Background: Few studies have investigated the ZIP proteins specifically expressed in keratinocytes.
Results: ZIP2 is highly expressed in differentiating keratinocytes, and their differentiation is inhibited by ZIP2 siRNA.
Conclusion: ZIP2 is essential for the differentiation of keratinocytes.
Significance: Understanding the regulation of keratinocyte differentiation by zinc and its transporters is crucial for developing new therapies against skin disease.
Keywords: Cell Differentiation, Epidermis, Keratinocyte, Transporter, Zinc
Abstract
Zinc is essential for the proper functioning of various enzymes and transcription factors, and its homeostasis is rigorously controlled by zinc transporters (SLC39/ZIP, importers; SLC30/ZnT, exporters). Skin disease is commonly caused by a zinc deficiency. Dietary and inherited zinc deficiencies are known to cause alopecia and the development of vesicular or pustular dermatitis. A previous study demonstrated that zinc played crucial roles in the survival of keratinocytes and their unique functions. High levels of zinc have been detected in the epidermis. Epidermal layers are considered to use a mechanism that preferentially takes in zinc, which is involved with the unique functions of keratinocytes. However, few studies have investigated the ZIP (Zrt- and Irt-like protein) proteins specifically expressed in keratinocytes and their functions. We explored the ZIP proteins specifically expressed in the epidermis and analyzed their functions. Gene expression analysis showed that the expression of ZIP2 was consistently higher in the epidermis than in the dermis. Immunohistochemistry analysis confirmed the expression of ZIP2 in differentiating keratinocytes. The expression of ZIP2 was found to be up-regulated by the differentiation induction of cultured keratinocytes. Intracellular zinc levels were decreased in keratinocytes when ZIP2 was knocked down by siRNA, and this subsequently inhibited the differentiation of keratinocytes. Moreover, we demonstrated that ZIP2 knockdown inhibited the normal formation of a three-dimensional cultured epidermis. Taken together, the results of this study suggest that ZIP2, a zinc transporter expressed specifically in the epidermis, and zinc taken up by ZIP2 are necessary for the differentiation of keratinocytes.
Introduction
Approximately 99% of elements that constitute the human body are carbon, oxygen, nitrogen, hydrogen, calcium, and phosphorus. Although zinc accounts for only 0.003% of elements in the human body, it is essential for the active center formation of over 300 types of enzymes and maintenance of transcription factors (1). Furthermore, according to recent studies on the human genome sequence, ∼4–10% of the proteins coded by all genes contain zinc-biding domains (2). Therefore, intracellular zinc levels are rigorously controlled by zinc transporters (3, 4). Zinc transporters have been roughly classified into two families: SLC39 (ZIP) and SLC30 (ZnT). SLC39 (ZIP) up-regulates, whereas SLC30 (ZnT) down-regulates zinc levels in the cytoplasm. Fourteen types of ZIP proteins and eight types of ZnT proteins have been identified in human cells (5).
Skin disease is commonly caused by a zinc deficiency. Dietary and inherited zinc deficiencies cause alopecia and the development of vesicular or pustular dermatitis. Although it has been detected in locations all over the body, zinc is known to be particularly abundantly in the skin (6). Taking these findings together, zinc is suspected of being a significant element in skin tissues.
The skin is composed of three layers: the epidermis, dermis, and subcutaneous adipose layer. The epidermis, the outermost layer of the skin, is primarily composed of keratinocytes (∼90% of all epidermal cells) (7). Keratinocytes differentiate from basal epidermal stem cells and migrate toward the surface of the epidermis as they mature. Therefore, the epidermis consists of several layers (basal, spinous, granular, and cornified layers) that are classified according to the stage of differentiation. Epidermal stem cells undergo a cycle of proliferation and differentiation when needed to supply keratinocytes to the epidermis, which is responsible for the continuance renewal of the skin. This process has been referred to as the turnover of the epidermis. Keratinocytes express various differentiation markers during their differentiation. For example, involucrin (IVL)2 is expressed in the upper spinous and granular layers in which differentiation is at an advanced stage, whereas IVL is not expressed in the basal layer (8). The abnormal proliferation and differentiation of keratinocytes cause various skin diseases. For example, psoriasis is characterized by epidermal hyperplasia because of the hyperproliferation and failure of differentiation of keratinocytes (9, 10). The mechanisms underlying the turnover of epidermal layers need to be clarified to elucidate the pathogenesis of skin diseases. We believe that trace metals (particularly zinc) are closely associated with the turnover of epidermal layers.
A number of studies have been conducted to investigate the effects of zinc on keratinocytes. For example, apoptosis has been reported in keratinocytes cultured under zinc-deficient conditions or high zinc conditions. Therefore, an appropriate concentration of zinc is crucial for the survival of keratinocytes (11, 12). Sakamoto et al. (13) demonstrated that zinc is involved in the polymerization of cytokeratin. Furthermore, Deters et al. (14) reported that zinc promoted the generation of IVL, a differentiation marker, when it was added to keratinocytes. These findings indicate that zinc plays crucial roles in the survival of keratinocytes and their unique functions. Zinc has also been shown to be present at high levels in the epidermis (15). Epidermal layers may use a mechanism that takes in zinc preferentially, which is involved with the unique functions of keratinocytes. However, few studies have investigated the ZIP proteins specifically expressed in keratinocytes and their functions. In this study, we explored the ZIP proteins specifically expressed in the epidermal layers and analyzed their functions.
EXPERIMENTAL PROCEDURES
Distribution Analysis of Skin Tissue Elements Using Synchrotron Radiation High Energy X-ray Fluorescence (SR-XRF)
The synchrotron radiation experiments were performed at the BL37XU of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI, Hyogo, Japan) (Proposals 2012B1394 and 2013A1327). Healthy human frozen skin (Transkin) purchased from KAC (Kyoto, Japan) was used for the analysis. The skin was cut into 1-cm-wide pieces with a scalpel and were then embedded in Tissue-Tek OCT (optimal cutting temperature) compound (Sakura Finetechnical, Tokyo, Japan) to prepare 20-μm-thick frozen sections with a microtome. The thinly sliced sections were pasted on 4 × 4 cm2 Kapton film 30EN (Toray Industries, Tokyo, Japan) and stored frozen as specimens for SR-XRF analysis. SR-XRF analysis was performed on the basis of the study by Kodera et al. (16). Specifically, specimens were returned to room temperature and used for SR-XRF analysis with a scanning x-ray microscope (BL37XU) at SPring-8. Specimens were irradiated with a 20-keV, 0.8(H) × 0.6(V) μm2 beam to determine the distribution and amounts of elements (zinc, iron, copper, and sulfur) in the skin sections. Cross-sections of skin specimens were scanned by a two-dimensional scanner with a step width of 1 μm to measure the fluorescent x-ray intensity (counts per second) of each metal element.
Preparation of Epidermal and Dermal Samples from HR-1 Mice
Dorsal skin tissues were collected from HR-1 mice (Japan SLC, Shizuoka, Japan) and were reacted overnight in 200 units/ml of Dispase II (Godo Shusei, Tokyo, Japan) at 4 °C. The epidermal sheet was peeled from the dermis the following day, and RNA was isolated from the tissues for gene expression analysis.
