Background: CatK plays a pivotal role in osteoclastic bone resorption.
Results: Autoprocessing of pro-CatK is enhanced by chondroitin sulfate (CS), and their co-localization is found within the ruffled border of osteoclasts.
Conclusion: Association with CS promotes autoactivation of CatK.
Significance: Understanding the mechanisms of CatK activation in osteoclasts may provide alternative designs of inhibitors for the treatment of osteoporosis.
Keywords: Bone; Chondroitin Sulfate; Osteoarthritis; Osteocyte; Osteoporosis; Cathepsin K; Activation; Collagenase; Osteoclast; Peptidase,
Abstract
Cathepsin K (CatK), a major lysosomal collagenase produced by osteoclasts, plays an important role in bone resorption. Evidence exists that the collagenase activity of CatK is promoted by chondroitin sulfate (CS), a sulfated glycosaminoglycan. This study examines the role of CS in facilitating CatK activation. We have demonstrated that chondroitin 4-sulfate (C4-S) promotes autoprocessing of the pro-domain of CatK at pH ≤ 5, leading to a fully matured enzyme with collagenase and peptidase activities. We present evidence to demonstrate this autoactivation process is a trans-activation event that is efficiently inhibited by both the covalent cysteine protease inhibitor E-64 and the reversible selective CatK inhibitor L-006,235. During bone resorption, CatK and C4-S are co-localized at the ruffled border between osteoclast bone interface, supporting the proposal that CatK activation is accomplished through the combined action of the acidic environment together with the presence of a high concentration of C4-S. Formation of a multimeric complex between C4-S and pro-CatK has been speculated to accelerate CatK autoactivation and promote efficient collagen degradation. Together, these results demonstrate that CS plays an important role in contributing to the enhanced efficiency of CatK collagenase activity in vivo.
Introduction
Cathepsin K (CatK)2 is a papain-like cysteine peptidase and the major collagenase produced in osteoclasts (1–3). It plays a pivotal role in bone resorption by removing demineralized collagen (2, 4–6). A balance between osteoclast activity and osteoblast activity is required to maintain bone mass. Excessive osteoclast activity that tilts the balance toward higher levels of bone resorption compared with bone formation has been implicated as the primary cause of osteoporosis (7). Evidence for the importance of CatK in osteoporosis is derived from the observation that: 1) disruption of CatK in mice leads to osteopetrosis (8–10), and 2) a human genetic defect of CatK function known as pycnodysostosis, a rare autosomal recessive osteosclerotic skeletal dysplasia, is associated with impaired osteoclastic bone resorption (11, 12). Conversely, transgenic mice that overexpress CatK exhibited reduced bone mass as a result of accelerated bone resorption (13).
CatK is unique among mammalian collagenases in that it is capable of efficiently cleaving the triple-helical regions of collagen as well as degrading the telopeptides. This enzyme is a highly active protease that is capable of catabolizing itself within minutes under physiological conditions (14, 15). However, the exact mechanism regulating CatK activation and stabilization during cellular function has not been fully elucidated. Cysteine proteases are expressed as inactive proenzymes that undergo activation by cleavage the pro-domain under acidic condition (pH 4.0–4.5) and release the catalytically active protease domain (16, 17). Structurally, human CatK (a 330-amino acid protein) is organized into an N-terminal signal peptide, followed by a pro-domain, and lastly a protease domain (18). A crystal structure of pro-CatK depicts the pro-domain making contacts with the active site of the enzyme to presumably prevent access of substrates in an autoinhibitory conformation (18). Bossard et al. (19) demonstrated autoactivation of pro-CatK in vitro by briefly incubating the pro-enzyme at high temperatures or by adding a small amount of preactivated CatK at 4 °C for 24 h. These conditions was also reported by McQueney et al. (15). Brömme et al. (20) showed that in vitro processing of pro-CatK is successful in the presence of pepsin. Although these reports provided valuable insights into the activation process for CatK in vitro, neither of these conditions closely mimic the local environment present for physiological activation.
Directional secretion toward bone surface is robust in osteoclasts during the resorption cycle, leading to formation of the ruffled border (21). Because of the ability of the cells to actively secrete acid and lysosomal contents to form the resorption lacunae, this compartment is occasionally referred to as an extracellular lysosome of an osteoclast (21). CatK is the predominant lysosomal protease that represents 98% of total cysteine protease activity in osteoclasts (22). Activation of CatK in vivo has been suggested to likely occur in the low pH environment of the resorption lacunae (15). The propeptide of CatK has been suggested to be cleaved by other peptidases, particularly the aspartic cathepsin D or by an autocatalytic process (22). In osteoclasts, other cathepsins are relatively minor lysosomal components compared with CatK (23). Gene deletion of cathepsin D, B, or L does not lead to defective processing of pro-CatK in osteoclasts, suggesting that these peptidases may not play a pivotal role in cleavage of pro-CatK. Moreover, cathepsin D is not co-localized with CatK in the same lysosomal vesicles in osteoclasts (21). Because the high demand for CatK collagenase activity for degradation of the bulk demineralized collagen type I, activation of CatK activity likely occurs in proximity to the proteoglycan-collagen fibril bundles within the resorption lacunae (24).
Chondroitin sulfate (CS) is a sulfated glycosaminoglycan composed of alternating sugar moieties N-acetylgalactosamine and glucuronic acid. The CS molecule carries biological information by interacting with a variety of molecules, such as growth factors, cytokines, chemokines, adhesion molecules, and lipoproteins and thus plays an important role in cell growth and neuronal development (25, 26). It also possesses important biological properties for tissue integration, such as anti-inflammatory activity and nutrient absorption (25, 26). The sulfated linear polysaccharide chondroitin 4-sulfate (C4-S) is present in bone and is abundantly detected in articular cartilage (27, 28). C4-S has recently been demonstrated to directly interact with mature CatK and to promote its triple-helical collagenase activity (29, 30). A model of beads on a string has been proposed to represent the complex formation with high molecular mass between multiple CatK molecules and a single strand of C4-S (31).
Numerous in vitro studies have demonstrated that activated CatK inefficiently degrades collagen in solution, requiring several hours for ∼2 μm of activated CatK at the optimal acidic pH to digest just a few micromolar concentration of collagen type I (2, 32, 33). This apparently weak collagenase activity of CatK in vitro is not consistent with the efficient removal of bulk collagen during osteoclastic bone resorption, suggesting that other factors may enhance the collagenase activity of CatK. The formation of a complex between mature CatK and CS, thereby increasing the proximity of substrate to enzyme, may explain the enhanced efficiency of CatK collagenase activity (24). In this study, we examined the role of C4-S in the early step of CatK activation, i.e. the autoprocessing of pro-CatK to mature CatK. We demonstrated that CS promotes a pH-dependent autoprocessing of pro-CatK, and the resultant matured enzyme exhibits both peptidase and collagenase activities. In addition, abundant amounts of C4-S and CatK are co-localized in the ruffled border of actively resorbing osteoclasts, providing the optimal environment to promote CatK activation.
EXPERIMENTAL PROCEDURES
Materials
All reagents used in this research were of analytical grade. Chondroitin 4-sulfate was purchased from W&J PharmaChem, Inc. (Silver Spring, MD). Human recombinant mature CatK (hCatK) was from Enzo Life Sciences (Farmingdale, NY), and pro-hCatK was from EMD Millipore Corporation (Billerica, MA). Type I collagen from calf skin was from USB Corporation (Cleveland, OH).
Expression and Purification of pro-hrCatK Proteins
Because of limitation of access to the human CatK in our screening program for CatK inhibitors, three human mutations (S163A, Y175D, and V274L) were substituted into the catalytic pocket of the rabbit CatK gene (NCBI reference sequence accession number NM_000396.3) to produce humanized rabbit (hr) CatK, which has overall 94.5% amino acids conserved and identical active site as compared with hCatK. Based on results from the internal screening database of small molecular mass inhibitors of CatK and crystallographic studies, hrCatK displays almost identical catalytic activity as the human enzyme. The full-length pro-hrCatK gene was then cloned into pTT3 vector and transfected into HEK293 cells. Expression vectors for mutant proteins were obtained by site-directed mutation of the wild type gene. The target protein was expressed and secreted into the culture medium within 72 h under standard conditions. Cells were harvested, and the culture medium was concentrated and exchanged into 20 mm sodium phosphate buffer (pH 6.8) to obtain unactivated pro-CatK (pro-hrCatK), or into 50 mm sodium acetate (pH 4.0) containing 2.5 mm EDTA to obtain activated or matured enzyme (hrCatK). In the later case, pro-hrCatK was allowed to process into the matured form before proceeding into purification. The protein was then purified by cation exchange chromatography with NaCl gradient elution. Purity was enhanced further by Superdex 200HR size exclusion chromatography into PBS (pH 7.4) for hrCatK or 50 mm sodium acetate, pH 4.0, 2.5 mm EDTA, 20 mm l-cysteine, 0.5 m NaCl for hrCatK.
