Background: ETS family transcription factors recognize DNA via structurally conserved DNA-binding domains that share limited amino acid homology.
Results: DNA recognition by the ETS domains of Ets-1 and PU.1, two extreme sequence-divergent paralogs, was compared.
Conclusion: Preferential hydration differentiates DNA recognition by Ets-1 and PU.1.
Significance: Preferential hydration represents a potential mechanism for PU.1 regulation and its activity as a pioneer transcription factor in vivo.
Keywords: ETS Transcription Factor Family, Isothermal Titration Calorimetry (ITC), Kinetics, Protein-DNA Interaction, Surface Plasmon Resonance (SPR), Thermodynamics, Ets-1, PU.1, Hydration, Osmotic Stress
Abstract
ETS family transcription factors regulate diverse genes through binding at cognate DNA sites that overlap substantially in sequence. The DNA-binding domains of ETS proteins (ETS domains) are highly conserved structurally yet share limited amino acid homology. To define the mechanistic implications of sequence diversity within the ETS family, we characterized the thermodynamics and kinetics of DNA site recognition by the ETS domains of Ets-1 and PU.1, which represent the extremes in amino acid divergence among ETS proteins. Even though the two ETS domains bind their optimal sites with similar affinities under physiologic conditions, their nature of site recognition differs strikingly in terms of the role of hydration and counter ion release. The data suggest two distinct mechanisms wherein Ets-1 follows a “dry” mechanism that rapidly parses sites through electrostatic interactions and direct protein-DNA contacts, whereas PU.1 utilizes hydration to interrogate sequence-specific sites and form a long-lived complex relative to the Ets-1 counterpart. The kinetic persistence of the high affinity PU.1·DNA complex may be relevant to an emerging role of PU.1, but not Ets-1, as a pioneer transcription factor in vivo. In addition, PU.1 activity is critical to the development and function of macrophages and lymphocytes, which present osmotically variable environments, and hydration-dependent specificity may represent an important regulatory mechanism in vivo, a hypothesis that finds support in gene expression profiles of primary murine macrophages.
Introduction
The ETS family of transcription factors comprises a major group of transcriptional regulators in the Metazoan kingdom (1). Humans express 28 ETS paralogs in addition to various oncogenic fusions associated with bone, breast, and prostate tumors (2, 3). All ETS proteins harbor eponymous DNA-binding domains that are highly conserved in structure, which is manifest in their overlapping selectivity of DNA sites around a 5′-GGA(A/T)-3′ consensus (4). The strong correspondence of sequence preference by ETS domains in vitro to genomic occupancy of native ETS proteins in vivo suggests that site recognition by ETS domains per se is essential to understanding how ETS proteins function in cells. Despite strong structural conservation, ETS domains are highly divergent in primary sequence (5). Moreover, ETS proteins are limited in terms of interchangeability in vivo, and ETS members that are co-expressed in the same cell direct distinct cohorts of target genes (6–8).
Recent genomic studies have identified the ETS member PU.1 as a pioneering transcription factor (9). Specifically, PU.1 can overcome chromatin restriction to bind DNA, including DNase I-inaccessible chromatin, initiates nucleosomal remodeling by promoting local histone modifications, and defines the localization of other transcription factors by cooperative recruitment (10–13). Interestingly, ETS proteins are not equivalent in this regard, because Ets-1 has been identified recently as a non-pioneer transcription factor (14). It is therefore of interest to understand how DNA recognition by PU.1 is differentiated from Ets-1 and other ETS proteins. Comparative crystallographic analyses of several ETS·DNA complexes (Ets-1, GABPα, SAP-1, Elk-1, and PU.1) have revealed paralog-specific interactions (15–17), but these differences do not appear sufficiently fundamental to account for the pioneering properties of PU.1. Thus, the need persists for studies to address the physical mechanisms of sequence recognition by ETS family transcription factors.
To date, the nature of sequence discrimination is best understood for PU.1 and Ets-1, whose interactions with a number of sequence-specific and nonspecific sites have been characterized. Recent studies on PU.1 have revealed an essential role for preferential hydration in sequence discrimination by PU.1 (18). Although co-crystal structures of ETS domains show various degrees of water-mediated contacts, it remains unclear whether osmotic sensitivity is restricted to PU.1 or whether it is a generally shared feature among ETS proteins. To define the mechanistic implications of sequence diversity among ETS proteins in solution, we compared the thermodynamics and kinetics of the ETS domain of Ets-1 and PU.1, which represent the extremes of primary sequence divergence. We found that, unlike PU.1, Ets-1 is only weakly sensitive to osmotic stress and lacks the distinctive sequence dependence observed with PU.1. These differences in preferential hydration give rise to a host of thermodynamic and kinetic features that qualitatively distinguish site recognition by these proteins and point to a mechanism by which the activity and specificity of ETS proteins may be differentially controlled through their osmotic environment in vivo.
MATERIALS AND METHODS
Molecular Cloning
The DNA sequence encoding the ETS domain of murine PU.1 (residues 167–272, termed ΔN167) was cloned into pQE60 as previously described (18). The minimal ETS domain of human Ets-1 (residues 311–440, termed ΔN311) was amplified by PCR from full-length Ets-1 (GenBankTM accession number AY888522.1) into the NcoI/BamHI sites of pET28b. A clone of the autoinhibited ETS domain of Ets-1 (residues 280–440, termed ΔN280) was a gift from Dr. Lawrence McIntosh (University of British Columbia). All three constructs contain vector-derived sequences encoding a thrombin cleavage site and C-terminal His6 tag (LVPR↓GSH6). Clones were verified directly by Sanger sequencing.
Protein Expression and Purification
The recombinant ETS domain of PU.1 was overexpressed in Escherichia coli and purified as previously described (18). The Ets-1 constructs were handled similarly. Briefly, BL21*(DE3) E. coli were transformed with the appropriate plasmid and grown at 37 °C. Cultures were induced at A600 = 0.6 with 0.5 mm isopropyl β-d-thiogalactopyranoside at 30 °C for ∼4 h, harvested by centrifugation, and stored at −80 °C until use. Purification on cobalt affinity resin, followed by thrombin cleavage and size exclusion chromatography, was performed as with PU.1, except preparative buffers for Ets-1 constructs also contained 0.5 mm Tris(2-carboxyethyl)phosphine hydrochloride to maintain reduced cysteines. Protein concentrations were determined spectrophotometrically at 280 nm based on the following extinction coefficients: 22,460, 32,430, and 39,880 m−1 cm−1 for PU.1ΔN167, Ets-1ΔN280, and Ets-1ΔN331, respectively.
DNA Constructs
Double-stranded DNA (21–23 bp) harboring various ETS binding sites were assembled from oligonucleotides purchased from IDT Technologies (Coralville, IA) by annealing at ∼0.5 mm duplex. DNA fragments (∼200 bp) harboring the same sites were amplified by PCR from pUC19 plasmids using M13-based primers that were modified to remove cryptic Ets-1 binding sites in the vector. Amplicons were purified by agarose gel electrophoresis. DNA concentration was determined spectrophotometrically using nearest neighbor methods (19) for oligonucleotides and using the nominal value of 50 ng/μl for fragments. The high affinity sites used for PU.1 are 5′-AGCGGAAGTG-3′ (20) and 5′-AAAGGAAGTG-3′ (the λB motif) (21), which share similar affinities under physiologically saline, normo-osmotic conditions (20, 22). The low affinity site for PU.1 is 5′-AAAGGAATGG-3′. The sites used for Ets-1 were GCCGGAAGTG (termed SC1, high affinity) and TCCGGAAACC (termed SC12, low affinity) (23).
Osmotic Stress Experiments
The hydration changes associated with DNA site recognition by ETS domains were measured and analyzed as described (18). Briefly, the effect of added osmolytes on the affinity of various ETS domains encoded by duplex oligonucleotides were determined as a competition with DNA fragments (1 nm) harboring a single specific site. Protein concentrations were chosen such that fragments were ∼90% bound in the absence of oligonucleotide competitor. The solution conditions were 10 mm Tris-HCl (pH 7.4 at 25 °C), 150 mm NaCl, 0.5 mm Tris(2-carboxyethyl)phosphine hydrochloride, 0.1 g/liter acetylated BSA (Promega), and various concentrations of osmolytes. Osmolality (Osm) was calculated from solution molality (m) using published values of osmotic coefficients (φ): Osm = φm as detailed previously (18). DNA-bound and free protein was separated by native polyacrylamide electrophoresis. Preferential hydration ΓPW was calculated from the osmotic dependence of the binding constant as described (24).
