Background: TORC2 is a conserved protein complex that regulates multiple aspects of cell survival and proliferation.
Results: Gad8 is regulated by the cAMP-dependent protein kinase A and the Pmk1 protein-mitogen-activated protein kinase.
Conclusion: Glucose is a major regulator of TORC2-Gad8 signaling.
Significance: Identification of a novel mode of regulation of TORC2-Gad8 in response to glucose and stress.
Keywords: Cyclic AMP (cAMP), Glucose, Mitogen-activated Protein Kinase (MAPK), Protein Kinase A (PKA), Stress Response, GAD8, S. pombe, TORC2
Abstract
The target of rapamycin (TOR) kinase belongs to the highly conserved eukaryotic family of phosphatidylinositol 3-kinase-related kinases. TOR proteins are found at the core of two evolutionary conserved complexes, known as TORC1 and TORC2. In fission yeast, TORC2 is dispensable for proliferation under optimal growth conditions but is required for starvation and stress responses. TORC2 has been implicated in a wide variety of functions; however, the signals that regulate TORC2 activity have so far remained obscure. TORC2 has one known direct substrate, the AGC kinase Gad8, which is related to AKT in human cells. Gad8 is phosphorylated by TORC2 at Ser-546 (equivalent to AKT Ser-473), leading to its activation. Here, we show that glucose is necessary and sufficient to induce Gad8 Ser-546 phosphorylation in vivo and Gad8 kinase activity in vitro. The glucose signal that activates TORC2-Gad8 is mediated via the cAMP/PKA pathway, a major glucose-sensing pathway. By contrast, Pmk1, similar to human extracellular signal-regulated kinases and a major stress-induced mitogen activated protein kinase (MAPK) in fission yeast, inhibits TORC2-dependent Gad8 phosphorylation and activation. Inhibition of TORC2-Gad8 also occurs in response to ionic or osmotic stress, in a manner dependent on the cAMP/PKA and Pmk1-MAPK signaling pathways. Our findings highlight the significance of glucose availability in regulation of TORC2-Gad8 and indicate a novel link between the cAMP/PKA, Pmk1/MAPK, and TORC2-Gad8 signaling.
Introduction
Target of rapamycin (TOR)2 is an atypical protein kinase that was isolated as the target of the immunosuppressive and anticancer drug rapamycin. TOR proteins play a central role in growth, proliferation, and survival and can be found in two distinct complexes, TORC1 and TORC2 (1–3). In many different eukaryotes, TORC1 positively regulates cell growth in response to various signals, including nutrients, growth factors, energy, and stress. TORC2 also affects proliferation, metabolism, and cell survival, yet its precise cellular functions are less well understood compared with TORC1. In several eukaryotes, TORC2 mediates its cellular functions by phosphorylation and activation of AGC kinases at their C-terminal hydrophobic and turn motifs. In mammalian cells, TORC2 was shown to phosphorylate the AGC kinases AKT/PKB (protein kinase B), serum and glucocorticoid-induced protein kinase, and protein kinase C (PKC), thereby contributing to cell survival and proliferation (3). In the fission yeast, Schizosaccharomyes pombe, TORC2 activates and phosphorylates the AGC kinase Gad8 (4, 5). Deletion of gad8+ results in a phenotype most similar to disruption of TORC2, suggesting that TORC2 mediates most of its known functions via Gad8 (4–6). S. pombe contains two TOR homologs, Tor1 and Tor2 (7), that were numbered based on the order of their discovery. Later, it was found that Tor1 interacts with Ste20 (Rictor) and Sin1 to form TORC2. Tor2 interacts with Mip1 (Raptor) to form TORC1 (8). Disruption of TORC2 (Δtor1, Δste20, or Δsin1) results in pleiotrophic defects, including elongated cell morphology, deregulation of mitotic entrance, sensitivity to osmotic and oxidative stress, inability to enter sexual development or acquire stationary phase physiology, and a decrease in amino acid uptake (5–7, 9–11). Recently, we have showed that TORC2 is required under DNA replication stress and for maintenance of telomere length and gene silencing (12). Interestingly, a role for TORC2 in maintenance of genome stability has also been reported in Saccharomyces cerevisiae (13), suggesting that the role of TORC2 in tolerance to DNA damage and genome integrity is conserved in evolution.
Disruption of TORC1 in fission yeast results in cells that highly resemble nitrogen-starved cells, in agreement with a role for TORC1 in regulating growth in response to nitrogen availability (14). Moreover, the Rag family of GTPases activates S. pombe TORC1 in response to amino acids, similar to previous findings in Drosophila and mammals (15–17). In contrast, reduction in TORC2 activity, using a tor1 hypomorph mutation, resulted in cells that are unable to grow under low glucose conditions (18), suggesting that TORC2 may sense low glucose (19). However, no further link between TORC2 and glucose sensing has been described to date.
Glucose is the preferred carbon source in most cells, including yeast. In S. pombe, glucose detection occurs mainly through the cAMP/PKA signaling pathway that shares many features with those of mammalian cells (20). The presence of glucose is mediated to the cAMP/PKA pathway via Git3, a G protein-coupled receptor at the plasma membrane and a heterotrimeric G protein composed of the Gpa2 (Gα), Git5 (Gβ), and Git11 (Gγ) (21). Glucose detection leads to activation of Gpa2, which binds and activates the adenylate cyclase Cyr1 (22). A transient increase in cAMP results in activation of the protein kinase A, Pka1 (20). Glucose starvation leads to a decrease in Pka1 activity, which allows entrance into sexual development and activation of gluconeogenesis, partially through phosphorylation of the Zn2+ finger transcription factor Rst2 (23). Another branch involved in glucose signaling is mediated by AMP kinase, called Ssp2 in S. pombe, which is activated under glucose-limiting condition. Ssp2 phosphorylates Scr1, a transcription repression factor involved in glucose-mediated transcription repression (24). Ssp2 is phosphorylated by Ssp1, a calmodulin-dependent kinase, and thereby recruited to the nucleus (25). These findings suggest a cross-talk between glucose-dependent signaling and the calcium/calmodulin pathway.
Although glucose availability activates the cAMP/PKA pathway, glucose depletion results in the activation of Pmk1, the downstream effector of a MAPK cascade module, which also includes Pek1/Skh1 (MAPKK) and Mkh1 (MAPKKK) (26–28). The Pmk1-MAPK pathway is known as a key kinase in the cell wall integrity pathway and is closely related to the Mpk1/Slt2 pathway in the budding yeast S. cerevisiae. It was previously suggested that the MAPK activation domain in Pmk1 is similar to that present in human extracellular signal-regulated kinase 1 and 2 (ERK1/2) (27), although sequence comparison suggests that the closest ortholog of Pmk1 in mammals is ERK5. The Pmk1-MAPK cascade is activated in response to variety of stresses, including glucose starvation, osmotic stress and cell wall damage (26–29). Accordingly, loss of function of Pmk1-dependent signaling impairs cell wall synthesis, ion homeostasis, vacuole fusion, cytokinesis, and morphogenesis (26, 28). In response to glucose starvation or osmotic stress, the small GTPase Rho2 positively regulates Pmk1-MAPK through the protein kinase C ortholog Pck2 (27). Additional regulators, including the small GTPase Rho1 and Pck1, a second PKC ortholog, have been implicated in the regulation of the Pmk1-MAPK signaling pathway in response to cell wall damage (26, 30).
Here, we demonstrate that glucose is necessary and sufficient for TORC2-dependent phosphorylation of Gad8 at Ser-546 and for activation of Gad8 kinase activity in vitro. The activation of Gad8 in response to glucose is rapid and independent of protein synthesis. The glucose signal is mediated to TORC2-Gad8 via positive regulation of the cAMP/PKA pathway and negative regulation by the Pmk1-MAPK pathway. Our data locate TORC2-Gad8 downstream of two major signaling pathways that respond to extracellular signals and provide the first insights into the mechanisms that underlie TORC2 activation.