Purification of Chondroitin 4-Sulfate
A ∼17-kDa C4-S was purified using methods described by Li et al. (31) without hyaluronidase added. This condition yielded a sufficient amount of ∼17-kDa C4-S fragments as needed. Approximately 600 mg of powdered C4-S was dissolved in 100 mm acetate buffer (pH 5.5) and then incubated overnight at 37 °C. The next day, the mixture was fractionated on HiPrep 16/60 Sephacryl S-200 HR (GE Healthcare), and the size distribution was monitored by dynamic light scattering using the DynaPRO plate reader (Wyatt Technologies). Fractions containing ∼17-kDa C4-S were pooled, concentrated ∼10-fold using Amicon Ultra centrifugal filter units with 5-kDa molecular mass cutoff. Concentration was estimated by UV-visible measurements using a stock of known concentration as standard. Aliquots were frozen at −20 °C.
Autoprocessing of pro-CatK
Wild type or mutant CatK enzyme (∼4 μm or otherwise indicated) was incubated at 37 °C with or without C4-S (18 μm) for the duration indicated, and the reaction was quenched with 1.4 mm E-64. The protein was then analyzed by SDS-PAGE using 16% Tris-glycine (Invitrogen), and the intensity of desired bands was quantified using Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE) whenever necessary.
Peptidase Activity after Autoprocessing
CatK peptidase activity was measured using the fluorogenic substrate Z-Leu-Arg-MCA from Bachem (Torrance, CA). After 4 μm of pro-hrCatK was allowed to undergo autoprocessing for the duration indicated, 2 μl of the reaction mixture was diluted with 3 ml of assay buffer (50 mm MES, pH 5.5, 10% DMSO, 2.5 mm DTT, 2.5 mm EDTA). The enzyme mixture was added 1:1 to 8 μm of Z-Leu-Arg-MCA, and the final reaction mixture was incubated at 25 °C for 1 h. Fluorescence emission measurements were taken at 460 nm upon excitation at 355 nm using the Wallac Victor 1420 Multilabel counter (PerkinElmer Life Sciences). The data were analyzed using Microsoft Excel software.
Collagenase Activity after Autoprocessing
Approximately 90% of the autoprocessing reaction mixture (containing < 4 μm of mature or pro-hrCatK) was mixed with 5 μg of soluble calf skin type I collagen in 100 mm sodium acetate buffer (pH 5.5) containing 2.5 mm DTT and 2.5 mm EDTA, and the mixture was incubated at 37 °C for 2 h unless otherwise stated. Samples were analyzed by SDS-PAGE using 16% Tris-glycine gels (Invitrogen) and Coomassie Blue staining.
Self-association of pro-hrCatK
Protein stock in PBS was concentrated ∼5-fold using Amicon Ultra centrifugal filter units with 10-kDa molecular mass cutoff (EMD Millipore Corporation). The resultant protein solution was then centrifuged at 14,000 rpm for 2 min to remove any aggregates. Concentration of the protein was estimated by UV-visible measurement using predicted molar extinction coefficient of the protein. Dynamic light scattering measurements were taken on the stock protein as well as 2-fold serial dilutions made on ice using DynaPro NanoStar (Wyatt Technology Corporation, CA). For dynamic light scattering measurements in different buffers, the stock protein was exchanged into respective buffers using protein desalting spin columns (Thermo Scientific, Rockford, IL). The resultant protein solution was then centrifuged at 14,000 rpm for 2 min to remove any aggregates, and the concentration was estimated as described above. All data were analyzed by Dynamics software.
Fluorescence Measurements of Conformational Change
Intrinsic fluorescence measurements of 1 μm pro-hrCatK (with or without C4-S) in acetate buffer at pH 5.0 (containing 100 mm NaCl and 1 mm CaCl2) were taken using FluoroMax4 channel cuvette system or the MicroMax 384 plate reader (Horiba Scientific, Edison, NJ). To avoid autoprocessing of the enzyme during titration, the measurements were taken at ∼22 °C. When using the micro cuvette system (250-μl capacity), readings were taken 2 min after mixing reagents to allow time for equilibration. Fluorescence measurements were corrected for absorption and emission contribution from buffer and reagents other than the fluorophore of interest.
Immunofluorescent Staining
Human osteoclast (OC) precursors at 2.5 × 103 cells/cm2 (Poietics, Lonza, Walkersville, MD) were cultured on Petri dishes with macrophage colony-stimulating factor and RANKL according to the manufacturer's protocol. After 5–6 days, matured human OC were harvested with 0.05% Trypsin/EDTA, seeded on bovine cortical bone slices for 2 days, and fixed in 4% paraformaldehyde (5). OC were incubated with either anti-human CatK mAb (EMD Millipore) or anti-C4-S mAb (EMD Millipore) for 1 h, followed with Alex Fluor 488 F(ab′)2 goat anti-mouse IgG (Invitrogen) for 30 min, and then co-stained with Alexa Fluor 568 Phalloidin (Invitrogen) for 30 min. For C4-S staining, cells were pretreated with 0.5 unit/ml of chondroitinase ABC (Sigma) for 30 min at room temperature before immunostaining. OC were viewed under Nikon confocal microscopy with a 60× oil objective. The confocal images of cells were Z-scanned and viewed by Velocity software (PerkinElmer Life Sciences).
RESULTS
Effect of Chondroitin Sulfate on Autoprocessing of pro-CatK
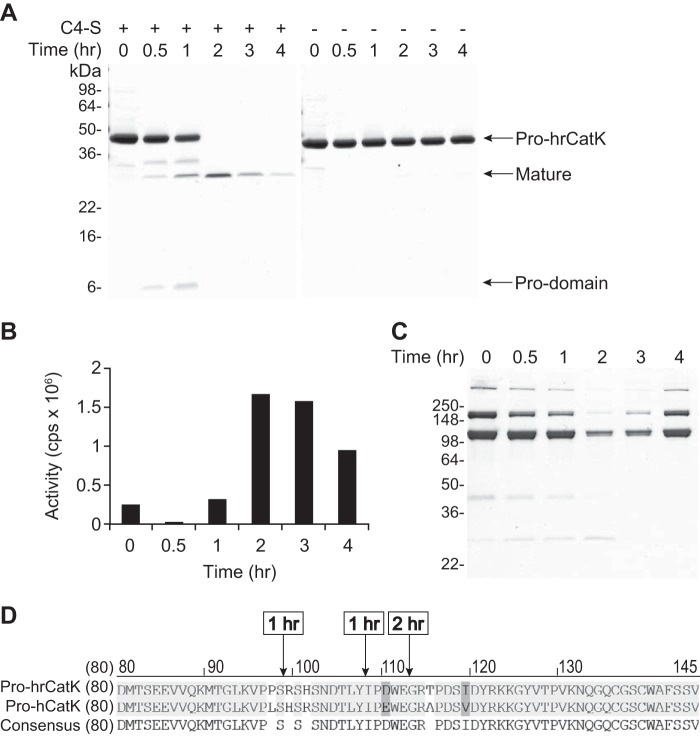
Because autoactivation of CatK has been reported to occur in acidic condition (pH ≤ 4.0) (26–28), we incubated pro-hrCatK in 100 mm of buffers with pH ranging from 4.0 to 7.5 at 37 °C for 6 h. Although processing of pro-hrCatK was observed only at pH 4.0 in the absence of C4-S, this was shifted to pH 5.0 in the presence of C4-S (Fig. 1A). To ensure that the observed processing is specific to CatK and not from contaminating proteases in the preparation of the recombinant enzyme, we compared autoprocessing of WT pro-hrCatK to the pro forms of human CatK (pro-hCatK) or of the mutant hrCatK(C139G), a mutant with substitution of the cysteine in the catalytic triad to glycine and the pycnodysostotic mutant hrCatK(Y212C). All recombinant pro-CatK proteins were purified using the same method. As shown in Fig. 1B, C4-S promotes processing of the pro-forms of hrCatK WT and hCatK at pH 5.0, but not the C139G mutant. Interestingly, the Y212C mutation, being remote from the active site (34), did not show any significant autoprocessing even in the presence of C4-S. Note that no accumulation of mature CatK was observed that was more pronounced with decreasing pH, because of the fully processed CatK also undergoing autoproteolysis. We also tested different sources of chondroitin sulfate containing type A, C, or D in singles or in mixtures or different lengths of sugar moieties and found these CS forms activated pro-hrCatK at similar rates (data not shown).
