Background: It has been proposed that DMP1 functions not only as an extracellular matrix protein but also as a transcriptional co-factor.
Results: The nuclear activities of DMP1 did not recover the Dmp1-null phenotype, whereas secreted DMP1 fully rescued the defects.
Conclusion: DMP1 functions mainly as an extracellular matrix protein in vivo.
Significance: This work demonstrates the biological functions of DMP1 in different subcellular locations.
Keywords: Biomineralization, Bone, Development, Osteoblast, Osteocyte, Autosomal Recessive Hypophosphatemic Rickets, Osteogenesis, Osteomalacia, Dentin Matrix Protein 1
Abstract
Dentin matrix protein 1 (DMP1) plays multiple roles in bone, tooth, phosphate homeostasis, kidney, salivary gland, reproductive cycles, and the development of cancer. In vitro studies have indicated two different biological mechanisms: 1) as a matrix protein, DMP1 interacts with αvβ3 integrin and activates MAP kinase signaling; and 2) DMP1 serves as a transcription co-factor. In vivo studies have demonstrated its key role in osteocytes. This study attempted to determine whether DMP1 functions as a transcription co-factor and regulates osteoblast functions. For gene expression comparisons using adenovirus constructs, we targeted the expression of DMP1 either to the nucleus only by replacing the endogenous signal peptide with a nuclear localization signal (NLS) sequence (referred to as NLSDMP1) or to the extracellular matrix as the WT type (referred to as SPDMP1) in MC3T3 osteoblasts. High levels of DMP1 in either form greatly increased osteogenic gene expression in an identical manner. However, the targeted NLSDMP1 transgene driven by a 3.6-kb rat Col 1α1 promoter in the nucleus of osteoblasts and osteocytes failed to rescue the phenotyope of Dmp1-null mice, whereas the SPDMP1 transgene rescued the rickets defect. These studies support the notion that DMP1 functions as an extracellular matrix protein, rather than as a transcription co-factor in vivo. We also show that DMP1 continues its expression in osteoblasts during postnatal development and that the deletion of Dmp1 leads to an increase in osteoblast proliferation. However, poor mineralization in the metaphysis indicates a critical role for DMP1 in both osteoblasts and osteocytes.
Introduction
Dentin matrix protein 1 (DMP1)4 originally was originally isolated from rat dentin matrix (1) and later was found to have a broader expression profile in many tissues such as bone, cartilage, salivary gland, kidney, pituitary gland, and blood vessels, as well as in cancer tissues, such as those found in the lung and breast (2–11). This acidic phosphorylated extracellular matrix (ECM) protein belongs to the SIBLING (small integrin-binding ligand, N-linked glycoprotein) family (12, 13). Similar to other SIBLING family members, the Dmp1 gene is located on chromosome 4q21 in humans and 5q21 in mice (4, 12). Mutations in this gene result in autosomal recessive hypophosphatemic rickets in humans (14–19), which is similar to that found in Dmp1-null mice (14). Additionally, it is known that DMP1 must be cleaved into the NH2-terminal and COOH-terminal fragments, of which the C terminus appears to be the key functional domain (20–24).
DMP1 has been considered to be an ECM protein since its discovery. An early in vitro mechanism study showed that DMP1 facilitates biomineralization of collagen fibers and crystal growth (25). Although phosphorylated bovine DMP1 expressed in marrow stromal cells shows an inhibitory effect on hydroxyapatite crystal formation and growth (26), the recombinant full-length nonphosphorylated DMP1 and the phosphorylated C terminus work as hydroxyapatite nucleators (26). Furthermore, an Arg-Gly-Asp (RGD) motif is present in the C terminus of DMP1, which can bind to integrin receptors. Based on this structure, Wu et al. (27), Eapen et al. (28) and our laboratory (29) have carried out various mechanistic studies, each of which have independently demonstrated that DMP1 activates the MAP kinase signaling pathway using the full-length or C-terminal fragments of recombinant DMP1 in different mesenchymal cell lines. All of these groups concluded that DMP1 functions as an ECM protein and controls cell function via the αvβ3-integrin-MAP kinase signaling pathway.
Other studies have suggested that DMP1 has dual functional roles, where in addition to functioning as an ECM protein involved in biomineralization, it may also function as a transcription co-factor in osteoblasts and odontoblasts. This transcription co-factor theory is mainly based on two lines of evidence: first, there is a functional NLS sequence in the C terminus of DMP1 that could enable its role as a transcription co-factor, regulating in vitro osteoblast and odontoblast functions (30); second, the N terminus of DMP1 can specifically bind to the GRP-78 (glucose-regulated protein-78) receptor on the cell membrane, triggering the internalization of full-length DMP1 via an endocytotic pathway (31). In addition, two laboratories have shown evidence for DMP1 expression in the nucleus both in vivo (based on immunostaining images) (32) and in vitro (DMP1 found in the cell) (33). These authors proposed that DMP1 is first internalized and transported into the nucleus to function as a transcription co-factor in osteoblasts, and later, during the maturation and differentiation of osteoblasts/osteocytes, DMP1 is phosphorylated in the nucleus, which triggers its transfer into the matrix where it initiates the nucleation of hydroxyapatite formation (30).
This dual function theory was challenged by the identification of a DMP1 mutation in several autosomal recessive hypophosphatemic rickets patients, namely a “Met1Val (M1V)” mutation, which had a biallelic nucleotide substitution in the DMP1 start codon (ATG to GTG, or A1→G). This mutation resulted in the disruption of the endoplasmic reticulum signal sequence in DMP1, as shown by Farrow and co-workers (16). After transfecting the construct of M1V DMP1 into UMR-106 cells (osteosarcoma-derived cell lines), they found the M1V DMP1 did not transfer to the trans-Golgi network for secretion, but rather, it filled the entire cytoplasm (16), implying that DMP1 mainly functions as an ECM protein. Furthermore, Kucka and co-workers (8) reported that gonadotropin-releasing (GnRH) hormone induced a 600-fold increase in Dmp1 expression in primary pituitary cells of cycling female rats and a 30-fold increase in Dmp1 expression in male pituitary cells. Their Western blot data clearly revealed DMP1 in the medium of cultured female pituitary cells after GnRH treatment, whereas no DMP1 protein was identified in the lysate, indicating that DMP1 had been secreted after being induced and little was internalized. In addition, immunohistochemical studies by Kucku et al. (8) demonstrated localization of DMP1 in the cytoplasm but not in the nucleus, and the co-localization of DMP1 with other secreted hormones (such as follicle-stimulating hormone β) further supported the notion that DMP1 is a secreted ECM protein rather than a protein translocating to the nucleus.
To further explore the role of DMP1 in bone biology, we first examined the expression of DMP1 in postnatal osteoblasts and osteocytes and its role in regulating osteoblasts in vivo. We further generated two constructs that either carried the original endoplasmic reticulum entry signal sequence of DMP1 (referred to as “SPDMP1”), or we replaced this secretory signal sequence with an NLS sequence (referred to as “NLSDMP1”). Results of in vitro cell transfection studies showed similar regulation of these two constructs, where there were up-regulated mRNA levels of bone sialoprotein (Bsp), as well as alkaline phosphatase (Alp). However, the expression levels of osteopontin (Opn), osteocalcin (Ocn), dentin sialophosphoprotein (Dspp), and sclerostin (Sost) were reduced significantly. However, evaluations of DMP1 using in vivo transgenic mice models revealed contrary results: NLSDMP failed to rescue the Dmp1-null phenotype, whereas SPDMP1 successfully reversed the defects. These findings suggest that 1) the transcription co-factor hypothesis based on the in vitro data may reflect a nonphysiological event in an extremely high concentration, and that 2) DMP1 mainly functions as an ECM protein during postnatal bone development.