Quantitative Real-time PCR Analysis
Total RNA was extracted from tissues and cells using TRIzol reagent (Invitrogen), and cDNA was synthesized by reverse transcription. Real-time PCR was performed with the SuperScript III Platinum two-step quantitative real-time PCR kit (Invitrogen), using the 7300 real-time PCR system (Applied Biosystems, Tokyo, Japan) according to the protocol of the manufacturer. The mouse primer sequences used were as follows: Gapdh, 5′-TGCACCACCAACTGCTTAGC-3′ (sense) and 5′-TCTTCTGGGTGGCAGTGATG-3′ (antisense); Ivl, 5′-GCAACCAACTCCACATCCTACA-3′ (sense) and 5′-CATGTTTGGGAAAGCCCTTCT-3′ (antisense); Col3a1, 5′-TTCCTGAAGATGTCGTTGATGTG-3′ (sense) and 5′-TGTTTTTGCAGTGGTATGTAATGTTC-3′ (antisense); Zip1, 5′-GAGCGACAGCAATGGAGTG-3′ (sense) and 5′-TGGCTGTGATAACTCGGTGAC-3′ (antisense); Zip2, 5′-GTGGCCTTACTCCCATCTACGTG-3′ (sense) and 5′-GACCCTGTGGTGATGACCTGTAG-3′ (antisense); Zip3, 5′-TGGGCGTGTTCTTCTTCATG-3′ (sense) and 5′-GTGCGCCTTCTCCAAGTCAG-3′ (antisense); Zip6, 5′-TACAGCAAGTGAGAAGAAGGCAG-3′ (sense) and 5′-CCAAGCCAGGCTATTTGTAAAG-3′ (antisense); Zip8, 5′-GCTGCACTTCAACCAGTGTTTG-3′ (sense) and 5′-GGTGACATTTGAGAAACCATGAAGA-3′ (antisense); Zip10, 5′-TCTGCATCTACTGCCCCATTC-3′ (sense) and 5′-CCGTGCGTATGCTGATGACTG-3′ (antisense); and Zip14, 5′-TTCCCAGCCCAAGGAAGGAC-3′ (sense) and 5′-GCAAAGAGGTCTCCAGAGCTAAAG-3′ (antisense). The human primer sequences used were as follows: 18 S rRNA, 5′-CCGAGCCGCCTGGATAC-3′ (sense) and 5′-CAGTTCCGAAAACCAACAAAATAGA-3′ (antisense); IVL, 5′-CCATCAGGAGCAAATGAAACAG-3′ (sense) and 5′-GCTCGACAGGCACCTTCTG-3′ (antisense); ZIP1, 5′-GCGCCTACCCTCATACCTAT-3′ (sense) and 5′-GAGGCCAGAGAAGATACCAAA-3′ (antisense); ZIP2, 5′-GGTCATCACCGGCGAGTC-3′ (sense) and 5′-TCCAGGGCTTCAGCAGTCATA-3′ (antisense); and ZIP3, 5′-CGGGAGTTGCTGGACTGAGA-3′ (sense) and 5′-CCAAGTCCCACGATGTGCTAC-3′ (antisense). The contents of the selected genes were normalized to Gapdh and 18 S rRNA. All PCR products were checked by melting curve analysis to exclude the possibility of multiple products or incorrect product sizes. PCR analyses were conducted in triplicate for each sample.
Immunohistochemistry
Formalin-fixed and paraffin-embedded sections prepared from skin biopsies were deparaffinized. The media with the anti-KRT14 rabbit polyclonal (Covance, Princeton, NJ), anti-IVL rabbit polyclonal (Spring Bioscience, Pleasanton, CA), and anti-ZIP2 rabbit polyclonal antibodies (Abnova Corp., Taipei, Taiwan) at a dilution of 1:200 were introduced into the skin sections, which were then incubated for 1 h at 37 °C. After being washed in PBS, the sections were stained with Alexa Fluor 594 donkey anti-rabbit IgG (Molecular Probes, Eugene, OR) at a dilution of 1:1000 for 30 min at 37 °C. Sections were then washed in PBS, and 1 μg/ml DAPI solution (Dojindo Laboratories, Kumamoto, Japan) was added to confirm the presence of cell nuclei.
Cell Culture
Immortalized human keratinocytes (human dermal keratinocyte 1, HDK1) were provided by Dr. Akihiro Umezawa (Department of Reproductive Biology, National Institute for Child Health and Development, Tokyo, Japan) and Dr. Tohru Kiyono (National Cancer Center Research Institute, Tokyo, Japan). Normal human epidermal keratinocytes (NHEKs) were purchased from Cell Applications Inc. (San Diego, CA). HDK1 and NHEKs were grown routinely in the low-Ca2+ medium Keratinocyte-SFM (serum-free medium) (KSFM, Invitrogen) for propagation. Differentiation was induced by elevating the calcium level in the cell culture medium by the addition of calcium chloride (Wako Pure Chemical Industries, Osaka, Japan).
Immunocytochemistry
HDK1 and NHEKs were washed with PBS, fixed in 4% paraformaldehyde in PBS for 1 h at 4 °C, and permeabilized in PBS containing 0.25% Triton X-100 (Sigma) and 1% bovine serum albumin (Sigma) for 30 min before the detection of IVL with immunofluorescence. Media with the anti IVL rabbit polyclonal antibody (Spring Bioscience) at a dilution of 1:200 were introduced into the culture dish, and cells were incubated for 1 h at 37 °C. After being washed in PBS, the cells were stained with Alexa Fluor 594 donkey anti-rabbit IgG (Molecular Probes) at a dilution of 1:1000 for 30 min at 37 °C. Cells were then washed in PBS, and 1 μg/ml DAPI solution (Dojindo Laboratories) was added to confirm the presence of cell nuclei.
Western Blot Analysis
Cells were lysed with 2% SDS. Total protein was measured using a BCA protein assay kit (Thermo Fisher Scientific, Yokohama, Japan). Samples were run on SDS-polyacrylamide gels, transferred onto nitrocellulose membranes, and reacted with the primary antibody, anti-IVL rabbit polyclonal antibody (Spring Bioscience, 1:1000), and anti-β-actin mouse monoclonal antibody (Abcam, Cambridge, England, 1:5000). Blots were then incubated with peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) and visualized by enhanced chemifluorescence (ECL Western blotting detection reagents, Amersham Biosciences) according to the protocol of the manufacturer.
Measurement of Intracellular Zinc Levels
To measure intracellular zinc levels in HDK1, cells were loaded with 1 μm cell-permeant FluoZin-3 AM (acetoxymethyl) (Invitrogen) in plain DMEM (Invitrogen) at 37 °C for 1 h. The cells were further incubated in fresh plain DMEM for 1 h prior to measuring intracellular zinc levels by flow cytometric analysis (FACSAria, BD Biosciences).
Measurement of Zinc in Cell Culture Medium
The chelexing process of culture medium (KSFM) was done by treatment with Chelex-100 (Bio-Rad) according to the protocol of the manufacturer. The concentration of zinc in chelexed KSFM was analyzed by atomic absorption spectrophotometry (furnace) (GFA-7000, Shimadzu, Tokyo, Japan) according to the protocol of the manufacturer. Differentiation was induced by elevating the calcium level in the chelexed KSFM by addition of calcium chloride.
siRNA Transfection
Cells were seeded in Keratinocyte-SFM the day before transfection. Transfection was performed with Lipofectamine RNAiMAX (Invitrogen) and with 20 nm of Stealth siRNAs (Invitrogen), HSS179136 (si-1), HSS179137 (si-2), and HSS179138 (si-3) against human ZIP2 with non-targeting (NT) siRNA as a control. The medium was changed to differentiation medium 4 h after transfection, and assays were performed after 4 days.
Giemsa Stain
Cells were fixed with methanol for 20 min and stained with Giemsa solution (Sigma) for 30 min.
Cell Proliferation Assay
HDK1 were seeded in 96-well plates (0.3 × 104 cells/well), and siRNA transfection was performed as mentioned above. The medium was changed to differentiation medium 4 h after transfection, and the cell proliferation assay was performed after 4 days. The relative cell number was measured with cell counting kit 8 (CCK-8, Dojindo Laboratories) according to the protocol of the manufacturer. In brief, after removing the medium, 100 μl of CCK-8 solution was added to cells, which were then incubated for another hour. Optical density values were tested at an absorbance of 450 nm using a microplate reader (Molecular Devices).
