Background: SIgM and SIgA are emerging as therapeutic agents, but their mode of action requires further definition.
Results: We provide a functional demonstration of SIgM and SIgA in maintaining the integrity of intestinal epithelial cells exposed to Shigella flexneri.
Conclusion: SIgM is superior to SIgA in ensuring the homeostasis of intestinal epithelial cells challenged with Shigella flexneri.
Significance: SIgM and SIgA have potential as mucosal therapeutic agents.
Keywords: Cell Invasion, Chemokine, Confocal Microscopy, Cytokine Induction, Epithelial Cell, Host-Pathogen Interaction
Abstract
Intravenous administration of polyclonal and monoclonal antibodies has proven to be a clinically valid approach in the treatment, or at least relief, of many acute and chronic pathologies, such as infection, immunodeficiency, and a broad range of autoimmune conditions. Plasma-derived IgG or recombinant IgG are most frequently used for intravenous or subcutaneous administration, whereas a few IgM-based products are available as well. We have established recently that secretory-like IgA and IgM can be produced upon association of plasma-derived polymeric IgA and IgM with a recombinant secretory component. As a next step toward potential future mucosal administration, we sought to unravel the mechanisms by which these secretory Igs protect epithelial cells located at the interface between the environment and the inside of the body. By using polarized epithelial Caco-2 cell monolayers and Shigella flexneri as a model enteropathogen, we found that polyspecific plasma-derived SIgA and SIgM fulfill many protective functions, including dose-dependent recognition of the antigen via formation of aggregated immune complexes, reduction of bacterial infectivity, maintenance of epithelial cell integrity, and inhibition of proinflammatory cytokine/chemokine production by epithelial cells. In this in vitro model devoid of other cellular or molecular interfering partners, IgM and secretory IgM showed stronger bacterial neutralization than secretory IgA. Together, these data suggest that mucosally delivered antibody preparations may be most effective when combining both secretory-like IgA and IgM, which, together, play a crucial role in preserving several levels of epithelial cell integrity.
Introduction
Mucosal surfaces constantly exposed to a large variety of pathogens are protected by multilayer defense mechanisms. Among these, specific humoral mucosal immunity is dominated by secretory antibodies (Abs)3: secretory immunoglobulin A (SIgA) and secretory immunoglobulin M (SIgM). Both secretory Abs result from the transport across the epithelium of J chain-containing polymeric IgA (mostly dimeric) and pentameric IgM by the polymeric immunoglobulin receptor and exert a role of neutralizing Abs (1, 2). The role of specific SIgA at mucosal surfaces has been studied extensively, and its multiple functional facets extend from immune exclusion to homeostatic control of epithelial integrity (3, 4). As the most conserved Ab among vertebrate species, the importance of IgM has been appreciated for several decades. It is present early in immune development (5) and is known to be crucial in the primary mucosal immune response (6). Moreover, IgM is able to compensate for the lack of IgA in IgA-deficient individuals (7).
In vitro and in vivo studies have established the potential of specific, antigen-induced IgM in the systemic neutralization of viruses (8–10), bacteria (11–13), fungi (14), and parasites (15–17). Important advances have come especially from the use of IgM-deficient mice (18), which exhibited a high sensitivity to bacterial and viral infections (19), a condition that could be partly controlled upon administration of normal mouse immune serum (8).
Immunotherapy on the basis of the passive administration of human plasma-derived IgG has been used for three decades in clinical applications, with improvement of a large panel of disease conditions like immunodeficiencies, infections, or autoimmune diseases (20, 21). Preclinical and clinical studies have underscored the efficacy against various infectious agents of polyclonal IgM-enriched preparations administered by the systemic route (22–26). Similar to SIgA, SIgM can be seen as a valid candidate immunoglobulin for mucosal application, given its ability to bind antigens with strong avidity, its potential to ensure long term protection (27), its capacity to survive low pH conditions (28), as well as its resistance to proteases (29). We have demonstrated recently that human plasma can serve as a source of polyreactive, polymeric IgA (pIgA) and IgM to generate secretory-like IgA and IgM Abs, the natural molecular form found in secretions. We found that plasma-derived purified pIgA and IgM can associate with a recombinant secretory component (SC) with a 1:1 stoichiometry and that this association delayed the degradation of pIgA or IgM by intestinal washes containing proteases. In addition to these essential biochemical features, we showed that pIgA and secretory-like IgA delayed damage to epithelial polarized Caco-2 cell monolayers induced by a virulent strain of enteropathogenic Shigella flexneri (29). However, how the plasma-derived Ab operates to block the bacterium and contributes to epithelial homeostasis was not addressed in this study.
To provide answers to these open questions, we here dissect the mechanisms of protection conferred by plasma-derived pIgA and secretory-like IgA and then extend this study by evaluating the functionality of human plasma IgM and secretory-like IgM in the same experimental setting. We found that IgM or secretory-like IgM demonstrates a superior ability to maintain transepithelial electrical resistance (TER) and to forestall damage of cell monolayers resulting from S. flexneri infection when compared with pIgA or secretory-like IgA. Bacterial aggregates formed with both plasma pIgA and secretory-like IgA. This phenomenon was amplified upon association with IgM and secretory-like IgM, consistent with the capacity of all polyreactive Ab molecules to recognize S. flexneri. The diminished intracellular bacterial load varied as a function of the Ab isotype and resulted in differential production of proinflammatory mediators by the Caco-2 cell monolayers. In addition, incubation of secretory-like IgA and IgM resulted in reduced secretion of the S. flexneri virulence factors invasion plasmid antigen (Ipa) B and IpaC, overall suggesting a dual mode of action of the Abs, combining disabling of the bacteria and shielding of the target epithelium.
EXPERIMENTAL PROCEDURES
Preparation of Human Plasma IgA-, IgM- and IgG-enriched Fractions
IgA and IgM were purified from process intermediates of immunoglobulins manufactured from human plasma (30) by affinity chromatography using CaptureSelect human IgA and CaptureSelect human IgM resins (Bioaffinity Co.). The starting material used was a chromatographic side fraction consisting of the strip fraction from an ion exchange chromatography column used in the large scale manufacture of IgG from human plasma. The starting material was diluted in PBS to a target protein (IgA or IgM) concentration of ∼1 mg/ml and then loaded onto a CaptureSelect human IgA or IgM column pre-equilibrated with PBS without exceeding the IgA- or IgM-binding capacity of the column. After loading, the column was washed with PBS, and IgA or IgM was eluted with glycine buffer at pH 3.0. The eluate was adjusted with 0.5 m Tris base (pH 8.0) to pH 4.5 and concentrated up to 16 mg/ml protein. Human plasma IgG preparations (IgPro10, Privigen) were prepared as described previously (30). Monoclonal IgAC5 specific for S. flexneri LPS serotype 5a was produced via hybridoma cells as described previously (31).
Separation of Plasma-derived pIgA and Monomeric IgA (mIgA) and Purification of Plasma-derived IgM
IgA-enriched preparations containing a mixture of mIgA and pIgA were diluted in PBS to a final volume of 10 ml, suitable for injection onto the ÄKTAprime chromatography system (GE Healthcare). The flow rate was set at 1 ml/min, with PBS as mobile phase for all runs. Separation of the two molecular forms of IgA was performed on two serially coupled 1-m-long columns filled with Sephacryl S-300 high resolution (HR) beads (32). The IgA content of 3.5-ml fractions was verified by immunodetection using polyclonal rabbit anti-human IgA/HRP (1/3000, Dako), and pools of mIgA and pIgA were obtained. IgM-enriched preparations run under identical conditions yielded a single fully excluded peak. The IgM content of 3.5-ml fractions was verified by immunodetection using polyclonal rabbit anti-human IgM/HRP (1/3000, Dako). Concentration was performed using the Labscale system (Millipore) connected to a 100-kDa cutoff cartridge and stored at 4 °C until further use.