Filter Binding
The effect of salt on ETS·DNA affinity was evaluated by filter binding experiments using 32P-labeled oligonucleotides harboring various sites and analyzed as previously described (25); protein concentration ranged up to 0.1 μm. The buffer used was the same as in osmotic stress experiments with the exception of added salmon sperm DNA (10 μm bp) to reduce background DNA binding to the filters. The number of neutralized phosphates (Z) was calculated from the observed salt dependence of the binding constant according to Record et al. (26).
Isothermal Titration Calorimetry
Purified protein was co-dialyzed extensively with various DNA constructs against analytical buffers (50 mm NaH2PO4/Na2HPO4, pH 7.4, 150 mm total Na+, 0.1 mm EDTA, 1 mm DTT). Titrations were performed by injecting DNA into protein in a Nano ITC instrument (TA Instruments). Model-dependent analysis was performed as described (27, 28). For PU.1, which undergoes self-dimerization in both DNA-free and -bound states at high concentrations (>10−5 m), we followed our previously described model to extract the enthalpy changes for the canonical 1:1 complex (29).
Biosensor Surface Plasmon Resonance
Kinetic and steady state measurements of DNA binding by PU.1 and Ets-1 were performed with a Biacore T200 biosensor (GE Healthcare) as described (30). Briefly, biotinylated hairpin DNA harboring sequence-specific sites was immobilized on streptavidin functionalized Biacore CM5 sensor chips. Purified protein was injected over the immobilized DNA at 100 μl/min in 25 mm NaH2PO4/Na2HPO4, pH 7.4, with 1 mm DTT and additional NaCl to achieve the desired [Na+]. Kinetic data were fitted to a 1:1 binding model with correction for residual mass transfer as previously described (30).
Computational Procedures
Model fitting to titration data were performed using the nonlinear regression engine in Origin (Northampton, MA). Structural alignment of published structures was performed using the RAPIDO server (31) accessible online.
Microarray Data Analysis
Microarray data sets were retrieved and analyzed using the NCBI GEO2R web tool. Data sets were screened according to the following criteria: 1) cellular background (unstimulated murine bone marrow-derived macrophages or progenitors); 2) data normalization by median centering; and 3) differential gene expression >4% based on a false discovery rate-adjusted p < 0.05. Differentially expressed genes from various data sets (as identified in the text) were tested for cross-correlation with differentially expressed genes in NFAT5 knock-outs (GEO accession number GSE26343). Statistical significance is inferred at p < 0.05 by the exact binomial test, using differential expression within their respective test data sets as the expected frequencies.
RESULTS
The Sequence-divergent ETS Domains of Ets-1 and PU.1 Share Strong Conformational Homology
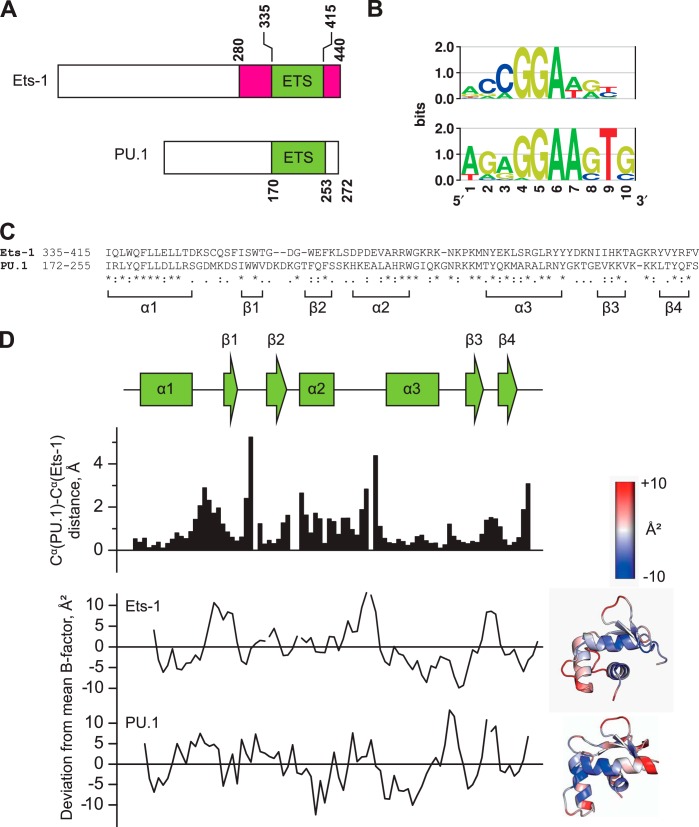
Members of the ETS family are categorized in terms of homology in their amino acid sequences (5) or DNA site selectivities (4). By either measure, the ETS domains of Ets-1 (residues 331–440) and PU.1 (residues 166–272) are most distantly related. Both ETS domains are located at the C terminus of their respective full-length proteins but share only 34% sequence identity and exhibit distinct DNA sequence preferences (Fig. 1, A–C). Despite these differences, the two domains trace similar backbone trajectories when bound to their respective high affinity sites, as revealed by a structural alignment of the DNA-bound co-crystal structures (Protein Data Bank codes 1K79 and 1PUE; Fig. 1D) (32, 33). The Cα atoms of the proteins align strongly with a root mean square deviation of 1.4 Å. Segments that deviate the most between the two structures occur primarily between assigned α-helices and β-sheets. However, these intervening segments are not disordered, as judged by their main chain B-factors, which deviate from the average value over the respective ETS domain by no more than ∼10 Å2. Nevertheless, the two domains show very different interactions with consensus DNA sequences (5′-GGAA-3′) in the DNA major groove. Whereas the PU.1 structure shows a coordinated network of interfacial water molecules mediating most contacts between the protein and DNA (18, 33), the Ets-1 structure shows a relatively dehydrated interface (32). If these crystal structures reflect differences in complex hydration under solution conditions, their divergent amino acid sequences may encode different mechanisms of site recognition by the two proteins.
FIGURE 1.
The sequence-divergent ETS domains of PU.1 and Ets-1 adopt homologous backbone conformations. A, domain structures of murine Ets-1 and PU.1. The ETS domains of mouse and human counterparts are identical in sequence. The autoinhibitory flanking regions in Ets-1 are colored in magenta. B, sequence preferences of human Ets-1 and PU.1 as determined by ChIP sequencing and presented as relative entropy-weighted DNA logos (68). Positions at which only a single base is found contribute 2 bits of information content and positions at which all bases are equiprobable contribute 0 bits. C, sequence alignment of the ETS domains of Ets-1 and PU.1. Asterisks (*), colons (:), and periods (.) represent identity, conservative, and semiconservative differences, respectively. D, structural alignment of the high affinity protein·DNA complexes formed by the minimal ETS domains of Ets-1 (Protein Data Bank code 1K79) and PU.1 (Protein Data Bank code 1PUE). Scalar distances between aligned Cα and the deviation from the mean B-factor over the entire domain (37 and 16 Å2 for Ets-1 and PU.1, respective) are shown. The B-factor deviations are also spatially annotated in cartoon structures of the two ETS domains. Note that alignment is lowest at segments adjoining secondary structure elements, but these segments are not disordered as judged by their B-factors.