FIGURE 1.
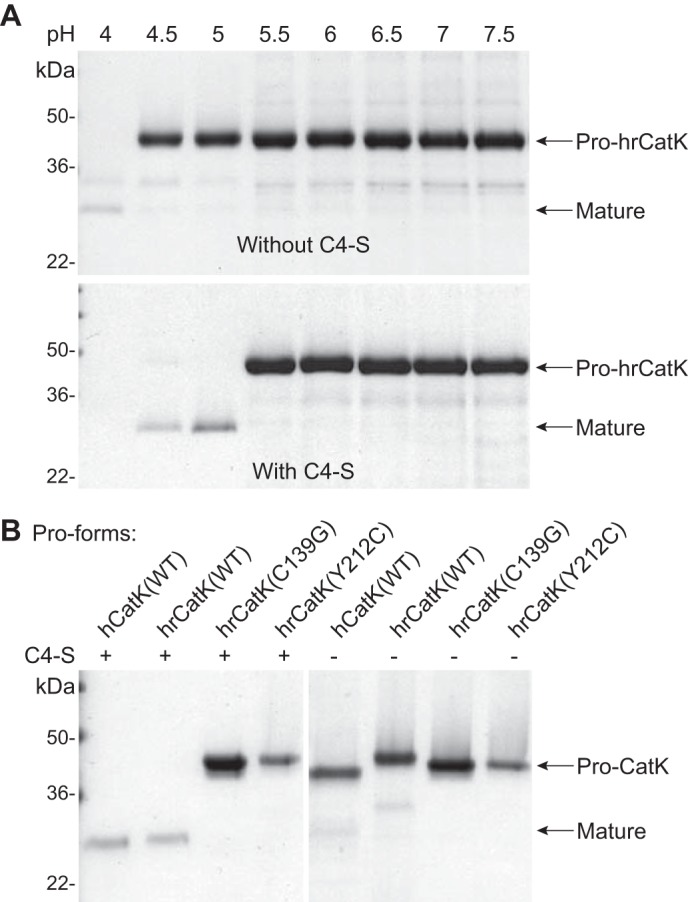
Autoprocessing of cathepsin K is promoted by acidic pH and chondroitin 4-sulfate. A, cleavage of the pro-domain from pro-hrCatK (4 μm) to mature enzymes was evaluated in buffers with pH ranging from 4.0 to 7.5 without (top panel) and with (bottom panel) 18 μm C4-S. The reaction mixture was incubated at 37 °C for 3 h in 100 mm of pH buffers containing 100 mm NaCl and 1 mm CaCl2. B, processing of the pro-domain from wild type pro-hrCatK(WT) was compared with that from the pro-forms of hrCatK mutants (as described under “Experimental Procedures”) and wild type human pro-CatK (pro-hCatK(WT)) at pH 5.0.
Peptidase and Collagenase Activity of Autoprocessed pro-hrCatK
Autoprocessing of hrCatK was further investigated at pH 5.0 to determine whether it leads to activation of the enzyme. As shown in Fig. 2A, autoprocessing occurred over time only in the presence of C4-S and appeared to reach completion within 2 h at 37 °C. After this time point, the intensity of the matured hrCatK band (labeled activated) decreased with time, supporting autoproteolysis of the matured enzyme continued post activation of the enzyme. The time course of autoprocessing correlated well with increases in peptidase activity (Fig. 2B) and collagenase activity (Fig. 2C). The highest peptidase and collagenase activities were observed at 2 h of autoprocessing reaction when the maximal amount of matured enzyme was detected and also when the pro-domain was completely removed. The observed proteolytic activities also decreased, correlating with the time course of autoproteolysis of the matured enzyme. The results, as summarized in Fig. 2, together confirmed that the observed autoprocessing of the pro-CatK led to activation of its catalytic activity.
FIGURE 2.
Peptidase and collagenase activity of autoactivated cathepsin K. A, time-dependent autoprocessing of pro-hrCatK (4 μm) to mature hrCatK in the presence (+) or absence (−) of 18 μm C4-S at pH 5.0. The volumes from reactions in A were transferred to the subsequent assays. B and C, peptidase activity (B) and collagenase activity (C) of the activated hrCatK enzyme produced in A in a time-dependent manner were demonstrated as described under “Experimental Procedures.” D, sequence mapping to identify N termini of products from autoprocessing of pro-hrCatK (4 μm) after 1 and 2 h of incubation at 37 °C in the presence of C4-S at pH 5.0.
N-terminal sequencing showed that autoactivation occurred via intermediates (Fig. 2D). At 1 h of incubation, autoprocessing of pro-hrCatK resulted in a mixture of an intermediate with Arg-99 as its N terminus and a partially matured enzyme with Ile-108 as its N terminus. When the highest peptidase and collagenase activities are detected at 2 h, a mixture of products with Gly-113 and Arg-114 N termini was seen that corresponded to the previously reported forms of fully matured CatK enzyme (15). Of note, Arg-99 amino acid residue is just outside the active site cleft-binding segment (Lys-89–Pro-96) and belongs to the C-terminal segment (Pro-97–Arg-114) of the pro-domain (18). We also found that the pro-domain fragment that migrated to ∼6 kDa on the SDS-PAGE, had residue Leu-1 as its N terminus. Meanwhile the pro-domain cleaved at Arg-99 (11.5 kDa) migrated to ∼13 kDa on SDS-PAGE gel (35). Hence, the proteolytic activity that took place within the first 2 h of the autoactivation reaction occurred sequentially at multiple sites within the pro-domain.
The observation that activity of the hrCatK was optimal after 2 h of autoprocessing underscored the importance of removing residues Ile-108–Glu-112 to achieve a fully functional matured CatK enzyme. Also, N-terminal sequencing of pro-forms of hrCatK, ratCatK, and hCatK after incubation at pH 4.0 with or without C4-S showed a mixture of Gly-113 and Arg-114 N termini. These proteins showed both peptidase and collagenase activities, confirming that they became fully matured (data not shown). Hence, we demonstrated that autoprocessing of pro-CatK occurring at pH ≤ 5.0 resulted in a matured enzyme capable of degrading collagen.
Inhibition of Autoactivation by Cathepsin K-specific Inhibitors
Next, the specificity of the autoactivation of pro-CatK was investigated further at pH 5.0 using a previously reported specific, reversible inhibitor of CatK, L-006,235 (L-235) (36) and the irreversible cysteine protease inhibitor E-64. The inhibitors L-235 and E-64 showed somewhat different modes of inhibition of pro-hrCatK autoactivation with the former displaying a stronger potency than the latter (Fig. 3). L-235 at 0.1 nm significantly inhibited the formation of both intermediates and matured hrCatK and achieved complete inhibition at 10 μm (Fig. 3A). Meanwhile, E-64 weakly inhibited the generation of both intermediates and matured hrCatK up to 100 nm, where the degree of inhibition was comparable with that with 0.1 nm L-235 (Fig. 3B). E-64 showed complete inhibition at 25 μm (data not shown). We previously demonstrated that L-235 is a potent and selective inhibitor of mature hrCatK with a Ki of ∼0.2 nm (36), and it binds specifically to the active site of mature hrCatK via x-ray crystallography (data not shown). Here, inhibition of autoactivation was achieved at substoichiometric concentrations of either inhibitor. Both fluorescence intensity and anisotropy measurements demonstrated that L-235 bound to preactivated hrCatK (hrCatK-Act) with a Kd of ∼50 nm but did not show any significant binding to the pro-enzyme (data not shown). Hence, it is likely that low concentrations of the inhibitors were sufficient to inactivate a small fraction of the preactivated hrCatK during protein preparation, which was responsible for initiating the autoactivation process.
FIGURE 3.