EXPERIMENTAL PROCEDURES
Adenovirus Constructs
The full-length murine Dmp1 cDNA was cloned into an adenovirus CMV construct (Ad5-CMV vector) by the Baylor College of Medicine (Vector Development Lab) with the endogenous signal peptide (MKTVILLVFLWGLSCAL, SPDMP1) or with this signal peptide replaced by a nuclear localization signal peptide (PPKKKRKV, NLSDMP1).
Cell Culture and Adenovirus Infection
MC3T3 cells were cultured in a 12-well plate with α-modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen). Infection was performed when the cells reached at least an 80% confluence level. Vectors in α-modified Eagle's medium with a concentration of 1 × 109 viral particles/well was added and then incubated in a CO2 incubator for 30 min before adding the culture medium. The infection efficiencies were tested under a fluorescent microscope that showed the highest efficiencies after 48 h. All the cells were cultured at 37 °C in a humidified 5% CO2 atmosphere.
Quantitative Real-time PCR
Total RNA was extracted from the infected MC3T3 cells using TRIzol reagent (Invitrogen) according to the manufacturer's protocol and then reverse-transcribed into cDNA, followed by real-time PCR analysis, as in our previous studies (34). The genes analyzed included Dmp1, Bsp, Alp, Opn, Ocn, Dspp, and Sost, with GAPDH as an internal control. The details of the primer sequences were as follows: Dmp1 primer, 5′-AGT GAG TCA TCA GAA GAA AGT CAA GC-3′ (forward) and 5′-CTA TAC TGG CCT CTG TCG TAG CC-3′ (reverse); Bsp primer, 5′-GAG ACG GCG ATA GTT CC-3′ (forward) and 5′-AGT GCC GCT AAC TCA A-3′ (reverse); Alp primer, 5′-CTT GCT GGT GGA AGG AGG CAG G-3′ (forward) and 5′-CAC GTC TTC TCC ACC GTG GGT C-3′ (reverse); Opn primer, 5′-TTT ACA GCC TGC ACC C-3′ (forward) and 5′-CTA GCA GTG ACG GTC T-3′ (reverse); Ocn primer, 5′-CTC TGT CTC TCT GAC CTC ACA G-3′ (forward) and 5′-GGA GCT GCT GTG ACA TCC ATA C-3′ (reverse); Dspp primer, 5′-AAC TCT GTG GCT GTG CCT CT-3′ (forward) and 5′-TAT TGA CTC GGA GCC ATT CC-3′ (reverse); Sost primer, 5′-AGC TCC TTC AGA GGG CTG AT-3′ (forward) and 5′-GAG GCA GGC ATT TCA GTA GC-3′ (reverse); and GAPDH primer, 5′-GGT GTG AAC CAC GAG AAA-3′ (forward) and 5′-TGA AGT CGC AGG AGA CAA-3′ (reverse).
Generation and Characterization of Transgenic Mice
To generate the Col1α1-NLSDmp1 transgene, the full-length mouse Dmp1 cDNA with its endoplasmic reticulum entry signal sequence replaced by a NLS sequence and a SV40 polyadenylation signal sequence were cloned into a mammalian expression vector (graciously provided by B. Kream and A. Lichtler, University of Connecticut Health Center, Farmington, CT, USA) (35) containing the 3.6-kb rat type 1 collagen promoter plus a 1.6-kb intron 1 at the EcoRV and SalI sites, which gave rise to the Col1α1-NLSDmp1 transgene. This transgene was released from the vector backbone by digestion with SacII and SalI and then purified for pronuclear injection. Transgenic founders with a C57B/L6 background were generated at the UT Southwestern Medical Center (Dallas, TX). A total of nine independent transgenic lines were obtained and partially characterized by radiographs, micro-computed tomography (Micro-CT), and H&E stain. Except for one line displaying an increase in femur trabecular bone volume, the rest of the transgenic mouse lines showed no apparent differences from the control mice (data not shown). As reported previously, the same 3.6-kb Col1α1 promoter was also used to generate the Col1α1-SPDmp1 transgenic mice (21).
Targeted Expression of the NLSDmp1 and SPDmp1 Transgenes into Dmp1-null Mice
Dmp1-null mice on a C57B/L6 genetic background using the lacZ knock-in target approach have been described previously (36). To target Dmp1 to the nucleus or matrix, the Dmp1-null mice were crossed, respectively, with SPDMP1 or three different NLSDMP1 lines (Table 1). Only line 95, whose expression level is 6-fold higher than that of the wild type (WT) Dmp1, was fully characterized using multiple approaches. Because no apparent phenotypic differences between the WT and heterozygous (HET) Dmp1 mice were found, the HET Dmp1 mice were used as controls. All of the mice were fed autoclaved Purina rodent chow (Ralston Purina, St. Louis, MO) containing 1% calcium, 0.67% phosphorus, and 4.4 international units (IU) of vitamin D/g. All the animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Texas A&M University Baylor College of Dentistry.
TABLE 1.
Three transgenic mouse lines and their DMP1 expression levels compared with WT
M, male.
| Line No. | mRNA Expression of Dmp1 | Sex |
|---|---|---|
| 95 | 6.27 | M |
| 97 | 118.48 | M |
| 98 | 4.96 | M |
PCR Genotyping
Genomic DNA was extracted from a tail biopsy and used for genotyping by PCR analysis. Primers p01 (5′-GAG TGC GAT CTT CCT GAG GCC GAT ACT GTC-3′) and p02 (5′-CGC GGC TGA AAT CAT CAT TAA AGC GAG TGG-3′) were used for targeting LacZ (490 bp); primers p03 (5′-GCC CCT GGA CAC TGA CCA TAG C-3′) and p04 (5′-CTG TTC CTC ACT CTC ACT GTC C-3′) were used for identification of endogenous Dmp1 (400 bp); primers p05 (5′-CAG CCG TTC TGA GGA AGA CAG TG-3′ from Dmp1 cDNA) and p06 (5′-TGT CCA AAC TCA TCA ATG TAT CT-3′ from the SV40 polyadenylation signal) were used to detect the transgenic NLSDMP1 (337 bp).
Protein Extraction and Western Blotting
To determine expression levels of NLSDMP1 in bone cells, total cell lysates and nuclear protein were extracted from the HET, Dmp1-knockout (KO)+NLSDMP1, Dmp1-KO+SPDMP1, and Dmp1-KO primary calvarial cells, respectively, using a nuclear extract kit (Active Motif, Carlsbad, CA). 20 μg of the total proteins of each group were then subjected to 10% SDS-PAGE gel, followed by blotting onto a polyvinylidene difluoride membrane. The membranes were subsequently immunoblotted with different antibodies, including anti-DMP1 polyclonal antibody (24); anti-lamin monoclonal antibody (Santa Cruz Biotechnology), anti-β-actin monoclonal antibody (Sigma-Aldrich). Alkaline phosphatase-conjugated anti-rabbit IgG and anti-mouse IgG (Sigma-Aldrich) were employed as the secondary antibody at a dilution of 1:3000. Following this procedure, the membranes were incubated in chemiluminescent substrate CDP-star (Ambion, Grand Island, NY) for 30 s and exposed to Kodak film (PerkinElmer Life Science, Waltham, MA) to visualize the bands.