The ETS Domains of Ets-1 and PU.1 Differ Profoundly in the Hydration of Their Protein-DNA Complexes
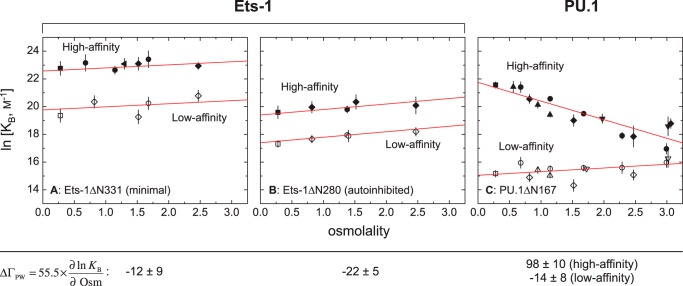
Using the osmotic stress technique (34), we have previously observed that DNA binding by the ETS domain of PU.1 (ΔN167) is sensitive to water activity in a sequence-dependent manner (18). With increasing osmolality, high affinity complexes are strongly destabilized, whereas low affinity complexes are only weakly sensitive. We have now determined the hydration change in sequence recognition by Ets-1 by measuring the binding of the minimal ETS domain of Ets-1 (ΔN331) to high affinity (termed SC1) and low affinity (SC12) specific sites under the same osmotic conditions, using six chemically distinct osmolytes (Fig. 2). In contrast to PU.1, both Ets-1 complexes are weakly stabilized, to equivalent extents, with increased osmolality. Thus, not only are sequence-specific Ets-1ΔN331·DNA complexes hydrated differently, they also lack the marked dependence on DNA sequence observed with PU.1ΔN167. The absence of systematic osmolyte-specific effects in all cases strongly supports the interpretation that the perturbations in binding are mediated by the coupled preferential interactions of water and the osmolytes with the macromolecules rather than osmolyte-dependent changes in physical solution properties. Moreover, SDS-PAGE and native electrophoresis confirmed that these observations were not due to heterogeneity in the protein preparations or osmolyte-induced aggregation, respectively (data not shown), but rather reflect differences in hydration changes in site recognition by the two structurally conserved ETS homologs.
FIGURE 2.
Site-specific ETS·DNA complexes are osmotically sensitive in a sequence-dependent manner for PU.1, but not Ets-1. The role of preferential hydration in sequence-specific binding to high (solid symbols) and low affinity (open symbols) is determined by osmotic stress. Affinity is expressed as the binding constant, KB in units of m−1. A, the minimal ETS domain of Ets-1 (Ets-1ΔN331). B, the autoinhibited ETS domain of Ets-1 (Ets-1ΔN280). C, the ETS domain of PU.1 (PU.1ΔN167). Negative controls (without added osmolyte) are shown as squares. The osmolytes used are: betaine (diamonds), triethylene glycol (circles), glycerol (hexagons), nicotinamide (up triangles), sucrose (down triangles), and maltose (left triangles). The data for high and low affinity binding by Ets-1 are fitted to a common slope for each construct. The data for PU.1 are from experiments performed by Poon (18) under identical solution conditions.
Autoinhibition Does Not Alter the Osmotic Insensitivity of Sequence-specific Ets-1·DNA Complexes
Unlike PU.1, the ETS domain of Ets-1 is flanked by structured elements (Fig. 1A) that unfold upon DNA binding and reduce affinity relative to the minimal ETS domain (35–40). Previous studies have defined residues 280–330 and the C-terminal residues 416–440 as necessary and sufficient for autoinhibition (38). The loss of either flanking segment abolishes autoinhibitory effects, such that Ets-1ΔN331 behaves identically to Ets-1(331–415) (40). To probe whether autoinhibition modifies the hydration changes in Ets1·DNA binding, we repeated the osmotic stress experiments with Ets-1ΔN280. We observed that the affinity of Ets-1Δ280 for SC1 and SC12 is reduced ∼10-fold relative to Ets-1ΔN331 but exhibits, within experimental uncertainty, the same osmotic response as Ets-1ΔN331 (Fig. 2, A and B). Thus, autoinhibition exerts no major effect on the hydration changes in sequence-specific DNA recognition by Ets-1.
Ets-1·DNA and PU.1·DNA Complexes Are Differentially Destabilized by Salt
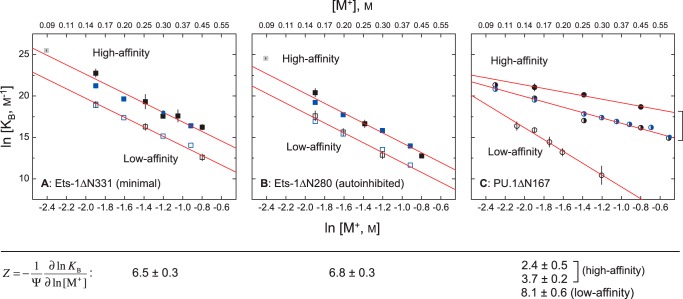
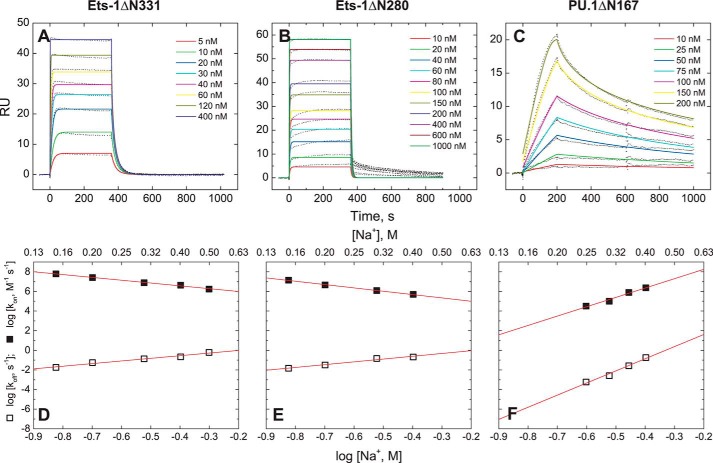
The high affinity co-crystal structures for PU.1 (Protein Data Bank code 1PUE) and Ets-1 (Protein Data Bank code 1K79) both show close contact of seven to eight backbone phosphates (32, 33). Previous measurements for high affinity binding by PU.1ΔN167 have revealed a considerably weaker salt dependence than predicted from structure (corresponding to only two to three phosphates neutralized), whether measured at equilibrium (22) or derived from kinetic rate constants (30). In addition, as with the osmotic stress data, the quantitative dependence varies with site identity, progressively increasing to the structure-predicted value for the lowest affinity specific site (18, 22). Here, we determined the salt dependence of binding by (minimal) Ets-1ΔN331 and (autoinhibited) Ets-1ΔN280 to both high affinity (SC1) and low affinity (SC12) sites at 25 °C. As confirmed by two different techniques (filter binding and SPR),6 the binding of both Ets-1 constructs to SC1 and SC12 exhibits salt dependence that corresponds closely to the number of contacted phosphates in the co-crystal structure (Fig. 3). Our data also extrapolate with good agreement to reported affinities from gel-mobility measurements by Graves and co-workers (40). Thus, Ets-1 contrasts sharply with PU.1 in that salt destabilizes both the high and low affinity complex of Ets-1 strongly and equally.
FIGURE 3.
The contribution of electrostatic interactions to site-specific ETS·DNA complex formation is sequence-dependent for PU.1, but not Ets-1. Affinity is expressed as the binding constant, KB in units of m−1. A, the minimal ETS domain of Ets-1 (Ets-1ΔN331). B, the autoinhibited domain of Ets-1 (Ets-1ΔN280). C, the ETS domain of PU.1 (PU.1ΔN167). Binding data measured independently by different techniques and monovalent cations are shown: filter binding (Na+; black symbols) (18), SPR (Na+; blue) (30), and electrophoretic gel mobility shift (TrisH+ and K+; gray) (40). The filter binding and SPR data for high and low affinity binding by Ets-1 are fitted to a common slope. The two high affinity PU.1-binding sequences tested were AGCGGAAGTG (filled circle) and the λB motif (AAAGGAAGTG, half-filled circle).
