which binds first to the DNA and only forms crosslinks to the histones when the nucleosome core is heavily loaded with platinum.
Keywords: chromatin, histones, antitumor drug, crosslinker, DNA
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Gonias S. L., Kam S. K., Wu K. C., Lippard S. J. Binding of the antitumor drug platinum uracil blue to closed and nicked circular duplex DNAs. Biochemistry. 1978 Mar 21;17(6):1060–1068. doi: 10.1021/bi00599a019. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Felsenfeld G. Histone H3 disulfide dimers and nucleosome structure. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5519–5523. doi: 10.1073/pnas.74.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. L., Bauer W. R., Barton J. K., Lippard S. J. Binding of cis- and trans-dichlorodiammineplatinum(II) to DNA: evidence for unwinding and shortening of the double helix. Science. 1979 Mar 9;203(4384):1014–1016. doi: 10.1126/science.370979. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Filipski J., Kohn K. W., Prather R., Bonner W. M. Thiourea reverses cross-links and restores biological activity in DNA treated with dichlorodiaminoplatinum (II). Science. 1979 Apr 13;204(4389):181–183. doi: 10.1126/science.571145. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., French M. F., Musso R., Busch H. Presence of protein A24 in rat liver nucleosomes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5492–5495. doi: 10.1073/pnas.74.12.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison R. C., Zeitler D. P., Murphy J. M., Chalkley R. Histone neighbors in nuclei and extended chromatin. Cell. 1977 Oct;12(2):417–427. doi: 10.1016/0092-8674(77)90118-0. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Rhodes D., Brown R. S., Klug A. A crystallographic study of metal-binding to yeast phenylalanine transfer RNA. J Mol Biol. 1977 Apr 15;111(3):315–328. doi: 10.1016/s0022-2836(77)80054-5. [DOI] [PubMed] [Google Scholar]
- Jackson V. Studies on histone organization in the nucleosome using formaldehyde as a reversible cross-linking agent. Cell. 1978 Nov;15(3):945–954. doi: 10.1016/0092-8674(78)90278-7. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lutter L. C. Kinetic analysis of deoxyribonuclease I cleavages in the nucleosome core: evidence for a DNA superhelix. J Mol Biol. 1978 Sep 15;124(2):391–420. doi: 10.1016/0022-2836(78)90306-6. [DOI] [PubMed] [Google Scholar]
- Lutter L. C. Precise location of DNase I cutting sites in the nucleosome core determined by high resolution gel electrophoresis. Nucleic Acids Res. 1979 Jan;6(1):41–56. doi: 10.1093/nar/6.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Prunell A., Kornberg R. D., Lutter L., Klug A., Levitt M., Crick F. H. Periodicity of deoxyribonuclease I digestion of chromatin. Science. 1979 May 25;204(4395):855–858. doi: 10.1126/science.441739. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Thomson A. J. The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol. 1979;22:71–133. doi: 10.1016/s0079-6603(08)60799-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg B., VanCamp L., Trosko J. E., Mansour V. H. Platinum compounds: a new class of potent antitumour agents. Nature. 1969 Apr 26;222(5191):385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Stone P. J., Kelman A. D., Sinex F. M. Specific binding of antitumour drug cis-Pt(NH3)2C12 to DNA rich in guanine and cytosine. Nature. 1974 Oct 25;251(5477):736–737. doi: 10.1038/251736a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Butler P. J. Characterization of the octamer of histones free in solution. J Mol Biol. 1977 Nov;116(4):769–781. doi: 10.1016/0022-2836(77)90270-4. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. Cleavable cross-links in the analysis of histone-histone associations. FEBS Lett. 1975 Oct 15;58(1):353–358. doi: 10.1016/0014-5793(75)80296-1. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. The study of histone--histone associations by chemical cross-linking. Methods Cell Biol. 1978;18:429–440. [PubMed] [Google Scholar]
- Zwelling L. A., Anderson T., Kohn K. W. DNA-protein and DNA interstrand cross-linking by cis- and trans-platinum(II) diamminedichloride in L1210 mouse leukemia cells and relation to cytotoxicity. Cancer Res. 1979 Feb;39(2 Pt 1):365–369. [PubMed] [Google Scholar]