Histology
For the decalcified bone analysis, the animals were injected twice with BrdU (5-bromo-2′-deoxyuridine, 100 μg/g mouse, intraperitoneal; Sigma-Aldrich). The first injection was made 24 h before mouse sacrifice, and the second was 2 h prior to sacrifice. For fixation, perfusion was performed using freshly prepared 4% paraformaldehyde in phosphate-buffered saline (pH 7.4). Next, the specimens were decalcified and embedded in paraffin as described previously (3). Sections were cut in 4.5-μm slices, mounted on slides, and air-dried. These slides were used for immunohistochemistry, in situ hybridization, Safranin O, and BrdU staining (BrdU staining kit, Invitrogen) as described previously (14). The antibodies used for immunohistochemistry are listed as follows: anti-DMP1-C-terminal polyclonal antibody (23), anti-E11 polyclonal antibody (37), anti-sclerostin polyclonal antibody (R&D Biosystems, Minneapolis, MN), anti-osterix monoclonal antibody (Abcam, Cambridge, MA), anti-decorin monoclonal antibody (38), and anti-sox9 polyclonal antibody (Santa Cruz Biotechnology). Two probes were used for in situ hybridization, including Runx2 and Shh (39). For the undecalcified bones, the specimens were dehydrated in ascending concentrations of ethanol (from 70 to 100%) and embedded in methyl methycrylate (Buehler, Lake Bluff, IL), then cut as 6-mm-thick slices using a Leica 2165 rotary microtome (Ernst Leitz Wetzlar). The undecalcified specimens or sections were used for Masson Goldner trichrome staining (14), Von Kossa staining, and Alizarin red/Alcian blue staining (36), separately.
β-Galactosidase (lacZ) Expression Assay
The method of β-galactosidase staining was described previously (40). The freshly fixed 3-week-old heterozygous mouse long bone was rinsed with phosphate-buffered saline after 15 min fixation, and then the specimens were stained for 48 h in freshly prepared X-gal solution (1 mg/ml) at 37 °C. The stained samples were washed with phosphate-buffered saline again, followed by refixation, decalcification, and embedding in paraffin for sectioning and counterstaining.
High-resolution Radiography, Micro-CT, Scanning Electron Microscopy, and Transmission Electron Microscopy
After surrounding muscle tissue was removed, radiographs of the bone specimens were taken using a Faxitron model MX-20 Specimen Radiography System with a digital camera (Faxitron X-Ray Corp., Lincolnshire, IL). Long bone micro-CT analyses were performed using the Scanco micro-CT35 (micro-CT35; Scanco Medical, Bassersdorf, Switzerland). The cortical thickness data were obtained at the midshaft of the bone by means of serial tomographic imaging at an energy level of 55 kV and an intensity of 145 μA. Thus, 100 lateral sections of slices above the midshaft of the femur were analyzed at a threshold of 283. The methyl methycrylate-embedded samples were cut, and the surfaces were polished using 1-μm and 0.3-μm Alumina Alpha micropolish II solution (Buehler), followed by acid etching with 37% phosphoric acid for 2 to 10 s, followed by 5% sodium hydrochloride for 5 min, and then the samples were gold-coated with gold and palladium. The specimens were scanned using a FEI/Philips XL30 field-emission environmental scanning electron microscopy (Hillsboro, OR) as described previously (21). For transmission electron microscopy, specimens were fixed with 4% paraformaldehyde and 1% glutaraldehyde in 0.1 m sodium cacodylate buffer (pH 7.2) and then postfixed with 1% osmium tetroxide in 0.1 m sodium cacodylate buffer, and processed for embedding in LR White acrylic resin (London Resin Company, Bershire, UK). Colloidal-gold immunostaining of DMP1 was performed, and specimens were examined by transmission electron microscopy as described previously (41).
Serum Biochemistry
A common cardiac puncture method was used to collect blood from the 6-week-old mice under anesthesia. The serum phosphorus was measured using the phosphomolybdate-ascorbic acid method, and serum FGF23 levels were determined using a full-length FGF23 ELISA kit (Kainos Laboratories, Tokyo, Japan), as described previously (14).
Statistical Analysis
Data analysis was performed using one-way analysis of variance for multiple group comparison. If significant differences were found with the one-way analysis of variance, the Bonferroni method was used to determine which groups were significantly different from the others. The quantified results are represented as the means ± S.E. of the mean (S.E.). We considered p < 0.05 as the statistical significance level.
RESULTS
Localization of DMP1 in Relation to Osteocytes and Studying the Role of DMP1 in Osteoblasts in Vivo
DMP1, highly expressed in osteocytes, plays a key role in osteocyte maturation (4, 36, 42), although its expression in the nucleus is debatable. Here, we used high-resolution immunogold labeling to show the ECM localization of DMP1 in the pericellular matrix surrounding osteocytes in 2-month-old murine bones. These results showed a strong localization of DMP1 in the ECM adjacent to both the osteocyte dendritic processes along the canalicular wall and around the cell body but not in the nucleus (Fig. 1a), supporting its key role as an ECM protein (see below for further discussion).
FIGURE 1.
DMP1, highly expressed by osteocytes (Ocy) and also expressed by postnatal osteoblasts (Ob), is likely responsible for regulating osteoblast proliferation and differentiation. a, immuno-gold labeling reveals the presence of DMP1 accumulation in the ECM of the canalicular wall adjacent to the dendrites/cell processes and surrounding the cell body (lacunar wall) of osteocytes. b, X-gal staining of HET long bones displays blue positive cells not only in osteocytes but also in osteoblasts in both embryonic day 16.5 (E16.5; inset) and the postnatal 3 weeks, especially in the metaphysis (right image). c, representative radiographs of 3-week-old long bones reveals the co-existence of an expanded metaphysis (arrows) and a delayed formation of the epiphysis (arrowhead). d and e, histological images showing that in the Dmp1-KO tibia metaphysis (right panels), there is a sharp expansion of bone mass with little residual cartilage (d, Safranin O stain, 6 weeks), indicating a lack of endochondral bone formation but the presence of active intramembranous bone formation; von Kossa staining (e, 3 months) shows poor mineralization of the bone (arrow) compared with age-matched control (left). f–h, in situ and immunohistochemical staining showing that in the KO tibia metaphysis (right), there are increased BrdU-positive cells (f), and mRNA levels of Runx2 (g), and OSX (h).
Recent in vitro studies suggested that DMP1 may also be expressed by osteoblasts and may regulate their function (32, 44). We then studied the postnatal Dmp1 expression profile using the X-gal staining approach, in which the blue lacZ staining reflects endogenous Dmp1 because the lacZ reporter is used to replace exon 6 of Dmp1 (40). Different stages of Dmp1-HET bones were used for the study, and we not only confirmed that the Dmp1-lacZ signal is mainly expressed in the osteoblast at embryonic day 16.5 (E16.5) (Fig. 1b, inset), but we also identified a strong Dmp1-lacZ signal in both osteocytes (Fig. 1b, left) and osteoblasts, with abundant blue osteoblasts in the metaphyseal region at 3 weeks of age (Fig. 1b, right).