Similar Thermal Stabilities of Ets-1·DNA and PU.1·DNA Complexes Disguise Major Thermodynamic Differences
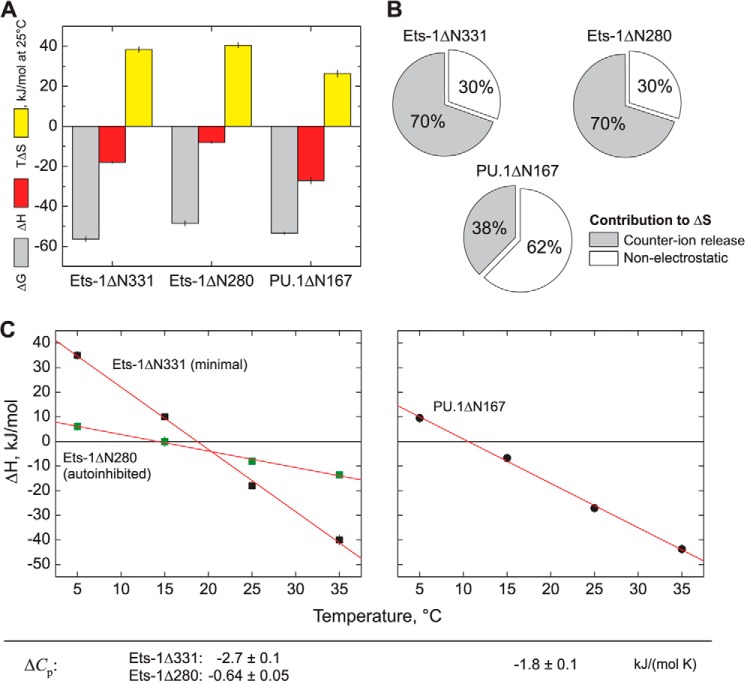
Remarkably, despite the disparate effects of water and salt activities on DNA binding by PU.1 and Ets-1, both PU.1ΔN167 and Ets-1ΔN331 bind their respective optimal sequences with similar affinities (KD = ∼10−10 m) under physiologically saline, normo-osmotic conditions (150 mm Na+, 0.3 osmolal). To dissect how these dependences are constituted in the underlying thermodynamic driving forces, we measured the heat of high affinity binding by PU.1ΔN167 and Ets-1ΔN331 directly by isothermal titration calorimetry (ITC). In our hands, purified Ets-1ΔN331 aggregated rapidly at 10−4 m at 150 mm Na+, precluding its use as titrant, but was stable at 10−5 m to serve as titrate. “Reverse” titrations were therefore performed with DNA injected into protein. Both Ets-1ΔN331 and (autoinhibited) Ets-1ΔN280 bound SC1 with 1:1 stoichiometry. For PU.1ΔN167, we have previously observed that it dimerizes in both DNA-bound and unbound states at concentrations required for ITC, and enthalpy changes for the canonical 1:1 PU.1·DNA complex were extracted from the calorimetric enthalpies according to our established model (29). The enthalpy change for each protein was then used to compute the corresponding entropic contribution to the observed free energy change (Fig. 4A). At 25 °C, high affinity DNA binding by both Ets-1ΔN331 and Ets-1ΔN280 was more entropically driven than for PU.1ΔN167. We further dissected the entropy changes for each protein using our salt data and found that the entropic contributions for high affinity Ets-1·DNA binding are primarily due to counter ion release from phosphate neutralization (Fig. 4B). In contrast, the entropic contributions to high affinity binding by PU.1ΔN167 are primarily nonelectrostatic in nature. Across a temperature span from 5 to 35 °C, high affinity binding by Ets-1ΔN331 was associated with a more negative change in heat capacity (ΔCp) than PU.1ΔN167 (Fig. 4C). Because both domains are well folded and have similarly sized binding surfaces, this difference is consistent with the net accumulation of ordered hydration water for PU.1ΔN167 relative to Ets-1ΔN331. In addition, the magnitude of ΔCp is greatly reduced for Ets-1ΔN280 compared with Ets-1ΔN331, consistent with unfolding of the autoinhibitory helices that have been observed by NMR spectroscopy.
FIGURE 4.
Thermodynamic dissection of high affinity DNA site recognition by the ETS domains of Ets-1 and PU.1. A, comparative thermodynamics for minimal Ets-1ΔN331, autoinhibited Ets-1ΔN280, and PU.1ΔN167 at 25 °C, 150 mm Na+, and normal osmolality. Free energy values are derived from equilibrium affinity measurements: ΔG = −RT ln KB where R is the gas constant. Enthalpy changes for PU.1 are extracted from calorimetric measurements as previously described (29). Entropic contributions are computed as −TΔS = ΔG − ΔH. B, electrostatic contribution to the observed entropy changes. The entropy change caused by counter ion release is calculated from the salt dependence data (Fig. 3) as: ΔSCR = −ΨZR ln [M+] (26). C, temperature dependence of the enthalpy change of binding, yielding heat capacity changes (ΔCp) as the slope.
The Kinetics of DNA Binding Reveal Major Mechanistic Differences in Site Recognition by Ets-1 and PU.1
To probe the mechanistic relevance of the foregoing energetic differences in Ets-1·DNA and PU.1·DNA binding more directly, we measured their kinetics by biosensor-SPR. All ETS constructs exhibited association and dissociation kinetics that were described by 1:1 binding. The lack of significant intermediates over the second time régime has been validated in the case of PU.1ΔN167, for which dissociation constants derived from kinetic rate constants (koff/kon) correspond quantitatively with equilibrium measurements across a broad range of salt concentrations (30). For Ets-1ΔN331 and Ets-1ΔN280, extrapolation of the kinetics down to 90 mm Na+ also yields good agreement with previous measurements by gel mobility shift (40). Comparison of PU.1ΔN167 and Ets-1ΔN331 reveals significant kinetic differences in high affinity DNA binding. At a common concentration of 300 mm Na+, Ets-1ΔN331 associates rapidly with SC1 (on rate constant kon = 7.8 × 106 m−1 s−1), ∼80 times faster than the association of PU.1 to the high affinity λB motif (kon = 1.0 × 105 m−1 s−1; Fig. 5, A and C). Moreover, the salt dependences of the on rate constant are opposite for the two ETS homologs (Fig. 5, D and F) under these conditions; whereas increasing [Na+] retards Ets-1ΔN331 association to high affinity DNA, association by PU.1ΔN167 is accelerated. These contrasting trends in the rate constants combine to give the more attenuated salt dependences in the equilibrium constants (Fig. 3). Although SPR measurements for PU.1ΔN167 could not be carried out below 250 mm Na+ because of strong affinity of the protein for the sensor chip matrix under low salt conditions (30), extrapolation of the data suggests an even wider gap in the DNA binding kinetics at physiologic salt concentrations and points to mechanistic differences in site recognition between PU.1ΔN167 and Ets-1ΔN331. Analysis of additional conditions and ETS proteins will be essential to fully understand these differences.
FIGURE 5.
Kinetic analysis of high affinity complex formation by the ETS domains of Ets-1 and PU.1. A–C, SPR sensorgrams for minimal Ets-1ΔN331 (A), autoinhibited Ets-1ΔN280 (both binding to SC1) (B), and PU.1ΔN167 binding to the λB motif (C) at 25 °C with 300 mm total Na+. Protein concentrations are as labeled in the panels. Colored lines are the best fits of a 1:1 binding model to the data. RU, response units. D–F, salt dependence of the association and dissociation rate constants. The data for PU.1 are from experiments performed by Munde et al. (30).
We also studied the kinetics of autoinhibition by comparing high affinity DNA binding by Ets-1ΔN331 and Ets-1ΔN280 (Fig. 5, B and E). Autoinhibited Ets-1 exhibits the same monophasic character of the kinetics as Ets-1ΔN331, suggesting that conformational changes by the inhibitory helices are either rapid in the second time scale or they occur in concert with DNA binding. Comparison of Ets-1ΔN331 and Ets-1ΔN280 shows that autoinhibition reduces binding affinity primarily through slowing down association kinetics. The similarity in dissociation rate constants for the two constructs suggests that the stability of the Ets-1·DNA complex, once formed, is insensitive to structural elements distal to its ETS domain.
PU.1 Target Genes Are Enriched among Genes That Are Sensitive to Cellular Osmotic Stress
Cells maintain volume regulatory mechanisms to limit the concentrations of impermeant cellular components (e.g. inorganic ions and macromolecules) within physiologic tolerance (41). A major component of volume regulation is the accumulation of compatible osmolytes (such as amino acids and inositol) to adjust intracellular osmotic pressure and control water flow across the cell membrane. Active accumulation of compatible osmolytes is mediated through up-regulation of biosynthetic enzymes and transporters by the transcription factor NFAT5 (42). Dynamic volume regulation thus gives rise to an osmotically labile environment in vivo. To probe the potential biological relevance of PU.1 osmotic sensitivity, we analyzed published microarray data to examine the effect of osmotic stress response on the gene regulatory functions of PU.1 in vivo. PU.1 is a critical transcriptional regulator in myelopoiesis (43), and PU.1 target genes harbor binding sites that correspond to high affinity sequences (44). We analyzed differential gene expression in bone marrow-derived macrophages from NFAT5 knock-out mice relative to their isogenic wild type and identified a core set of 24 genes (NCBI GEO accession number GSE26343; adjusted p < 0.05) (45). We then screened these NFAT5-dependent genes against data sets in which PU.1 or another (control) protein is induced, knocked down, or knocked out in similar cellular backgrounds under resting (unstimulated) conditions (Fig. 6). PU.1 is not known to directly interact with NFAT5, nor is the PU.1 (Sfpi1) gene a target of NFAT5 regulation in the NFAT5-KO data set (p = 0.52). If gene regulation by PU.1 is osmotically sensitive in vivo, PU.1 target genes would be observed more frequently among NFAT5-regulated genes than expected based on the number of genes with altered expression in the PU.1 data set.