In Vitro Association of Polymeric Ig and Free SC
Recombinant human SC was produced from a CHO clone stably transfected with an expression cassette coding for the protein (33). Plasma-derived SIgA molecules were obtained by combining, in vitro, 10 μg of purified pIgA or IgM molecules with 2.5 or 1.5 μg of recombinant human SC, respectively. Mouse monoclonal SIgAC5 was obtained by combining, in vitro, 10 μg of purified pIgAC5 molecules with 2.5 μg of recombinant mouse SC. Association and characterization of SIgA and SIgM Abs were performed in PBS for 30 min at room temperature as described previously (29).
Caco-2 Cell Culture and Growth as a Polarized Monolayer
The human colonic adenocarcinoma epithelial Caco-2 cells (ATCC) were grown in complete DMEM consisting of DMEM-Glutamax (Invitrogen) supplemented with 10% FCS (Sigma), 10 mm HEPES (Invitrogen), 1% nonessential amino acids (Invitrogen), 1% sodium pyruvate (Invitrogen), 1% l-glutamine (Sigma), 1% penicillin/streptomycin (Sigma), and 0.1% transferrin (Invitrogen) and used between passages 32 and 40. Cells cultivated up to 80% confluency were seeded on polyester Snapwell filters (diameter, 12 mm; pore size, 0.4 μm; Corning Costar) at a density of 0.8 × 105 cells/cm2. At week 3, the Caco-2 cell monolayer integrity was checked by measuring the TER using the Millicell-electrical resistance system (ERS) device (Millipore) (34). TER values of well differentiated monolayers were in the range of 400–500 ohm/cm2.
Bacterial Strain and Culture Conditions
Bacteria used were the virulent strain of serotype 5a LPS S. flexneri M90T constitutively expressing GFP (35). Bacteria from frozen stock were grown on a Luria-Bertani (LB) agar plate containing 0.1‰ Congo red (Applichem) and 50 μg/ml ampicillin (Sigma-Aldrich) for 30 h at 37 °C. Three red colonies were amplified in 10 ml of LB liquid broth supplemented with 50 μg/ml ampicillin for 16 h at 37 °C and 200 rpm. The culture was centrifuged at 2000 × g for 5 min, resuspended in PBS, diluted 1:10 in 10 ml of LB liquid broth/ampicillin, and then cultured for 2 h at 37 °C with shaking (200 rpm). Finally, bacteria in the exponential phase were washed twice in PBS by centrifugation at 2000 × g for 5 min and resuspended in PBS. Assessment of cfu/ml was carried out by measurement of the optical density at 600 nm with the knowledge that 1 optical density unit at 600 nm corresponds to 5 × 108 cfu/ml.
Incubation of Bacteria with Different Ab Preparations
2 × 107 bacteria were mixed with 0.049 μm SIgAC5 specific for S. flexneri LPS serotype 5a or with human plasma-derived pIgA (0.61 μm), reconstituted SIgA (0.61 μm), mIgA (0.61 μm), IgM (0.61 μm), reconstituted SIgM (0.61 μm), or IgG (0.61 μm). All mixtures were prepared in a final volume of 500 μl of plain DMEM (DMEM complemented with 10 mm HEPES, 1% nonessential amino acids, 1% sodium pyruvate, 1% l-glutamine, and 0.1% transferrin). The mixtures were incubated for 1 h at room temperature under gentle agitation.
Protection Assay
One hour before the use of polarized Caco-2 cell monolayers, complete DMEM was replaced by plain DMEM in both the apical and basolateral compartments. Polarized Caco-2 cell monolayers were infected apically with S. flexneri serotype 5a alone or in combination with the Ab preparations. Exposure of Caco-2 cells to antigens or the various immune complexes was performed overnight (O/N), and pathogen-induced damage was tracked by measuring TER decrease over time.
Counting of Associated Bacteria with Cell Monolayers
To count internalized bacteria, cells on Snapwell filters were washed with PBS and incubated for 30 min with 50 μg/ml gentamicin, followed by incubation in 500 μl of cold lysis buffer (10 mm Tris-HCl (pH 7), 0.2% Nonidet P-40, 50 mm NaCl, and 2 mm EDTA (pH 8)) for 5 min on ice and lysis by up-and-down pipetting. S. flexneri present in cell lysates were counted from serial dilutions seeded onto LB agar plates. In control experiments, we checked that Ab-mediated aggregation did not result in an artifactual decrease in bacterial numbering, as determined by plating of S. flexneri alone or in complex with SIgA or SIgM (data not shown).
Laser-scanning Confocal Microscopy (LSCM) Observation of Caco-2 Cell Monolayers
To examine the integrity of Caco-2 cell monolayers, Snapwells were washed twice with PBS prior to fixation O/N with 5 ml of 4% paraformaldehyde at 4 °C. After washing, filters were permeabilized, and nonspecific binding sites were blocked with PBS containing 5% FCS and 0.2% Triton X-100 for 30 min at room temperature. All Abs were diluted in PBS containing 0.05% Tween 20. Filters were incubated with phalloidin associated to fluoprobes 547H (1:200, Interchim) for 90 min at room temperature and washed in PBS. To visualize cells, filters were incubated with 200 ng/ml of DAPI (Invitrogen) in PBS for 30 min. Filters were cut out of their holders and mounted in Vectashield solution (Vector Laboratories) for observation using a Zeiss LSM 710 Meta confocal microscope (Carl Zeiss, Germany) equipped with a ×40 objective. Snapshots of x-plan slices were performed with the Zeiss ZEN 2009 light software.
Quantification of the Infected Areas and the Number of Infection Foci
Observation of whole filters was carried out with the ×10 objective and the Zeiss ZEN 2009 light software. The sum of the infected areas and the number of infection foci were determined with the particle analysis tool of the ImageJ software applied onto the channel associated with the bacteria.
LSCM Observations of Immune Complexes
The formation of immune complexes was verified after incubation with biotinylated mouse anti-human IgA1/IgA2 (1:10, BD Biosciences) for 30 min at room temperature under gentle agitation, followed by cyanine 5-conjugated streptavidin (1:400, GE Healthcare) for 30 min at room temperature under gentle agitation. Three washes with PBS were performed between each step, and all Abs were diluted in PBS/5% FCS. Labeled immune complexes were laid onto glass slides (Thermo Scientific), mounted, and visualized immediately using a Zeiss LSM 710 Meta confocal microscope (Carl Zeiss) equipped with a ×40 objective. Images were processed with the Zeiss ZEN 2009 light software.
ELISA
Human CXCL8, TNF-α, and CCL3 in the basolateral compartment of polarized Caco-2 cell monolayers infected by S. flexneri alone or combined with Abs were measured by ELISA with commercial kits (BD Biosciences and R&D Systems). To examine the binding capacity of human plasma IgA/IgM to bacteria, 96-well plates (MaxiSorp, Nunc) were coated with 4 × 107 cfu/well of S. flexneri serotype 5a in PBS O/N at 4 °C. After three washes with PBS/0.05% Tween 20, wells were blocked PBS/0.05% Tween 20 containing 1% BSA (Fluka) for 1 h at room temperature. Serial dilutions of human plasma IgA, SIgA, IgM, SIgM, IgG, or mouse SIgAC5 (from 0.61 μm) were incubated in wells for 2 h at room temperature, washed with PBS/0.05% Tween 20, and then detection was performed by incubation with isotype-specific Abs (mouse anti-human IgA1/IgA2 biotinylated IgG (BD Biosciences, 1:1000), goat anti-human μ chain biotinylated IgG (KPL, 1:1000), goat anti-human γ chain biotinylated IgG (Sigma, 1:1000), or goat anti-mouse α chain biotinylated IgG (KPL, 1:1000 dilution) for 2 h at room temperature followed by Extravidin-HRP (Sigma, 1:5000 dilution) for 1 h at room temperature. All samples and Ab dilutions were performed in PBS/0.05% Tween 20 containing 0.1% BSA. Finally, detection was performed with citrate/phosphate solution (44.4 mm citric acid, 103 nm Na2HPO4 (pH 5.0)) containing 1 mg/ml O-phenylenediamine (Sigma) and 0.01% H2O2. The reactions were stopped with 1 m H2SO4. Absorbance was read at 490 nm with 630 nm as a reference.