To test the role of DMP1 in osteoblasts in vivo, we then re-examined the phenotype of the expanded bone mass in the Dmp1-null metaphysis (Fig. 1c). The Safranin O-stained images showed a lack of residual cartilage in the Dmp1-null metaphysis because of a sharp reduction of apoptosis in the hypertrophic chondrocyte zone resulting from the low Pi level (34), compared with the control trabecula, in which there were numerous cartilage remnants indicating normal endochondral ossification (Fig. 1d). However, the mineralization in this expanded Dmp1-null metaphysis was poor, based on the von Kossa stained images (Fig. 1e). We also evaluated the molecular changes in the Dmp1-null metaphyses and observed high levels of BrdU, Runx2, and osterix (OSX) compared with the control (Fig. 1, f–h). These results support the notion that in addition to its role in osteocytes DMP1 also regulates osteoblasts by slowing down cell proliferation and accelerating mineralization.
Overexpression of SPDMP1 or NLSDMP1 Greatly Enhances Osteoblast Activities in Vitro in an Identical Manner
One of the ways to test the dual function of DMP1 is to generate two different forms of DMP1 to ensure its different subcellular localizations: SPDMP1, the secreted DMP1 form containing its own endoplasmic reticulum entry signal sequence, and NLSDMP1, the endogenous endoplasmic reticulum entry signal sequence was replaced by a nuclear localization signal sequence to ensure the nuclear localization of DMP1, using the adenovirus CMV (Ad-CMV) approach (Fig. 2a). Both constructs, plus an empty Ad-CMV construct (as a control), were used to infect MC3T3 (a pre-osteoblast cell line) for 48 h. Quantitative real-time PCR was then performed to examine the mRNA expression levels of the targeted Dmp1 in the infected cells. As expected, there were significantly high expressions of Dmp1 mRNAs in both forms, compared with the control (Fig. 2b). Further analyses of the common markers linked to mineralization revealed an identical response pattern in both experimental groups, compared with the control. For example, Bsp and Alp sharply increased, whereas Ocn, Opn, Dspp, and Sost were significantly reduced in both infected groups (Fig. 2b and Table 2). These findings indicated that the NLSDMP1 transgene exhibits the same biological response as that of the SPDMP1 transgene when highly expressed in vitro, in agreement with a previous report (30).
FIGURE 2.
Transfection of Ad-CMV-NLSDMP1 or Ad-CMV-SPDMP1 greatly changes the expression of bone markers in MC3T3 cells. a, schematic constructs of NLSDMP1 and SPDMP1, in which the CMV promoter was used to drive the full-length Dmp1 cDNA. In NLSDMP1, the endogenous DMP1 signal peptide amino acids 1–16 (MKTVILLVFLWGLSCAL) was replaced by the nuclear localization signal peptide (NLS, PPKKKRKV, upper panel) in contrast to the SPDMP1 with its endogenous signal peptide (lower panel). b, >1000-fold increases in NLSDMP1 and SPDMP1 were induced by adenovirus transinduction. There were significant up-regulations of mRNA levels for Bsp, Alp, Ocn, Opn, Dspp, and Sost in both experimental groups with all showing an identical trend. The real-time PCR data, normalized to Gapdh (as an internal control), are presented as mean ± S.E. (n = 4 in each group; *, p < 0.05; **, p < 0.01, compared with the control).
TABLE 2.
RNA expression levels after transfection
Values were expressed as mean ± S.E. from at least four samples per group. Comparisons were performed using one-way analysis of variance and post hoc Fisher test. S.D. indicates significant differences (*, p < 0.05 vs. control; **, p < 0.01 vs. control).
| Control | NLSDMP1 | SPDMP1 | S.D. between NLS and SP | |
|---|---|---|---|---|
| DMP1 | 1.05 ± 0.11 | 3070 ± 137.50** | 2425 ± 248.10** | None |
| BSP | 1.09 ± 0.24 | 2.68 ± 0.26* | 12.92 ± 1.81** | ** |
| ALP | 0.99 ± 0.01 | 10.19 ± 0.85** | 27.18 ± 2.60** | * |
| OCN | 0.95 ± 0.03 | 0.37 ± 0.06** | 0.16 ± 0.02** | * |
| OPN | 1.01 ± 0.13 | 0.17 ± 0.12* | 0.18 ± 0.13* | None |
| DSPP | 0.68 ± 0.08 | 0.11 ± 0.01** | 0.14 ± 0.10** | None |
| SOST | 1.34 ± 0.08 | 0.40 ± 0.07** | 0.28 ± 0.05** | None |
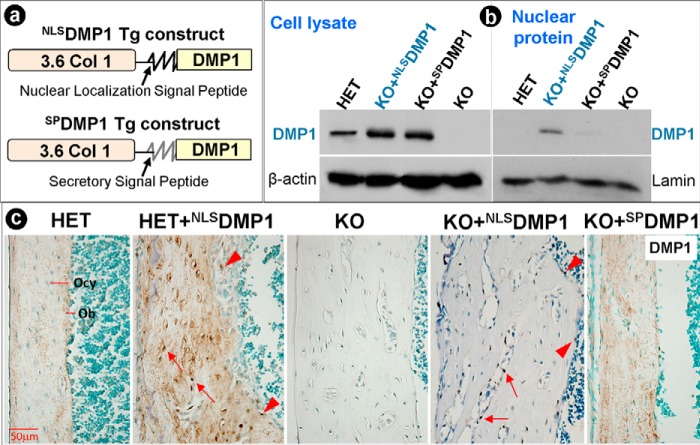
Specific Targeting of DMP1 to the Nuclei of Osteoblasts and Osteocytes or Secretion into the Matrix in Vivo
To test the in vivo function of both the NLSDMP1 and SPDMP1 transgenes, we generated transgenic mice, which individually express these two transgenes in osteoblasts and osteocytes, using a 3.6 kb Col1α1 promoter (Fig. 3a). Three independent lines among nine partially characterized lines of NLSDMP1 transgenic mice (Table 1) were crossed to the Dmp1-null mice separately (only line 95 was fully characterized), and compared with the Dmp1-null with the SPDMP1 transgene group. Western blotting of cell lysates and nuclear proteins, extracting from primary calvarial cells obtained separately from HET, KO+NLSDMP1, KO+SPDMP1, and KO, showed DMP1 expression (∼115 kDa) in the whole-cell extracts of each group except for the KO (Fig. 3b, left). However, in the nuclear protein extract, detectable expression of DMP1 was only found in the KO+NLSDMP1 group (Fig. 3b, right).
FIGURE 3.
NLSDMP1 localizes to the nucleus and SPDMP1 is secreted into the matrix. a, schematic structure of NLSDMP1 and SPDMP1 transgenes. In the NLSDMP1 construct, the full-length Dmp1 cDNA is driven by the 3.6-kb Col 1α1 promoter with its endogenous signal peptide replaced by a NLS peptide, whereas in the SPDMP1 construct, the same 3.6-kb Col 1α1 promoter was used to drive the full-length Dmp1 cDNA with its endogenous secretory signal peptide. b, proteins extracted from cell lysate (left) or nuclei (right) of different groups were blotted for the expression level of DMP1. Results show that DMP1 can be found in the cell lysate of all these groups except the Dmp1-null group; however, in the nuclear protein extract, it was only detectable in the KO+NLSDMP1 group. c, immunohistochemical staining of DMP1 in 3-week-old mouse long bone. DMP1 is mainly expressed in the HET matrix with little signal in the nucleus, compared with no positive signal in the matrix in KO mice. In the NLSDMP1 transgenic bone, DMP1 is detected in both the matrix and the nucleus of osteocytes (arrows) and osteoblasts (arrowheads), whereas the SPDMP1 is highly expressed in the matrix and the osteoblasts. These data demonstrate that the transgenes are successfully targeted to the nucleus or to the matrix (of bone cells only).