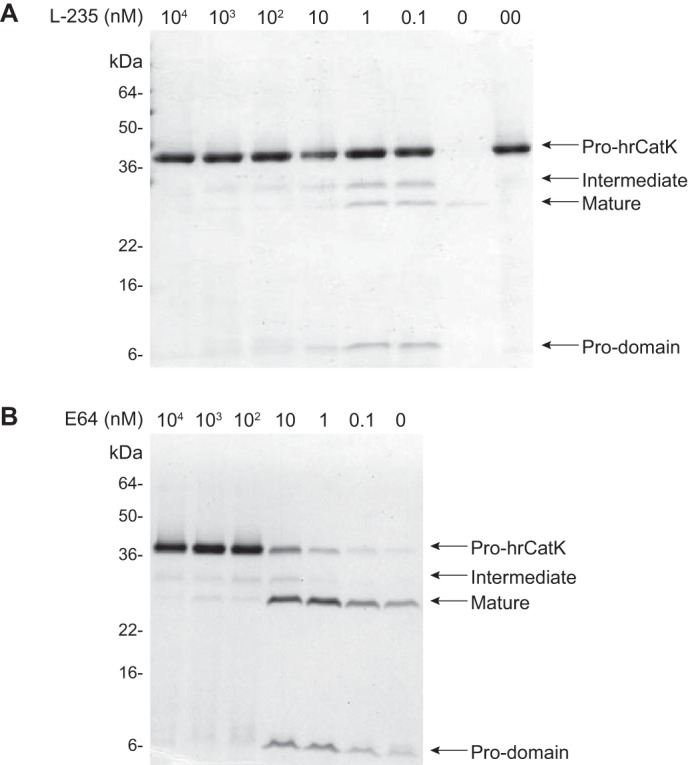
Inhibition of autoactivation by cathepsin K specific inhibitors. Inhibition of processing of pro-hrCatK (4 μm) was demonstrated by varied concentrations of the reversible CatK specific inhibitor L-235 (A) and covalent cathepsin inhibitor E-64 (B). Reaction mixture containing 18 μm C4-S was incubated at 37 °C for 6 h. Lane 0, pro-hrCatK without L-235 after incubation at 37 °C; lane 00, pro-hrCatK without L-235, stored at 0 °C prior processed for Western blot.
Effects of Complex Formation on Autoactivation of pro-hrCatK
To understand whether self-association of pro-hrCatK molecules is the main driver of autoactivation, we examined the oligomeric state of the unactivated pro-enzyme in its storage buffer (PBS) and at pH 4 where both C4-S-dependent and C4-S-independent autoactivation was observed (Fig. 4). Note that the molecular masses of complexes were estimated by dynamic light scattering measurements correcting for solvent effect. Although pro-hrCatK showed a concentration-dependent increase in molecular mass to ∼170 kDa consistent with formation of an octamer in PBS, it showed a much weaker self-association to ∼70 kDa consistent with a dimer in acetate buffer at pH 4.0 (Fig. 4A). When salt 100 mm NaCl and 1 mm CaCl2 was added to this buffer at pH 4.0, self-association was eliminated, suggesting that it was mediated by ionic interactions (Fig. 4A). Moreover, self-association of pro-hrCatK molecules alone did not necessarily promote enzyme activation because we did not see autoprocessing in PBS at neutral pH. As shown in Fig. 4B, the addition of a salt increased the rate of autoactivation of pro-hrCatK by ∼3-fold (2 pmol/min in the absence of C4-S versus 6 pmol/min in the presence of C4-S). On the other hand, C4-S by itself already increased the rate of pro-hrCatK autoactivation by ∼10-fold, and the addition of salt did not change this rate (23 pmol/min with C4-S alone, compared with 20 pmol/min with C4-S and salt) (Fig. 4B).
FIGURE 4.
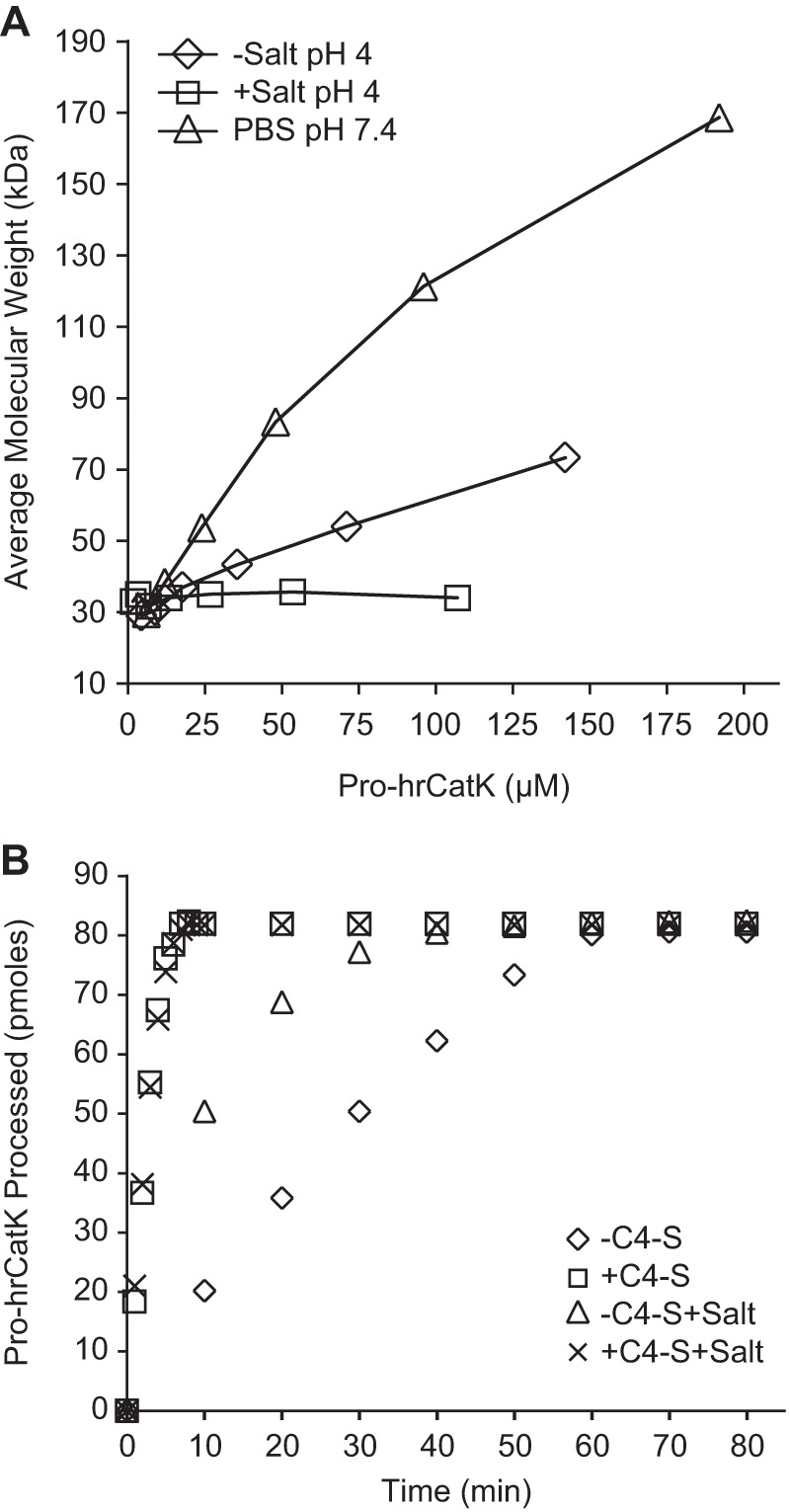
Effects of complex formation on autoactivation of cathepsin K. A, self-association of pro-hrCatK(WT) measured by dynamic light scattering in PBS at pH 7.4 compared with that in 100 mm sodium acetate at pH 4.0 with (+) or without (−) salt (100 mm NaCl and 1 mm CaCl2). B, effect of salt condition and C4-S (18 μm) on the rate of autoactivation of pro-hrCatK (4 μm) at pH 4.0. Activity was measured by scanning densitometry of the remained “unactivated” pro-hrCatK bands on SDS-PAGE gel at a given duration of the reaction. The rates of autoactivation were then estimated from the slope of the early phase of the reaction when only ∼20% of substrate converted to product.
Effect of C4-S on the Conformation of pro-hrCatK
Because structural studies have shown that residues Lys-89–Pro-96 of the pro-domain of CatK interact with the active site in an apparent autoinhibition conformation (18), we investigated whether C4-S can induce a conformational change in pro-CatK that could expose its active site to substrates. There are eight tryptophan residues in pro-hrCatK: four located in the pro-domain and four located in the catalytic domain. The effect of C4-S on the conformational of CatK was measured by the intrinsic fluorescence of the tryptophan residues in pro-hrCatK. As shown in Fig. 5A, a gain in pro-hrCatK intrinsic fluorescence was detected as the concentration of C4-S increased (i.e. a decrease in the [pro-hrCatK]/[C4-S] ratio). The maximum gain in pro-hrCatK intrinsic fluorescence was observed at a [pro-hrCatK]/[C4-S] ratio of 20. Interestingly, a loss of pro-hrCatK intrinsic fluorescence was observed below a [pro-hrCatK]/[C4-S] ratio of 6 (Fig. 5B). We also examined whether the autoactivation is dependent on the stoichiometry of pro-hrCatK on C4-S. As shown in Fig. 5B, autoactivation of hrCatK remained at the same rate as the ratio of [pro-hrCatK]/[C4-S] was titrated up to 6. However, processing of pro-hrCatK to mature enzyme became less efficient because this ratio increased beyond 6. Thus, C4-S induces changes in intrinsic tryptophan fluorescence of pro-hrCatK at [pro-hrCatK]/[C4-S] ratios that enhance autoactivation.