Three-dimensional Human Keratinocyte Culture
The culture was made on the basis of the Millicell hanging cell culture insert (polyethylene terephthalate, 0.4 μm, Millipore, Billerica, MA). HDK1 cells were seeded at a density of 6 × 104 cells/insert in 0.4 ml of expansion medium (CNT-07, CELLnTEC, Bern, Switzerland). The inserts were then placed in a 24-well plate with 1 ml of CNT-07 medium added to the well. The culture was maintained for 24 h before siRNA transfection. Transfection was performed with Lipofectamine RNAiMAX (Invitrogen) and 100 nm of Stealth siRNAs (Invitrogen) against human ZIP2 with NT siRNA as a control. Cells were first grown in CNT-07 medium for 48–72 h until 100% confluence and were then maintained in three-dimensional medium (CNT-02-3DP5, CELLnTEC). The medium in the insert was aspirated after 16 h, and the cells were exposed to air. The medium in the wells was also refreshed. The three-dimensional culture was then maintained for 5 days by changing the medium every other day. Samples were then collected for immunohistological analysis.
Statistical Analysis
Student's t test was used for statistical analysis. Multiple groups were evaluated by a one-way analysis of variance, followed by Dunnett's multiple comparisons.
RESULTS
Distribution Analysis of Elements in the Skin Tissue
SR-XRF analysis using human skin tissue was initially conducted to identify the distribution patterns of trace metal elements. The results obtained showed that zinc was localized in the epidermal layers and that iron was localized abundantly in the basal layer. On the other hand, the distribution of both copper and sulfur in the epidermis and dermis was nearly uniform (Fig. 1). We confirmed that zinc was specifically localized in the epidermal layers and, therefore, hypothesized the presence of ZIP proteins that exclusively take up zinc in the epidermal layers.
FIGURE 1.
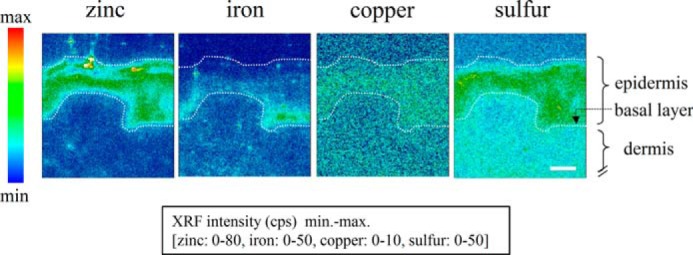
Elemental distribution in cross-sections of human skin tissue. Two-dimensional mapping (pixel size, 1 × 1 μm2; time, 1 s/point) indicating the localizations of various elements (zinc, iron, copper, and sulfur) in human skin tissue on the basis of fluorescent x-ray intensities (counts per second, cps) detected by SR-XRF analysis. White dotted lines mark the epidermis. Scale bar = 40 μm.
Expression Analysis of Zinc Transporters in the Skin Tissue
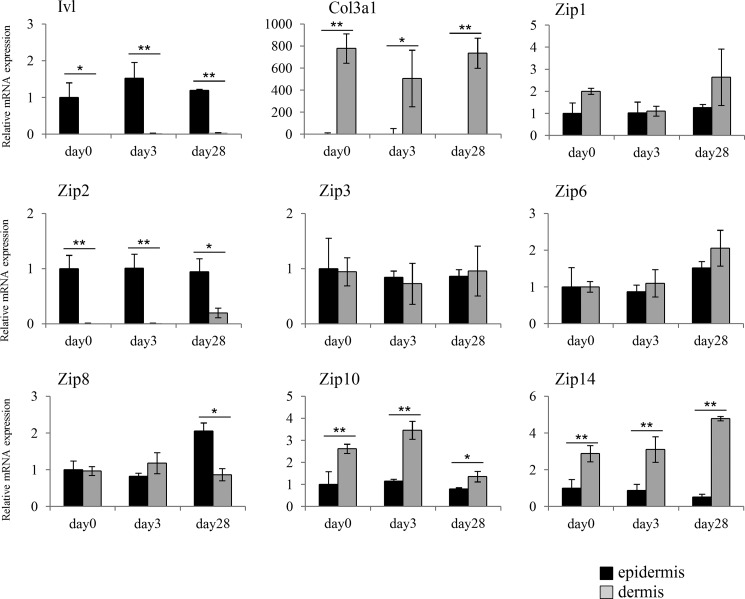
Gene expression analysis was conducted by real-time PCR using the skin tissue of HR-1 male mice aged 0, 3, and 28 days to analyze the expression of ZIP (Zip) in epidermal and dermal tissues. The analysis was conducted specifically on Zip (Zip1-Zip6, Zip8, Zip10, and Zip14) associated with the uptake of zinc into cells (17).
The dorsal skins were collected from HR-1 mice, and the epidermis and dermis were separated using Dispase II. RNA was then isolated from the tissues for use in gene expression analysis.
Markers for the epidermis and dermis, Ivl and Col3a1, respectively, were confirmed to be specifically expressed in each tissue, which confirmed that each tissue was separated properly. Among the nine types of Zip, the expression of Zip2 only was higher in the epidermis than in the dermis, regardless of aging (Fig. 2). Therefore, this result suggests that ZIP2 (Zip2) may be associated with the specific functions of keratinocytes. The expression of Zip4 and Zip5 was not detected in either the epidermis or dermis (data not shown).
FIGURE 2.
Expression patterns of Zip proteins. Gene expression analysis of Zip proteins in the epidermis and dermis of mice aged 0, 3, and 28 days. The expression of Zip2 was higher in the epidermis than in the dermis, regardless of aging. Data are expressed as the mean ± S.D. of three experiments. *, p < 0.05; **, p < 0.01; significantly different from the epidermis.
Localization Analysis of ZIP2 (Zip2) in Skin Tissue Sections
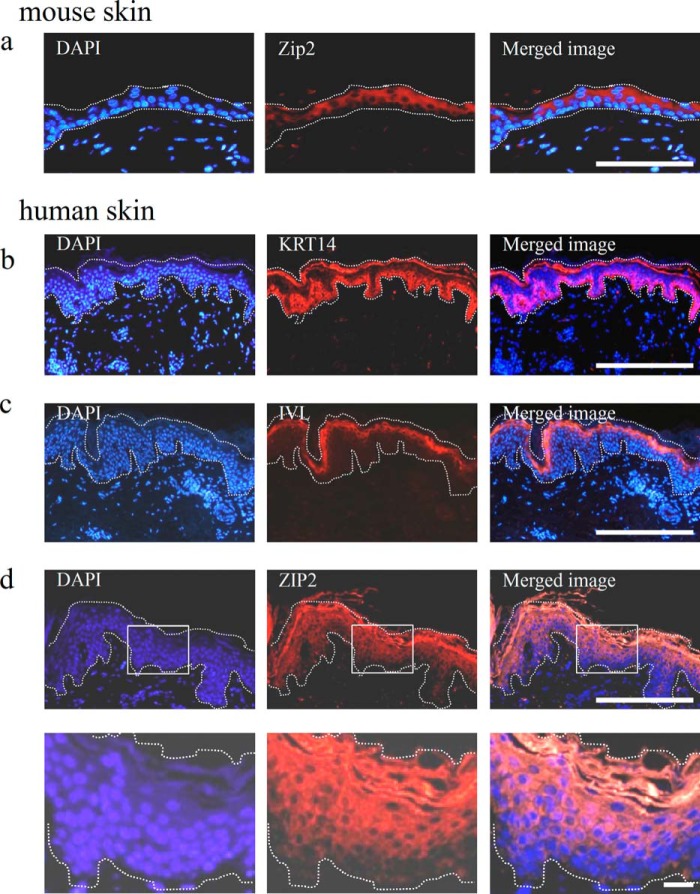
Mouse skin sections were immunostained to analyze the localization of Zip2 in the skin tissue. The results obtained showed that Zip2 was specifically expressed in the epidermis, which was consistent with the results of the gene expression analysis (Fig. 3a). The expression of Zip2 was not confirmed in the basal layer but was in the differentiated keratinocytes in the upper layer.