EXPERIMENTAL PROCEDURES
Yeast Strains, Growth Conditions, and Chemicals
S. pombe strains are described in Table 1. All experiments were performed by using standard genetic and molecular yeast techniques as described previously (31). Yeast cells were cultured in rich YE medium supplemented with adenine and uracil at 30 °C, as described previously (7), or in Edinburgh minimal medium (EMM, 5 g/liter NH4Cl), as described before (31). Gene deletions were performed by standard PCR-based methods (32). Rapamycin (Sigma, R0395) was dissolved in 50% methanol, 50% DMSO. FK506 (Abcam, AB120223) was dissolved in DMSO at a final concentration of 10 mg/ml. To assay sexual differentiation, 5 × 106 or 5 × 105 cells were mixed and spotted onto EMM-N (EMM lacking nitrogen source) or YE medium before incubation at 25 °C. Mating efficiency was determined after 4 days of growth as described previously (33). For stress sensitivity, cells were grown in YE to a cell density of 5 × 106 cells/ml. 10-Fold dilution series, starting with 5 × 105 cells, were spotted on different media as indicated.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| TA2 | leu1-32 ura4-D18 ade6-M210 h− | Laboratory stock |
| TA16 | leu1-32 ura4-D18 ade6-M216 h90 | Laboratory stock |
| TA1253 | sat1::KanMX leu1-32 h− | 35 |
| TA1125 | tor1::ura4+gad8::ura4+≪kanMX-gad8–6HA leu1-32 ura4-D18 ade6-M210 h90 | YGRCa |
| TA2027 | sat1::KanMX gad8::ura4+≪kanMX-gad8–6HA leu1-32 ade6 h90 | This study |
| TA1126 | gad8::ura4+≪kanMX-gad8–6HA leu1-32 ura4-D18 ade6-M210 h90 | YGRC |
| TA1754 | git3::ura4+ ura4-D18 h90 | YGRC |
| TA1920 | gpb1::kanMX6 leu1-32 ura4-D18 ade6-M216 h90 | This study |
| TA1773 | git3::ura4+ gad8::ura4+≪kanMX-gad8–6HA leu1-32 ura4-D18 ade6 h90 | This study |
| TA1772 | gpa2::ura4+ gad8::ura4+≪kanMX-gad8–6HA leu1-32 ura4-D18 ade6 h90 | This study |
| TA1972 | gpb1::kanMX6 gad8::ura4+≪kanMX-gad8–6HA leu1-32 ura4-D18 ade6 h90 | This study |
| TA500 | pka1::ura4+ ura4-D18 leu1-32 ade6-M210 his7-366 h90 | 63 |
| TA1728 | pka1::ura4+ gad8::ura4+≪kanMX-gad8–6HA leu1-32 ura4-D18 ade6 h90 | This study |
| TA1029 | gad8::ura4+ ade6-M216 leu1-32 ura4-D18, h− | 5 |
| TA1753 | gpa2::ura4+ leu1-32 ura4-D18 h90 | YGRC |
| TA512 | pka1::ura4+ leu1-32 ura4-D18 ade6-M216 his1-102 h90 | Laboratory stock |
| TA1954 | rho2::kanMX6 gad8::ura4+≪kanMX-gad8–6HA leu1-32 ura4-D18 ade6 h90 | This study |
| TA1903 | pck2::kanMX6 gad8::ura4+≪kanMX-gad8–6HA leu1-32 ura4-D18 ade6 h90 | This study |
| TA1886 | pmk1::kanMX6 gad8::ura4+≪kanMX-gad8–6HA leu1-32 ura4-D18 ade6 h90 | This study |
| TA1905 | pde1::kanMX6 gad8::ura4+≪kanMX-gad8–6HA leu1-32 ura4-D18 ade6 h90 | This study |
| TA808 | gad8::ura4+ leu1-32 ura4-D18 ade6-M216 h90 | 5 |
| TA1598 | Gad8:kanMX6 leu1-32 ura4-D18 ade6-M210 h− | This study |
| TA156 | tor1::ura4+ leu1-32 ura4-D18 ade6–216 h90 | Laboratory stock |
| TA1805 | git3::ura4+ Gad8:kanMX6 leu1 ura4-D18 ade6-M216 h90 | This study |
| TA1806 | gpa2::ura4+ Gad8:kanMX6 leu1 ura4-D18 ade6-M216 h90 | This study |
| TA1847 | pka1::ura4+ Gad8:kanMX6 leu1-32 ura4-D18 ade6-M216 h90 | This study |
| TA2080 | tor1::his1+ git3::ura4+ leu1-32 ura4-D18 ade6-M210 his1-102 h90 | This study |
| TA2060 | gpa2::ura4+ tor1::his1+ leu1-32 ura4-D18 ade6 his1-102 h90 | This study |
| TA2081 | tor1::his1+ pka1::ura4+ leu1-32 ura4-D18 ade6-M210 his1-102 h90 | This study |
a YGRC is Yeast Genetic Resource Center, Japan.
Protein Extraction and Immunoprecipitation Assays
Cells were grown to mid-logarithmic phase, washed once with water, and resuspended in lysis buffer (20 mm Tris-HCl, pH 7.5, 0.5 mm EGTA, 0.5 mm EDTA, 1 mm DTT, 125 mm potassium acetate, 12.5% glycerol, 0.1% Triton X-100, protease inhibitor mixture, and 1 mm phenylmethylsulfonyl fluoride). Cells were broken for 20 min with glass beads and centrifuged for 10 min at 10,000 × g, and the supernatant was collected. 20 μg of total protein extract was resolved on SDS-PAGE using 10% acrylamide gels. For immunoprecipitations, 500–1,000 μg of proteins were prepared and pre-cleared with 20 μl of protein A-Sepharose and protein G-Sepharose beads mixture (GE Healthcare). 2 μl of hemagglutinin (HA) antibodies were added to the cleared extract and incubated overnight at 4 °C. The beads were washed once with lysis buffer, once with lysis buffer containing 0.5 m NaCl, and twice with buffer A (50 mm Tris-HCl, pH 7.5, 0.1 mm EGTA, 0.1% β-mercaptoethanol). The resulting immunoprecipitates were used for in vitro kinase assays.
In Vitro Kinase Assays
We have previously reported a nonradioactive in vitro kinase assay for Gad8 (34), based on the use of GST-Fkh2 as a substrate (4). For the Gad8 kinase assay, a DNA fragment encoding amino acid residues 291 (Gln) to 411 (Pro) of Fkh2 was expressed in Escherichia coli BL21 strain as GST fusion, using the pGEX-4T1 expression vector and purified. Cells expressing Gad8-HA extracts were immunoprecipitated, and the resultant immunocomplexes were resuspended in 30 μl of kinase buffer (10 mm MgAc, 100 mm ATP, and phosphatase inhibitor mixture, Sigma) containing 0.1 μg of GST-Fkh2. After incubation for 10 min at 30 °C, the reaction was terminated by addition of 7 μl of 5× SDS-PAGE sample buffer and incubated for 5 min at 80 °C. The reaction was detected by Western blot analysis using anti-phospho-AKT substrate antibody (Cell Signaling Technology). The level of Gad8–6HA was detected by anti-HA antibody (Santa Cruz Biotechnology). The experiments were repeated at least three times, and representative pictures are shown.
Western Blotting
Proteins were resolved by SDS-PAGE 10–15% acrylamide gels and transferred to nitrocellulose membranes, blocked with 5% milk in TBST, and immunoblotted with the indicated antibodies. Detection was carried out using the ECL SuperSignal detection system (Thermo Scientific). Gad8 Ser-546 phosphorylation was detected using total protein extracts by phosphospecific antibodies raised against the Gad8 phosphopeptide CRFANWpSYQRPT as described previously (34).