FIGURE 5.
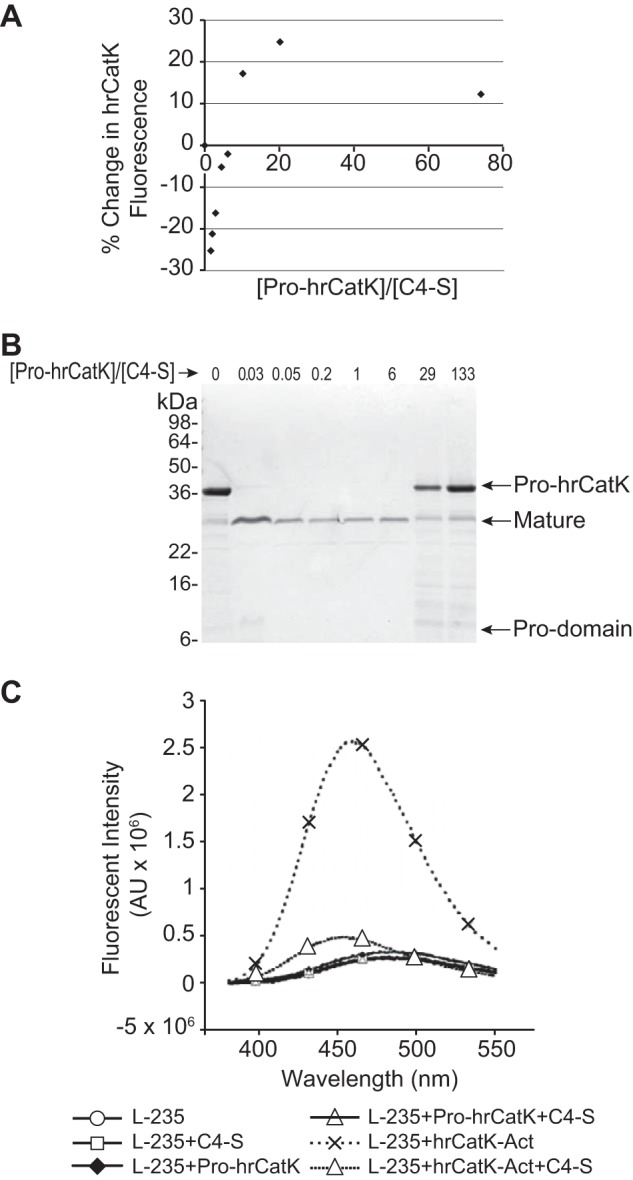
Effect of chondroitin 4-sulfate on the conformation of cathepsin K. A, stoichiometric-dependent changes in pro-hrCatK (1 μm) in the presence of C4-S was monitored by the intrinsic fluorescence of tryptophan moieties. Measurements were taken in acetate buffer at pH 5.0 and with salt (100 mm NaCl, 1 mm CaCl2) and at 22 °C where autoactivation is insignificant over the duration of titration and measurements. The reaction was mixed after each addition of C4-S and allowed to equilibrate for 2 min. B, stoichiometry-dependent autoactivation of 4 μm pro-hrCatK in the presence of C4-S. The reaction mixture was incubated at 37 °C for 6 h also in acetate buffer at pH 5.0 and salt. C, fluorescence changes of the CatK inhibitor L-235 (6 μm) in the presence of C4-S (6 μm) and pro-hrCatK (6 μm) or preactivated CatK (hrCatK-Act). Measurements were taken at 37 °C with excitation at 315 nm using a Micromax 384 plate reader.
We demonstrated above that L-235 inhibited the conversion of pro-hrCatK to mature enzyme in the presence of C4-S, suggesting that this inhibitor is capable of binding to activated enzyme in complex with C4-S (Fig. 3). Here, we relied on the autofluorescence property of L-235 (Emax = 315 nm) (37) to investigate whether complex formation of the pro-enzyme with C4-S induces a conformational change in CatK to open its active site for catalysis. As shown in Fig. 5C, L-235 binding to activated hrCatK resulted in ∼10-fold enhancement in its fluorescence emission intensity and a 25-nm blue shift of its Emax wavelength. However, in the presence of C4-S, we observed ∼6-fold loss of L-235 fluorescence and a further 6-nm blue shift in L-235 Emax, suggesting a significant change in the environment of L-235 in the active site of mature hrCatK induced by complex formation with C4-S. Note that C4-S alone did not affect the fluorescence emission of L-235. Unlike activated hrCatK, the fluorescence emission of L-235 increased very slightly in the presence of pro-CatK. When C4-S was introduced to pro-hrCatK in the presence of L-235, fluorescence emission reversed to that of L-235 alone. Because the propeptide blocks the active site of the pro-enzyme based on the published structure (18), it is thus not surprising that the active site of pro-hrCatK in the absence or presence of C4-S is not accessible to L-235. Hence, the observed slight loss of L-235 fluorescence when C4-S was introduced may be due to a small amount of preactivated hrCatK present in the preparation.
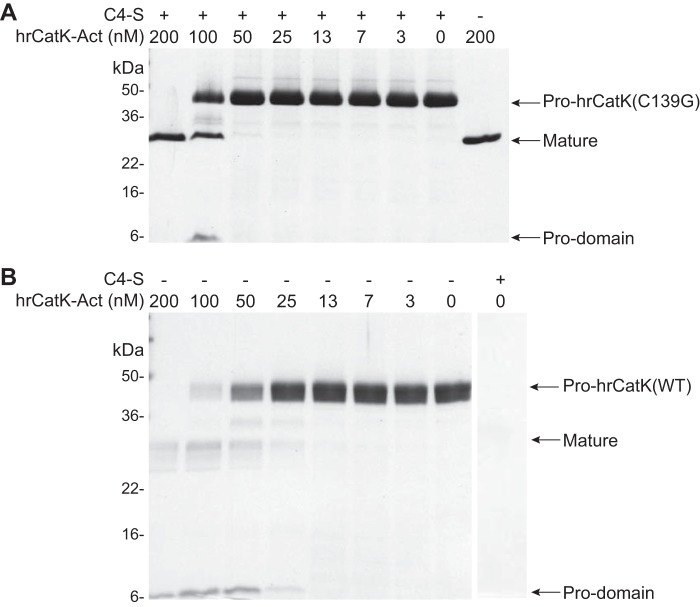
Contribution from Transmechanism to Autoactivation
We then examined whether the observed conformational change induced by C4-S could drive a trans-mechanism of autoprocessing. The catalytically inactive pro-hrCatK mutant C139G (4 μm) was incubated with increasing amounts of the hrCatK-Act at 37 °C for 6 h, and the cleavage of the pro-enzyme was monitored by SDS-PAGE. As shown in Fig. 6A, a significant level of processing of the pro-domain of hrCatK(C139G) occurred with 100 nm of hrCatK-Act, but complete processing of pro-hrCatK was achieved with 200 nm of hrCatK-Act. Interestingly, 200 nm of hrCatK-Act achieved the same level of processing of pro-hrCatK(C139G) in the absence of C4-S as in the presence of C4-S. This indicates that processing of the pro-domain occurred via direct trans-interaction of CatK molecules that was independent of C4-S. It should also be noted that ∼20% of the total band of processed protein on the gel was contributed by the activated enzyme. Hence, the continuing degradation of the processed hrCatK(C139G) was also via a trans-mechanism. In Fig. 6B, although significant processing of the pro-domain of hrCatK(WT) occurred with 50 nm of hrCatK-Act, the higher concentration of hrCatK-Act (200 nm) was still required to achieve complete processing of the pro-domain of hrCatK(WT). The subsequent degradation of the mature enzyme was more extensive with hrCatK(WT) than with hrCatK(C139G), suggesting that the processed hrCatK(WT) at any given stage of the reaction contributed to the active pool of enzyme. This finding also indicated that the observed processing by hrCatK-Act continuously generated activated hrCatK(WT).
FIGURE 6.
Autoprocessing of the pro-domains from wild type and mutant cathepsin K isoforms demonstrates trans-mechanism of cleavage. Processing of catalytically inactive mutant pro-hrCatK(C139G) (A) or pro-hrCatK(WT) (B) at 4 μm was demonstrated by incubating with increasing concentrations (0–200 nm) of the preactivated enzyme (hrCatK-Act) without (−) and with (+) 18 μm C4-S. The reaction mixture was incubated at 37 °C for 6 h.