FIGURE 3.
Gene expression profiles of mouse and human skin. a, immunostaining images of mouse skin sections stained for the Zip2 antibody. As with the gene expression analysis, the expression of Zip2 was not detected in the dermis but was in the epidermis. More specifically, the expression of Zip2 was not detected in undifferentiated keratinocytes in the basal layer but was in differentiated keratinocytes in the upper layer. b–d, immunostaining images of human skin sections stained for the KRT14, IVL, and ZIP2 antibodies. The expression of KRT14 and IVL was confirmed in the basal layer and outer living layer of the epidermis, respectively. The expression of ZIP2 was not detected in undifferentiated keratinocytes in the basal layer but was in differentiated keratinocytes in the upper layer. The bottom panels in d are magnified views of the top panels. White dotted lines mark the epidermis. Scale bars = 100 μm (a), 200 μm (b–d, top panels), and 20 μm (d, bottom panels).
Furthermore, the expression patterns of ZIP2 were analyzed in detail using human skin tissue. The basal layer of human skin tissue was composed of undifferentiated keratinocytes that expressed KRT14 (Fig. 3b). Differentiated keratinocytes expressed IVL, a differentiation marker, in the upper layer (Fig. 3c). The expression of ZIP2 was specifically confirmed in the upper layer of the epidermis, as in the study using the mouse skin (Fig. 3d). Taken together, these results suggest that ZIP2 (Zip2) may be associated with the differentiation of keratinocytes.
Changes in the Expression of ZIP2 Associated with the Differentiation of Human Keratinocytes
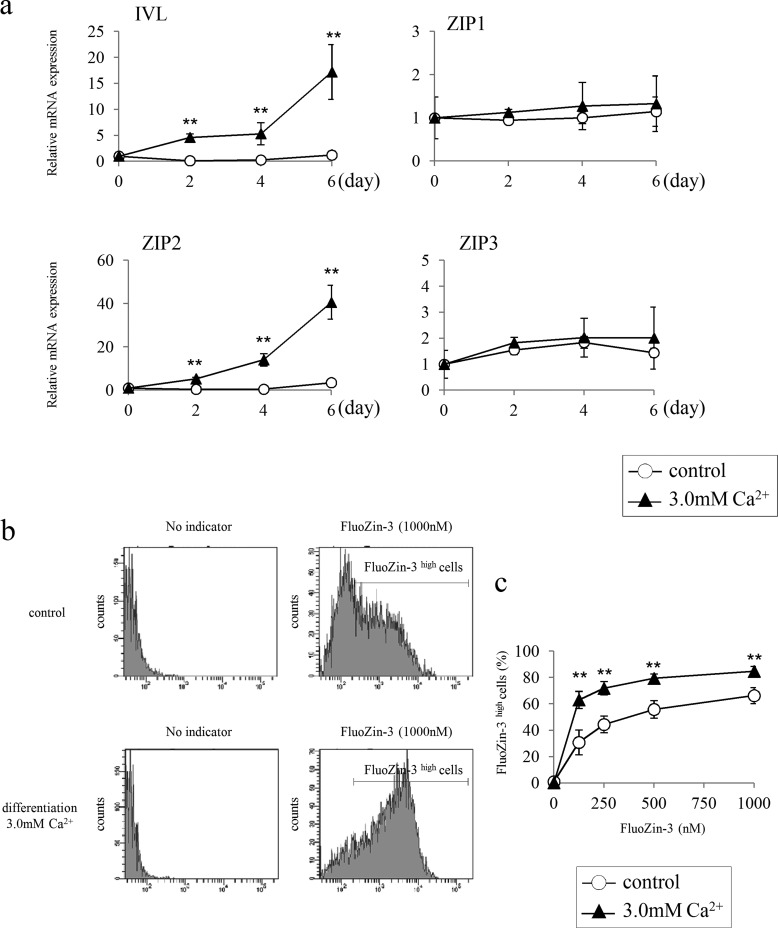
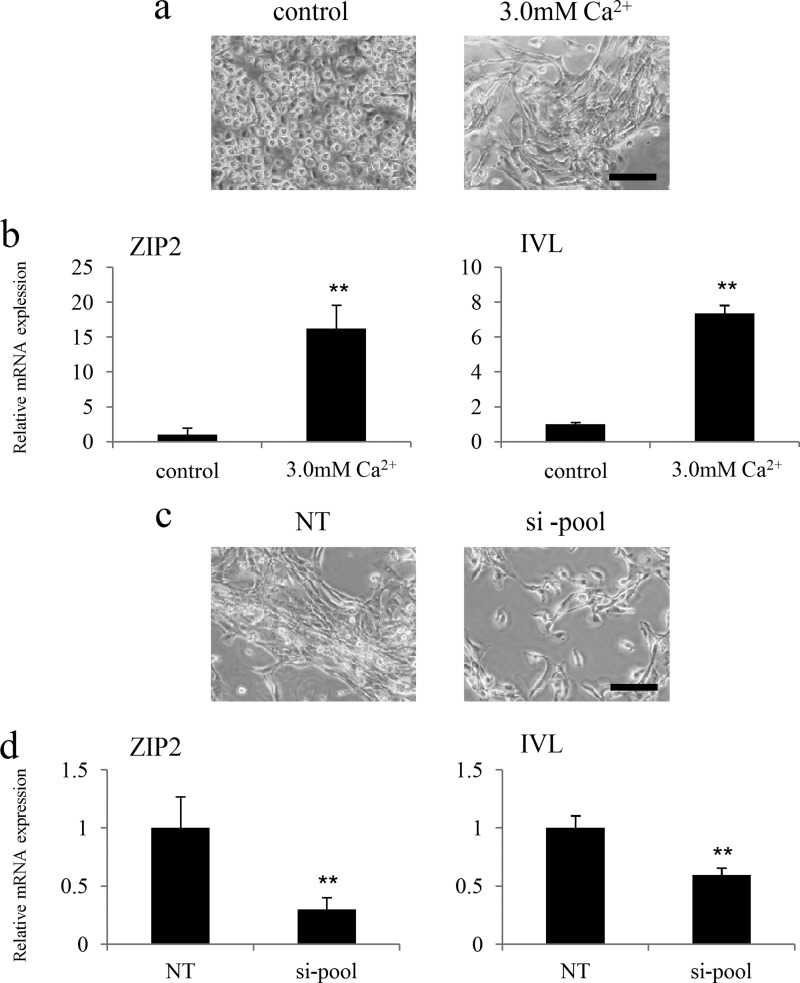
HDK1, immortalized human keratinocytes, were induced to differentiate under high calcium conditions (1.5 mm or 3.0 mm) to examine changes in the expression of ZIP2 associated with the differentiation of keratinocytes (18). HDK1 exhibited a flattened and cobblestone morphology during the induction of differentiation (Fig. 4a). Western blotting and immunostaining revealed that the expression of IVL was up-regulated by the induction of differentiation (Fig. 4, b and c). Gene expression analysis by real-time PCR showed that the expression of ZIP2 was up-regulated, similar to that of IVL (Fig. 5a). On the other hand, the expression of other ZIP proteins (ZIP1 and ZIP3) remained unchanged. Taken together, these results suggest that ZIP2 may be associated with the differentiation of keratinocytes. Staining with the zinc indicator FluoZin-3 revealed that intracellular free zinc levels were significantly higher in HDK1 that highly expressed ZIP2 because of differentiation induction (Fig. 5, b and c).