Measurement of Virulence Factors IpaB and IpaC Secreted by S. flexneri
To examine the impact of Abs on the expression of virulence factors, the immune complexes bacteria-Abs were formed as described above. To induce type 3 secretion system-mediated secretion of Ipas, bacteria and immune complexes were exposed to 6 μg/ml Congo red for 10 min at 37 °C (36, 37). The supernatants were recovered by centrifugation and kept at −20 °C prior to use. The presence of secreted IpaB (42 kDa) and IpaC (62 kDa) in the supernatant was analyzed by immunodetection with 1:1000 dilutions of specific mouse monoclonal Abs (provided by Dr. Edwin Oaks, Walter Reed Army Institute of Research (WRAIR), Silver Spring, MD), followed by HRP-conjugated rabbit anti-mouse IgG (Sigma, 1:3000). In parallel, probing of outer Shigella protein F (OspF) (27.5 kDa), similarly secreted in a T3SS-dependent manner, was used as a loading control of gels (38). Densitometric analyses were standardized to the signal produced by OspF, and secretion of IpaB and IpaC was depicted arbitrarily as 100% under the condition S. flexneri alone (Sf).
Statistical Analysis
Results are expressed as mean ± S.E. of n determinations. Differences in TER, cytokine secretion, number of foci, infected areas, and secretion of IpaB and IpaC between the control group (Sf alone) and in the presence of Abs were tested by analysis of variance (parametric, two-tailed). Differences were considered as significant when p < 0.05. Graph generation and statistical analyses were performed using GraphPad Prism software version 6.
RESULTS
Human Plasma pIgA and SIgA Abs Agglutinate Bacteria Lead to a Decrease of the Bacterial Load in Caco-2 Cell Monolayers and of Proinflammatory Cytokines/Chemokines
We demonstrated previously that human plasma-derived pIgA and reconstituted SIgA, but not plasma mIgA, significantly maintained Caco-2 cell intestinal epithelial monolayer integrity after O/N infection with S. flexneri by preventing the reduction of TER, the disruption of the tight junction network, and the detachment of filter-bound Caco-2 cells (29). However, the underlying mechanisms explaining protection were not addressed. To better define the mode of action of human plasma pIgA and SIgA, we first focused on the nature of the interaction between the bacteria and the Abs. The binding capacity of the various molecular forms of plasma IgA to S. flexneri was compared by ELISA. All molecular forms of IgA demonstrated a concentration-dependent ability to bind bacteria. At identical concentrations, the signal for pIgA or SIgA was 3- to 4-fold stronger than the signal observed with mIgA (Fig. 1A), emphasizing the avidity effect associated with polymeric Abs. To gain further insight into the nature of the interaction, immune complexes between cyanine 5-labeled Abs and GFP-expressing bacteria were formed and visualized directly by LSCM. Specific SIgAC5 and plasma-derived IgG were assessed for comparison. All molecular forms bound to S. flexneri, but bacterial aggregates of increasing size formed upon association with human plasma pIgA and SIgA, demonstrating that only polymeric IgA was capable of engaging bacteria in complex lattices (Fig. 1B). Interestingly, the large aggregate pattern resembles that formed with S. flexneri LPS-specific SIgAC5 (31).
FIGURE 1.
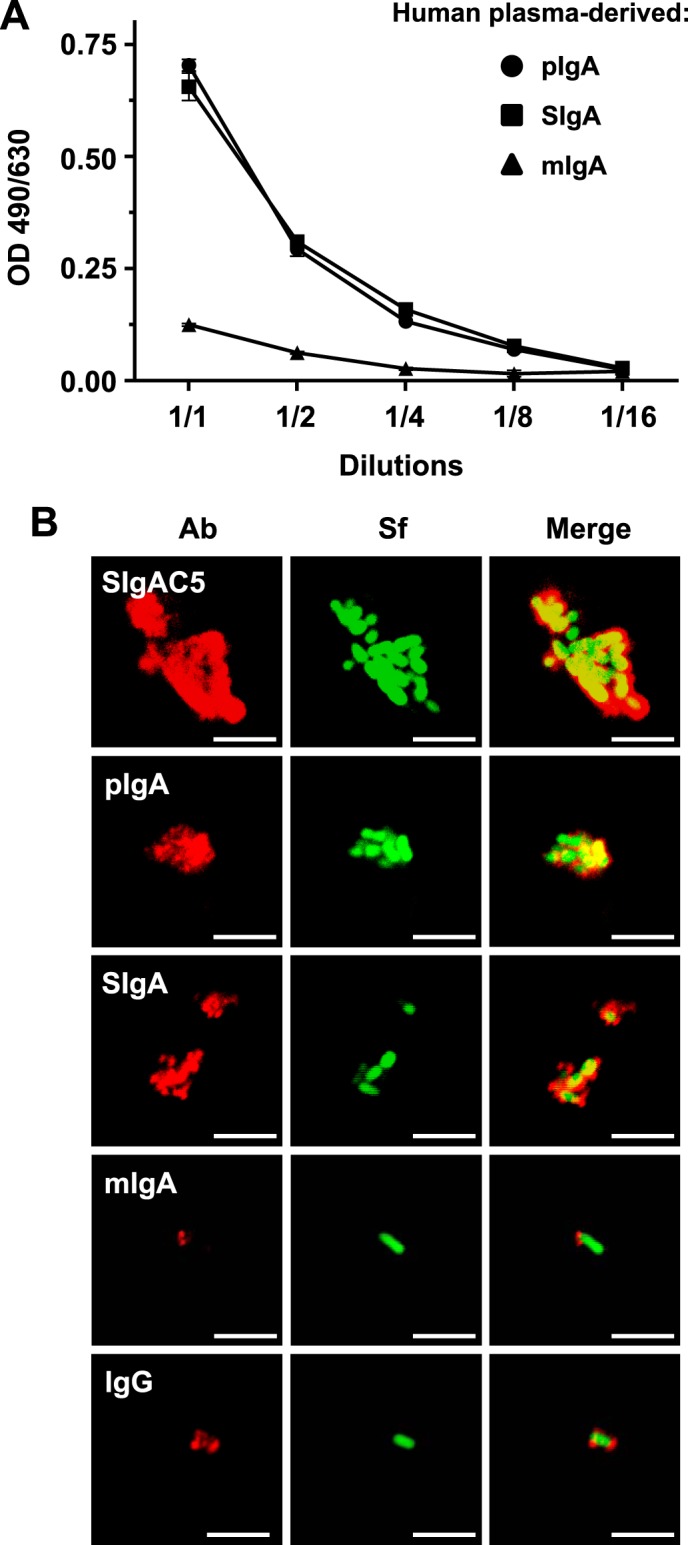
Association of human plasma-derived IgA/SIgA with S. flexneri. A, binding of equimolar concentrations of pIgA, reconstituted SIgA, or mIgA to immobilized S. flexneri as determined by ELISA. Successive dilutions of the various molecular forms of IgA were assessed, with the 1:1 ratio corresponding to 0.61 μm of each respective Ab. Data are representative of two independent experiments performed in duplicates. B, LSCM images of immune complexes of bacteria associated with human plasma-derived pIgA, SIgA, mIgA, IgG, or anti-S. flexneri LPS-specific SIgAC5 monoclonal Ab. Bacteria constitutively expressing GFP appear in green. Bound Abs were detected by antisera directed against the α or γ chain, followed by Abs conjugated to fluorophores, yielding red signals after image processing. Images are representative of one representative field obtained from 15 observations from three independent slides per experiment. Scale bars = 10 μm.
We next examined whether sequestering bacteria in immune complexes would influence the number of infecting bacteria found in Caco-2 cells. Although specific SIgAC5 monoclonal Abs were most effective at blocking bacterial internalization, we found that the presence of either of the plasma-derived Abs reduced the number of internalized bacteria 2-fold on average (Fig. 2A). The effect has to be considered as a trend only because statistical significance was not reached. In the same experimental setting, we then studied the effect of the molecular forms of plasma IgA on the inflammatory response of infected cell monolayers. In the presence of pIgA or SIgA, basolateral secretion of TNF-α, CCL3, and CXCL8 was less than half the level of production measured upon infection with S. flexneri alone (Fig. 2B). This inhibition of cytokine/chemokine production was in the same range as that obtained with specific SIgAC5, whereas mIgA and (monomeric) IgG had no effect on the secretion of the three proinflammatory mediators (Fig. 2B). We conclude that the various molecular forms of plasma IgA and plasma IgG directly bind to bacteria but with distinct consequences on infection or responsiveness of target epithelial cells, depending on the size of formed immune complexes.