RESULTS
TORC2-dependent Gad8 Phosphorylation and Gad8 Kinase Activity Are Decreased in Response to Glucose Depletion, Ionic or Osmotic Stress
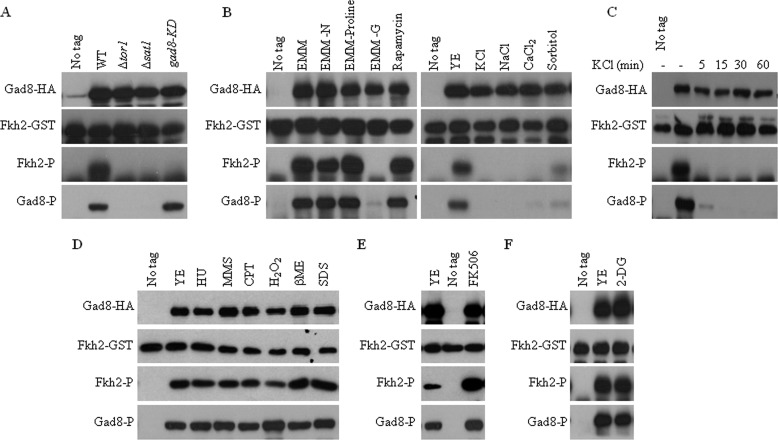
To monitor TORC2-Gad8 activity in response to extracellular changes, we developed a simple, nonradioactive in vitro kinase assay for Gad8 (34). Gad8 is activated in a two-step mechanism, in which it is first phosphorylated by TORC2 at serine 527 and serine 546 and then by the PDK homolog Ksg1 at threonine 387 (5). We therefore raised antibodies that specifically recognize the in vivo phosphorylation of Gad8 by TORC2 at Ser-546 (34). These two assays allow us to monitor both Gad8 activation and activity. As expected, the in vitro kinase activity of Gad8 was abolished in mutant cells lacking Tor1, the catalytic subunit of TORC2 (Δtor1), or in cells lacking Sat1, a positive regulator of the Rab-GTPase Ryh1 that is required for TORC2 activity (Δsat1) (35). Loss of Gad8 kinase activity was also observed in cells carrying a kinase-dead version of Gad8 (Gad8K259D (5)) (Fig. 1A). Loss of Gad8 kinase activity in Δtor1 or Δsat1 correlated with loss of the phosphorylation of Gad8 at Ser-546 (Fig. 1A).
FIGURE 1.
Gad8 Ser-546 phosphorylation and Gad8 activity are dependent on glucose availability and are diminished in response to stresses. A, Gad8 kinase activity is dependent on TORC2 activity. A wild type strain expressing no tagged gad8+ or wild type (WT), Δtor1, Δsat1, or gad8-KD (gad8K259D, a kinase-dead allele) strains carrying the gad8-HA allele were grown to mid-log phase in YE medium. Gad8-HA was immunoprecipitated and assayed for its activity using a peptide of Fkh2 as a substrate (Fkh2-GST). Phosphorylation of Fkh2 or phosphorylation of Gad8 at Ser-546 was detected with anti-phospho-AKT substrate or anti-Gad8 phosphospecific antibodies, respectively. B, Gad8 kinase activity and Gad8 Ser-546 phosphorylation are diminished in the absence of glucose or in response to stresses. Wild type cells with no tag or cells expressing gad8-HA were grown to mid-log phase and left untreated in rich (YE) or minimal (EMM) media or treated for 1 h with 1 m KCl, 1 m NaCl, 0.2 m CaCl2, 1 m sorbitol, 200 nm rapamycin or transferred for 1 h to EMM containing proline as the only nitrogen source (EMM-proline), EMM with no nitrogen source (EMM-N), or no carbon source (EMM-G). C, Gad8 kinase activity and Gad8 Ser-546 phosphorylation are rapidly reduced in response to KCl. Wild type cells with no tag or cells expressing gad8-HA were grown to mid-log phase in YE and treated for the indicated times with 1 m KCl. D, Gad8 activity and phosphorylation at Ser-546 are not affected by DNA stress, oxidative stress, reducing conditions, or cell wall stress. Wild type cells with no tag or cells expressing the gad8-HA were grown to mid-log phase and left untreated in rich (YE) media or treated for 1 h with 12 mm hydroxyurea, 0.03% methyl-methanesulfonate (MMS), 40 μm CPT, 1 μm H2O2, 15 mm β-mercaptoethanol (βME), or 0.01% SDS. E, calcineurin inhibitor FK506 activates Gad8. Cells were grown as described above and left untreated (YE) or treated for 1 h with FK506 (2 μg/ml). F, metabolic suppressor 2-deoxyglucose has no effect on Gad8 activity. Cells were grown as described above and left untreated (YE) or treated for 1 h with 100 μg/ml 2-DG.
We used our Gad8 in vitro kinase assay to screen for environmental conditions that may regulate Gad8 activity (Fig. 1B). Gad8 kinase activity or TORC2-dependent phosphorylation of Gad8 at Ser-546 was unchanged upon shift to media containing a poor nitrogen source (EMM-proline) or no nitrogen source (EMM-N). Rapamycin also had no effect on Gad8 activity or phosphorylation, consistent with the current notion that rapamycin specifically targets the TORC1 complex (34, 36, 37). In a remarkable contrast, a shift to a medium lacking glucose (EMM-G) abolished Gad8 activity or Gad8 Ser-546 phosphorylation (Fig. 1B). This finding suggests that the presence of glucose is required for TORC2-dependent Gad8 activity.
Treating cells for 1 h with NaCl, KCl, CaCl2, or sorbitol reduced the kinase activity of Gad8 and the level of Gad8 Ser-546 phosphorylation (Fig. 1B). Time course analysis showed that Gad8 phosphorylation and Gad8 kinase activity are lost following 5 min of treatment with KCl, indicating a rapid response to ionic or osmotic stress (Fig. 1C). In contrast, DNA damage or replication stress induced by hydroxyurea, methylmethane sulfonate, or camptothecin (CPT) did not affect Gad8 kinase activity. Also, exposure to oxidative stress (H2O2), reducing stress (β-mercaptoethanol), or cell membrane stress (SDS) had no effect on Gad8 activity (Fig. 1D).
Salt stress can activate the calcineurin pathway (38). Indeed, FK506, a specific inhibitor of calcineurin (38), led to a sharp increase in Gad8 kinase activity (Fig. 1E), consistent with the possibility that calcineurin inhibits TORC2-dependent phosphorylation and activation. However, because the activity of TORC2-Gad8 is also down-regulated by sorbitol (Fig. 1B), which does not affect cells via the calcineurin pathway, we presume that the activity of TORC2-Gad8 is reduced in response to osmostress or salt stress at least partially independent of the calcineurin pathway.
Because glucose is essential for TORC2-dependent activation of Gad8 (Fig. 1B), we examined whether this effect is due to a drop in the energy level of the cells and activation of the AMP kinase pathway. For this purpose, we treated cells with the metabolic inhibitor 2-deoxyglucose (2-DG). Cells treated with 100 μg/ml 2-DG, a concentration that was shown to cause cell death after an initial period of normal growth in S. pombe (39), had no effect on either Gad8 activity or its TORC2-dependent phosphorylation (Fig. 1F).
Glucose Is Required for Activation of TORC2-Gad8
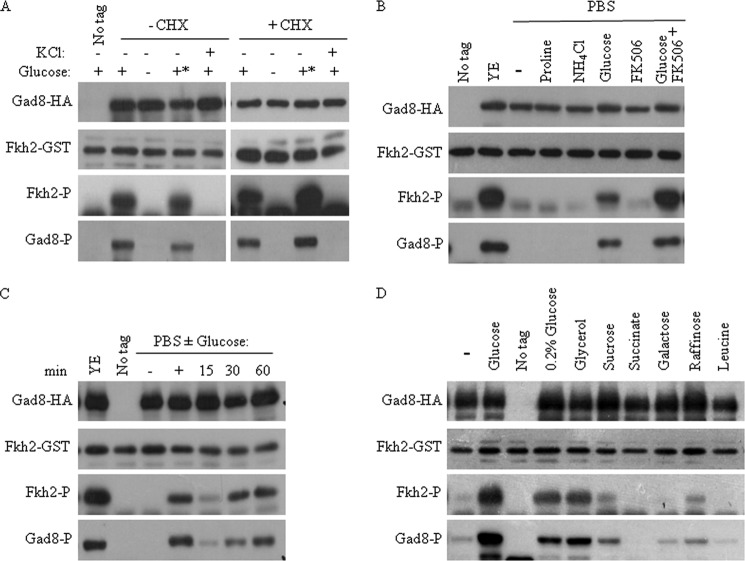
We next examined the ability of re-addition of glucose to re-activate the TORC2-Gad8 pathway. Complete loss of Gad8 Ser-546 phosphorylation and kinase activity was observed following 15 min of glucose starvation (Fig. 2A). We observed full restoration of Gad8 phosphorylation and activity within 15 min of glucose re-addition (Fig. 2B), indicating that the de-activation of the TORC2-Gad8 is reversible. The fast changes in Gad8 activity and phosphorylation upon glucose depletion or KCl treatment (Figs. 1C and 2A) suggest a post-translational mode of regulation. Indeed, the addition of cycloheximide, a protein synthesis inhibitor, did not affect Gad8 Ser-546 phosphorylation or activation in response to glucose or KCl (Fig. 3A).