FIGURE 4.
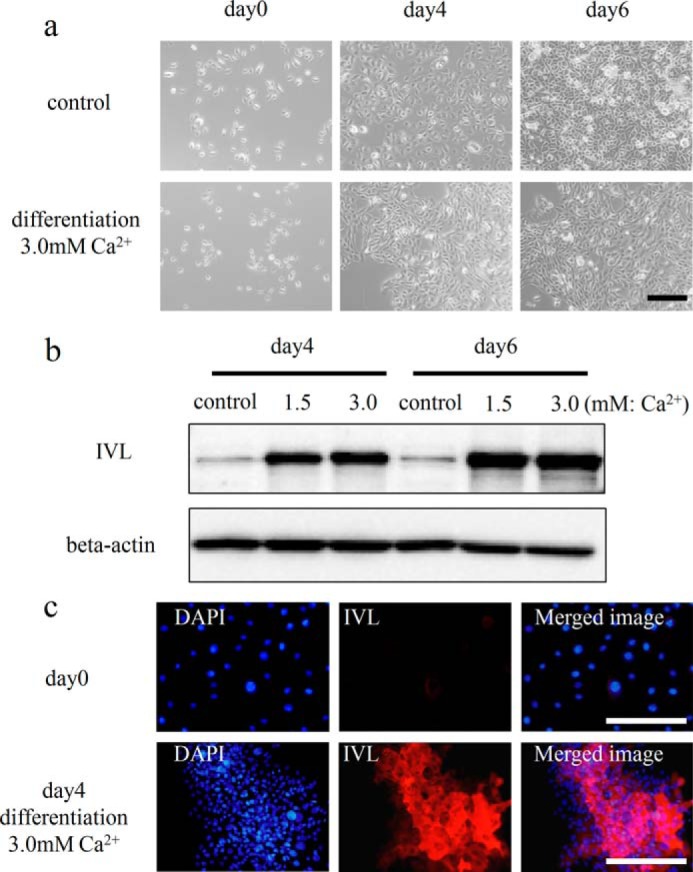
Differentiation induction of HDK1. a, differentiation process of HDK1. The differentiation of HDK1 was induced by elevating the extracellular calcium levels of KSFM to 3.0 mm. b, expression analysis of IVL by Western blotting. The differentiation of HDK1 was induced by elevating the calcium levels of KSFM to 1.5 and 3.0 mm. Cells were collected on day 4 of the culture and analyzed for the expression of IVL. c, immunostaining images of HDK1 stained for the IVL antibody after differentiation induction. The expression of IVL was confirmed after a 4-day induction. Scale bars: 200 μm (a) and 200 μm (c).
FIGURE 5.
HDK1 differentiation-related changes in the expression of IVL and ZIP. a, gene expression analysis by real-time PCR. The expression of IVL and ZIP2 were increased simultaneously with differentiation. No change was detected in the expression of ZIP1 or ZIP3. b and c, intracellular zinc levels in HDK1 (control and differentiation) on day 4 of differentiation. FluoZin-3 high cells were increased simultaneously with differentiation. Data are mean ± S.D. of three experiments. **, p < 0.01; significantly different from the control.
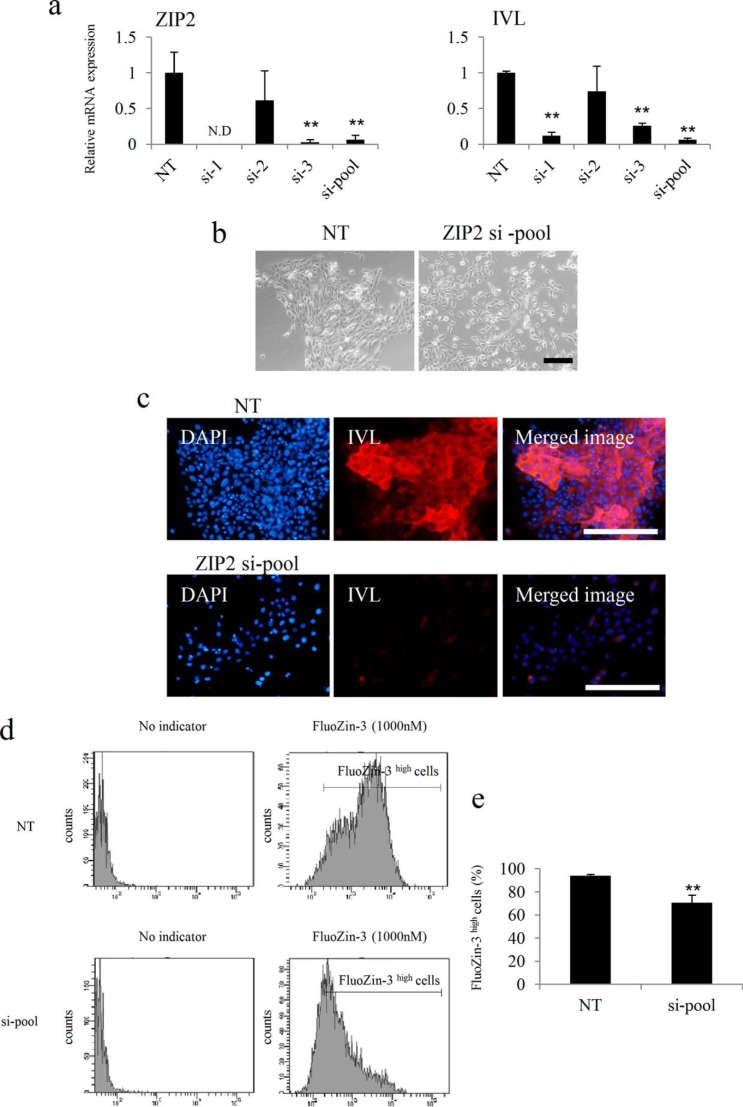
Analysis of the Effects of ZIP2 Knockdown on the Differentiation and Proliferation of Keratinocytes
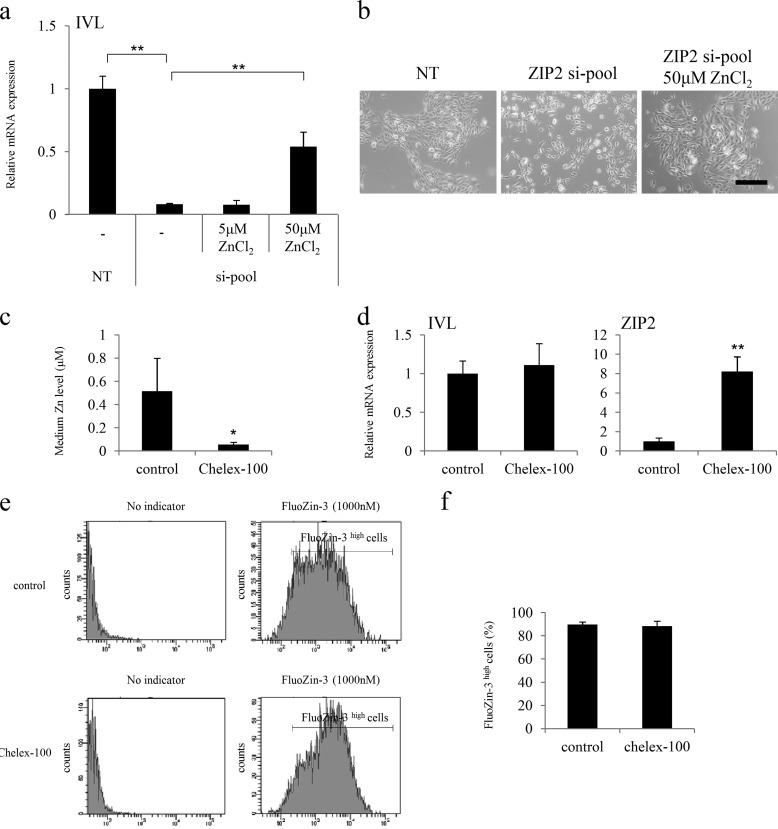
ZIP2 was knocked down by siRNA to clarify the functions of ZIP2 that affect the differentiation of keratinocytes. As a result, HDK1 exhibited a cobblestone morphology after differentiation, as usual in the negative control (NT siRNA) group, whereas HDK1 did not exhibit a cobblestone morphology in the ZIP2 knockdown group (Fig. 6, a and b). Gene expression analysis by real-time PCR revealed that the expression of IVL was down-regulated significantly by ZIP2 knockdown (Fig. 6a), and this was also confirmed by immunostaining (Fig. 6c). Intracellular zinc levels were significantly lower in the si-pool group than in the NT group (Fig. 6, d and e). Next, excessive amounts of zinc, which were 10 times (5 μm) and 100 times (50 μm) the amount originally contained in the medium, were added to ZIP2 knockdown HDK1 to examine whether the differentiation could be restored. The result showed that 50 μm of ZnCl2 (Kanto Chemical, Tokyo, Japan) restored the differentiation of HDK1 (Fig. 7, a and b). These results suggest that ZIP2 and zinc taken up by ZIP2 are necessary for the differentiation of keratinocytes.