FIGURE 2.
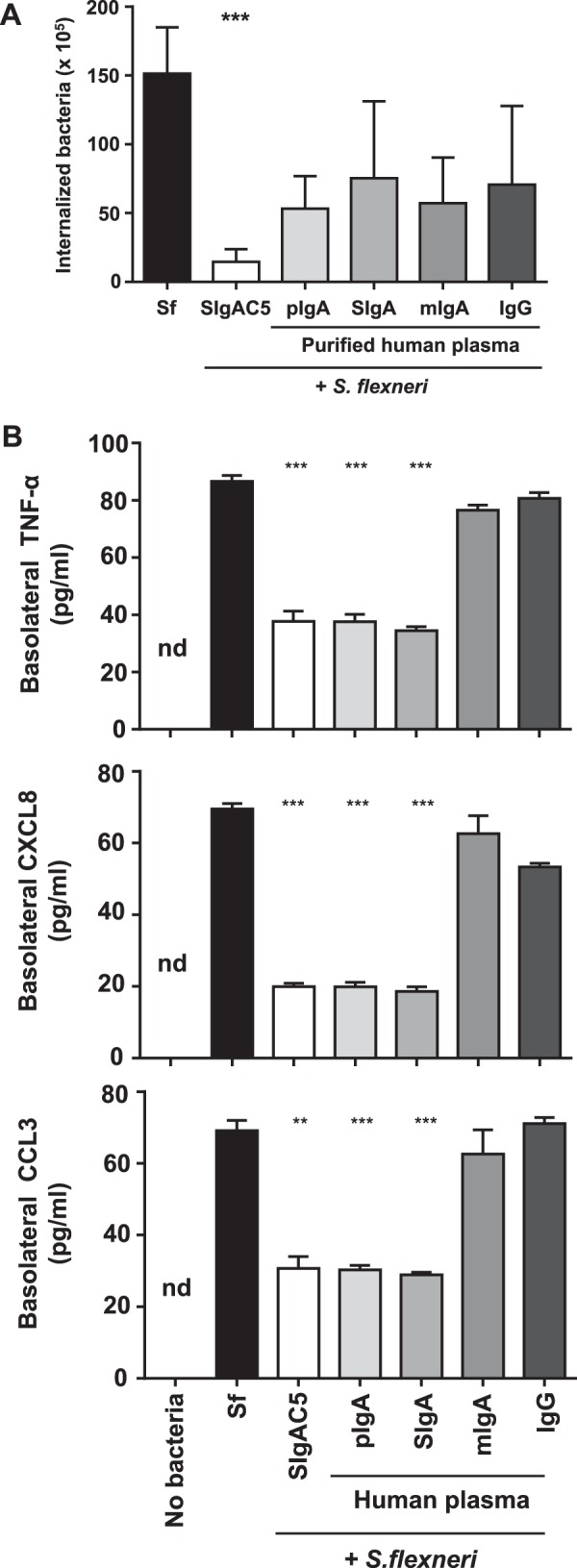
Modulatory effect of various human plasma-derived IgA/SIgA preparations on Caco-2 cells infected by S. flexneri. A, internalization of S. flexneri alone or associated with SIgAC5 monoclonal Ab and with plasma-derived pIgA, reconstituted SIgA, mIgA, or IgG to polarized Caco-2 cell monolayers, as determined after O/N incubation, with addition of gentamicin for the last 30 min. Data are expressed on a “per filter” basis and correspond to a pool of two independent experiments (n = 4 or 5) for each tested condition. B, basolateral secretion of TNF-α, CXCL8, and CCL3 by polarized Caco-2 cell monolayers after O/N incubation with S. flexneri alone or associated to plasma-derived pIgA, SIgA, mIgA, IgG, or anti-Shigella LPS-specific SIgAC5 monoclonal Ab. The concentration of proinflammatory mediators was determined by ELISA. Non-infected Caco-2 cell monolayers (No bacteria) served as a control. Data are the pool of two experiments performed in triplicates (n = 6). When obtained, significant statistical differences were calculated by comparison with the condition Sf. **, p < 0.01; ***, p < 0.001; nd, non-detectable.
Human plasma IgM and SIgM Abs Induce Bacterial Agglutination, Reducing Interactions between S. flexneri and Caco-2 Cell Monolayers
Because the avidity of polyspecific pIgA/SIgA seems to be crucial to limit Caco-2 cell monolayer infection by S. flexneri, we sought to determine whether plasma-derived IgM would achieve similar functions. As shown in Fig. 3A, IgM and SIgM demonstrated concentration-dependent binding to S. flexneri. LSCM imaging revealed large aggregates comprising bacteria and either IgM or SIgM Abs (Fig. 3B), whose sizes were well above that detected previously after incubation with SIgA. This prompted us to speculate that such a strong agglutination capacity may result in blocking the internalization of S. flexneri by Caco-2 cell monolayers. After O/N infection with the bacteria alone, or in complex with IgM, SIgM or SIgA, cells were treated with gentamicin for 30 min, lysed, and then the lysate was plated on a selective medium for numeration. In comparison with bacteria alone, an at least 15-fold reduction in the number of cell-internalized bacteria was measured when associated with either IgM or SIgM (Fig. 4A), whereas a 2- to 3-fold decrease was observed in the presence of SIgA (Figs. 2A and Fig. 4A). These results illustrate a strong ability of IgM and SIgM to neutralize the bacteria via aggregation.
FIGURE 3.
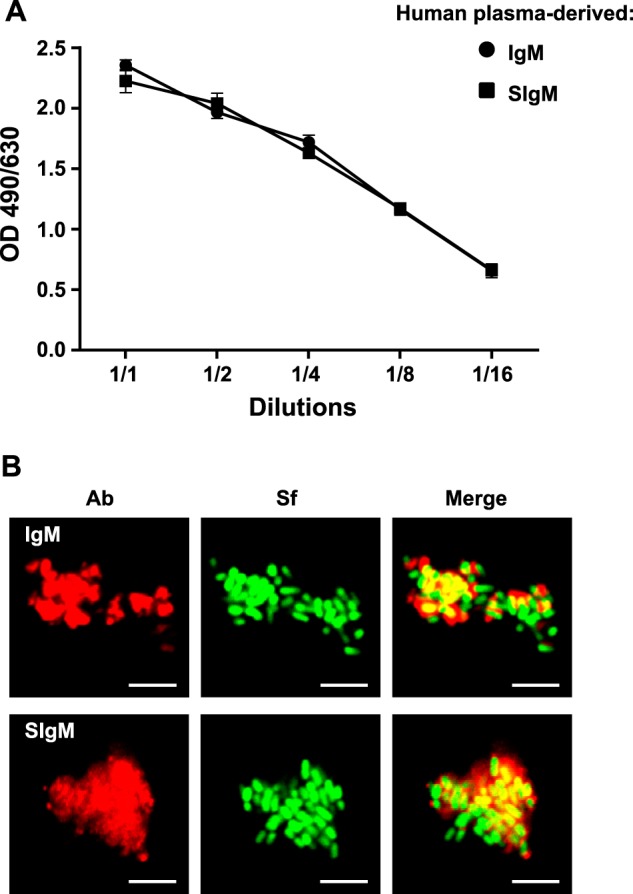
Association of human plasma-derived IgM/SIgM with S. flexneri. A, binding of IgM and SIgM to S. flexneri determined by ELISA. Bacteria coated in well plates were incubated with IgM or reconstituted SIgM at decreasing concentrations, with the 1:1 ratio corresponding to 0.61 μm of either Ab. Data are representative of two experiments performed in duplicates. B, LSCM pictures of immune complexes formed between bacteria and human plasma-derived IgM or SIgM. Bacteria constitutively expressing GFP are shown in green, whereas IgM and SIgM detected μ chain-specific and fluorescent Abs appear in red. Images are representative of one representative field obtained from 10 observations from two independent slides per experiment. Scale bars = 10 μm.
FIGURE 4.