FIGURE 2.
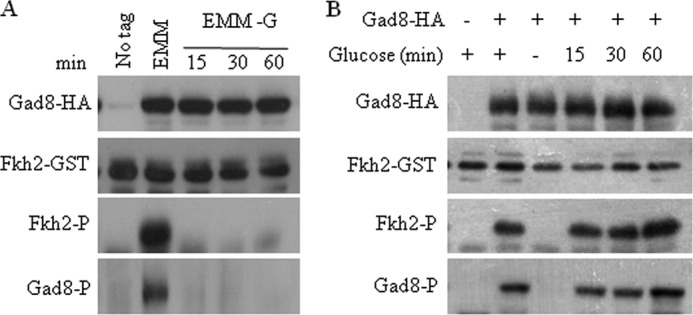
Gad8 Ser-546 phosphorylation and Gad8 activity rapidly respond to changes in glucose availability. A, Gad8 activity and phosphorylation at Ser-546 are rapidly reduced in the absence of glucose. Wild type cells with no tag or cells expressing gad8-HA were grown to mid-log phase in YE and then shifted for 1 h to EMM with 2% glucose or to EMM without glucose for the indicated time (minutes). B, re-feeding of glucose to starved cells re-activates Gad8. Wild type cells with no tag or cells expressing gad8-HA were grown to mid-log phase and shifted to EMM with or without glucose. After 1 h of starvation, 2% glucose was added for the indicated times (minutes).
FIGURE 3.
Glucose is the minimal requirement for Gad8. A, regulation of Gad8 phosphorylation and activity in response to glucose or KCl is independent of protein synthesis. Cells were grown to mid-log and shifted to EMM without glucose or to EMM containing 1 m KCl for 1 h. Following glucose starvation, 2% glucose was re-added for 1 h (+*). When indicated, cycloheximide (100 μg/ml) was added for 30 min. Gad8 in vitro kinase activity and Ser-546 phosphorylation were detected as described above. B, glucose is necessary for Gad8 activation. Cells were grown to mid-log and left untreated (YE) or washed and incubated for 1 h in PBS supplemented with proline (10 mm), NH4Cl (5 mm), glucose (2%), FK506 (2 μg/ml), or glucose (2%) and FK506 (2 μg/ml). Gad8 in vitro kinase activity and phosphorylation at Ser-546 were detected as described above. C, re-feeding of glucose to cells incubated in PBS is enough to re-activate Gad8. Cells were grown to mid-log phase and then incubated for 1 h in PBS. 2% glucose was added for the indicated times. Gad8 in vitro kinase activity and phosphorylation status at Ser-546 were determined as above. D, glucose is the most efficient carbon source for activation of Gad8. Cells were incubated for 1 h in EMM with no carbon source (−) or EMM supplemented with glucose (2%), low glucose (0.2%), glycerol (3%), sucrose (2%), succinate (2%), galactose (2%), raffinose (2%) or leucine (2%). Gad8 in vitro kinase activity and Ser-546 phosphorylation were determined as above.
We further analyzed Gad8 activity and Gad8 Ser-546 phosphorylation following starvation in phosphate-buffered saline (PBS). PBS containing glucose was sufficient to support Gad8 activity, although PBS containing proline or ammonium chloride failed to support Gad8 activity (Fig. 3B). Thus, glucose is necessary and sufficient for TORC2-dependent Gad8 Ser-546 phosphorylation and Gad8 kinase activity. Addition of FK506 to cells incubated in PBS did not activate Gad8, but addition of FK506 to cells incubated in PBS in the presence of glucose further increased Gad8 activity (Fig. 3B), recapitulating the effect observed in rich (YE) medium (Fig. 1E). This result indicates that initial activation of Gad8 by glucose is required for further activation by FK506. Glucose was also sufficient to restore Gad8 phosphorylation and activity following starvation in PBS, albeit with slower kinetics compared with starvation in EMM-G (Fig. 3C compared with 1A).
We next asked whether glucose is the only carbon source that can support Gad8 Ser-546 phosphorylation and Gad8 kinase activity. Cells were grown in standard growth medium (2% glucose) and shifted for 1 h to media containing low glucose (0.2%) or other carbon sources (Fig. 3D). The kinase activity of Gad8 was reduced upon shift to 0.2% glucose or to 3% glycerol, and a further reduction was observed upon shift to 2% sucrose or 2% raffinose, and no Gad8 kinase activity was detected in galactose (2%), succinate (2%), or leucine (2%). These results indicate that glucose is the most effective carbon source for the activation of TORC2-Gad8.
cAMP/PKA Pathway Is Essential for TORC2-dependent Gad8 Activation
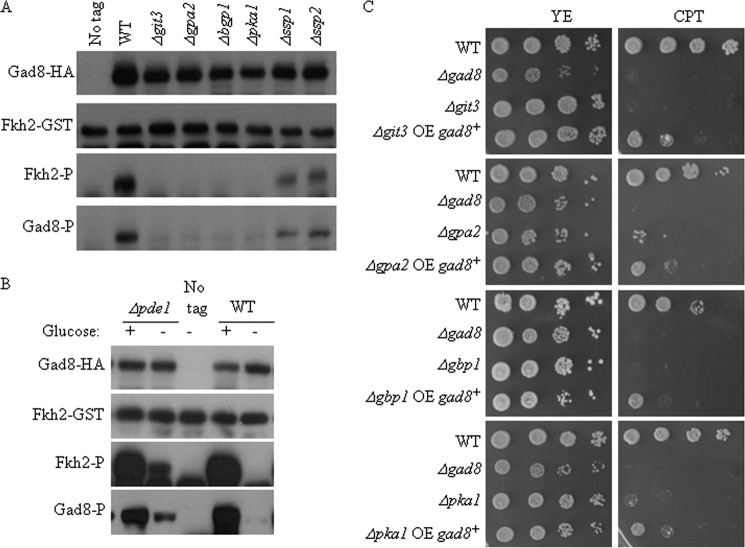
Cells sense the availability of glucose via Git3, a G protein-coupled receptor. Git3 is coupled to a heterotrimeric G protein composed of Gpa2 (Gα), Gbp1 (Gβ, also known as Git5), and Git11 (Gγ). Upon binding of an agonist, Git3 triggers the activation of Gpa2 by promoting the release of the GDP nucleotide bound to Gpa2 and allowing GTP binding. The Gpa2-GTP form binds and activates the Cyr1 adenylate cyclase protein to produce a transient cAMP signal that activates Pka1, the cAMP-dependent protein kinase A (PKA) (20–22). Remarkably, Gad8 Ser-546 phosphorylation and Gad8 kinase activity were completely abolished in Δgit3, Δgpa2, Δgpb1, or Δpka1 (Fig. 4A), suggesting that glucose activates TORC2-Gad8 via the cAMP/PKA pathway. Consistently, disruption of the cAMP phosphodiesterase, pde1+, which leads to hyperactivation of the cAMP/PKA pathway (40), resulted in Gad8 Ser-546 phosphorylation and Gad8 kinase activation in the absence of glucose (Fig. 4B). Thus, although disruption of the cAMP/PKA pathway results in de-activation of TORC2-Gad8, constitutive activation of the cAMP/PKA pathway results in TORC2-Gad8 hyperactivation.
FIGURE 4.
Gad8 activity depends on the PKA pathway. A, wild type, Δgit3, Δgpa2, Δgpb1, Δpka1, Δssp1, or Δssp2 mutant cells were grown to mid-log phase. Gad8 in vitro kinase activity and phosphorylation status at Ser-546 were determined as above. B, suppression of Gad8 activity in glucose-depleted conditions is reversed by constitutive activation of the PKA pathway. Wild type (WT) cells or cells lacking pde1+ (Δpde1), encoding for phosphodiesterase, were grown to mid-log phase and incubated for 1 h in EMM with or without glucose (2%). Gad8 in vitro kinase activity and phosphorylation status at Ser-546 was determined as above. C, overexpression of gad8+ suppresses the genotoxic sensitivity of mutant cells in the PKA pathway. Serial dilutions of exponentially growing wild type (WT), Δgad8, Δgit3, Δgpa2, Δgpb1, or Δpka1 strains transformed with empty vector (pREP1) or pREP1-gad8+ were spotted on rich medium (YE) with or without CPT (7.5 μm).