FIGURE 6.
Effects of ZIP2 knockdown on the differentiation of HDK1. a, gene expression analysis of ZIP2 and IVL on day 4 of differentiation induction. The expression of IVL was down-regulated significantly by the knockdown of ZIP2. b, images of HDK1 in which ZIP2 was knocked down by siRNA. On day 4 of differentiation, HDK1 did not exhibit a cobblestone morphology by the knockdown of ZIP2. c, immunostaining images of ZIP2 knockdown HDK1 stained with the IVL antibody on day 4 of differentiation. The expression of IVL was down-regulated significantly by the knockdown of ZIP2. d and e, intracellular zinc levels in HDK1 (NT and si-pool) on day 4 of differentiation. The ratio of FluoZin-3 high cells was decreased by ZIP2 knockdown. Scale bars = 200 μm (b) and 200 μm (c). Data are mean ± S.D. of three experiments. **, p < 0.01; significantly different from the control.
FIGURE 7.
Effects of the addition of excessive amounts of zinc and the removal of zinc on the differentiation of HDK1. a, gene expression analysis of IVL on day 4 of differentiation induction. The expression of IVL was restored by adding 50 μm of ZnCl2. b, images of HDK1 in which ZIP2 was knocked down by siRNA. On day 4 of differentiation, HDK1 did not exhibit a cobblestone morphology by knockdown of ZIP2. However, HDK1 exhibited a cobblestone morphology by addition 50 μm of ZnCl2. c, quantitative analysis of zinc in the culture medium by atomic absorption spectrophotometry. The zinc level in the culture medium was depleted by treatment with Chelex-100. d, gene expression analysis of IVL and ZIP2 on day 4 of differentiation induction. The expression of ZIP2 was enhanced notably by removing zinc in the culture medium by treatment with Chelex-100. e and f, intracellular zinc levels in HDK1 (control and Chelex-100) on day 4 of differentiation. The ratio of FluoZin-3 high cells was not changed by treatment with Chelex-100. Scale bar = 200 μm. Data are mean ± S.D. of three experiments. *, p < 0.05; **, p < 0.01; significantly different from the control.
On the other hand, when differentiation of HDK1 was induced in the culture medium depleted of zinc by treatment with Chelex-100 (Bio-Rad), the expression of IVL was not decreased (Fig. 7, c and d). No change was detected in the intracellular zinc level (Fig. 7, e and f). Meanwhile, the expression of ZIP2 was enhanced notably (Fig. 7d).
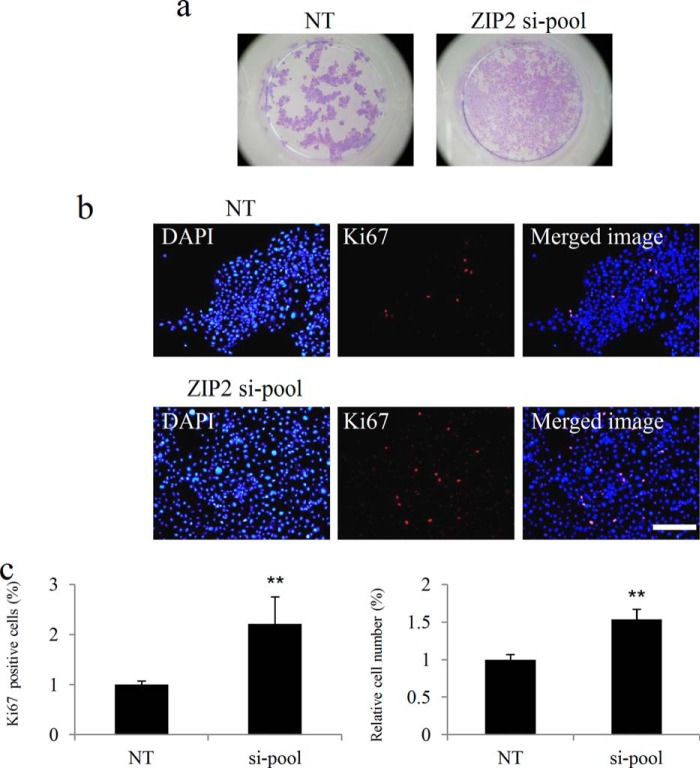
Giemsa staining showed that HDK1 did not exhibit a cobblestone morphology but was diffused in the entire dish in the ZIP2 knockdown group (Fig. 8a). In addition, the ratio of proliferation marker Ki67-positive cells was increased by ZIP2 knockdown, which promoted the proliferation of HDK1 (Fig. 8, b and c). An experiment using NHEKs revealed that the expression of ZIP2 increased simultaneously with differentiation (Fig. 9, a and b), whereas differentiation was inhibited when ZIP2 was knocked down (Fig. 9, c and d).
FIGURE 8.
Effects of ZIP2 knockdown on the proliferation of HDK1. a, images of Giemsa staining. ZIP2 knockdown HDK1 were cultured and Giemsa-stained. b, HDK1 in which ZIP2 was knocked down by siRNA and stained for the Ki67 antibody. c, percentages of Ki67-positive cells and relative cell numbers on day 4 of differentiation induction. The percentage of Ki67-positive cells and cell proliferation rate were elevated because of the knockdown of ZIP2. Scale bar = 200 μm. Data are mean ± S.D. of three experiments. **, p < 0.01; significantly different from the control.
FIGURE 9.
Effects of ZIP2 knockdown on the differentiation of NHEKs. a, morphological changes associated with the differentiation of NHEKs on day 4 of differentiation. The differentiation of NHEKs was induced by elevating the extracellular calcium levels of KSFM to 3.0 mm. b, gene expression analysis by real-time PCR on day 4 of differentiation. The expression of ZIP2 was increased simultaneously with differentiation, similar to that of IVL. c, NHEKs in which ZIP2 was knocked down by siRNA. On day 4 of differentiation, NHEKs did not exhibit a cobblestone morphology by the knockdown of ZIP2. d, gene expression analysis of ZIP2 and IVL on day 4 of differentiation induction. The expression of IVL was down-regulated significantly by the knockdown of ZIP2. Scale bars = 250 μm. Data are mean ± S.D. of three experiments. **, p < 0.01; significantly different from the control.