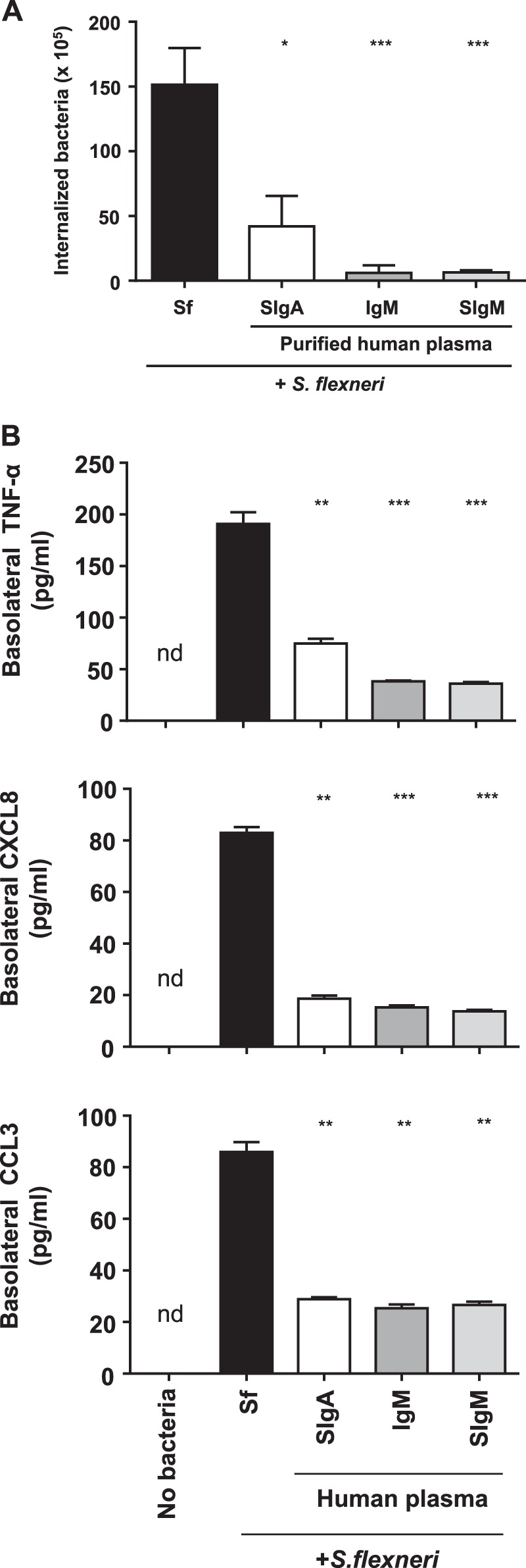
Modulatory effect of various human plasma-derived IgM/SIgM preparations on Caco-2 cells infected by S. flexneri. A, bacteria internalized within Caco-2 cell monolayers determined after O/N infection by S. flexneri alone or in complex with human plasma IgM, reconstituted SigM, or SIgA. Bacterial counts were carried out after addition of gentamicin for the last 30 min of incubation. Data are expressed on a per filter basis and correspond to a pool of two independent experiments (n = 4 or 5) for each tested condition. B, basolateral secretion of TNF-α, CXCL8, and CCL3 by polarized Caco-2 cell monolayers after O/N incubation with S. flexneri alone or associated with plasma-derived IgM, SigM, or SIgA. The concentration of proinflammatory mediators was determined by ELISA. Non-infected Caco-2 cell monolayers (No bacteria) served as a control. Data are the pool of two experiments performed in triplicates (n = 6). When obtained, significant statistical differences were calculated by comparison with the condition Sf. *, p < 0.01; **, p < 0.01; ***, p < 0.001; nd, non-detectable.
Human Plasma IgM and SIgM Abs Diminish the Secretion of Proinflammatory Chemokines and Cytokines by Caco-2 Cell Monolayers
The finding that plasma IgM and SIgM Abs appear superior to SIgA for all parameters tested so far led us to hypothesize that this should hold true when examining the proinflammatory responsiveness of Caco-2 cell monolayers exposed to the bacterium alone or in complex with Abs. Measurement of the basolateral secretion of TNF-α, CCL3, and CXCL8 by ELISA after O/N incubation showed that cell monolayers infected by SIgA-, IgM- or SIgM-S. flexneri complexes released at least 3-fold less TNF-α, CXCL8, and CCL3 than monolayers infected by the bacteria alone (Fig. 4B). Hence, neutralization of S. flexneri by either IgM or SIgM had the most marked effect on the proinflammatory response of the polarized Caco-2 cell monolayer of all Abs tested, and, importantly, this occurs in the absence of any other cell partner that could have biased the analysis.
Human Plasma IgM and SIgM Abs Efficiently Prevent Damage to Epithelial Caco-2 Cell Monolayers Infected by S. flexneri
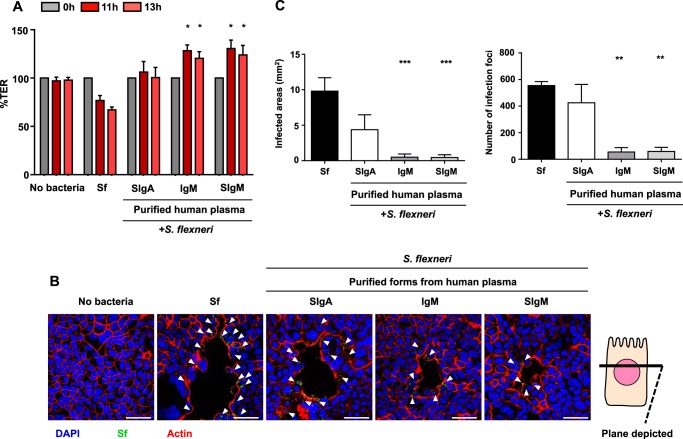
Human plasma-derived IgM and reconstituted SIgM at the same molar concentration as SigA, serving as a reference control, were combined with S. flexneri and incubated O/N with Caco-2 cell monolayers. The integrity of the cell monolayers was assessed by TER measurement, cell morphology, and the number of, and total surface of, infected foci on the whole filters. In contrast to S. flexneri alone, TER was maintained when the bacteria were mixed with IgM or SIgM in a range similar to that observed with SIgA (Fig. 5A). Representative snapshots of transversal sections obtained along the x axis of the monolayers showed that the infected areas were systematically smaller after incubation with IgM and SIgM compared with incubation with SIgA or with S. flexneri alone (Fig. 5B). Detection of the preserved, well organized actin network in IgM-treated samples confirmed the improved monolayer integrity (Fig. 5B), as exemplified by limited binding and spreading of S. flexneri. When compared with bacteria alone, complexes with plasma SIgA diminished the total surface of infection foci 2-fold while slightly reducing their number (Fig. 5, C and D). Plasma IgM and SIgM showed a 15-fold and 10-fold reduction for these two parameters, respectively, reflecting lower damage of the monolayer (Fig. 5, C and D). Therefore, both IgM and SIgM prevent the destruction of Caco-2 cell monolayers exposed to infectious S. flexneri to a degree superior to SIgA.
FIGURE 5.
Integrity of Caco-2 cell monolayers infected with S. flexneri alone or in combination with human plasma-derived IgM/SIgM. A, TER of Caco-2 cell monolayers exposed O/N to S. flexneri alone or associated with human IgM, reconstituted SIgM, and plasma-derived SIgA (control), as determined at three time points. The TER values for each condition and each time point were normalized to the TER values at time 0 and are expressed as percentages. Data are a pool of two independent experiments (n = 4). Significant statistical differences were calculated by comparison with the condition Sf. *, p < 0.05. B, LSCM pictures (snapshots) of transversal sections obtained along the x axis of Caco-2 cell monolayers exposed O/N to S. flexneri alone or in complex with IgM, SigM, or SIgA. Actin fibers are visualized by phalloidin labeling (red), Caco-2 cells are visualized via nuclear staining with DAPI (blue), and bacteria constitutively expressing GFP stained green. Arrowheads indicate the loci of dense S. flexneri. Scale bar = 50 μm. C and D, for quantitative analysis, the sum of infected areas (C) and the number of infection foci (D) were determined from LSCM pictures of whole filters using ImageJ software. When obtained, significant statistical differences were calculated by comparison with the condition Sf. **, p < 0.005; ***, p < 0.001. The data in C and D are a pool of two independent experiments (n = 4 or 5).