A decrease in glucose is sensed by the AMP-dependent kinase Ssp2 (24), which was recently shown to be activated by the calmodulin-dependent kinase Ssp1 (25). To examine whether the AMP kinase pathway is involved in the regulation of Gad8 activity, we monitored Gad8 activity in mutants lacking ssp1+ or ssp2+. We observed a reduction in Gad8 kinase activity and TORC2-dependent phosphorylation in Δssp1 or Δssp2 mutant strains (Fig. 4A). Thus, glucose starvation does not lead to inactivation of TORC2-Gad8 via activation of Ssp2-Ssp1. Rather, the Ssp2-Ssp1 module is required for full activity of TORC2-Gad8 under conditions of glucose sufficiency.
PKA Pathway Genetically Interacts with Gad8
To explore the biological significance of activation of TORC2-Gad8 by the cAMP/PKA pathway, we examined the effect of overexpression of gad8+ in the absence of a functional cAMP/PKA pathway. Recently, mutant cells of the cAMP/PKA pathway were isolated in a genome-wide screen for mutant cells sensitive to camptothecin (41). CPT forms a toxic complex with topoisomerase I that prevents DNA re-ligation and therefore causes DNA damage. We have previously demonstrated that cells disrupted for any component of TORC2 (Δtor1, Δste20, or Δsin1) or disruption of gad8+ lead to sensitivity to CPT (12). Overexpression of gad8+ from a multicopy plasmid partially suppressed the CPT sensitivity of Δgit3, Δgpa2, Δgpb1, or Δpka1 cells (Fig. 4C) but did not suppress the sensitivity of cAMP/PKA mutant cells to KCl (data not shown). The partial effect of overexpression of gad8+ may reflect a partial activation of Gad8-dependent signaling, because the overexpressed Gad8 protein is only partially activated in the absence of increased activity of TORC2, as discussed previously (6). Overexpression of git3+, gpa2+, gpb1+, or pka1+ did not suppress the sensitivity of Δgad8 mutant cells to CPT (data not shown), consistent with the possibility that the cAMP/PKA pathway lies upstream of TORC2-Gad8.
Loss of function of the cAMP/PKA pathway, including mutations of pka1+ or positive upstream regulators git3+, gpa2+, gpb1+, or cyr1+, results in mutant cells that are “hyper-maters,” i.e. they are able to enter sexual development in rich medium (Table 2) (20). In contrast, disruption of TORC2-Gad8 (Δtor1, Δste20, Δsin1, or Δgad8) results in mutant cells that are highly sterile (4). Thus, TORC2-Gad8 and the cAMP/PKA pathways act to oppositely regulate sexual development. Combining mutations in the cAMP/PKA, Δgit3, Δgpa2, or Δpka1, together with Δtor1 or Δgad8, resulted in double mutant cells that were as sterile as single Δtor1 or Δgad8 mutant cells, (Table 2). Thus, cells mutated in the cAMP/PKA pathway require a functional TORC2-Gad8 pathway to execute sexual development. At present, the opposite effect of TORC2-Gad8 and the cAMP/PKA pathway on sexual development is difficult to interpret. It appears that the cAMP/PKA positively regulates TORC2-Gad8 but also negatively regulates sexual development in a TORC2-Gad8-independent mechanism.
TABLE 2.
The hyper-mating phenotype of cAMP/PKA mutant cells is reversed by mutations in the TORC2-Gad8 pathway
| Strain | % mating efficiency |
|
|---|---|---|
| YE | EMM-N | |
| WT | 70 ± 6 | |
| Δgad8 | ||
| Δtor1 | ||
| Δgit3 | 23 ± 2 | 79 ± 1 |
| Δgpa2 | 27 ± 3 | 76 ± 4 |
| Δpka1 | 23 ± 4 | 69 ± 4 |
| Δgit3Δgad8 | ||
| Δgpa2Δgad8 | ||
| Δpka1Δgad8 | ||
| Δgit3Δtor1 | ||
| Δgpa2Δtor1 | ||
| Δpka1Δtor1 | ||
Cell Wall Integrity Pathway Inhibits Gad8 Activity
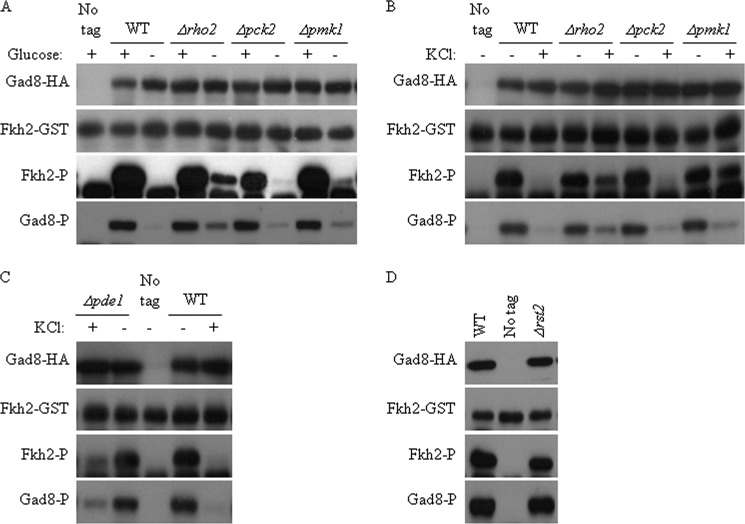
Several signaling pathways are activated in response to glucose starvation. One of these is the Pmk1-MAPK pathway (27). Therefore, we examined the effect of perturbing the Pmk1-MAPK pathway on TORC2-Gad8 activation. Previous studies demonstrated that Pmk1 phosphorylation and activation in response to glucose starvation is mediated via Pck2, one of the two orthologs of PKC (8, 18, 19). Rho2, one of the six Rho GTPases, is a main positive regulator upstream of the Pmk1 in some stress conditions (26, 42), although its role in activation of Pmk1 in response to glucose starvation is debatable (27). Under normal growth conditions, disruption of rho2+, pck2+, or pmk1+ did not have any effect on Gad8 Ser-546 phosphorylation or Gad8 kinase activity (Fig. 5A). However, in response to glucose starvation, the disruption of rho2+ or pmk1+, and to a lesser extent pck2+, partially alleviated the inhibition of Gad8 Ser-546 phosphorylation and kinase activity under glucose starvation (Fig. 5A), suggesting that the Rho2-Pck2-Pmk1 pathway inhibits the TORC2-Gad8 pathway in the presence of glucose (see our model in Fig. 6). Similar to the effect of Δrho2, Δpck2, or Δpmk1 mutant cells under glucose starvation, we observed alleviation of the inhibition of Gad8 Ser-546 phosphorylation and kinase activity in the presence of KCl (Fig. 5B). Because disruption of rho2+ or pmk1+ had a more pronounced effect compared with disruption of pck2+ (Fig. 5B), Rho2 may mediate its effect to Pmk1 in a Pck2-independent mechanism or another Rho2 effector may act in redundancy with Pck2.
FIGURE 5.
Pmk1-MAPK pathway negatively regulates Gad8 activity. A, Pmk1-MAPK pathway negatively regulates Gad8 activity in response to glucose depletion. Wild type (WT) cells or cells lacking rho2+ (Δrho2), pck2+ (Δpck2), or pmk1+ (Δpmk1) were grown as described by mid-log phase, washed, and incubated for 1 h in EMM with or without glucose (2%). Gad8 in vitro kinase activity and Ser-546 phosphorylation were determined as above. B, Pmk1-MAPK pathway negatively regulates Gad8 activity in response to osmotic stress. Wild type cells or cells lacking rho2+ (Δrho2), pck2+ (Δpck2), or pmk1+ (Δpmk1) were grown to mid-log phase, washed, and incubated for 1 h in YE with or without KCl (1 m), C, constitutive activation of the PKA relieves the suppression of Gad8 activity in salt stress. Wild type cells or cells lacking pde1+ (Δpde1) were grown to mid-log phase, washed, and incubated for 1 h in YE with or without KCl (1 m) as indicated. D, Rst2, a transcription factor downstream of Pka1, is not involved in the regulation of Gad8 activity. Wild type or Δrst2 cells were grown to mid-log phase. Gad8 in vitro kinase activity and Ser-546 phosphorylation were determined as above.
FIGURE 6.