FIGURE 6.
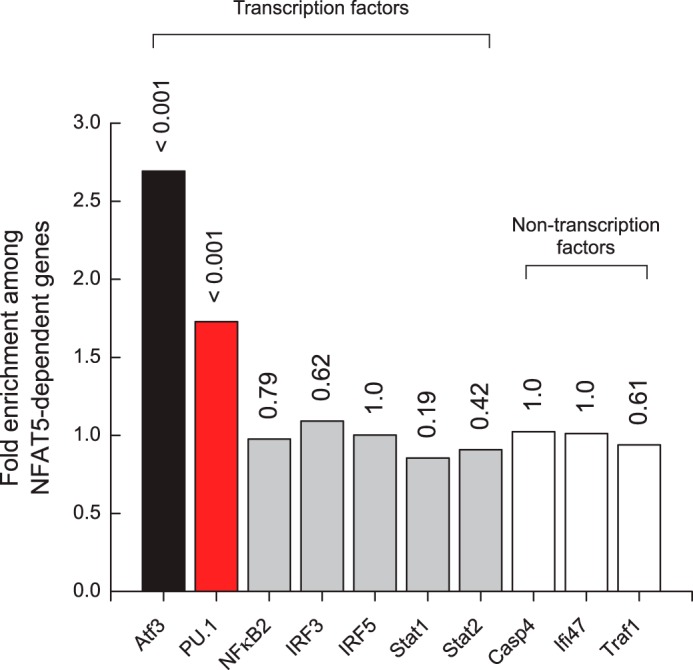
PU.1 target genes are correlated with genes sensitive to cellular hyperosmotic stress. Differential gene expression (p < 0.05, adjusted for false discovery) in primary bone marrow-derived macrophages from NFAT5-KO mice was compared with data sets in which the gene indicated in the abscissa was knocked out, knocked down, or induced in the same cellular background. The ordinate shows the occurrence of target genes for factors shown in the abscissa among NFAT5-regulated genes, normalized to the expected frequency for the respective genes. The numbers above the bars indicate the p values for the correlation between the lists of target genes for the factors shown in the abscissa with the NFAT5 list as calculated from the binomial distribution.
To validate our approach, we screened the NFAT5-dependent genes against differentially expressed genes from bone marrow-derived macrophages in which ATF3, a direct genetic target of NFAT5 (46), has been knocked out (GSE32574) (47). As negative controls, we examined data from bone marrow-derived macrophages in which NFκB2, an NFAT family paralog with no known role in osmo-regulation (48), as well as several other unrelated proteins, have been knocked down (GSE14534) (49). As expected, ATF3-dependent genes are strongly over-represented (2.7-fold enrichment; p = 7.2 × 10−4) among NFAT5-regulated genes, whereas genes from the negative controls are uncorrelated. PU.1 target genes from macrophage progenitor cells harboring an inducible PU.1 gene (GSE13125) (50) are also significantly over-represented (1.7-fold enrichment; p = 3.3 × 10−4) in the NFAT5 list. Repeating the analysis with a more relaxed list of NFAT5-dependent genes (43 genes; adjusted p < 0.1) did not affect the results of the statistical inferences.
DISCUSSION
The sequence preferences of the DNA-binding (ETS) domains of ETS family transcription factors are central to the functions of their parent ETS proteins in vivo. Genomic studies on the site occupancy by native, full-length ETS proteins in vivo have confirmed the sequence preferences determined with isolated ETS domains in vitro (4). To discover how the primary sequence diversity among ETS proteins preserves structural homology but encodes potentially distinct mechanisms of sequence recognition, we characterized the ETS domains of Ets-1 and PU.1, two paralogs at the extremes of amino acid divergence in the ETS family. Despite being strong structural conformers and binding their respective optimal sequences with similar affinities under physiologic conditions, the two ETS domains exhibit striking differences in their underlying thermodynamics and kinetics, suggesting correspondingly major differences in their mechanisms of DNA recognition.
Preferential Hydration Defines the Heterogeneity in DNA Recognition by the ETS Domains of Ets-1 and PU.1
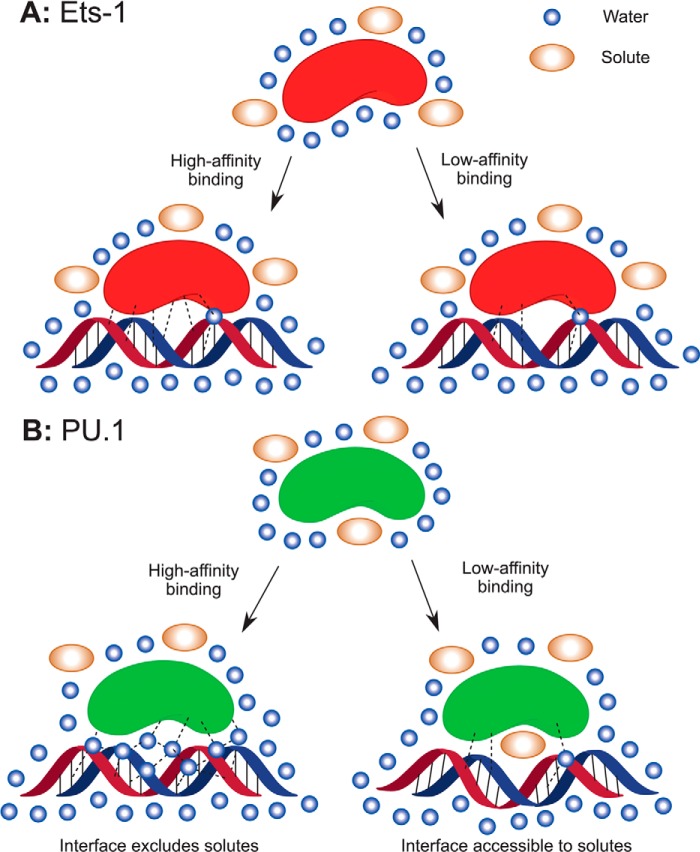
Data from previous studies (18, 51) strongly implicate preferential hydration as the defining feature in DNA discrimination by the ETS domain of PU.1. The present thermodynamic and kinetic data in DNA binding by Ets-1 provide a unified description for DNA recognition by two structurally homologous ETS domains (Fig. 7). Central to the model is the spectroscopic evidence (25, 35, 37, 52) that minimal ETS domains are well folded monomers in the bound and unbound states at concentrations used in our experiments, except for ITC titrations from which data corresponding to the canonical 1:1 complex for PU.1 have been extracted according to a validated model (29). The small magnitudes in the heat capacity changes observed for both Ets-1 and PU.1 are consistent with this feature (53). Thus, the strong observed differences in hydration changes between Ets-1ΔN331 and PU.1ΔN167 are not attributable to the gain or loss of major crevices or self-association.
FIGURE 7.
Proposed mechanisms of DNA site recognition by the ETS domains of Ets-1 and PU.1. A, DNA binding by Ets-1 is electrostatically driven with only minor changes in hydration. Site discrimination is based on rapid parsing of different sequence variants for optimal protein to DNA contacts. B, in contrast, PU.1 interrogates sequence variants through excess hydration in a slow, incremental process. Optimal binding involves both the uptake of interfacial water and possibly the release of preferential bound solutes. Suboptimal sequences are tolerated through compensatory interactions that cause additional distortions in bound DNA (29).