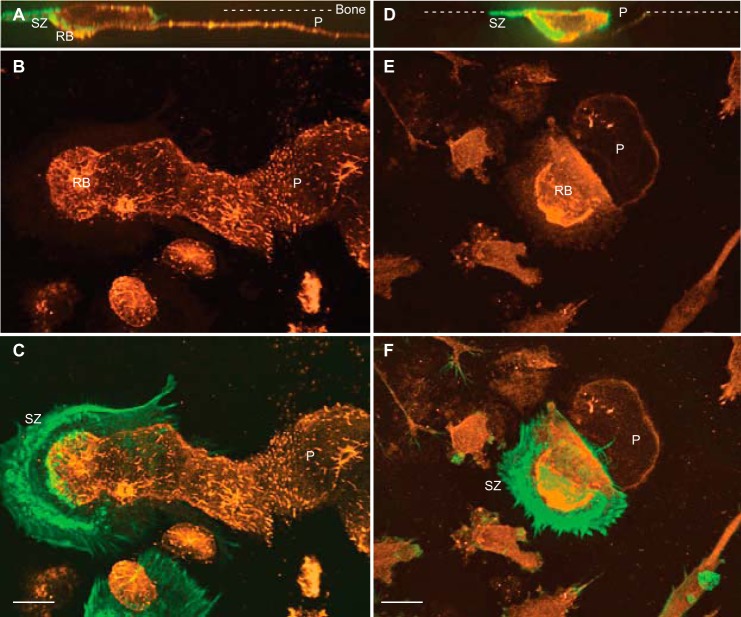
Co-localization of C4-S and Cathepsin K in Osteoclast Ruffled Border and Bone Resorption Lacunae
To investigate the localization of C4-S and CatK during osteoclastic bone resorption, mature human OC were cultured on bovine cortical bone slices for 2 days and followed by immunostaining separately with antibodies to C4-S (in orange in Fig. 7, A–C) or anti-CatK monoclonal antibody (in orange in Fig. 7, D–F). Note that the anti-CatK monoclonal antibody used in this study recognizes both pro- and mature CatK. Actively resorbing OCs were also identified by staining for the F-actin-rich sealing zone structure and the ruffled border (in green in Fig. 7). Under the same treatment with chondroitinase to enhance accessibility of C4-S antigenic site, confocal images showed that C4-S was essentially undetectable on nonresorbed cortical bone surface and was weakly detected in OC cytoplasm (Fig. 7, A–C). However, C4-S was detected highly at the ruffled border and became accessible on the surface of the resorption pits generated by these cells (Fig. 7, A–C). We purposely viewed an OC with a clear leading edge associated with formation of a sealing zone and a lifted trailing edge to view the newly formed resorption pit (Fig. 7, D–F). CatK was detected in intracellular punctate vesicles and clearly intensely localized in the ruffled border of osteoclasts. However, CatK was very weakly detected in the newly formed resorbed surface underneath the cell as compared with that in the ruffled border, suggesting that very little of immunodetectable intact CatK is left behind after the resorption cycle completed. Furthermore, the apparent co-localization of CatK to C4-S in the ruffled border within the sealing zone suggested that the resorption lacunae, i.e. low pH (5.0–5.5) and high concentration of C4-S and calcium salt, likely provide the optimal environment favoring autoactivation of newly secreted pro-CatK to promote efficient collagen degradation on the demineralized bone surface.
FIGURE 7.
Localization of C4-S and cathepsin K in human OCs during bone resorption. Multinucleated OCs were cultured on bone slices, fixed, and immunostained with either anti-C4-S or anti-CatK antibodies (orange) and then with phalloidin for F-actin (green). A, B, D, and E, confocal images of XZ sections (A and D), merged Z sections of C4-S and CatK stained OCs (B and E), respectively. C and F, overlaid images of C4-S or CatK and F-actin are shown. Active OCs form the sealing zone (SZ) on bone surface (dashed lines) and generate deep and long bone resorption pits (P). C4-S is weakly stained in the cytoplasm but strongly localized in the ruffled border (RB) and bone resorption pits (A–C). Total CatK is detected in the cytoplasm and resorption pits but highly accumulated in deep ruffled border (RB) of OCs (D–F). Bars, 12 μm.
DISCUSSION
In adults, bone remodeling is a process to maintain normal healthy bone whereby old bone is removed by osteoclast bone resorption and subsequently replaced with new bone via osteoblast bone formation. When the delicate remodeling balance is tilted toward a higher rate of resorption than formation, bone mass is reduced, which is associated with progressive breakdown of skeletal microarchitecture, leading to an increased risk of fractures in patients with osteoporosis. Excessive CatK activity accompanied with elevated osteoclast number is primarily responsible for this increased bone loss in osteoporosis. Hence, a better understanding of how this enzyme is regulated during osteoclastic bone resorption may assist the design of novel bone resorption inhibitors. C4-S has been previously shown to increase the efficiency of collagenase activity of the mature CatK by promoting interactions of CatK and collagen fibers (29, 30, 32). However, the exact role of C4-S in the earlier process of pro-CatK activation has yet to be elucidated.
We have demonstrated that the pro-forms of hrCatK and hCatK isoforms are self-activated at pH ≤5.0, conditions that are readily attainable within the acidic environment of lysosomes or the resorption lacunae. These osteoclastic acidic cellular compartments are where the newly synthesized and secreted CatK is localized during resorption (38). In the resorption lacunae, because of the activities of H+-ATPase and chloride channel at the ruffled border, the environment in proximity to this membrane conceivably reaches a highly acidic pH (<4.0), and the pH increases as a function of distance from the ruffled border. Dissolution of hydroxyapatite can buffer the region of demineralized collagen toward the neutral pH. We thus propose that the glycosaminoglycan-derived C4-S embedded within the bone matrix (39) may facilitate the activation of the secreted pro-CatK and maintains high collagenase activity as the resorption process proceeds without self-catabolism. Indeed, Silver et al. (40) have used pH microelectrodes to demonstrate that the pH in the attachment zone of cultured osteoclast and macrophages is as low as 3.0. In that study, using H+ and Ca2+ double-barreled electrodes, the pH of osteoclasts in situ in osteoporotic bone fragments in rabbit ear chambers drops to a lower limit of 4.7.
It is known that the Ki of inhibition of CatK catalytic activity by a peptide corresponding to its pro-domain increases with a decrease in the pH from 6 to 4 (35). This is consistent with our finding that the efficiency of pro-hrCatK autoprocessing increased as the pH became more acidic. At pH 4.0, C4-S-independent autoactivation was significant; however, the rate of autoprocessing was still 10-fold less than in the presence of C4-S. This finding suggests that a conformational change of pro-CatK is regulated by acidic pH, which is further enhanced by binding to C4-S. Additionally, stabilization of matured CatK by binding of C4-S could contribute to the autoactivation process. Studies by Horkay et al. (41) showed that varied concentrations of NaCl and CaCl2 modify the structural dynamics of CS, which in turn directs how CS interacts with proteins. Silver et al. (40) also demonstrated that the calcium concentration of the resorption lacunae could reach as high as 40 mm. Meanwhile, calcium binds to CS with association constant of 14 mm (42), suggesting that the calcium concentration in the lacunae may have significant effect on the conformation of CS and its resultant effect on collagen degradation. In this study, we found that although salt concentration results in changes in pro-hrCatK conformation favoring enzyme activation, C4-S showed predominant regulation of pro-hrCatK to promote autoprocessing. We also demonstrated that the autoprocessing is a CatK-specific event, because the pro-hrCatK(C139G) mutant of the catalytic cysteine moiety is not able to undergo autoprocessing, and this process is inhibited efficiently by the CatK inhibitor L-235 even in the presence of C4-S. Interestingly, the pycnodysostotic mutant pro-hrCatK(Y212C) did not show any significant autoprocessing even in the presence of C4-S under the same assay conditions. Although this Y212C mutation in human CatK is remote from the active site, it has been suggested to potentially compromise the active cysteine moiety within the enzyme catalytic site (32). The resistance of pro-hrCatK(Y212C) mutant to autoactivation is consistent with a previous observation suggesting that this mutant may eliminate the ability of the enzyme to interact with C4-S (32). This provides another piece of evidence suggesting that disruption of C4-S binding may result in the disease phenotype.