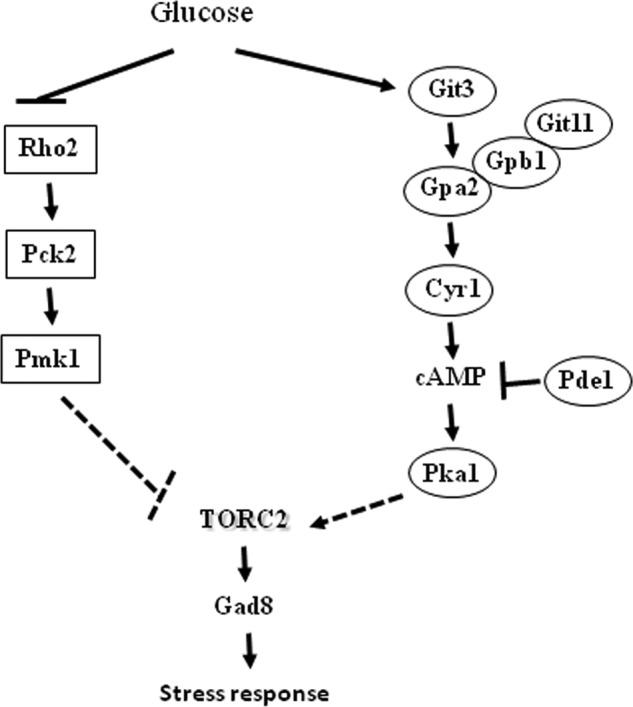
Working model. The TORC2-Gad8 pathway is positively regulated by cAMP/PKA1 and negatively regulated by the PmK1-MAPK pathway. In the presence of glucose, the PKA pathway is activated in a cAMP-dependent manner, leading to the activation of TORC2-Gad8. The Pmk1-MAPK pathway is activated under glucose starvation conditions, leading to inhibition of TORC2-Gad8, via inhibition of the Pka1 pathway or via an independent mechanism.
Like the Pmk1-MAPK pathway, the cAMP/PKA pathway is also required for adaptation to KCl stress (43). Deletion of pde1+, the cAMP phosphodiesterase, results in hyperactivation of the cAMP/PKA pathway and alleviated the inhibitory effect of KCl on Gad8 Ser-546 phosphorylation and Gad8 kinase activity (Fig. 5C). It was previously shown that the activation of Pmk1 in response to glucose starvation requires a functional cAMP/PKA pathway but is independent of the Pka1-dependent transcriptional regulator Rst2 (27). Interestingly, Gad8 phosphorylation and activation are also independent of Rst2 (Fig. 5D). Thus, similar to the Pmk1-MAPK pathway, the TORC2-Gad8 pathway requires a functional Pka1, but not its downstream transcriptional activator Rst2. Our data suggest a cross-talk between the cAMP/PKA and Pmk1-MAPK pathway, but its detailed mechanism has yet to be determined.
DISCUSSION
Nutrients are well known effectors of TOR-dependent signaling. Here, we demonstrate that glucose, but not nitrogen, is required and sufficient for activation of TORC2-Gad8 in fission yeast. Accordingly, we show that Gad8, the direct AGC-like kinase downstream of TORC2, is phosphorylated at Ser-546 and activated in response to the presence of glucose. The regulation of TORC2-dependent Gad8 phosphorylation and activation in response to glucose availability is fast and does not require protein translation, suggesting a post-translational mode of regulation of TORC2. Fig. 6 summarizes our current working model of the regulation of TORC2-Gad8 by glucose. We demonstrate that glucose availability is mediated to TORC2-Gad8 via the cAMP/PKA pathway, a major glucose-sensing pathway. Loss of function mutations in Pka1, the catalytic subunit of PKA, or its upstream positive regulators resulted in loss of Ser-546 phosphorylation and loss of the kinase activity of Gad8. In contrast, constitutive activation of the cAMP/PKA pathway by disruption of the cAMP phosphodiesterase pde1+ resulted in hyperactivation of TORC2-Gad8 under glucose starvation or KCl stress. Consistent with our findings showing a rapid and translation-independent response of TORC2-Gad8 to glucose, the stimulating effect of the cAMP/PKA pathway on TORC2-Gad8 was independent of the Pka1-regulated transcription factor Rst2. We have recently identified a role for TORC2-Gad8 in survival under DNA-damaging conditions, in particular those occurring during DNA replication (6, 12). A screen for CPT-sensitive mutant cells has recently identified mutations in the cAMP/PKA pathway as sensitive to this drug (41). In agreement with the possibility that Gad8 lies downstream to the cAMP/PKA pathway, we found that overexpression of gad8+ can partially suppress the CPT sensitivity of cAMP/PKA mutant cells. Our data thus suggest a possible link between glucose-sensing pathways and tolerance to DNA damage via TORC2-Gad8 regulation.
Another pathway that has a positive effect on TORC2-Gad8 activity is the AMP-dependent pathway, composed of Ssp1-Ssp2 module (24, 25). Deletion mutants in ssp1+ or ssp2+ resulted in down-regulation of Gad8 activity and Ser-546 phosphorylation. Addition of the toxic glucose analog 2-DG had no effect on Gad8 activity or its phosphorylation status. Therefore, we suggest that the inactivation of TORC2-Gad8 in response to glucose withdrawal is not due to a drop in energy level but involves direct sensing of glucose, possibly via the Git3 receptor. In this respect, it may be interesting to note that SNF1, the S. cerevisiae AMP kinase homolog, is activated in response to glucose withdrawal but is also required for normal growth rates and G1 to S phase transition under normal growth conditions (44), suggesting that AMP kinase-dependent signaling also positively regulates growth under high glucose concentration.
In contrast with the cAMP/PKA pathway, we found that the stress-induced pathway Pmk1-MAPK, which consists of Rho2-Pck2-Pmk1, negatively regulates TORC2-Gad8. The Pmk1 kinase is regulated by many stress conditions, among them hypertonic or hypotonic stress or glucose limitation, which activates Pmk1 through the Rho2-Pck2 module (26). Interestingly, hydrogen peroxide, which activates Pmk1 in a Rho2/Pck2-independent manner, had no effect on Gad8 activity. These results are in agreement with our model suggesting that the Rho2-Pck2-Pmk1 pathway inhibits Gad8 activity (Fig. 6) but suggest that activation of Pmk1 by the Rho2/Pck2-independent manner does not lead to TORC2-Gad8 activation, possibly due to activation of Pmk1 toward a different set of substrates. Either the hyperactivation of the PKA pathway by deletion of pde1+ or inactivation of the Pmk1-MAPK pathway partially alleviated Gad8 activity in response to high concentration of salts, suggesting a possible cross-talk between cAMP/PKA and Pmk1-MAPK signaling. A link between PKC and TORC2 signaling was previously suggested in S. cerevisiae and human cells. Knockdown of mTORC2-specific components resulted in alteration of the actin cytoskeleton via a Rho-GTPases and PKC-dependent mechanism (45, 46). However, PKCα was also suggested to act upstream of mTORC2. Partovian et al. (47) showed that the syndecan-4 receptor recruits PKCα to the plasma membrane, which in turn is required for mTORC2 localization to lipid rafts at the plasma membrane and subsequent AKT activation.
Disruption of TORC2 in fission yeast results in a complex phenotype that includes defects in survival under a wide variety of stress conditions. Somewhat surprisingly, TORC2 is required for cell survival under certain conditions in which Gad8 Ser-546 phosphorylation and Gad8 kinase activity are down-regulated. For example, disruption of TORC2 or gad8+ results in sensitivity to stress by KCl, NaCl (4), sorbitol, CaCl2 (35), or low glucose (18). These stresses resulted in down-regulation of TORC2-Gad8 (Fig. 1). Why should cells down-regulate TORC2-Gad8 activity in response to external stresses that require a functional TORC2-Gad8 pathway? One possibility is that TORC2-Gad8 activity is required to prepare cells for adverse conditions but that a subsequent dampening of TORC2-Gad8 signaling is also necessary. Another possibility is that a shift to low glucose or osmotic or ionic stress may result in a surge in TORC2-Gad8 activity, which is too rapid to be detected by our experiments. The notion that too much or too little of TORC2-dependent activity can result in similar adverse effects has already been considered. Thus, for example, either disruption of tor1+ or a hyperactive tor1 mutation results in reduced sexual development efficiencies (48).