For Ets-1, the strong salt dependence of binding affinity in agreement with the number of DNA phosphate contacts in co-crystal structures, the dominant contribution of the polyelectrolyte effect to the observed binding entropy, and the reduction in association rate with increasing salt all suggest site-specific interactions that are primarily electrostatically driven. Hydration plays a manifestly negligible role in driving DNA binding or discerning between high and low affinity specific sites. Sequence preference is driven by favorable direct protein to DNA contacts in a relatively dehydrated protein·DNA interface, with fast kinetics, consistent with crystallographic structures of Ets-1ΔN331 bound to SC1 and a suboptimal DNA site (32).
With Ets-1 as a well defined backdrop, the thermodynamics and kinetics for PU.1 may be interpreted in a structurally meaningful manner. In contrast with Ets-1, excess hydration participates directly in the cohesive forces (enthalpy change) that stabilize the high affinity PU.1·DNA complex. The less negative heat capacity change for PU.1 is consistent with the sequestration of well ordered water molecules in the high affinity complex (54). Together with our earlier observation (18) of a solute-excluding cavity in the high affinity PU.1·DNA interface, but not the low affinity one, the data suggest a “wet” model of site discrimination in which PU.1 interrogates sequence-specific sites to optimize interfacial hydration. The more stringent site selectivity and slow association kinetics for PU.1 relative to Ets-1 therefore arise from the more demanding requirements for optimal hydration (involving many molecules) versus optimal protein-DNA contacts. Taking the low affinity PU.1·DNA complex as baseline, optimal hydration accounts for ∼17 kJ/mol of additional stabilizing free energy under normo-osmotic conditions.
Linkage Control of PU.1·DNA Interactions
The linkage relation that describes the effect of osmolytes on ETS·DNA binding gives a change in preferential hydration of ΔΓPW ∼ 100 for the high affinity PU.1·DNA complex. If interpreted strictly as stoichiometric waters, this quantity exceeds the apparent capacity of the interfacial cavity in the high affinity PU.1·DNA co-crystal structure (∼337 Å3) (18). The absence of major coupled folding or oligomerization appears to preclude significant water binding outside the PU.1·DNA interface. Formally, ΔΓPW represents all thermodynamically detected water (24), as well as the linkage between hydration Δv̄w and solute (osmolyte) interactions Δv̄x with the macromolecules,
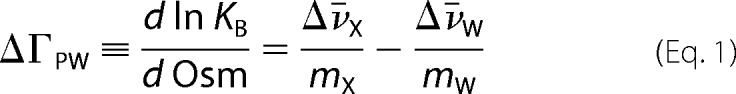 |
where mx and mw = 55.6 are the molal concentrations of solute and water in the solution (55). Thus, the destabilizing effect of osmolytes on high affinity PU.1·DNA binding may involve the release of bound solute from the DNA contact interface of PU.1 (Δv̄x < 0), as well as water uptake (Δv̄w < 0). The observed lack of significant dependence on osmolyte identity is compatible with Δv̄x = 0 or a small value of Δv̄x. The latter situation may arise because any Δv̄x is expected to be small (∼1 per protein), and fractional differences among the osmolytes tested could be obscured within experimental uncertainty.
Interestingly, even though the addition of salt necessarily increases solution osmolality, the high affinity PU.1·DNA complex is less sensitive to salt than the low affinity one (Fig. 3). This observation recapitulates the perturbing nature of ionic solutes on macromolecular complexes relative to compatible net neutral osmolytes. The observed salt dependence of the high affinity, osmotically sensitive PU.1·DNA complex is ∼25% of that for the low affinity complex, as well as that predicted by the co-crystal structure of the high affinity PU.1 complex. The mechanism by which hydration buffers the PU.1·DNA complex against ionic solutes is as yet uncertain. For other protein·DNA systems, weaker than expected salt dependence has been attributed to cation uptake by unpaired protein surface charges in the DNA-bound state (56, 57). In the case of PU.1, some of the DNA contacts in the high affinity complex are water-mediated and may be incompletely neutralized, as proposed based on a structural analysis (18) of the PU.1·DNA co-crystal structure (33).
The Thermodynamics and Kinetics of Ets-1 Autoinhibition
The structure and dynamics of autoinhibition in Ets-1 (35–40), as well as other autoinhibited ETS paralogs (58, 59), have been extensively studied by solution NMR. The folded helices immediately adjacent to the ETS domain of Ets-1 are only marginally stable in the unbound state and unfold upon DNA binding. Our data comparing the minimal Ets-1ΔN331 and autoinhibited Ets-1ΔN280 suggest that autoinhibitory helices decrease the free energy of unbound Ets-1 without significant effect on the bound state. Interestingly, the heat capacity change in high affinity DNA binding by Ets-1ΔN280 is significantly reduced compared with Ets-1ΔN331 but is not coupled to an attendant change in osmotic sensitivity. These thermodynamic signatures echo hydrogen exchange data, showing only modest protection at residues in the N-terminal inhibitory helices in free Ets-1 (36).
In Vivo Implications of Osmotic Sensitive PU.1·DNA Recognition
Under our experimental conditions and time scale, the kinetics of site recognition by Ets-1 and PU.1 are described by a 1:1 model. Although the apparent kinetics necessarily embody more complex microscopic events, they predict valid thermodynamics and provide a quantitative basis for comparison for the two proteins. The observed lack of intermediates does not preclude the possibility of early discrete species with lifetimes shorter than the time resolution (67 ms) of SPR detection. Alternatively, the slow, monophasic kinetics for PU.1 may represent the evolution of the ensemble of partially hydrated microstates, separated by small activation barriers, toward the final, fully hydrated complex. Whichever the route, comparison with Ets-1 indicates that excess hydration confers a significantly long-lived PU.1·DNA complex, a feature that may be critical to a role for PU.1 (9–13), but not Ets-1 (14), as a pioneer transcription factor. In the dynamic nucleosomal environment, DNA accessibility in the exit-entry region of nucleosomes where cognate transcription factor binding sites are concentrated is determined by the DNA unwrapping rate (60, 61). Although the relatively slow on rate for PU.1 to high affinity DNA would hinder its association to transiently accessible promoter or enhancer sites in heterochromatin, once formed, the PU.1·DNA complex could persist long enough to recruit other transcription factors or remodeling proteins to initiate transcription. Thus, dissociation kinetics may represent a more relevant parameter than equilibrium affinity in determining transcription regulatory activity.
In the broader context, NFAT5-mediated adaptation to hyperosmotic stress is crucial to the development and function of macrophages (45) and lymphocytes (62). The current paradigm of cellular osmotic regulation is focused on the activation of target genes, post-translational modification or other direct interactions of NFAT5, which mediates this program (63–67). Because cellular osmotic stress response perturbs intracellular water activity through the accumulation of osmolytes, macromolecular interactions that are tightly coupled to water molecules should be highly sensitive to this environment. Our analysis of gene expression data shows that PU.1 target genes are significantly over-represented among NFAT5-sensitive genes in murine macrophages and their precursors, even though PU.1 and NFAT5 are not known to interact physically or genetically, suggesting that gene regulation by PU.1 is osmotically sensitive in vivo. Although macromolecular interactions with water and solutes have been a classic area of study in biophysics and cell physiology, osmotic sensitivity as a transduction mechanism for responsiveness and specificity in gene regulation has received little attention. Our data suggest that PU.1 is a promising example of osmotically sensitive transcription factors that warrants further biophysical investigation.
This work was supported, in whole or in part, by National Institutes of Health Grant AI064200 (to W. D. W.). This work was also supported by National Science Foundation Grant MCB 1411502 (to G. M. K. P.).
- SPR
- surface plasmon resonance
- ITC
- isothermal titration calorimetry.