Analysis of the Effects of ZIP2 Knockdown on Differentiation in a Three-dimensional Cultured Epidermis
To analyze the effects of ZIP knockdown on differentiation of keratinocytes, a three-dimensional cultured epidermis was constructed using ZIP2 knockdown HDK1.
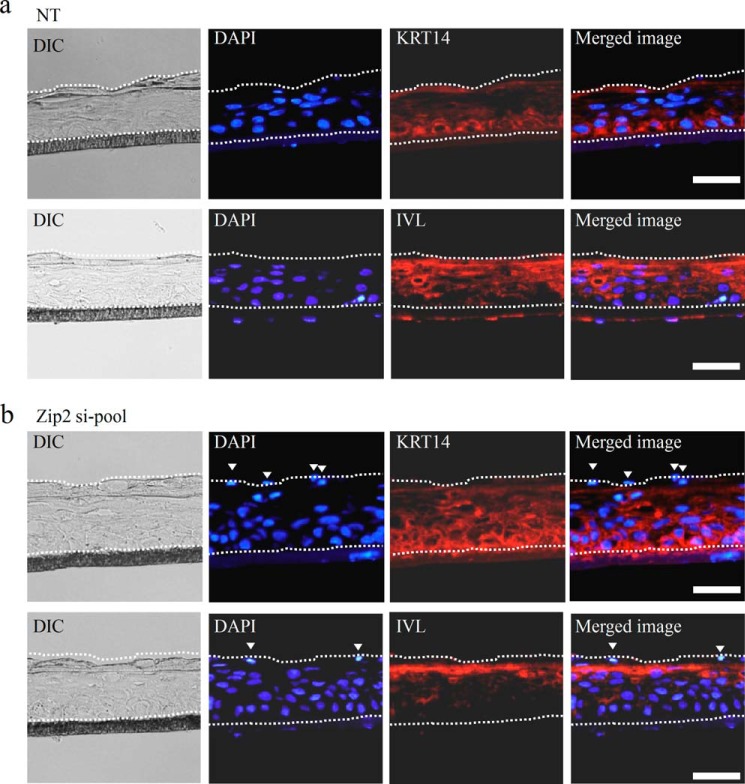
In the negative control group, the expression of KRT14, a marker of an undifferentiated keratinocyte, was confirmed only in the basal layer, and the expression of IVL, a differentiation marker, was confirmed in the upper layer. In addition, the nuclei of keratinocytes in the cornified layer disappeared, which suggests that keratinocytes underwent normal differentiation (Figs. 3, b and c, and 10a).
FIGURE 10.
Effects of ZIP2 knockdown on differentiation of HDK1 in a three-dimensional cultured epidermis. a, immunostaining images of the three-dimensional cultured epidermis constructed using HDK1 (negative control group). The expression of KRT14 was confirmed in the basal layer of the three-dimensional cultured epidermis (top panels). The expression of IVL was confirmed in the upper layer of the three-dimensional cultured epidermis (bottom panels). b, immunostaining images of the three-dimensional cultured epidermis constructed using HDK1 (ZIP2 knockdown group). The expression area of KRT14 was expanded in the ZIP2 knockdown group compared with the negative control group (top panels). The expression area of IVL was smaller in the ZIP2 knockdown group than in the negative control group (bottom panels). The presence of cell nuclei was confirmed around the cornified layer (top and bottom panels). The arrowheads indicate nuclei that are retained in cells in the cornified layers. White dotted lines mark the three-dimensional cultured epidermis constructed using HDK1. Scale bars = 50 μm.
On the other hand, in the ZIP2 knockdown group, the expression of KRT14 was confirmed not only in the basal layer but also in the upper layer. The expression region of IVL was decreased compared with the negative control group. Furthermore, the ZIP2 knockdown group exhibited poor keratinization and nuclei that are supposed to disappear in the cornified layer in keratinocytes (Fig. 10b). These results demonstrate that the differentiation of keratinocytes is suppressed by ZIP2 knockdown.
DISCUSSION
Each element exhibited its own unique distribution pattern in the SR-XRF analysis performed in this study (Fig. 1). Zinc in particular was localized abundantly in the epidermal layers rather than the dermal layers. A neutron activation analysis showed previously that zinc concentrations in the epidermis were approximately six times greater than those in the dermis (15). Iron has also been shown to be localized in the area around the basal layer. Previous studies have also suggested the localization of iron around the basal layer (19, 20), which is consistent with the results obtained in this study. Iron is known to play an important role in cell proliferation (21). Because the basal layer is composed of keratinocytes that possess a high proliferative potential, these keratinocytes may aggressively take in iron. Further studies are needed to clarify the effects of iron on keratinocytes in the basal layer. In this study, we investigated the roles of zinc localized in the epidermis.
Gene expression analysis showed that the expression of ZIP2 (Zip2), which plays a role in the uptake of zinc into a cell, was consistently high in the epidermis (Fig. 2). The expression of ZIP2 (Zip2) was confirmed in differentiating keratinocytes in the human and mouse epidermis. The expression of ZIP2 was up-regulated by differentiation induction of HDK1 and NNEK cells. Moreover, this differentiation was inhibited by a down-regulation in the expression of ZIP2 (Figs. 3–6, 9, and 10), and the inhibited differentiation was restored by the addition of zinc (Fig. 7, a and b). Therefore, ZIP2, which is located upstream of the pathway of differentiation of keratinocytes, and zinc taken up by ZIP2 are necessary for the terminal differentiation of keratinocytes.
On the other hand, when differentiation of HIDK1 was induced in the culture medium depleted of zinc by treatment with Chelex-100 (Bio-Rad), the expression of IVL was not decreased (Fig. 7, c and d). No change was detected in the intracellular zinc level (Fig. 7, e and f). Interestingly, the expression of ZIP2 was enhanced notably (Fig. 7d). This is probably because when keratinocytes sensed the decreased extracellular zinc level, the expression of the zinc uptake protein ZIP2 was enhanced to maintain homeostasis, which resulted in maintaining homeostasis of intracellular zinc. Therefore, the differentiation ability was maintained. In fact, it has been reported that, when enterocytes are deficient in zinc, the expression of the zinc uptake protein ZIP4 is increased (22). As for ZIP2, a similar phenomenon was seen in a study using monocytic cells (23).
The expression of Zip2 has been reported previously in mouse epidermal tissue during embryogenesis, and Zip2 knockout mice exhibit skin blistering during early embryogenesis. However, the underlying mechanism for this remains unknown (24). Considering the results of our study, it is likely that Zip2 knockout mice developed a deficiency in the skin because of the abnormal differentiation of keratinocytes.
ZIP2 is known to exist in the cell membrane and transport zinc into cells, which consequently increases zinc cellular levels (25). Because the expression of ZIP2 increased simultaneously with the differentiation of keratinocytes, we speculated that zinc may be taken into a cell simultaneously with differentiation by the mediation of ZIP2. In fact, intracellular zinc levels were increased with the differentiation of HDK1 and decreased by ZIP2 knockdown (Figs. 5, b and c, and 6, d and e). Zinc has been shown to play a role in maintaining the conformation of various proteins, including transcriptional factors and enzymes. Therefore, it is conceivable that the zinc taken up by ZIP2 may control the activation of the zinc-requiring transcriptional factors related to the differentiation of keratinocytes. For example, KLF4, a zinc-requiring transcriptional factor, is known to be expressed in differentiating keratinocytes in the epidermis (26), which coincides with an increase in the expression of ZIP2. As with the case of ZIP2 knockdown, the expression of IVL has been shown to be inhibited when KLF4 was knocked down by siRNA, thereby inhibiting the normal formation of the three-dimensional cultured epidermis (27). Taken together, these findings suggest that zinc-requiring proteins, such as KLF4, which controls the differentiation of keratinocytes, may exist downstream of ZIP2. More studies should be carried out to identify factors lying downstream and upstream of ZIP2. The expression of Zip10 and Zip14 was higher in the dermis than in the epidermis, regardless of aging (Fig. 2), which indicated that Zip10 and Zip14 may be associated with the specific functions of fibroblasts.