Human Plasma Secretory-like Abs Impact the Secretion of the Virulence Factors IpaB and IpaC Released by S. flexneri
In addition to its protective properties, the specific anti-S. flexneri IgAC5 monoclonal Ab is known to mediate a transient suppression of the type 3 secretion system of the bacterium, leading to a decrease in secretion of the virulence factor known as IpaB (37). To examine whether polyreactive human plasma-derived IgA or IgM could act via a similar mechanism, we evaluated the secretion of IpaB and IpaC under identical conditions as those used to form immune complexes in protection experiments. S. flexneri was associated with human plasma pIgA, SIgA, mIgA, IgM, SIgM, IgG, or specific IgAC5 monoclonal Ab for 1 h or left as such. Although bacteria not exposed to Congo red secreted a low basal level of IpaB and no IpaC, exposure to Congo red led to secretion of various levels of IpaB and IpaC in supernatants as a function of the complexing Ab. The level of secretion of both IpaB and IpaC by bacteria in complex with human plasma SIgA or SIgM was decreased (Fig. 6, A and B). In comparison with the bacterium alone, the other molecular forms of IgA, IgM, as well as IgG and SIgAC5, did not lead to measurable changes (Fig. 6C). This result suggests that the secretory form of plasma-derived IgA or IgM may contribute indirectly to protection of target epithelial surfaces through its impact on bacterial virulence.
FIGURE 6.
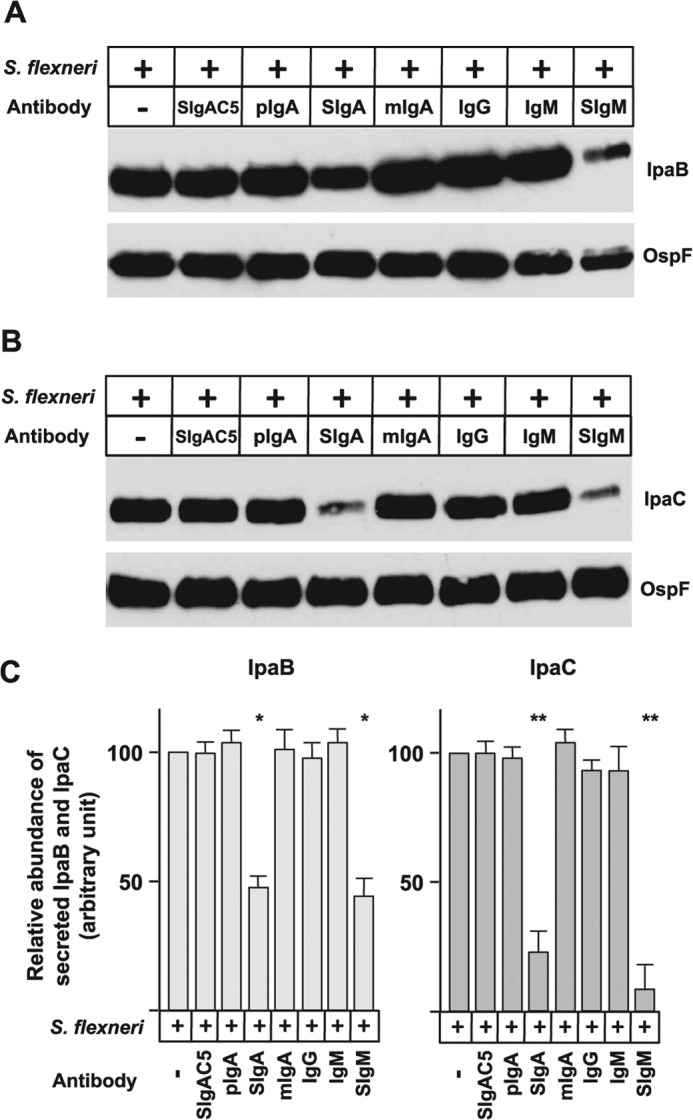
Impact of human plasma-derived IgA and IgM preparations on the secretion of virulence factors IpaB and IpaC by S. flexneri. Immune complexes between bacteria and human plasma-derived pIgA, SIgA, mIgA, IgG, IgM, SigM, or anti-Shigella LPS-specific SIgAC5 monoclonal Ab were formed for 1 h as described under “Experimental procedures,” and the expression of virulence factors was induced by Congo red. A and B, the secretion of IpaB (A) and IpaC (B) was examined by immunoblot analysis using monoclonal Abs directed against IpaB and IpaC, with immunodetection of OspF serving as a loading control. The images are representative of one individual experiment performed three times in duplicates. C, densitometric analysis of replicated immunoblots (n = 6) exposed for optimal times to avoid saturation of the photographic film. The intensity of the signals reached with S. flexneri alone was fixed arbitrarily at 100%. When obtained, significant statistical differences were calculated by comparison with the condition Sf. *, p < 0.02; **, p < 0.005.
DISCUSSION
We established previously that human plasma-derived pIgA and IgM can be assembled into secretory-like Abs (29). However, how the various molecular forms of the Ab displayed differential protection was not evaluated at the cellular and molecular levels. This study addresses these issues and further extends the analysis to plasma-derived IgM and SIgM. We found that pIgA and IgM or the secretory form of the Ab recognized S. flexneri to the same extent and that the interaction led to the formation of aggregates with a size dependent on the valence of the Ab. Although reduced bacteria internalization into the cell monolayer occurred with all Ab isotypes and molecular forms tested, bacteria-induced damage to the monolayer organization was diminished significantly with polymeric and secretory-like Abs only, as identified by TER measurement, cell imaging, quantification of infected foci, and assessment of areas exhibiting monolayer destruction as well as secretion of proinflammatory mediators. Moreover, plasma-derived Abs were found to disable S. flexneri in its capacity to produce IpaB and IpaC, two proteins involved in the invasion of epithelial cells. A direct comparison with SIgA led to the conclusion that IgM and SIgM Ab molecules demonstrate a superior activity in preserving the integrity and responsiveness to infection of polarized Caco-2 cell monolayers used as a mimic of the gut mucosal epithelium.
Similar in vitro models have been used previously to compare the neutralizing function of antigen-specific monoclonal IgG and IgA/SIgA (31, 39, 40). Although one has to acknowledge that the Caco-2 cell line may not recapitulate all functions of normal intestinal epithelial cells, their pattern of cytokines following exposure to S. flexneri was consistent with that of other cell lines and that found in vivo (41, 42). The availability of polyreactive IgA and IgM Abs with well characterized biochemical properties (29) allowed us to directly address their modes of action in the absence of any other cellular and molecular partners involved in clearance of S. flexneri, therefore allowing a comparison of their respective functional characteristics.
Remarkably, for most of the parameters examined, in comparison with the specific protective SIgAC5 monoclonal Ab, polyreactive pIgA and SIgA displayed similar degrees of protection at a 10-fold higher concentration only. Both IgM and SIgM proved to be even more potent. This is particularly true for blocking of internalization, for maintenance of the polarized Caco-2 cell monolayer integrity, and, to a lesser extent, for reduction in the production of proinflammatory TNF-α. This may be explained by the observation that apical immune exclusion appears to be the most potent function of multivalent IgM Abs. Strikingly, agglutination mediated by pIgA and SIgA did not reduce the internalization of bacteria with a better efficiency than mIgA and IgG. This contrasts with the observation that LPS-specific monomeric IgAC5 and IgGC20 monoclonal Abs perform more poorly than their SIgA counterpart recognizing the same epitope (31). This suggests that the polyclonal nature of plasma-derived mIgA and IgG masking both LPS and bacterial adhesins impairs bacterial entry more efficiently. Alternatively, it may block intracellular bacterial proliferation without impacting epithelial cell responsiveness, such as morphological changes and secretion of proinflammatory mediators. In addition, this reveals that the sensing of antigens by epithelial cells might differ depending on the molecular form of the Abs participating in the immune complexes as, for instance, intracellular processing pathways (43).