Our findings suggest an interesting differential mode of nutrient-dependent regulation of TORC1 and TORC2. Although TORC1 responds to nitrogen availability (14, 49), we show here that TORC2 is tightly regulated by glucose. The idea that TORC1 may regulate growth in response to nitrogen, although TORC2 may be important for the response to glucose, has recently been considered by Yanagida and co-workers (18, 19) following the observation that tor1 mutant cells are unable to respond to glucose starvation by cell size shortening. As carbon and nitrogen are two major macronutrients required for cellular growth, a cross-talk between TORC1 and TORC2 is strongly anticipated. Indeed, a line of recent studies unraveled complex inter-links between TORC1 and TORC2 in higher eukaryotes (50–52). In fission yeast, TORC1 and TORC2 oppositely regulate amino acid uptake via transcriptional regulation of amino acid permeases (49). More recently, it was shown that Gad8 is involved in inhibitory phosphorylation of Tor1 (TORC2) and Tor2 (TORC1), which may provide a mechanism for co-regulation of the complexes (48).
In mammalian cells, mTORC1 is strongly regulated by the availability of amino acids, in a mechanism that is not fully understood but that involves mTORC1 activation at the lysosome surface by the Rag GTPases and requires the activity of the Rheb GTPase (16, 17, 53). The regulation of TORC1 in fission yeast by Rheb and Rag homologs, Rhb1 and Gtr1/2, in response to nitrogen availability or amino acids is strikingly conserved (15). A recent paper (54) demonstrated that glucose but not amino acids is required for mTORC2 integrity and for mTORC2-dependent AKT phosphorylation on the turn motif at Thr-450, although the effect of glucose on this mTORC2-dependent activity is likely mediated via detection of ATP levels. mTORC2 has been implicated in glucose homeostasis in several higher eukaryotic model systems. Thus, for example, a specific knock-out of rictor in the muscles of mice impaired insulin-stimulated glucose uptake and enhanced glycogen synthesis (55). Chronic administration of rapamycin impairs glucose tolerance and insulin action via inhibition of mTORC2 (56, 57). The SGK1 kinase, which is also phosphorylated and activated by mTORC2, is implicated in sodium, potassium, and glucose homeostasis (58, 59). Interestingly, the human ERK5 kinase, an ortholog of Pmk1, is induced in response to hyperosmotic stress (60) and is involved in SGK1 phosphorylation (61). A cross-talk between ERK5 and cAMP signaling has also been reported (62).
Our results demonstrating that TORC2-Gad8 is activated in response to glucose implies that TORC2 plays a role in regulating processes in response to glucose availability and may suggest a basic mode for TORC2-mediated glucose response in single cell organisms.
Acknowledgments
We thank C. Hoffmann, K. Shiozaki, and M. Yamamoto for strains and members of the Kupiec laboratory for encouragement and support.
This work was supported by Association for International Research Grant 11-0281 and by Open University of Israel Research Fund Grant 37076 (to R. W.).
- TOR
- target of rapamycin
- TORC1 and -2
- TOR complex 1 and 2, respectively
- CPT
- camptothecin
- 2-DG
- 2-deoxyglucose
- EMM
- Edinburgh minimal medium.
REFERENCES
- 1. Wullschleger S., Loewith R., Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 2. Loewith R. (2011) A brief history of TOR. Biochem. Soc. Trans. 39, 437–442 [DOI] [PubMed] [Google Scholar]
- 3. Cybulski N., Hall M. N. (2009) TOR complex 2: a signaling pathway of its own. Trends Biochem. Sci. 34, 620–627 [DOI] [PubMed] [Google Scholar]
- 4. Ikeda K., Morigasaki S., Tatebe H., Tamanoi F., Shiozaki K. (2008) Fission yeast TOR complex 2 activates the AGC-family Gad8 kinase essential for stress resistance and cell cycle control. Cell Cycle 7, 358–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsuo T., Kubo Y., Watanabe Y., Yamamoto M. (2003) Schizosaccharomyces pombe AGC family kinase Gad8p forms a conserved signaling module with TOR and PDK1-like kinases. EMBO J. 22, 3073–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schonbrun M., Laor D., López-Maury L., Bähler J., Kupiec M., Weisman R. (2009) TOR complex 2 controls gene silencing, telomere length maintenance, and survival under DNA-damaging conditions. Mol. Cell. Biol. 29, 4584–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weisman R., Choder M. (2001) The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J. Biol. Chem. 276, 7027–7032 [DOI] [PubMed] [Google Scholar]
- 8. Hayashi T., Hatanaka M., Nagao K., Nakaseko Y., Kanoh J., Kokubu A., Ebe M., Yanagida M. (2007) Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells 12, 1357–1370 [DOI] [PubMed] [Google Scholar]
- 9. Petersen J., Nurse P. (2007) TOR signalling regulates mitotic commitment through the stress MAP kinase pathway and the Polo and Cdc2 kinases. Nat. Cell Biol. 9, 1263–1272 [DOI] [PubMed] [Google Scholar]
- 10. Urano J., Sato T., Matsuo T., Otsubo Y., Yamamoto M., Tamanoi F. (2007) Point mutations in TOR confer Rheb-independent growth in fission yeast and nutrient-independent mammalian TOR signaling in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 104, 3514–3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weisman R., Roitburg I., Nahari T., Kupiec M. (2005) Regulation of leucine uptake by tor1+ in Schizosaccharomyces pombe is sensitive to rapamycin. Genetics 169, 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schonbrun M., Kolesnikov M., Kupiec M., Weisman R. (2013) TORC2 is required to maintain genome stability during S phase in fission yeast. J. Biol. Chem. 288, 19649–19660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimada K., Filipuzzi I., Stahl M., Helliwell S. B., Studer C., Hoepfner D., Seeber A., Loewith R., Movva N. R., Gasser S. M. (2013) TORC2 signaling pathway guarantees genome stability in the face of DNA strand breaks. Mol. Cell 51, 829–839 [DOI] [PubMed] [Google Scholar]
- 14. Matsuo T., Otsubo Y., Urano J., Tamanoi F., Yamamoto M. (2007) Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol. Cell. Biol. 27, 3154–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Valbuena N., Guan K. L., Moreno S. (2012) The Vam6 and Gtr1-Gtr2 pathway activates TORC1 in response to amino acids in fission yeast. J. Cell Sci. 125, 1920–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sancak Y., Peterson T. R., Shaul Y. D., Lindquist R. A., Thoreen C. C., Bar-Peled L., Sabatini D. M. (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim E., Goraksha-Hicks P., Li L., Neufeld T. P., Guan K. L. (2008) Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 10, 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ikai N., Nakazawa N., Hayashi T., Yanagida M. (2011) The reverse, but coordinated, roles of Tor2 (TORC1) and Tor1 (TORC2) kinases for growth, cell cycle and separase-mediated mitosis in Schizosaccharomyces pombe. Open Biol. 1, 110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yanagida M., Ikai N., Shimanuki M., Sajiki K. (2011) Nutrient limitations alter cell division control and chromosome segregation through growth-related kinases and phosphatases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 3508–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoffman C. S. (2005) Glucose sensing via the protein kinase A pathway in Schizosaccharomyces pombe. Biochem. Soc. Trans. 33, 257–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Welton R. M., Hoffman C. S. (2000) Glucose monitoring in fission yeast via the Gpa2 Gα, the git5 Gβ and the git3 putative glucose receptor. Genetics 156, 513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ivey F. D., Hoffman C. S. (2005) Direct activation of fission yeast adenylate cyclase by the Gpa2 Gα of the glucose signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 102, 6108–6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higuchi T., Watanabe Y., Yamamoto M. (2002) Protein kinase A regulates sexual development and gluconeogenesis through phosphorylation of the Zn finger transcriptional activator Rst2p in fission yeast. Mol. Cell. Biol. 22, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuzawa T., Fujita Y., Tohda H., Takegawa K. (2012) Snf1-like protein kinase Ssp2 regulates glucose derepression in Schizosaccharomyces pombe. Eukaryot. Cell 11, 159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valbuena N., Moreno S. (2012) AMPK phosphorylation by Ssp1 is required for proper sexual differentiation in fission yeast. J. Cell Sci. 125, 2655–2664 [DOI] [PubMed] [Google Scholar]