In accordance with these findings, immunohistochemistry of DMP1 (Fig. 3c) revealed that in the Dmp1-HET control, endogenous DMP1 was localized in the bone matrix surrounding the osteocyte and its dendrites, whereas in the NLSDMP1 transgene with the HET background, DMP1 was detected in both the bone matrix (the endogenous) and the nuclei (the transgene) of the osteoblasts and osteocytes. As expected, there was no DMP1 in the KO group. DMP1 was detected in the nuclei of the KO+NLSDMP1 group, and in the bone matrices of KO+SPDMP1 group.
Dmp1-null Mice Display Hypophosphatemic Rickets with High Levels of Cell Proliferation in the Prehypertrophic Zones, which Is Rescued by SPDMP1 but Not by NLSDMP1
We previously demonstrated that the short stature of the Dmp1-null mice is secondary to hypophosphatemia caused by the elevated FGF23 (14). The low level of Pi inhibited cell apoptosis in the hypertrophic chondrocytes (45), leading to a sharp reduction in bone growth with severe skeletal deformities (46).
If DMP1 has a function as a transcription co-factor, the targeted expression of DMP1 in the nucleus would likely restore the FGF23 and Pi levels. However, only the SPDMP1 transgene but not the NLSDMP1 apparently normalized the serum levels of FGF23 and Pi at the age of 6 weeks, favoring the role of DMP1 as an ECM protein (Fig. 4, a and b).
FIGURE 4.
NLSDMP1 does not rescue the rachitic phenotypes in Dmp1-null mice; however, SPDMP1 fully rescues the defects. a and b, quantitative data show a greater than 18-fold up-regulation of serum FGF23 levels and a decrease of >20% of the Pi (phosphorus) in Dmp1-null mice. The overexpression of NLSDMP1 has no effect on these parameters in either the HET control or KO group; however, the SPDMP1 is fully rescued. d, Safranin O-stained images of 3-week-old tibias, in which there are two expanded regions in the KO growth plate: the proliferation zone (PZ; white lines) and the hypertrophic zone. The NLSDMP1 transgene has no effect in both regions, but SPDMP1 recovers this abnormality. e, BrdU-stained images reveal an increase in the number of BrdU-positive cells in the KO and KO+NLSDMP1 groups. Statistical results demonstrate the above changes in proliferation zone length (c) and cell proliferation numbers (f) in the KO groups, which were significantly different from the control groups., the NLSDMP1 has no apparent effect on the above changes in either the HET or the KO background, but SPDMP1 rescues the KO phenotype. g--j, in situ and immunohistochemical staining shows the molecular changes of Runx2 mRNA (g), sonic hedgehog mRNA (Shh; h), SOX9 (i), and OSX (j). The data indicate an increase in cell proliferation and differentiation in the Dmp1-null mice, most likely attributable to hypophosphatemia (data are presented as mean ± S.E.; n = 5; *, p < 0.05; compared with the control). k, representative radiography shows short femurs with poor mineral remodeling in all three Dmp1-KO groups (3 weeks, 6 weeks, and 1 year). The targeted expression of NLSDMP1 has no rescue effects on the length, accumulated bone masses, expanded and malformed growth plates (arrows), and distorted tuberosities (arrowheads), all of which are fully rescued by SPDMP1.
Unexpectedly, we identified a significant expansion of the proliferation zone in the Dmp1-null mice as seen after Safranin O staining (Fig. 4, c and d). This expansion may attributable to the abnormal activity of chondrocytes because the BrdU analysis confirmed significantly high levels of cell proliferation in the KO growth plate (Fig. 4, e and f). In addition, further analyses indicated that molecular markers important for chondrocyte differentiation are up-regulated in the null chondrocytes, including Runx2 (Fig. 4g, in situ), sonic hedgehog (Shh, Fig. 4h, in situ), SOX9 (Fig. 4i, IHC), and OSX (Fig. 4j, IHC). However, this abnormality could be greatly rescued after re-expression of SPDMP1 but not by NLSDMP1 (Fig. 4, c–f).
We also compared the x-ray images of mice at the ages of 3 weeks, 6 weeks, and 1 year, attempting to determine which form of DMP1 transgene may correct the remodeling defect in the Dmp1-KO mice because Dmp1-null phenotypes become more severe with age (46). Again, the representative radiographs (Fig. 4k) showed no apparent improvement in the KO+NLSDMP1 group but fully rescued the KO+SPDMP1 group.
Here, we proved an up-regulation of chondrocyte proliferation and their function in the proliferation zone of the Dmp1-null growth plate, which contributed to the expanded growth plate phenotype. More importantly, we also demonstrated that the NLSDmp1 transgene had no apparent rescue effect on the rickets phenotype, whereas SPDMP1 did.
Targeted Expression of SPDMP1 in Dmp1-null Mice Rescues Mineralization Defects, whereas the NLSDMP1 Does Not
To test whether the NLSDMP1 or the SPDMP1 rescues ossification processes, we stained both bone (using Alizarin red) and cartilage (with Alcian blue) of 10-day-old mouse long bones. The deformed skeleton in the Dmp1-null mice was essentially the same as it is in the KO+NLSDMP1 group (Fig. 5a). In contrast, the bone in the KO+SPDMP1 was fully rescued (Fig. 5a). Additional μ-CT analyses, including overall bone images (Fig. 5b) and statistical analysis of bone histomorphometry changes (Table 3), showed a porous cortical bone in the Dmp1-null bone with or without the expression of NLSDMP1 at the age of 3 weeks but fully restored porosity in the KO+SPDMP1 cortical bone. Additionally, Goldner-stained images (Fig. 5c) also revealed no apparent rescue effect on the osteomalacia phenotype in the KO+ NLSDMP1 group.
FIGURE 5.

The NLSDMP1 transgene has no rescue effects on Dmp1-null mice but the SPDMP1 transgene fully rescues the Dmp1-null bone phenotype. a–c, compared with SPDMP1, NLSDMP1 has no effect on bone shape and secondary ossification (a, Alizarin red/Alcian blue staining, arrows), bone porosity and mineralization reflected by micro-CT images (b, arrowheads), and Goldner staining (c, red indicating a lack of mineral). d–h, the NLSDMP1 had no apparent effect on the osteocyte (Ocy)-lacunocanalicular system (d, S.E.), and expression of decorin (e, an inhibitory factor in mineralization), OSX (f, a marker for Ob), E11 (g, an early marker for osteocytes), and SOST (h, a marker for mature osteocytes).
TABLE 3.