The presence of SC in reconstituted SIgA and SIgM did not modify in any way the function of the Ab molecule in our model, with the notable exception of the effect on IpaB and IpaC secretion by S. flexneri. However, in vivo, the presence of bound SC is essential for the stability and anchoring of the molecule at mucosal surfaces (3). Furthermore, in addition to interfering with bacterial targeting of epithelial cells via carbohydrates abundantly found on its surface (44), SC associated with pIgA and IgM appears to intervene negatively in the secretion of S. flexneri virulence factors crucial for the infection of epithelial cells (45, 46). These results suggest the unexpected role of polyclonal SIgA and SIgM mostly in altering bacterial metabolism, which adds to recognized mechanisms of protection effective at the level of the mucosal epithelium.
Previous animal studies have demonstrated that administration of polyclonal IgM molecules, especially derived from human plasma, could be beneficial in case of sepsis. Lachmann et al. (25) showed that IgM-enriched preparations reduced Klebsiella pneumoniae infection using a distress syndrome rat model. Stehr et al. (26) emphasized the benefits of polyclonal IgM-enriched solution by using a rabbit model of bacteremia. Clinical trials showed that IgM-enriched preparations were able to significantly decrease endotoxin levels in plasma (23) and even reduce mortality (22) during the early phase of septic shock. Norrby-Teglund et al. (47) showed that IgM-enriched preparations were able to inhibit specific streptococcal antigens. However, as a limitation to the interpretation of these results, IgM-enriched preparations contained significant amounts of IgG and/or IgA Ab molecules, making it difficult to strictly assign the protective effect to the IgM moiety of the product. These in vivo studies, together with our current demonstration of epithelial cell protection with IgM/SigM, may suggest that mucosal passive delivery of IgM via the gastrointestinal and nasal routes are worth considering.
Although systemic IgM-based immune complexes may lead to the activation of complement, mucosal delivery along the gut is not expected to trigger such a process because the antibody distribution will be restricted to the lumen. In addition, previous research has shown that epithelial cells and follicular DCs in germinal centers producing cell membrane complement regulatory proteins (protectin (CD59), membrane cofactor protein, and decay-accelerating factor (CD55) are protected from the action of complement (48, 49). Together with SIgA exhibiting an important reduction in cytokine release as well, this would combine protection and low proinflammatory responses by the epithelial cells located at the interface between the environment and the inside of the body. In addition to potential clinical applications against infectious agents, it may be appealing to apply IgA and IgM preparations topically to patients with primary immune deficiency, which is associated with chronic infection and autoimmunity (50).
In the in vivo context, one can argue that commensal bacteria may act as a “trap” for exogenously delivered SIgA/SIgM. However, the fact that a large percentage of bacteria are coated with endogenous SIgA (51) at a steady state suggests that administration of milligram amounts of either SIgA or SIgM should leave enough operative Ab molecules available. A plausible explanation would be that both non-antigen-specific (possibly mediated by carbohydrate moieties (52) and specific interactions contribute to the recognition of bacterial antigens by the polyreactive Abs.
Acknowledgment
We thank Marlies Illi (CSL Behring) for purification of IgA and IgM.
This work was supported by Swiss Science Research Foundation Grant 3100-138422.
- Ab
- antibody
- SIgA
- secretory IgA
- SIgM
- secretory IgM
- pIgA
- polymeric IgA
- SC
- secretory component
- TER
- transepithelial electrical resistance
- Ipa
- invasion plasmid antigen
- mIgA
- monomeric IgA
- LB
- Luria-Bertani
- O/N
- overnight
- LSCM
- laser-scanning confocal microscopy
- Sf
- S. flexneri alone
- OspF
- outer Shigella protein F.
REFERENCES
- 1. Brandtzaeg P., Prydz H. (1984) Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature 311, 71–73 [DOI] [PubMed] [Google Scholar]
- 2. Klimovich V. B. (2011) IgM and its receptors: structural and functional aspects. Biochemistry 76, 534–549 [DOI] [PubMed] [Google Scholar]
- 3. Corthésy B. (2013) Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 10.3389/fimmu.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brandtzaeg P. (2013) Secretory IgA: designed for anti-microbial defense. Front. Immunol. 10.3389/fimmu.2013.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gronwall C., Vas J., Silverman G. J. (2012) Protective roles of natural IgM antibodies. Front. Immunol. 10.3389/fimmu.2012.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Croft N. M., Hodges M. (2005) IgM: mucosal response in acute diarrhoeal disease of infants. Scand. J. Gastroenterol. 40, 965–971 [DOI] [PubMed] [Google Scholar]
- 7. Brandtzaeg P., Karlsson G., Hansson G., Petruson B., Björkander J., Hanson L. A. (1987) The clinical condition of IgA-deficient patients is related to the proportion of IgD- and IgM-producing cells in their nasal mucosa. Clin. Exp. Immunol. 67, 626–636 [PMC free article] [PubMed] [Google Scholar]
- 8. Baumgarth N., Herman O. C., Jager G. C., Brown L. E., Herzenberg L. A., Chen J. (2000) B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192, 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diamond M. S., Sitati E. M., Friend L. D., Higgs S., Shrestha B., Engle M. (2003) A critical role for induced IgM in the protection against West Nile virus infection. J. Exp. Med. 198, 1853–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seiler P., Kalinke U., Rülicke T., Bucher E. M., Böse C., Zinkernagel R. M., Hengartner H. (1998) Enhanced virus clearance by early inducible lymphocytic choriomeningitis virus-neutralizing antibodies in immunoglobulin-transgenic mice. J. Virol. 72, 2253–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boes M., Prodeus A. P., Schmidt T., Carroll M. C., Chen J. (1998) A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188, 2381–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salinas-Carmona M. C., Pérez-Rivera I. (2004) Humoral immunity through immunoglobulin M protects mice from an experimental actinomycetoma infection by Nocardia brasiliensis. Infect. Immun. 72, 5597–5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alugupalli K. R., Gerstein R. M., Chen J., Szomolanyi-Tsuda E., Woodland R. T., Leong J. M. (2003) The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 170, 3819–3827 [DOI] [PubMed] [Google Scholar]
- 14. Subramaniam K. S., Datta K., Quintero E., Manix C., Marks M. S., Pirofski L. A. (2010) The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J. Immunol. 184, 5755–5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Couper K. N., Roberts C. W., Brombacher F., Alexander J., Johnson L. L. (2005) Toxoplasma gondii-specific immunoglobulin M limits parasite dissemination by preventing host cell invasion. Infect. Immun. 73, 8060–8068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Konishi E., Nakao M. (1992) Naturally occurring immunoglobulin M antibodies: enhancement of phagocytic and microbicidal activities of human neutrophils against Toxoplasma gondii. Parasitology 104, 427–432 [DOI] [PubMed] [Google Scholar]
- 17. Baral T. N., De Baetselier P., Brombacher F., Magez S. (2007) Control of Trypanosoma evansi infection is IgM mediated and does not require a type I inflammatory response. J. Infect. Dis. 195, 1513–1520 [DOI] [PubMed] [Google Scholar]
- 18. Ehrenstein M. R., O'Keefe T. L., Davies S. L., Neuberger M. S. (1998) Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc. Natl. Acad. Sci. U.S.A. 95, 10089–10093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boes M., Esau C., Fischer M. B., Schmidt T., Carroll M., Chen J. (1998) Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160, 4776–4787 [PubMed] [Google Scholar]