- 26. Barba G., Soto T., Madrid M., Núñez A., Vicente J., Gacto M., Cansado J., and Yeast Physiology Group (2008) Activation of the cell integrity pathway is channelled through diverse signalling elements in fission yeast. Cell. Signal. 20, 748–757 [DOI] [PubMed] [Google Scholar]
- 27. Madrid M., Fernández-Zapata J., Sánchez-Mir L., Soto T., Franco A., Vicente-Soler J., Gacto M., Cansado J. (2013) Role of the fission yeast cell integrity MAPK pathway in response to glucose limitation. BMC Microbiol. 13, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madrid M., Soto T., Khong H. K., Franco A., Vicente J., Pérez P., Gacto M., Cansado J. (2006) Stress-induced response, localization, and regulation of the Pmk1 cell integrity pathway in Schizosaccharomyces pombe. J. Biol. Chem. 281, 2033–2043 [DOI] [PubMed] [Google Scholar]
- 29. Sengar A. S., Markley N. A., Marini N. J., Young D. (1997) Mkh1, a MEK kinase required for cell wall integrity and proper response to osmotic and temperature stress in Schizosaccharomyces pombe. Mol. Cell. Biol. 17, 3508–3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sánchez-Mir L., Soto T., Franco A., Madrid M., Viana R. A., Vicente J., Gacto M., Pérez P., Cansado J. (2014) Rho1 GTPase and PKC Ortholog Pck1 are upstream activators of the cell integrity MAPK pathway in fission yeast. PLoS One 9, e88020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moreno S., Klar A., Nurse P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 [DOI] [PubMed] [Google Scholar]
- 32. Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 33. Weisman R., Choder M., Koltin Y. (1997) Rapamycin specifically interferes with the developmental response of fission yeast to starvation. J. Bacteriol. 179, 6325–6334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laor D., Cohen A., Pasmanik-Chor M., Oron-Karni V., Kupiec M., Weisman R. (2014) Isp7 is a novel regulator of amino acid uptake in the TOR signaling pathway. Mol. Cell. Biol. 34, 794–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tatebe H., Morigasaki S., Murayama S., Zeng C. T., Shiozaki K. (2010) Rab-family GTPase regulates TOR complex 2 signaling in fission yeast. Curr. Biol. 20, 1975–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takahara T., Maeda T. (2012) TORC1 of fission yeast is rapamycin-sensitive. Genes Cells 17, 698–708 [DOI] [PubMed] [Google Scholar]
- 37. Rallis C., Codlin S., Bähler J. (2013) TORC1 signaling inhibition by rapamycin and caffeine affect lifespan, global gene expression, and cell proliferation of fission yeast. Aging Cell 12, 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sugiura R., Sio S. O., Shuntoh H., Kuno T. (2002) Calcineurin phosphatase in signal transduction: lessons from fission yeast. Genes Cells 7, 619–627 [DOI] [PubMed] [Google Scholar]
- 39. Megnet R. (1965) Effect of 2-deoxyglucose on Schizosaccharomyces pombe. J. Bacteriol. 90, 1032–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mochizuki N., Yamamoto M. (1992) Reduction in the intracellular cAMP level triggers initiation of sexual development in fission yeast. Mol. Gen. Genet. 233, 17–24 [DOI] [PubMed] [Google Scholar]
- 41. Han T. X., Xu X. Y., Zhang M. J., Peng X., Du L. L. (2010) Global fitness profiling of fission yeast deletion strains by barcode sequencing. Genome Biol. 11, R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ma Y., Kuno T., Kita A., Asayama Y., Sugiura R. (2006) Rho2 is a target of the farnesyltransferase Cpp1 and acts upstream of Pmk1 mitogen-activated protein kinase signaling in fission yeast. Mol. Biol. Cell 17, 5028–5037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stiefel J., Wang L., Kelly D. A., Janoo R. T., Seitz J., Whitehall S. K., Hoffman C. S. (2004) Suppressors of an adenylate cyclase deletion in the fission yeast Schizosaccharomyces pombe. Eukaryot. Cell 3, 610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Busti S., Coccetti P., Alberghina L., Vanoni M. (2010) Glucose signaling-mediated coordination of cell growth and cell cycle in Saccharomyces cerevisiae. Sensors 10, 6195–6240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M. A., Hall A., Hall M. N. (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 46. Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 [DOI] [PubMed] [Google Scholar]
- 47. Partovian C., Ju R., Zhuang Z. W., Martin K. A., Simons M. (2008) Syndecan-4 regulates subcellular localization of mTOR Complex2 and Akt activation in a PKCα-dependent manner in endothelial cells. Mol. Cell 32, 140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hálová L., Du W., Kirkham S., Smith D. L., Petersen J. (2013) Phosphorylation of the TOR ATP binding domain by AGC kinase constitutes a novel mode of TOR inhibition. J. Cell Biol. 203 595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weisman R., Roitburg I., Schonbrun M., Harari R., Kupiec M. (2007) Opposite effects of tor1 and tor2 on nitrogen starvation responses in fission yeast. Genetics 175, 1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu P., Gan W., Inuzuka H., Lazorchak A. S., Gao D., Arojo O., Liu D., Wan L., Zhai B., Yu Y., Yuan M., Kim B. M., Shaik S., Menon S., Gygi S. P., Lee T. H., Asara J. M., Manning B. D., Blenis J., Su B., Wei W. (2013) Sin1 phosphorylation impairs mTORC2 complex integrity and inhibits downstream Akt signalling to suppress tumorigenesis. Nat. Cell Biol. 15, 1340–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Humphrey S. J., Yang G., Yang P., Fazakerley D. J., Stöckli J., Yang J. Y., James D. E. (2013) Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 17, 1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xie J., Proud C. G. (2013) Crosstalk between mTOR complexes. Nat. Cell Biol. 15, 1263–1265 [DOI] [PubMed] [Google Scholar]
- 53. Jewell J. L., Russell R. C., Guan K. L. (2013) Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 14, 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen C. H., Kiyan V., Zhylkibayev A. A., Kazyken D., Bulgakova O., Page K. E., Bersimbaev R. I., Spooner E., Sarbassov dos D. (2013) Autoregulation of the mechanistic target of rapamycin (mTOR) complex 2 integrity is controlled by an ATP-dependent mechanism. J. Biol. Chem. 288, 27019–27030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kumar A., Harris T. E., Keller S. R., Choi K. M., Magnuson M. A., Lawrence J. C., Jr. (2008) Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances basal glycogen synthase activity. Mol. Cell. Biol. 28, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lamming D. W., Ye L., Katajisto P., Goncalves M. D., Saitoh M., Stevens D. M., Davis J. G., Salmon A. B., Richardson A., Ahima R. S., Guertin D. A., Sabatini D. M., Baur J. A. (2012) Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Robida-Stubbs S., Glover-Cutter K., Lamming D. W., Mizunuma M., Narasimhan S. D., Neumann-Haefelin E., Sabatini D. M., Blackwell T. K. (2012) TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 15, 713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lang F., Cohen P. (2001) Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci. STKE 2001, re17. [DOI] [PubMed] [Google Scholar]
- 59. Lang F., Böhmer C., Palmada M., Seebohm G., Strutz-Seebohm N., Vallon V. (2006) (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 86, 1151–1178 [DOI] [PubMed] [Google Scholar]
- 60. Kato Y., Tapping R. I., Huang S., Watson M. H., Ulevitch R. J., Lee J. D. (1998) Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 395, 713–716 [DOI] [PubMed] [Google Scholar]
- 61. Hayashi M., Tapping R. I., Chao T. H., Lo J. F., King C. C., Yang Y., Lee J. D. (2001) BMK1 mediates growth factor-induced cell proliferation through direct cellular activation of serum and glucocorticoid-inducible kinase. J. Biol. Chem. 276, 8631–8634 [DOI] [PubMed] [Google Scholar]
- 62. Dodge-Kafka K. L., Soughayer J., Pare G. C., Carlisle Michel J. J., Langeberg L. K., Kapiloff M. S., Scott J. D. (2005) The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 437, 574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jin M., Fujita M., Culley B. M., Apolinario E., Yamamoto M., Maundrell K., Hoffman C. S. (1995) sck1, a high copy number suppressor of defects in the cAMP-dependent protein kinase pathway in fission yeast, encodes a protein homologous to the Saccharomyces cerevisiae SCH9 kinase. Genetics 140, 457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]