This study demonstrates that various kinds of trace metals, which exhibit specific distribution patterns, are found in the skin. In particular, zinc and its transporter ZIP2 are present specifically in the epidermis and are closely associated with the differentiation of keratinocytes and turnover of epidermal layers. More studies to elucidate the roles of trace metal elements, including zinc, and their transporters, which affect the maintenance of skin homeostasis, will provide insights into the mechanisms underlying the maintenance of skin homeostasis and various skin diseases.
Acknowledgments
We thank Dr. Yasuko Terada (Research and Utilization Division, JASRI) for advice regarding the SR-XRF analysis at Spring-8 and Ms. Makiko Hori (Cellisis Co., Ltd., Aichi, Japan) for supporting the analysis of our experimental data.
Footnotes
- IVL
- involucrin
- NHEK
- normal human epidermal keratinocyte
- NT
- non-targeting
- SFM
- serum-free medium.
REFERENCES
- 1. Maret W., Li Y. (2009) Coordination dynamics of zinc in proteins. Chem Rev. 109, 4682–4707 [DOI] [PubMed] [Google Scholar]
- 2. Andreini C., Banci L., Bertini I., Rosato A. (2006) Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 5, 196–201 [DOI] [PubMed] [Google Scholar]
- 3. Kambe T., Yamaguchi-Iwai Y., Sasaki R., Nagao M. (2004) Overview of mammalian zinc transporters. Cell. Mol. Life Sci. 61, 49–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fukada T., Kambe T. (2011) Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 3, 662–674 [DOI] [PubMed] [Google Scholar]
- 5. Eide D. J. (2006) Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta 1763, 711–722 [DOI] [PubMed] [Google Scholar]
- 6. Underwood E. J. (1977) Trace Elements in Human and Animal Nutrition, 4th Ed., pp. 196–242, Academic Press, New York [Google Scholar]
- 7. Sun T. T., Shih C., Green H. (1979) Keratin cytoskeletons in epithelial cells of internal organs. Proc. Natl. Acad. Sci. U.S.A. 76, 2813–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamazaki T., Nakano H., Hayakari M., Tanaka M., Mayama J., Tsuchida S. (2004) Differentiation induction of human keratinocytes by phosphatidylethanolamine-binding protein. J. Biol. Chem. 279, 32191–32195 [DOI] [PubMed] [Google Scholar]
- 9. Hodge L., Comaish J. S. (1977) Psoriasis: current concepts in management. Drugs 13, 288–296 [DOI] [PubMed] [Google Scholar]
- 10. Voorhees J. J. (1977) Pathophysiology of psoriasis. Annu. Rev. Med. 28, 467–473 [DOI] [PubMed] [Google Scholar]
- 11. Wilson D., Varigos G., Ackland M. L. (2006) Apoptosis may underlie the pathology of zinc-deficient skin. Immunol. Cell Biol. 84, 28–37 [DOI] [PubMed] [Google Scholar]
- 12. Stork C. J., Martorano L. M., Li Y. V. (2010) UVB radiation induces an increase in intracellular zinc in human epidermal keratinocytes. Int. J. Mol. Med. 26, 463–469 [DOI] [PubMed] [Google Scholar]
- 13. Sakamoto M., Tzeng S., Fukuyama K., Epstein W. L. (1980) Light-scattering studies of cation-stimulated filament assembly of newborn rat epidermal keratin. Biochim. Biophys Acta 624, 205–210 [DOI] [PubMed] [Google Scholar]
- 14. Deters A., Schnetz E., Schmidt M., Hensel A. (2003) Effects of zinc histidine and zinc sulfate on natural human keratinocytes. Forsch. Komplementarmed. Klass. Naturheilkd. 10, 19–25 [DOI] [PubMed] [Google Scholar]
- 15. Michaëlsson G., Ljunghall K., Danielson B. G. (1980) Zinc in epidermis and dermis in healthy subjects. Acta Derm. Venereol. 60, 295–299 [PubMed] [Google Scholar]
- 16. Kodera H., Nishioka H., Muramatsu Y., Terada Y. (2008) Distribution of lead in the lead-accumulating pteridophyte Blechnum nipponicum measured by synchrotron radiation micro X-ray fluorescence. Anal. Sci. 24, 1545–1549 [DOI] [PubMed] [Google Scholar]
- 17. Fukada T., Yamasaki S., Nishida K., Murakami M., Hirano T. (2011) Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 16, 1123–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xue M., Thompson P., Kelso I., Jackson C. (2004) Activated protein C stimulates proliferation, migration and wound closure, inhibits apoptosis and upregulates MMP-2 activity in cultured human keratinocytes. Exp. Cell Res. 299, 119–127 [DOI] [PubMed] [Google Scholar]
- 19. Pinheiro T., Silva R., Fleming R., Gonçalves A., Barreiros M. A., Silva J. N., Morlière P., Santus R., Filipe P. (2014) Distribution and quantitation of skin iron in primary haemochromatosis: correlation with total body iron stores in patients undergoing phlebotomy. Acta Derm. Venereol. 94, 14–19 [DOI] [PubMed] [Google Scholar]
- 20. Ylva W., Linde J., Pallon, Forslind B. (1998) Physiologically important trace elements of paralesional psoriatic skin. Scanning Microsc. 12, 599–608 [Google Scholar]
- 21. Hoffbrand A. V., Ganeshaguru K., Hooton J. W., Tattersall M. H. (1976) Effect of iron deficiency and desferrioxamine on DNA synthesis in human cells. Br. J. Haematol. 33, 517–526 [DOI] [PubMed] [Google Scholar]
- 22. Kambe T., Andrews G. K. (2009) Novel proteolytic processing of the ectodomain of the zinc transporter ZIP4 (SLC39A4) during zinc deficiency is inhibited by acrodermatitis enteropathica mutations. Mol. Cell. Biol. 29, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao J., Bobo J. A., Liuzzi J. P., Cousins R. J. (2001) Effects of intracellular zinc depletion on metallothionein and ZIP2 transporter expression and apoptosis. J. Leukocyte Biol. 70, 559–566 [PubMed] [Google Scholar]
- 24. Peters J. L., Dufner-Beattie J., Xu W., Geiser J., Lahner B., Salt D. E., Andrews G. K. (2007) Targeting of the mouse Slc39a2 (Zip2) gene reveals highly cell-specific patterns of expression, and unique functions in zinc, iron, and calcium homeostasis. Genesis 45, 339–352 [DOI] [PubMed] [Google Scholar]
- 25. Rezaei K. A., Chen Y., Cai J., Sternberg P. (2008) Modulation of Nrf2-dependent antioxidant functions in the RPE by Zip2, a zinc transporter protein. Invest. Ophthalmol. Vis. Sci. 49, 1665–1670 [DOI] [PubMed] [Google Scholar]
- 26. Segre J. A., Bauer C., Fuchs E. (1999) Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 22, 356–360 [DOI] [PubMed] [Google Scholar]
- 27. Chew Y. C., Adhikary G., Xu W., Wilson G. M., Eckert R. L. (2013) Protein kinase C δ increases Kruppel-like factor 4 protein, which drives involucrin gene transcription in differentiating keratinocytes. J. Biol. Chem. 288, 17759–17768 [DOI] [PMC free article] [PubMed] [Google Scholar]