Bone Histomorphometry Findings in the femurs of 3-week-old mouse
Values are expressed as mean ± S.E. from at least five mice per group. Comparisons were performed using one-way analysis of variance and post hoc Fisher test. *, p < 0.05 vs. DMP1−/−; **, p < 0.01 vs. DMP1−/− of femur length, cross-section area, total volume (TV), bone volume (BV), BV/TV, cortical porosity. *, p < 0.05 vs. NLSDMP1, DMP1−/−; **, p < 0.01 vs. NLSDMP1, DMP1−/− of ectopic BV.
| HET | HET + NLSDMP1 | KO | KO + NLSDMP1 | |
|---|---|---|---|---|
| Femur length | 9.44 ± 0.15 | 9.59 ± 0.19 | 8.18 ± 0.18** | 7.93 ± 0.35** |
| Cross-section area | 1.03 ± 0.10 | 1.31 ± 0.08 | 1.61 ± 0.08** | 2.23 ± 0.20** |
| TV (mm3) | 0.12 ± 0.01 | 0.20 ± 0.03 | 0.31 ± 0.08 | 0.28 ± 0.01 |
| BV (mm3) | 0.11 ± 0.01 | 0.17 ± 0.03 | 0.12 ± 0.01 | 0.15 ± 0.01* |
| BV/TV | 0.95 ± 0.01 | 0.88 ± 0.01 | 0.46 ± 0.10** | 0.51 ± 0.01** |
| Cortical porosity | 0.05 ± 0.01 | 0.12 ± 0.01 | 0.54 ± 0.10** | 0.49 ± 0.01** |
| Ectopic BV (mm3) | 0.01 ± 0.01 | 0.03 ± 0.01** | 0.01 ± 0.01** | 0.05 ± 0.01** |
Previously, we reported that the mineralization defect in the Dmp1-null mice was directly linked to the pathological changes in osteocyte morphology, including an enlarged cell body and a reduction of dendrite number (34, 47). In this study, we reanalyzed the SEM image data and found great improvement in cell morphology after the re-expression of SPDMP1. Comparing the identical malformation of osteocytes in the Dmp1-null mice with and without NLSDMP1 (Fig. 5d), this indicated that the secretion of DMP1 into the matrix is important for osteocyte morphology and function. In accordance with this interpretation, the analysis of mineralization-related markers also suggested that it is the secreted DMP1, but not the nuclear-targeted DMP1, that restores the expression pattern of: 1) decorin (Fig. 5e, an inhibitory factor for mineralization); 2) OSX (Fig. 5f, a critical transcription co-factor for osteoblast function); 3) E11/gp38 (Fig. 5g, E11, a marker linked to the newly formed osteocytes or immature osteocytes), and 4) sclerostin (Fig. 5h, SOST, an inhibitory matrix protein against Wnt-β-catenin, mainly expressed in mature osteocytes). Collectively, the overexpression of the NLSDMP1 in the Dmp1-null osteoblast and osteocyte failed to rescue the osteomalacia, which challenges the dual-function hypothesis.
DISCUSSION
It has been proposed that DMP1 has a dual function (as a transcription co-factor and an ECM protein) in osteogenesis (30, 31), which is indeed in agreement with our in vitro studies (Fig. 1 and Table 2). However, our in vivo studies do not support this hypothesis.
The key evidence against the dual-function hypothesis include the following. 1) The DMP1-immunogold labeling clearly shows no nuclear labeling but a high abundance of DMP1 in the ECM surrounding the WT osteocyte cell body and dendrites/cell processes (Fig. 2a). 2) The NLSDMP1 failed to rescue the rachitic phenotype in Dmp1-null mice but the SPDMP1 transgene did, essentially based on all assays used in this study. 3) The M1V mutant DMP1, which is trapped in the cell due to an interruption of the secretory signal sequence, retains all of its functions except being secreted (14, 30); however, the patient with the DMP1 M1V mutation showed typical autosomal recessive hypophosphatemic rickets symptoms (14). 4) The DMP1 transgene with a mutation in Asp-213, which is the DMP1 cleavage site, but carrying no change in other amino acid sequence, also failed to rescue the rachitic phenotypes of the Dmp1-null mice (24, 48, 49).
Therefore, we believe that the dual-function hypothesis may reflect a nonphysiological phenomenon in an extremely high in vitro concentration. Furthermore, this contrast may also be related to differences between in vivo studies and in vitro studies. Generally, in vivo studies are more complex and sometimes even lead to different conclusions when the results are compared with in vitro findings. For example, in vivo osteogenic cells grow three-dimensionally, which leads to different results from in vitro two-dimensional studies (43).
We previously generated several independent lines of the SPDMP1 transgene with different expression levels. None of them displayed apparent phenotypes in the WT background, but the mouse lines with high or low expression levels fully rescued the Dmp1-null mice separately (20, 21, 41). Based on these findings, the same approach and promoter were used to generate the NLSDMP1 mouse lines in this study. Interestingly, the NLSDMP1 transgenic line 95 with a low expression level displayed accelerated trabecular bone formation (Fig. 5b), but line 97 with a much higher expression level presented no bone phenotype (data not shown). These findings suggest the transgene dosage effect is limited in vivo.
Previous work studying DMP1 was mainly focused on its role in osteocytes because DMP1 is highly expressed in the osteocytes during postnatal development (4, 36, 42). In this work, we showed the continuous expression of a high level of Dmp1-lacZ in the osteoblasts of the metaphysis during early postnatal development (Fig. 1b). Furthermore, we demonstrated that the deletion of Dmp1 leads to the acceleration of bone cell proliferation (as assessed by BrdU staining) and osteoblast differentiation (from the expression of Runx2 and OSX) compared with the control. As a result, an expanded metaphysis appeared, although the mineralization was poor (Fig. 1, c--e). In addition, the expression of SPDMP1 rescued the metaphyseal phenotype (20, 21) and the differentiation of osteoblasts, supporting the role of DMP1 in osteoblasts (reducing osteoblast cell proliferation and accelerating the differentiation of osteoblasts into osteocytes).
In summary, the in vitro overexpression of SPDMP1 (the secreted form) or NLSDMP1 (the nucleus form) greatly changed the gene expression profile in the osteoblast in an identical manner. However, in vivo studies using genetic rescue approaches to target DMP1 either in the Dmp1-null nucleus or in the null ECM demonstrated that DMP1 functions as an ECM protein rather than as a transcription co-factor, which challenges the dual-function theory. Based on the new findings and the publications from our laboratory and others (27, 29, 46), we propose that DMP1 is secreted into the ECM, where it binds to αvβ3 integrin, leading to activation of the MAP kinase signaling pathway in the cell (Fig. 6a). In addition, the deletion of Dmp1 results in two novel phenotypes: 1) an increase in osteoblast cell proliferation, along with an inhibition in osteoblast differentiation into osteocytes, which causes the formation of abundant, but poorly mineralized intramembranous bone in the metaphysis (reflecting the direct role of DMP1 in osteoblasts); and 2) an increase in prechondrocyte activity (an indirect role likely due to hypophosphatemia) (Fig. 6b).
FIGURE 6.

The in vivo working model. a, DMP1 secreted from the cell binds to integrin via its RGD domain, followed by activation of the MAP kinase signaling pathway (27, 29). The targeted expression of DMP1 in the nucleus has no direct role in osteogenesis in vivo. b, DMP1 expressed in the osteoblast (Ob) facilitates cell proliferation and transformation (from osteoblasts into osteocytes (Ocy)). Deletion of Dmp1 leads to an increase in cell proliferation and a reduction in osteoblast cell differentiation. Because of the great increase of FGF23 in Dmp1-KO osteocytes, the reduced Pi causes an increase in cell proliferation and differentiation in chondrocytes (a new indirect role of hypophosphatemia in the growth plate), in addition to the abnormality in the apoptosis pathway (46).