REFERENCES
- 1. Degnan B. M., Degnan S. M., Naganuma T., Morse D. E. (1993) The ets multigene family is conserved throughout the Metazoa. Nucleic Acids Res. 21, 3479–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arvand A., Denny C. T. (2001) Biology of EWS/ETS fusions in Ewing's family tumors. Oncogene 20, 5747–5754 [DOI] [PubMed] [Google Scholar]
- 3. Clark J. P., Cooper C. S. (2009) ETS gene fusions in prostate cancer. Nat. Rev. Urol. 6, 429–439 [DOI] [PubMed] [Google Scholar]
- 4. Wei G. H., Badis G., Berger M. F., Kivioja T., Palin K., Enge M., Bonke M., Jolma A., Varjosalo M., Gehrke A. R., Yan J., Talukder S., Turunen M., Taipale M., Stunnenberg H. G., Ukkonen E., Hughes T. R., Bulyk M. L., Taipale J. (2010) Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. EMBO J. 29, 2147–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graves B. J., Petersen J. M. (1998) Specificity within the ets family of transcription factors. Adv. Cancer Res. 75, 1–55 [DOI] [PubMed] [Google Scholar]
- 6. DeKoter R. P., Singh H. (2000) Regulation of B lymphocyte and macrophage development by graded expression of PU. 1. Science 288, 1439–1441 [DOI] [PubMed] [Google Scholar]
- 7. Ross I. L., Yue X., Ostrowski M. C., Hume D. A. (1998) Interaction between PU. 1 and another Ets family transcription factor promotes macrophage-specific basal transcription initiation. J. Biol. Chem. 273, 6662–6669 [DOI] [PubMed] [Google Scholar]
- 8. Kopp J. L., Wilder P. J., Desler M., Kim J. H., Hou J., Nowling T., Rizzino A. (2004) Unique and selective effects of five Ets family members, Elf3, Ets1, Ets2, PEA3, and PU. 1, on the promoter of the type II transforming growth factor-beta receptor gene. J. Biol. Chem. 279, 19407–19420 [DOI] [PubMed] [Google Scholar]
- 9. Zaret K. S., Carroll J. S. (2011) Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 25, 2227–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pham T. H., Benner C., Lichtinger M., Schwarzfischer L., Hu Y., Andreesen R., Chen W., Rehli M. (2012) Dynamic epigenetic enhancer signatures reveal key transcription factors associated with monocytic differentiation states. Blood 119, e161–e171 [DOI] [PubMed] [Google Scholar]
- 11. Heinz S., Benner C., Spann N., Bertolino E., Lin Y. C., Laslo P., Cheng J. X., Murre C., Singh H., Glass C. K. (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pham T. H., Minderjahn J., Schmidl C., Hoffmeister H., Schmidhofer S., Chen W., Langst G., Benner C., Rehli M. (2013) Mechanisms of in vivo binding site selection of the hematopoietic master transcription factor PU. 1. Nucleic Acids Res. 41, 6391–6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghisletti S., Barozzi I., Mietton F., Polletti S., De Santa F., Venturini E., Gregory L., Lonie L., Chew A., Wei C. L., Ragoussis J., Natoli G. (2010) Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity 32, 317–328 [DOI] [PubMed] [Google Scholar]
- 14. Sherwood R. I., Hashimoto T., O'Donnell C. W., Lewis S., Barkal A. A., van Hoff J. P., Karun V., Jaakkola T., Gifford D. K. (2014) Discovery of directional and nondirectional pioneer transcription factors by modeling DNase profile magnitude and shape. Nat. Biotechnol. 32, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Batchelor A. H., Piper D. E., de la Brousse F. C., McKnight S. L., Wolberger C. (1998) The structure of GABPα/β: an ETS domain-ankyrin repeat heterodimer bound to DNA. Science 279, 1037–1041 [DOI] [PubMed] [Google Scholar]
- 16. Mo Y., Vaessen B., Johnston K., Marmorstein R. (1998) Structures of SAP-1 bound to DNA targets from the E74 and c-fos promoters: insights into DNA sequence discrimination by Ets proteins. Mol. Cell 2, 201–212 [DOI] [PubMed] [Google Scholar]
- 17. Mo Y., Vaessen B., Johnston K., Marmorstein R. (2000) Structure of the elk-1-DNA complex reveals how DNA-distal residues affect ETS domain recognition of DNA. Nat. Struct. Biol. 7, 292–297 [DOI] [PubMed] [Google Scholar]
- 18. Poon G. M. K. (2012) Sequence discrimination by DNA-binding domain of ETS family transcription factor PU. 1 is linked to specific hydration of protein-DNA interface. J. Biol. Chem. 287, 18297–18307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tataurov A. V., You Y., Owczarzy R. (2008) Predicting ultraviolet spectrum of single stranded and double stranded deoxyribonucleic acids. Biophys. Chem. 133, 66–70 [DOI] [PubMed] [Google Scholar]
- 20. Poon G. M., Macgregor R. B., Jr. (2003) Base coupling in sequence-specific site recognition by the ETS domain of murine PU. 1. J. Mol. Biol. 328, 805–819 [DOI] [PubMed] [Google Scholar]
- 21. Eisenbeis C. F., Singh H., Storb U. (1993) PU. 1 is a component of a multiprotein complex which binds an essential site in the murine immunoglobulin lambda 2–4 enhancer. Mol. Cell. Biol. 13, 6452–6461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poon G. M., Macgregor R. B., Jr. (2004) A thermodynamic basis of DNA sequence selectivity by the ETS domain of murine PU. 1. J. Mol. Biol. 335, 113–127 [DOI] [PubMed] [Google Scholar]
- 23. Nye J. A., Petersen J. M., Gunther C. V., Jonsen M. D., Graves B. J. (1992) Interaction of murine ets-1 with GGA-binding sites establishes the ETS domain as a new DNA-binding motif. Genes Dev. 6, 975–990 [DOI] [PubMed] [Google Scholar]
- 24. Timasheff S. N. (2002) Protein-solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. Proc. Natl. Acad. Sci. U.S.A. 99, 9721–9726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poon G. M., Groβ P., Macgregor R. B., Jr. (2002) The sequence-specific association of the ETS domain of murine PU. 1 with DNA exhibits unusual energetics. Biochemistry 41, 2361–2371 [DOI] [PubMed] [Google Scholar]
- 26. Record M. T., Jr., Lohman M. L., De Haseth P. (1976) Ion effects on ligand-nucleic acid interactions. J. Mol. Biol. 107, 145–158 [DOI] [PubMed] [Google Scholar]
- 27. Poon G. M. (2010) Explicit formulation of titration models for isothermal titration calorimetry. Anal. Biochem. 400, 229–236 [DOI] [PubMed] [Google Scholar]
- 28. Poon G. M. (2011) Probing solution thermodynamics by microcalorimetry. In Thermodynamics: Interaction Studies: Solids, Liquids and Gases (Moreno-Piraján J. C., ed) pp. 871–890, InTech, Rijeka, Croatia [Google Scholar]
- 29. Poon G. M. K. (2012) DNA binding regulates the self-association of the ETS domain of PU. 1 in a sequence-dependent manner. Biochemistry 51, 4096–4107 [DOI] [PubMed] [Google Scholar]
- 30. Munde M., Poon G. M., Wilson W. D. (2013) Probing the electrostatics and pharmacological modulation of sequence-specific binding by the DNA-binding domain of the ETS family transcription factor PU. 1: a binding affinity and kinetics investigation. J. Mol. Biol. 425, 1655–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mosca R., Schneider T. R. (2008) RAPIDO: a web server for the alignment of protein structures in the presence of conformational changes. Nucleic Acids Res. 36, W42–W46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garvie C. W., Hagman J., Wolberger C. (2001) Structural studies of Ets-1/Pax5 complex formation on DNA. Mol. Cell 8, 1267–1276 [DOI] [PubMed] [Google Scholar]
- 33. Kodandapani R., Pio F., Ni C. Z., Piccialli G., Klemsz M., McKercher S., Maki R. A., Ely K. R. (1996) A new pattern for helix-turn-helix recognition revealed by the PU. 1 ETS-domain-DNA complex. Nature 380, 456–460 [DOI] [PubMed] [Google Scholar]
- 34. Parsegian V. A., Rand R. P., Rau D. C. (1995) Macromolecules and water: probing with osmotic stress. Methods Enzymol. 259, 43–94 [DOI] [PubMed] [Google Scholar]
- 35. Donaldson L. W., Petersen J. M., Graves B. J., McIntosh L. P. (1996) Solution structure of the ETS domain from murine Ets-1: a winged helix-turn-helix DNA binding motif. EMBO J. 15, 125–134 [PMC free article] [PubMed] [Google Scholar]