- 20. Orange J. S., Hossny E. M., Weiler C. R., Ballow M., Berger M., Bonilla F. A., Buckley R., Chinen J., El-Gamal Y., Mazer B. D., Nelson R. P., Jr., Patel D. D., Secord E., Sorensen R. U., Wasserman R. L., Cunningham-Rundles C., Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology (2006) Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J. Allergy. Clin. Immunol. 117, S525–S553 [DOI] [PubMed] [Google Scholar]
- 21. Durandy A., Kaveri S. V., Kuijpers T. W., Basta M., Miescher S., Ravetch J. V., Rieben R. (2009) Intravenous immunoglobulins: understanding properties and mechanisms. Clin. Exp. Immunol. 158, 2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schedel I., Dreikhausen U., Nentwig B., Höckenschnieder M., Rauthmann D., Balikcioglu S., Coldewey R., Deicher H. (1991) Treatment of Gram-negative septic shock with an immunoglobulin preparation: a prospective, randomized clinical trial. Crit. Care Med. 19, 1104–1113 [DOI] [PubMed] [Google Scholar]
- 23. Behre G., Schedel I., Nentwig B., Wörmann B., Essink M., Hiddemann W. (1992) Endotoxin concentration in neutropenic patients with suspected Gram-negative sepsis: correlation with clinical outcome and determination of anti-endotoxin core antibodies during therapy with polyclonal immunoglobulin M-enriched immunoglobulins. Antimicrob. Agents Chemother. 36, 2139–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Norrby-Teglund A., Haque K. N., Hammarström L. (2006) Intravenous polyclonal IgM-enriched immunoglobulin therapy in sepsis: a review of clinical efficacy in relation to microbiological aetiology and severity of sepsis. J. Intern. Med. 260, 509–516 [DOI] [PubMed] [Google Scholar]
- 25. Lachmann R. A., van Kaam A. H., Haitsma J. J., Verbrugge S. J., Delreu F., Lachmann B. (2004) Immunoglobulin M-enriched intravenous polyclonal immunoglobulins reduce bacteremia following Klebsiella pneumoniae infection in an acute respiratory distress syndrome rat model. Exp. Lung Res. 30, 251–260 [DOI] [PubMed] [Google Scholar]
- 26. Stehr S. N., Knels L., Weissflog C., Schober J., Haufe D., Lupp A., Koch T., Heller A. R. (2008) Effects of IGM-enriched solution on polymorphonuclear neutrophil function, bacterial clearance, and lung histology in endotoxemia. Shock 29, 167–172 [DOI] [PubMed] [Google Scholar]
- 27. Racine R., McLaughlin M., Jones D. D., Wittmer S. T., MacNamara K. C., Woodland D. L., Winslow G. M. (2011) IgM production by bone marrow plasmablasts contributes to long-term protection against intracellular bacterial infection. J. Immunol. 186, 1011–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mueller M., Loh M. Q., Tscheliessnig R., Tee D. H., Tan E., Bardor M., Jungbauer A. (2013) Liquid formulations for stabilizing IgMs during physical stress and long-term storage. Pharm. Res. 30, 735–750 [DOI] [PubMed] [Google Scholar]
- 29. Longet S., Miled S., Lötscher M., Miescher S. M., Zuercher A. W., Corthésy B. (2013) Human plasma-derived polymeric IgA and IgM antibodies associate with secretory component to yield biologically active secretory-like antibodies. J. Biol. Chem. 288, 4085–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cramer M., Frei R., Sebald A., Mazzoletti P., Maeder W. (2009) Stability over 36 months of a new liquid 10% polyclonal immunoglobulin product (IgPro10, Privigen) stabilized with l-proline. Vox Sang. 96, 219–225 [DOI] [PubMed] [Google Scholar]
- 31. Mathias A., Longet S., Corthésy B. (2013) Agglutinating secretory IgA preserves intestinal epithelial cell integrity during apical infection by Shigella flexneri. Infect. Immun. 81, 3027–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Favre L. I., Spertini F., Corthésy B. (2003) Simplified procedure to recover recombinant antigenized secretory IgA to be used as a vaccine vector. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 786, 143–151 [DOI] [PubMed] [Google Scholar]
- 33. Phalipon A., Cardona A., Kraehenbuhl J. P., Edelman L., Sansonetti P. J., Corthésy B. (2002) Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17, 107–115 [DOI] [PubMed] [Google Scholar]
- 34. Cottet S., Corthésy-Theulaz I., Spertini F., Corthésy B. (2002) Microaerophilic conditions permit to mimic in vitro events occurring during in vivo Helicobacter pylori infection and to identify Rho/Ras-associated proteins in cellular signaling. J. Biol. Chem. 277, 33978–33986 [DOI] [PubMed] [Google Scholar]
- 35. Rathman M., Jouirhi N., Allaoui A., Sansonetti P., Parsot C., Tran Van Nhieu G. (2000) The development of a FACS-based strategy for the isolation of Shigella flexneri mutants that are deficient in intercellular spread. Mol. Microbiol. 35, 974–990 [DOI] [PubMed] [Google Scholar]
- 36. Bahrani F. K., Sansonetti P. J., Parsot C. (1997) Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect. Immun. 65, 4005–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Forbes S. J., Bumpus T., McCarthy E. A., Corthésy B., Mantis N. J. (2011) Transient suppression of Shigella flexneri type 3 secretion by a protective O-antigen-specific monoclonal IgA. MBio 10.1128/mBio.00042-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zurawski D. V., Mitsuhata C., Mumy K. L., McCormick B. A., Maurelli A. T. (2006) OspF and OspC1 are Shigella flexneri type III secretion system effectors that are required for postinvasion aspects of virulence. Infect. Immun. 74, 5964–5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Renegar K. B., Small P. A., Jr., Boykins L. G., Wright P. F. (2004) Effects of short-term sleep deprivation on murine immunity to influenza virus in young adult and senescent mice. J. Immunol. 173, 1978–1986 [PubMed] [Google Scholar]
- 40. Stubbe H., Berdoz J., Kraehenbuhl J. P., Corthésy B. (2000) Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J. Immunol. 164, 1952–1960 [DOI] [PubMed] [Google Scholar]
- 41. Yang J. Y., Lee S. N., Chang S. Y., Ko H. J., Ryu S., Kweon M. N. (2014) A mouse model of shigellosis by intraperitoneal infection. J. Infect. Dis. 209, 203–215 [DOI] [PubMed] [Google Scholar]
- 42. Fiorentino M., Levine M. M., Sztein M. B., Fasano A. (2014) Effect of wild-type Shigella species and attenuated Shigella vaccine candidates on small intestinal function, antigen trafficking, and cytokine release. PLoS ONE 10.1371/journal.pone.0085211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blander J. M., Sander L. E. (2012) Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nat. Rev. Immunol. 12, 215–225 [DOI] [PubMed] [Google Scholar]
- 44. Perrier C., Sprenger N., Corthésy B. (2006) Glycans on secretory component participate in innate protection against mucosal pathogens. J. Biol. Chem. 281, 14280–14287 [DOI] [PubMed] [Google Scholar]
- 45. Clerc P., Sansonetti P. J. (1987) Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect. Immun. 55, 2681–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Watarai M., Funato S., Sasakawa C. (1996) Interaction of Ipa proteins of Shigella flexneri with α5β1 integrin promotes entry of the bacteria into mammalian cells. J. Exp. Med. 183, 991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Norrby-Teglund A., Ihendyane N., Kansal R., Basma H., Kotb M., Andersson J., Hammarström L. (2000) Relative neutralizing activity in polyspecific IgM, IgA, and IgG preparations against group A streptococcal superantigens. Clin. Infect. Dis. 31, 1175–1182 [DOI] [PubMed] [Google Scholar]
- 48. Berstad A. E., Brandtzaeg P. (1998) Expression of cell membrane complement regulatory glycoproteins along the normal and diseased human gastrointestinal tract. Gut 42, 522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lampert I. A., Schofield J. B., Amlot P., Van Noorden S. (1993) Protection of germinal centres from complement attack: decay-accelerating factor (DAF) is a constitutive protein on follicular dendritic cells: a study in reactive and neoplastic follicles. J. Pathol. 170, 115–120 [DOI] [PubMed] [Google Scholar]
- 50. Singh K., Chang C., Gershwin M. E. (2014) IgA deficiency and autoimmunity. Autoimmun. Rev. 13, 163–177 [DOI] [PubMed] [Google Scholar]
- 51. Rol N., Favre L., Benyacoub J., Corthésy B. (2012) The role of secretory immunoglobulin A in the natural sensing of commensal bacteria by mouse Peyer's patch dendritic cells. J. Biol. Chem. 287, 40074–40082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mestecky J., Russell M. W. (2009) Specific antibody activity, glycan heterogeneity and polyreactivity contribute to the protective activity of SIgA at mucosal surfaces. Immunol. Lett. 124, 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]