Acknowledgments
We thank Professors Barbara Kream and Alexander Lichtler (University of Connecticut Health Center, Farmington, CT) for graciously providing the mammalian expression vector, Professor Larry Fisher (National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD) for generously supplying the anti-decorin monoclonal antibody, Professor Lynda Bonewald (School of Dentistry, University of Missouri at Kansas City, Kansas City, MO) for kindly providing the anti-E11 polyclonal antibody, and Dr. Gerard Karsenty (Columbia University Medical Center, New York, NY) for kindly providing the in situ hybridization probes for Runx2 and Shh. We also gratefully acknowledge Jeanne Santa Cruz for checking grammar.
This work was supported by National Institutes of Health Grants DE018486 and R56DE022789 (to J. Q. F.) and Scholarship NSF 2010627108 under the State Scholarship Fund of China (to S. L.).
- DMP1
- dentin matrix protein 1
- NLS
- nuclear localization signal
- ECM
- extracellular matrix
- micro-CT
- micro-computed tomography
- Ad
- adenovirus
- HET
- heterozygous
- OSX
- osterix
- BSP
- bone sialoprotein
- ALP
- alkaline phosphatase
- OPN
- osteopontin
- OCN
- osteocalcin
- DSPP
- dentin sialophosphoprotein
- SOST
- sclerostin
- E11
- E11/gp38.
REFERENCES
- 1. George A., Sabsay B., Simonian P. A., Veis A. (1993) Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J. Biol. Chem. 268, 12624–12630 [PubMed] [Google Scholar]
- 2. D'Souza R. N., Cavender A., Sunavala G., Alvarez J., Ohshima T., Kulkarni A. B., MacDougall M. (1997) Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J. Bone Miner. Res. 12, 2040–2049 [DOI] [PubMed] [Google Scholar]
- 3. Feng J. Q., Zhang J., Dallas S. L., Lu Y., Chen S., Tan X., Owen M., Harris S. E., MacDougall M. (2002) Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J. Bone Miner Res. 17, 1822–1831 [DOI] [PubMed] [Google Scholar]
- 4. Hirst K. L., Ibaraki-O'Connor K., Young M. F., Dixon M. J. (1997) Cloning and expression analysis of the bovine dentin matrix acidic phosphoprotein gene. J. Dent. Res. 76, 754–760 [DOI] [PubMed] [Google Scholar]
- 5. MacDougall M., Simmons D., Luan X., Nydegger J., Feng J., Gu T. T. (1997) Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4: Dentin phosphoprotein DNA sequence determination. J. Biol. Chem. 272, 835–842 [DOI] [PubMed] [Google Scholar]
- 6. Rangiani A., Cao Z., Sun Y., Lu Y., Gao T., Yuan B., Rodgers A., Qin C., Kuro-O M., Feng J. Q. (2012) Protective roles of DMP1 in high phosphate homeostasis. PLoS One 7, e42329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun Y., Ma S., Zhou J., Yamoah A. K., Feng J. Q., Hinton R. J., Qin C. (2010) Distribution of small integrin-binding ligand, N-linked glycoproteins (SIBLING) in the articular cartilage of the rat femoral head. J. Histochem. Cytochem. 58, 1033–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kucka M., Bjelobaba I., Clokie S. J., Klein D. C., Stojilkovic S. S. (2013) Female-specific induction of rat pituitary dentin matrix protein-1 by GnRH. Mol. Endocrinol. 27, 1840–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lv K., Huang H., Lu Y., Qin C., Li Z., Feng J. Q. (2010) Circling behavior developed in Dmp1 null mice is due to bone defects in the vestibular apparatus. Int. J. Biol. Sci. 6, 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaplet M., De Leval L., Waltregny D., Detry C., Fornaciari G., Bevilacqua G., Fisher L. W., Castronovo V., Bellahcène A. (2003) Dentin matrix protein 1 is expressed in human lung cancer. J. Bone Miner Res. 18, 1506–1512 [DOI] [PubMed] [Google Scholar]
- 11. Bucciarelli E., Sidoni A., Bellezza G., Cavaliere A., Brachelente G., Costa G., Chaplet M., Castronovo V., Bellahcène A. (2007) Low dentin matrix protein 1 expression correlates with skeletal metastases development in breast cancer patients and enhances cell migratory capacity in vitro. Breast Cancer Res. Treat 105, 95–104 [DOI] [PubMed] [Google Scholar]
- 12. Fisher L. W., Torchia D. A., Fohr B., Young M. F., Fedarko N. S. (2001) Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem. Biophys. Res. Commun. 280, 460–465 [DOI] [PubMed] [Google Scholar]
- 13. Kaartinen M. T., Sun W., Kaipatur N., McKee M. D. (2005) Transglutaminase crosslinking of SIBLING proteins in teeth. J. Dent. Res. 84, 607–612 [DOI] [PubMed] [Google Scholar]
- 14. Feng J. Q., Ward L. M., Liu S., Lu Y., Xie Y., Yuan B., Yu X., Rauch F., Davis S. I., Zhang S., Rios H., Drezner M. K., Quarles L. D., Bonewald L. F., White K. E. (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38, 1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lorenz-Depiereux B., Bastepe M., Benet-Pagès A., Amyere M., Wagenstaller J., Müller-Barth U., Badenhoop K., Kaiser S. M., Rittmaster R. S., Shlossberg A. H., Olivares J. L., Loris C., Ramos F. J., Glorieux F., Vikkula M., Jüppner H., Strom T. M. (2006) DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat. Genet. 38, 1248–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farrow E. G., Davis S. I., Ward L. M., Summers L. J., Bubbear J. S., Keen R., Stamp T. C., Baker L. R., Bonewald L. F., White K. E. (2009) Molecular analysis of DMP1 mutants causing autosomal recessive hypophosphatemic rickets. Bone 44, 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turan S., Aydin C., Bereket A., Akcay T., Güran T., Yaralioglu B. A., Bastepe M., Jüppner H. (2010) Identification of a novel dentin matrix protein-1 (DMP-1) mutation and dental anomalies in a kindred with autosomal recessive hypophosphatemia. Bone 46, 402–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koshida R., Yamaguchi H., Yamasaki K., Tsuchimochi W., Yonekawa T., Nakazato M. (2010) A novel nonsense mutation in the DMP1 gene in a Japanese family with autosomal recessive hypophosphatemic rickets. J. Bone Miner Metab. 28, 585–590 [DOI] [PubMed] [Google Scholar]
- 19. Mäkitie O., Pereira R. C., Kaitila I., Turan S., Bastepe M., Laine T., Kröger H., Cole W. G., Jüppner H. (2010) Long-term clinical outcome and carrier phenotype in autosomal recessive hypophosphatemia caused by a novel DMP1 mutation. J. Bone Miner Res. 25, 2165–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu Y., Qin C., Xie Y., Bonewald L. F., Feng J. Q. (2009) Studies of the DMP1 57-kDa functional domain both in vivo and in vitro. Cell Tissues Organs 189, 175–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu Y., Yuan B., Qin C., Cao Z., Xie Y., Dallas S. L., McKee M. D., Drezner M. K., Bonewald L. F., Feng J. Q. (2011) The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa C-terminal fragment. J. Bone Miner Res. 26, 331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qin C., Baba O., Butler W. T. (2004) Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit. Rev. Oral Biol. Med. 15, 126–136 [DOI] [PubMed] [Google Scholar]