To provide additional insights into the mechanism of pro-CatK autoprocessing, we sequenced the N termini of intermediates and products from a time course of the activation process. We observed sequential removal of N-terminal residues of the pro-hrCatK in a time-dependent manner. Structural organization of pro-forms of hrCatK (data not shown) and hCatK demonstrated that the active site or cleft-binding segment of the pro-domain (Arg-74–Pro-96) interacts with the active site residues in an orientation opposite to what is proposed for a typical substrate for a papain-like protease (18, 43). Assembly of pro-CatK molecules on C4-S does not necessarily promote self-activation of the enzyme. It is consistent with the observation that the apparent rate of autoactivation is independent of stoichiometry of pro-hrCatK on C4-S. This finding also suggests that the conformational change induced by C4-S is the main driver of autoactivation.
Other cathepsin endopeptidases, including cathepsin B, S, or L, also undergo autoactivation (44–47). Furthermore, glycosaminoglycans have been found to facilitate the autocatalytic processing of these cathepsins to their mature forms (44, 45). A model for autocatalytic activation of these cysteine cathepsins has been proposed to be first initiated by an unimolecular mechanism, involving dissociation of the propeptide from the active site cleft (44, 47). In the case of cathepsin L and B, low pH or glycosaminoglycans facilitate the disruption of propeptide-mature enzyme interactions, leading to low catalytic activity of the pro-enzymes (45, 47). This conformational change is followed by a bimolecular (intra- or trans-) proteolytic removal of the pro-peptide (45, 47).
Although our results cannot completely discount the possible involvement for a unimolecular or cis-mechanism of CatK autoprocessing, the contribution of a bimolecular or trans-mechanism to the activation of CatK is supported by complete processing of the pro-domain of pro-hrCatK(WT) or pro-hrCatK(C139G) using only a small amount of preactivated hrCatK (hrCatK-Act) and also by the extensive proteolysis of the mature hrCatK(WT) post-processing. Curiously, the trans-mechanism also occurs in the absence of C4-S when the pro-hrCatK(C139G) mutant was used as a substrate. The same amount of hrCatK-Act was required to achieve complete processing of the pro-domain of pro-hrCatK(WT) or pro-hrCatK(C139G) mutant. On the other hand, in the presence of C4-S, the preactivated hrCatK-Act was not required for complete processing of the pro-forms of WT and C139G mutant enzymes. Together, these observations suggest that C4-S drives an alternative mechanism for activation of CatK without fully opening the active site to external substrate. This is most likely a conformation- and pH-dependent event that facilitates the presentation of preactivated CatK in the required orientation to access pro-CatK. It has been speculated that an acidic environment may weaken or break the interaction of the pro-domain with the catalytic domain, and also the pro-domain may be in a slow binding equilibrium with the catalytic domain (35). However, the conformational change implied in the active site of CatK, as demonstrated by changes in L-235 fluorescence when C4-S was introduced, supports the proposal of a rearrangement in the active site of CatK upon binding C4-S. Although no conformational change was inferred from the co-crystal of C4-S and mature CatK compared with that of pro-CatK protein only (33), this potential conformational rearrangement may be elucidated by co-crystallization of C4-S and pro-hrCatK. Taken together, we have concluded that CatK autoactivation is accomplished in acidic environment via a trans-mechanism, which is facilitated by a conformational change in the pro-CatK induced by C4-S.
Acknowledgments
Many thanks to Dr. Stephen M. Soisson (Merck) for support. We also thank Boyd Scott (Merck) and Jennifer Pawlowski (Merck) for submitting the manuscript.
This work was supported by Merck & Co. All authors are employees of Merck, the studies' sponsor, and may own stock/stock options in this company.
- CatK
- cathepsin K
- hrCatK
- humanized rabbit cathepsin K
- hCatK
- human cathepsin K
- hrCatK-Act
- preactivated humanized rabbit cathepsin K
- CS
- chondroitin sulfate
- C4-S
- chondroitin 4-sulfate
- L-235
- L-006,235
- Z
- benzyloxycarbonyl
- MCA
- 7-amido-4-methylcoumarin
- OC
- osteoclast
- RANKL
- receptor activator of nuclear factor κ-B ligand.
REFERENCES
- 1. Rawlings N. D., Waller M., Barrett A. J., Bateman A. (2014) MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 42, D503–D509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garnero P., Borel O., Byrjalsen I., Ferreras M., Drake F. H., McQueney M. S., Foged N. T., Delmas P. D., Delaissé J. M. (1998) The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J. Biol. Chem. 273, 32347–32352 [DOI] [PubMed] [Google Scholar]
- 3. Inaoka T., Bilbe G., Ishibashi O., Tezuka K., Kumegawa M., Kokubo T. (1995) Molecular cloning of human cDNA for cathepsin K: novel cysteine proteinase predominantly expressed in bone. Biochem. Biophys. Res. Commun. 206, 89–96 [DOI] [PubMed] [Google Scholar]
- 4. Borel O., Gineyts E., Bertholon C., Garnero P. (2012) Cathepsin K preferentially solubilizes matured bone matrix. Calcif. Tissue Int. 91, 32–39 [DOI] [PubMed] [Google Scholar]
- 5. Leung P., Pickarski M., Zhuo Y., Masarachia P. J., Duong L. T. (2011) The effects of the cathepsin K inhibitor odanacatib on osteoclastic bone resorption and vesicular trafficking. Bone 49, 623–635 [DOI] [PubMed] [Google Scholar]
- 6. Everts V., Korper W., Hoeben K. A., Jansen I. D., Bromme D., Cleutjens K. B., Heeneman S., Peters C., Reinheckel T., Saftig P., Beertsen W. (2006) Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: differences between calvaria and long bone. J. Bone Miner Res. 21, 1399–1408 [DOI] [PubMed] [Google Scholar]
- 7. Costa A. G., Cusano N. E., Silva B. C., Cremers S., Bilezikian J. P. (2011) Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat. Rev. Rheumatol. 7, 447–456 [DOI] [PubMed] [Google Scholar]
- 8. Saftig P., Hunziker E., Wehmeyer O., Jones S., Boyde A., Rommerskirch W., Moritz J. D., Schu P., von Figura K. (1998) Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 95, 13453–13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saftig P., Hunziker E., Everts V., Jones S., Boyde A., Wehmeyer O., Suter A., von Figura K. (2000) Functions of cathepsin K in bone resorption. Lessons from cathepsin K deficient mice. Adv. Exp. Med. Biol. 477, 293–303 [DOI] [PubMed] [Google Scholar]
- 10. Kiviranta R., Morko J., Alatalo S. L., NicAmhlaoibh R., Risteli J., Laitala-Leinonen T., Vuorio E. (2005) Impaired bone resorption in cathepsin K-deficient mice is partially compensated for by enhanced osteoclastogenesis and increased expression of other proteases via an increased RANKL/OPG ratio. Bone 36, 159–172 [DOI] [PubMed] [Google Scholar]
- 11. Gelb B. D., Shi G. P., Chapman H. A., Desnick R. J. (1996) Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 273, 1236–1238 [DOI] [PubMed] [Google Scholar]
- 12. Everts V., Hou W. S., Rialland X., Tigchelaar W., Saftig P., Brömme D., Gelb B. D., Beertsen W. (2003) Cathepsin K deficiency in pycnodysostosis results in accumulation of non-digested phagocytosed collagen in fibroblasts. Calcif. Tissue Int. 73, 380–386 [DOI] [PubMed] [Google Scholar]
- 13. Morko J., Kiviranta R., Mulari M. T., Ivaska K. K., Väänänen H. K., Vuorio E., Laitala-Leinonen T. (2009) Overexpression of cathepsin K accelerates the resorption cycle and osteoblast differentiation in vitro. Bone 44, 717–728 [DOI] [PubMed] [Google Scholar]
- 14. Novinec M., Kovacic L., Lenarcic B., Baici A. (2010) Conformational flexibility and allosteric regulation of cathepsin K. Biochem. J. 429, 379–389 [DOI] [PubMed] [Google Scholar]
- 15. McQueney M. S., Amegadzie B. Y., D'Alessio K., Hanning C. R., McLaughlin M. M., McNulty D., Carr S. A., Ijames C., Kurdyla J., Jones C. S. (1997) Autocatalytic activation of human cathepsin K. J. Biol. Chem. 272, 13955–13960 [DOI] [PubMed] [Google Scholar]