- 36. Lee G. M., Donaldson L. W., Pufall M. A., Kang H.-S., Pot I., Graves B. J., McIntosh L. P. (2005) The structural and dynamic basis of Ets-1 DNA binding autoinhibition. J. Biol. Chem. 280, 7088–7099 [DOI] [PubMed] [Google Scholar]
- 37. Petersen J. M., Skalicky J. J., Donaldson L. W., McIntosh L. P., Alber T., Graves B. J. (1995) Modulation of transcription factor Ets-1 DNA binding: DNA-induced unfolding of an alpha helix. Science 269, 1866–1869 [DOI] [PubMed] [Google Scholar]
- 38. Skalicky J. J., Donaldson L. W., Petersen J. M., Graves B. J., McIntosh L. P. (1996) Structural coupling of the inhibitory regions flanking the ETS domain of murine Ets-1. Protein Sci. 5, 296–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Graves B. J., Cowley D. O., Goetz T. L., Petersen J. M., Jonsen M. D., Gillespie M. E. (1998) Autoinhibition as a transcriptional regulatory mechanism. Cold Spring Harb. Symp. Quant. Biol. 63, 621–629 [DOI] [PubMed] [Google Scholar]
- 40. Jonsen M. D., Petersen J. M., Xu Q. P., Graves B. J. (1996) Characterization of the cooperative function of inhibitory sequences in Ets-1. Mol. Cell. Biol. 16, 2065–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Neill W. C. (1999) Physiological significance of volume-regulatory transporters. Am. J. Physiol. 276, C995–C1011 [DOI] [PubMed] [Google Scholar]
- 42. Cheung C. Y., Ko B. C. (2013) NFAT5 in cellular adaptation to hypertonic stress: regulations and functional significance. J. Mol. Signal. 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson K. L., Smith K. A., Conners K., McKercher S. R., Maki R. A., Torbett B. E. (1998) Myeloid development is selectively disrupted in PU. 1 null mice. Blood 91, 3702–3710 [PubMed] [Google Scholar]
- 44. Szymczyna B. R., Arrowsmith C. H. (2000) DNA binding specificity studies of four ETS proteins support an indirect read-out mechanism of protein-DNA recognition. J. Biol. Chem. 275, 28363–28370 [DOI] [PubMed] [Google Scholar]
- 45. Buxadé M., Lunazzi G., Minguillón J., Iborra S., Berga-Bolaños R., del Val M., Aramburu J., López-Rodríguez C. (2012) Gene expression induced by Toll-like receptors in macrophages requires the transcription factor NFAT5. J. Exp. Med. 209, 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maallem S., Wierinckx A., Lachuer J., Kwon M. H., Tappaz M. L. (2008) Gene expression profiling in brain following acute systemic hypertonicity: novel genes possibly involved in osmoadaptation. J. Neurochem. 105, 1198–1211 [DOI] [PubMed] [Google Scholar]
- 47. Gold E. S., Ramsey S. A., Sartain M. J., Selinummi J., Podolsky I., Rodriguez D. J., Moritz R. L., Aderem A. (2012) ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol-induced lipid body formation. J. Exp. Med. 209, 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Macian F. (2005) NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 [DOI] [PubMed] [Google Scholar]
- 49. Lacaze P., Raza S., Sing G., Page D., Forster T., Storm P., Craigon M., Awad T., Ghazal P., Freeman T. C. (2009) Combined genome-wide expression profiling and targeted RNA interference in primary mouse macrophages reveals perturbation of transcriptional networks associated with interferon signalling. BMC Genomics 10, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weigelt K., Lichtinger M., Rehli M., Langmann T. (2009) Transcriptomic profiling identifies a PU. 1 regulatory network in macrophages. Biochem. Biophys. Res. Commun. 380, 308–312 [DOI] [PubMed] [Google Scholar]
- 51. Munde M., Wang S., Kumar A., Stephens C. E., Farahat A. A., Boykin D. W., Wilson W. D., Poon G. M. (2014) Structure-dependent inhibition of the ETS-family transcription factor PU. 1 by novel heterocyclic diamidines. Nucleic Acids Res. 42, 1379–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jia X., Lee L. K., Light J., Palmer A. G., 3rd, Assa-Munt N. (1999) Backbone dynamics of a short PU. 1 ETS domain. J. Mol. Biol. 292, 1083–1093 [DOI] [PubMed] [Google Scholar]
- 53. Spolar R. S., Record M. T., Jr. (1994) Coupling of local folding to site-specific binding of proteins to DNA. Science 263, 777–784 [DOI] [PubMed] [Google Scholar]
- 54. Sharp K. A., Madan B. (1997) Hydrophobic effect, water structure, and heat capacity changes. J. Phys. Chem. B 101, 4343–4348 [Google Scholar]
- 55. Courtenay E. S., Capp M. W., Anderson C. F., Record M. T. (2000) Vapor pressure osmometry studies of osmolyte-protein interactions: implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry 39, 4455–4471 [DOI] [PubMed] [Google Scholar]
- 56. Ha J.-H., Capp M. W., Hohenwalter M. D., Baskerville M., Record M. T., Jr. (1992) Thermodynamic stoichiometries of participation of water, cations and anions in specific and non-specific binding of lac repressor to DNA: possible thermodynamic origins of the “glutamate effect” on protein-DNA interactions. J. Mol. Biol. 228, 252–264 [DOI] [PubMed] [Google Scholar]
- 57. Holbrook J. A., Tsodikov O. V., Saecker R. M., Record M. T., Jr. (2001) Specific and non-specific interactions of integration host factor with DNA: thermodynamic evidence for disruption of multiple IHF surface salt-bridges coupled to DNA binding. J. Mol. Biol. 310, 379–401 [DOI] [PubMed] [Google Scholar]
- 58. De S., Chan A. C., Coyne H. J., 3rd, Bhachech N., Hermsdorf U., Okon M., Murphy M. E., Graves B. J., McIntosh L. P. (2013) Steric mechanism of auto-inhibitory regulation of specific and non-specific DNA binding by the ETS transcriptional repressor ETV6. J. Mol. Biol. 426, 1390–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Green S. M., Coyne H. J., 3rd, McIntosh L. P., Graves B. J. (2010) DNA binding by the ETS protein TEL (ETV6) is regulated by autoinhibition and self-association. J. Biol. Chem. 285, 18496–18504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Forties R. A., North J. A., Javaid S., Tabbaa O. P., Fishel R., Poirier M. G., Bundschuh R. (2011) A quantitative model of nucleosome dynamics. Nucleic Acids Res. 39, 8306–8313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. North J. A., Shimko J. C., Javaid S., Mooney A. M., Shoffner M. A., Rose S. D., Bundschuh R., Fishel R., Ottesen J. J., Poirier M. G. (2012) Regulation of the nucleosome unwrapping rate controls DNA accessibility. Nucleic Acids Res. 40, 10215–10227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Go W. Y., Liu X., Roti M. A., Liu F., Ho S. N. (2004) NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc. Natl. Acad. Sci. U.S.A. 101, 10673–10678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ferraris J. D., Burg M. B. (2007) Tonicity-regulated gene expression. Methods Enzymol. 428, 279–296 [DOI] [PubMed] [Google Scholar]
- 64. Dahl S. C., Handler J. S., Kwon H. M. (2001) Hypertonicity-induced phosphorylation and nuclear localization of the transcription factor TonEBP. Am. J. Physiol. Cell Physiol. 280, C248–C253 [DOI] [PubMed] [Google Scholar]
- 65. Lee S. D., Woo S. K., Kwon H. M. (2002) Dimerization is required for phosphorylation and DNA binding of TonEBP/NFAT5. Biochem. Biophys. Res. Commun. 294, 968–975 [DOI] [PubMed] [Google Scholar]
- 66. Ferraris J. D., Persaud P., Williams C. K., Chen Y., Burg M. B. (2002) cAMP-independent role of PKA in tonicity-induced transactivation of tonicity-responsive enhancer/osmotic response element-binding protein. Proc. Natl. Acad. Sci. U.S.A. 99, 16800–16805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Irarrazabal C. E., Liu J. C., Burg M. B., Ferraris J. D. (2004) ATM, a DNA damage-inducible kinase, contributes to activation by high NaCl of the transcription factor TonEBP/OREBP. Proc. Natl. Acad. Sci. U.S.A. 101, 8809–8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Workman C. T., Yin Y., Corcoran D. L., Ideker T., Stormo G. D., Benos P. V. (2005) enoLOGOS: a versatile web tool for energy normalized sequence logos. Nucleic Acids Res. 33, W389–W392 [DOI] [PMC free article] [PubMed] [Google Scholar]