- 23. Qin C., Brunn J. C., Cook R. G., Orkiszewski R. S., Malone J. P., Veis A., Butler W. T. (2003) Evidence for the proteolytic processing of dentin matrix protein 1: Identification and characterization of processed fragments and cleavage sites. J. Biol. Chem. 278, 34700–34708 [DOI] [PubMed] [Google Scholar]
- 24. Sun Y., Prasad M., Gao T., Wang X., Zhu Q., D'Souza R., Feng J. Q., Qin C. (2010) Failure to process dentin matrix protein 1 (DMP1) into fragments leads to its loss of function in osteogenesis. J. Biol. Chem. 285, 31713–31722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He G., Dahl T., Veis A., George A. (2003) Dentin matrix protein 1 initiates hydroxyapatite formation in vitro. Connect. Tissue Res. 44, 240–245 [PubMed] [Google Scholar]
- 26. Tartaix P. H., Doulaverakis M., George A., Fisher L. W., Butler W. T., Qin C., Salih E., Tan M., Fujimoto Y., Spevak L., Boskey A. L. (2004) In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J. Biol. Chem. 279, 18115–18120 [DOI] [PubMed] [Google Scholar]
- 27. Wu H., Teng P. N., Jayaraman T., Onishi S., Li J., Bannon L., Huang H., Close J., Sfeir C. (2011) Dentin matrix protein 1 (DMP1) signals via cell surface integrin. J. Biol. Chem. 286, 29462–29469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eapen A., Ramachandran A., Pratap J., George A. (2011) Activation of the ERK1/2 mitogen-activated protein kinase cascade by dentin matrix protein 1 promotes osteoblast differentiation. Cells Tissues Organs 194, 255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang B., Cao Z., Lu Y., Janik C., Lauziere S., Xie Y., Poliard A., Qin C., Ward L. M., Feng J. Q. (2010) DMP1 C-terminal mutant mice recapture the human ARHR tooth phenotype. J. Bone Miner. Res. 25, 2155–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Narayanan K., Ramachandran A., Hao J., He G., Park K. W., Cho M., George A. (2003) Dual functional roles of dentin matrix protein 1: Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J. Biol. Chem. 278, 17500–17508 [DOI] [PubMed] [Google Scholar]
- 31. Ravindran S., Narayanan K., Eapen A. S., Hao J., Ramachandran A., Blond S., George A. (2008) Endoplasmic reticulum chaperone protein GRP-78 mediates endocytosis of dentin matrix protein 1. J. Biol. Chem. 283, 29658–29670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maciejewska I., Qin D., Huang B., Sun Y., Mues G., Svoboda K., Bonewald L., Butler W. T., Feng J. Q., Qin C. (2009) Distinct compartmentalization of dentin matrix protein 1 fragments in mineralized tissues and cells. Cells Tissues Organs 189, 186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siyam A., Wang S., Qin C., Mues G., Stevens R., D'Souza R. N., Lu Y. (2012) Nuclear localization of DMP1 proteins suggests a role in intracellular signaling. Biochem. Biophys. Res. Commun. 424, 641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang R., Lu Y., Ye L., Yuan B., Yu S., Qin C., Xie Y., Gao T., Drezner M. K., Bonewald L. F., Feng J. Q. (2011) Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J. Bone Miner. Res. 26, 1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Braut A., Kalajzic I., Kalajzic Z., Rowe D. W., Kollar E. J., Mina M. (2002) Col1a1-GFP transgene expression in developing incisors. Connect. Tissue Res. 43, 216–219 [DOI] [PubMed] [Google Scholar]
- 36. Feng J. Q., Huang H., Lu Y., Ye L., Xie Y., Tsutsui T. W., Kunieda T., Castranio T., Scott G., Bonewald L. B., Mishina Y. (2003) The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J. Dent. Res. 82, 776–780 [DOI] [PubMed] [Google Scholar]
- 37. Zhang K., Barragan-Adjemian C., Ye L., Kotha S., Dallas M., Lu Y., Zhao S., Harris M., Harris S. E., Feng J. Q., Bonewald L. F. (2006) E11/gp38 selective expression in osteocytes: Regulation by mechanical strain and role in dendrite elongation. Mol. Cell Biol. 26, 4539–4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Corsi A., Riminucci M., Fisher L. W., Bianco P. (2001) Achondrogenesis type IB: agenesis of cartilage interterritorial matrix as the link between gene defect and pathological skeletal phenotype. Arch. Pathol. Lab. Med. 125, 1375–1378 [DOI] [PubMed] [Google Scholar]
- 39. Sato S., Kimura A., Ozdemir J., Asou Y., Miyazaki M., Jinno T., Ae K., Liu X., Osaki M., Takeuchi Y., Fukumoto S., Kawaguchi H., Haro H., Shinomiya K., Karsenty G., Takeda S. (2008) The distinct role of the Runx proteins in chondrocyte differentiation and intervertebral disc degeneration: findings in murine models and in human disease. Arthritis Rheum. 58, 2764–2775 [DOI] [PubMed] [Google Scholar]
- 40. Ye L., MacDougall M., Zhang S., Xie Y., Zhang J., Li Z., Lu Y., Mishina Y., Feng J. Q. (2004) Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J. Biol. Chem. 279, 19141–19148 [DOI] [PubMed] [Google Scholar]
- 41. Lu Y., Ye L., Yu S., Zhang S., Xie Y., McKee M. D., Li Y. C., Kong J., Eick J. D., Dallas S. L., Feng J. Q. (2007) Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev. Biol. 303, 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toyosawa S., Shintani S., Fujiwara T., Ooshima T., Sato A., Ijuhin N., Komori T. (2001) Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J. Bone Miner. Res. 16, 2017–2026 [DOI] [PubMed] [Google Scholar]
- 43. Barron M. J., Tsai C. J., Donahue S. W. (2010) Mechanical stimulation mediates gene expression in MC3T3 osteoblastic cells differently in 2D and 3D environments. J. Biomech. Eng. 132, 041005 [DOI] [PubMed] [Google Scholar]
- 44. Huang B., Maciejewska I., Sun Y., Peng T., Qin D., Lu Y., Bonewald L., Butler W. T., Feng J., Qin C. (2008) Identification of full-length dentin matrix protein 1 in dentin and bone. Calcif. Tissue Int. 82, 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sitara D., Kim S., Razzaque M. S., Bergwitz C., Taguchi T., Schüler C., Erben R. G., Lanske B. (2008) Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 4, e1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ye L., Mishina Y., Chen D., Huang H., Dallas S. L., Dallas M. R., Sivakumar P., Kunieda T., Tsutsui T. W., Boskey A., Bonewald L. F., Feng J. Q. (2005) Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J. Biol. Chem. 280, 6197–6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burra S., Nicolella D. P., Francis W. L., Freitas C. J., Mueschke N. J., Poole K., Jiang J. X. (2010) Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc. Natl. Acad. Sci. U.S.A. 107, 13648–13653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun Y., Chen L., Ma S., Zhou J., Zhang H., Feng J. Q., Qin C. (2011) Roles of DMP1 processing in osteogenesis, dentinogenesis and chondrogenesis. Cells Tissues Organs 194, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sun Y., Lu Y., Chen L., Gao T., D'Souza R., Feng J. Q., Qin C. (2011) DMP1 processing is essential to dentin and jaw formation. J. Dent. Res. 90, 619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]