- 16. Vernet T., Tessier D. C., Richardson C., Laliberté F., Khouri H. E., Bell A. W., Storer A. C., Thomas D. Y. (1990) Secretion of functional papain precursor from insect cells. Requirement for N-glycosylation of the pro-region. J. Biol. Chem. 265, 16661–16666 [PubMed] [Google Scholar]
- 17. Brömme D., Nallaseth F. S., Turk B. (2004) Production and activation of recombinant papain-like cysteine proteases. Methods 32, 199–206 [DOI] [PubMed] [Google Scholar]
- 18. LaLonde J. M., Zhao B., Janson C. A., D'Alessio K. J., McQueney M. S., Orsini M. J., Debouck C. M., Smith W. W. (1999) The crystal structure of human procathepsin K. Biochemistry 38, 862–869 [DOI] [PubMed] [Google Scholar]
- 19. Bossard M. J., Tomaszek T. A., Thompson S. K., Amegadzie B. Y., Hanning C. R., Jones C., Kurdyla J. T., McNulty D. E., Drake F. H., Gowen M., Levy M. A. (1996) Proteolytic activity of human osteoclast cathepsin K. Expression, purification, activation, and substrate identification. J. Biol. Chem. 271, 12517–12524 [DOI] [PubMed] [Google Scholar]
- 20. Brömme D., Okamoto K., Wang B. B., Biroc S. (1996) Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. Functional expression of human cathepsin O2 in Spodoptera frugiperda and characterization of the enzyme. J. Biol. Chem. 271, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 21. Zhao H. (2012) Membrane trafficking in osteoblasts and osteoclasts: new avenues for understanding and treating skeletal diseases. Traffic 13, 1307–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lecaille F., Brömme D., Lalmanach G. (2008) Biochemical properties and regulation of cathepsin K activity. Biochimie 90, 208–226 [DOI] [PubMed] [Google Scholar]
- 23. Drake F. H., Dodds R. A., James I. E., Connor J. R., Debouck C., Richardson S., Lee-Rykaczewski E., Coleman L., Rieman D., Barthlow R., Hastings G., Gowen M. (1996) Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J. Biol. Chem. 271, 12511–12516 [DOI] [PubMed] [Google Scholar]
- 24. Panwar P., Du X., Sharma V., Lamour G., Castro M., Li H., Brömme D. (2013) Effects of cysteine proteases on the structural and mechanical properties of collagen fibers. J. Biol. Chem. 288, 5940–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haylock-Jacobs S., Keough M. B., Lau L., Yong V. W. (2011) Chondroitin sulphate proteoglycans: extracellular matrix proteins that regulate immunity of the central nervous system. Autoimmun. Rev. 10, 766–772 [DOI] [PubMed] [Google Scholar]
- 26. Salbach J., Rachner T. D., Rauner M., Hempel U., Anderegg U., Franz S., Simon J. C., Hofbauer L. C. (2012) Regenerative potential of glycosaminoglycans for skin and bone. J. Mol. Med. 90, 625–635 [DOI] [PubMed] [Google Scholar]
- 27. Coscarelli P. G., Silva L. C., Mourao P. A. (1991) Chondroitin 4- and 6-sulfate in growth and articular cartilage of young and adult humans. Mem. Inst. Oswaldo Cruz 86, 99–100 [DOI] [PubMed] [Google Scholar]
- 28. Mourão P. A. (1988) Distribution of chondroitin 4-sulfate and chondroitin 6-sulfate in human articular and growth cartilage. Arthritis Rheum. 31, 1028–1033 [DOI] [PubMed] [Google Scholar]
- 29. Li Z., Hou W. S., Brömme D. (2000) Collagenolytic activity of cathepsin K is specifically modulated by cartilage-resident chondroitin sulfates. Biochemistry 39, 529–536 [DOI] [PubMed] [Google Scholar]
- 30. Li Z., Yasuda Y., Li W., Bogyo M., Katz N., Gordon R. E., Fields G. B., Brömme D. (2004) Regulation of collagenase activities of human cathepsins by glycosaminoglycans. J. Biol. Chem. 279, 5470–5479 [DOI] [PubMed] [Google Scholar]
- 31. Li Z., Kienetz M., Cherney M. M., James M. N., Brömme D. (2008) The crystal and molecular structures of a cathepsin K:chondroitin sulfate complex. J. Mol. Biol. 383, 78–91 [DOI] [PubMed] [Google Scholar]
- 32. Li Z., Hou W. S., Escalante-Torres C. R., Gelb B. D., Bromme D. (2002) Collagenase activity of cathepsin K depends on complex formation with chondroitin sulfate. J. Biol. Chem. 277, 28669–28676 [DOI] [PubMed] [Google Scholar]
- 33. Cherney M. M., Lecaille F., Kienitz M., Nallaseth F. S., Li Z., James M. N., Brömme D. (2011) Structure-activity analysis of cathepsin K/chondroitin 4-sulfate interactions. J. Biol. Chem. 286, 8988–8998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hou W. S., Brömme D., Zhao Y., Mehler E., Dushey C., Weinstein H., Miranda C. S., Fraga C., Greig F., Carey J., Rimoin D. L., Desnick R. J., Gelb B. D. (1999) Characterization of novel cathepsin K mutations in the pro and mature polypeptide regions causing pycnodysostosis. J. Clin. Invest. 103, 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Billington C. J., Mason P., Magny M. C., Mort J. S. (2000) The slow-binding inhibition of cathepsin K by its pro-peptide. Biochem. Biophys. Res. Commun. 276, 924–929 [DOI] [PubMed] [Google Scholar]
- 36. Palmer J. T., Bryant C., Wang D. X., Davis D. E., Setti E. L., Rydzewski R. M., Venkatraman S., Tian Z. Q., Burrill L. C., Mendonca R. V., Springman E., McCarter J., Chung T., Cheung H., Janc J. W., McGrath M., Somoza J. R., Enriquez P., Yu Z. W., Strickley R. M., Liu L., Venuti M. C., Percival M. D., Falgueyret J. P., Prasit P., Oballa R., Riendeau D., Young R. N., Wesolowski G., Rodan S. B., Johnson C., Kimmel D. B., Rodan G. (2005) Design and synthesis of tri-ring P3 benzamide-containing aminonitriles as potent, selective, orally effective inhibitors of cathepsin K. J. Med. Chem. 48, 7520–7534 [DOI] [PubMed] [Google Scholar]
- 37. Falgueyret J. P., Desmarais S., Oballa R., Black W. C., Cromlish W., Khougaz K., Lamontagne S., Massé F., Riendeau D., Toulmond S., Percival M. D. (2005) Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional selectivity. J. Med. Chem. 48, 7535–7543 [DOI] [PubMed] [Google Scholar]
- 38. Baron R. (1989) Molecular mechanisms of bone resorption by the osteoclast. Anat. Rec. 224, 317–324 [DOI] [PubMed] [Google Scholar]
- 39. Toole B. P., Linsenmayer T. F. (1977) Newer knowledge of skeletogenesis: macromolecular transitions in the extracellular matrix. Clin. Orthop. Relat. Res. 258–278 [PubMed] [Google Scholar]
- 40. Silver I. A., Murrills R. J., Etherington D. J. (1988) Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 175, 266–276 [DOI] [PubMed] [Google Scholar]
- 41. Horkay F., Basser P. J., Hecht A. M., Geissler E. (2012) Chondroitin sulfate in solution: effects of mono- and divalent salts. Macromolecules 45, 2882–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hunter G. K. (1987) Chondroitin sulfate-derivatized agarose beads: a new system for studying cation binding to glycosaminoglycans. Anal. Biochem. 165, 435–441 [DOI] [PubMed] [Google Scholar]
- 43. Drenth J., Kalk K. H., Swen H. M. (1976) Binding of chloromethyl ketone substrate analogues to crystalline papain. Biochemistry 15, 3731–3738 [DOI] [PubMed] [Google Scholar]
- 44. Pungercar J. R., Caglic D., Sajid M., Dolinar M., Vasiljeva O., Pozgan U., Turk D., Bogyo M., Turk V., Turk B. (2009) Autocatalytic processing of procathepsin B is triggered by proenzyme activity. FEBS J. 276, 660–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Caglic D., Pungercar J. R., Pejler G., Turk V., Turk B. (2007) Glycosaminoglycans facilitate procathepsin B activation through disruption of propeptide-mature enzyme interactions. J. Biol. Chem. 282, 33076–33085 [DOI] [PubMed] [Google Scholar]
- 46. Vasiljeva O., Dolinar M., Pungercar J. R., Turk V., Turk B. (2005) Recombinant human procathepsin S is capable of autocatalytic processing at neutral pH in the presence of glycosaminoglycans. FEBS Lett. 579, 1285–1290 [DOI] [PubMed] [Google Scholar]
- 47. Ménard R., Carmona E., Takebe S., Dufour E., Plouffe C., Mason P., Mort J. S. (1998) Autocatalytic processing of recombinant human procathepsin L. Contribution of both intermolecular and unimolecular events in the processing of procathepsin L in vitro. J. Biol. Chem. 273, 4478–4484 [DOI] [PubMed] [Google Scholar]