Abstract
Background
Fibroblast growth factor 23 (FGF23) levels are elevated in chronic kidney disease (CKD) and elevated values have been associated with both heart disease and mortality. Recent studies show that FGF23, a protein synthesized by osteocytes, is also present in calcified atherosclerotic plaques and may be induced by heart disease. Whether vascular expression of FGF23 is associated with progressive CKD, however, remains unknown. Therefore, the relationship between kidney function, vascular calcification and FGF23 expression was evaluated in patients with heart disease.
Methods
Immunohistochemistry for FGF23 was performed in coronary arteries of all patients undergoing heart transplantation at UCLA between February 2008 and 2010. Immunohistochemical staining for Klotho, DMP1, FGFR1, and FGFR3; calcium deposition; and RNA expression of Klotho and DMP1 were assessed in a subset of eight samples.
Results
FGF23 was detected by immunohistochemistry in 56% of the coronary artery specimens. Vascular FGF23 expression correlated with declining kidney function, as evidenced by reduced creatinine clearance. FGFR1 and FGFR3 were detected throughout the vascular tissue and in calcified plaques. Calcium deposition, Klotho expression and DMP1 expression correlated with FGF23 immunoreactivity.
Conclusions
The findings suggest that the Klotho-FGF23-FGFR system is active in coronary arteries and its upregulation correlates with impaired renal function and matrix calcium deposition.
Keywords: arteriosclerosis, immunohistochemistry, kidney
INTRODUCTION
Fibroblast growth factor 23 (FGF23) is an osteocyte-derived phosphaturic hormone which, in combination with its cofactor, Klotho, inhibits production of 1,25(OH)2 vitamin D by reducing the activity of renal 1α-hydroxylase [1]. In patients with chronic kidney disease (CKD), FGF23 levels rise in response to a combination of factors, including decreasing filtration and/or degradation by the diseased kidney [2, 3], phosphate retention [4] and Klotho deficiency [5]. In addition to its endocrine actions at the level of the kidney, several recent epidemiologic studies suggest that elevated circulating levels of FGF23 may be an independent risk factor for heart disease and early mortality in patients with all stages of CKD [6–9]. Recent in vivo and in vitro experiments have demonstrated that these ‘off-target’ effects of FGF23 on the myocardium are mediated, at least in part, through a direct, Klotho-independent mechanism [10].
Cardiovascular disease is the leading cause of death in the CKD population [11, 12]. The development of cardiovascular calcification, specifically the transformation of smooth muscle cells to an osteoblastic phenotype, has been implicated as a factor in the pathogenesis of CKD-mediated vascular disease [13]. Circulating levels of FGF23 are derived primarily from osteocytic expression in bone [14]; however, heart failure itself has recently been shown to induce FGF23 expression in cardiac myocytes, mediated through macrophage-induced oncostatin M production [15]. Also, FGF23 has been identified in calcified carotid atherosclerotic lesions from subjects with normal renal function [16]. The relative contributions of heart failure, CKD and Klotho deficiency to the presence of FGF23 expression in vascular tissue, however, have yet to be evaluated. Thus, the current study was performed to evaluate the relationship between renal function, vascular calcification and osteocytic proteins, including FGF23 expression, in the coronary arteries of subjects with heart failure across a wide spectrum of renal function.
MATERIALS AND METHODS
Patients and biochemical markers
All patients undergoing cardiac transplantation between February 2008 and 2010 at UCLA were considered for the current study. Explanted hearts were sent to the Department of Pathology Department, where the hearts and coronary arteries were fixed in 10% formalin for ∼24 h and then dissected, examined, processed routinely and embedded in paraffin. The sections of coronary arteries were re-reviewed and two to four blocks of tissue from discrete areas of coronary artery were obtained from each study subject and were sectioned at 4 µm for this study. Pre-transplant demographic and biochemical data were obtained by chart review. Biochemical measures, including serum ionized calcium, phosphorus, alkaline phosphatase, high-sensitivity C-reactive protein (HS-CRP), hemoglobin A1C and serum triglycerides, were ascertained in the UCLA clinical laboratory between 1 and 6 months before transplantation. Creatinine clearance was formally assessed by 24-h urine collection; values were normalized to a standard body surface area of 1.73 m2. The study was approved by the UCLA Institutional Review Board.
Immunohistochemical assessment of FGF23 protein in coronary artery tissue
In order to assess for the presence of FGF23 protein in tissue, sections of coronary artery tissue were deparaffinized, boiled in a sodium citrate buffer (10 mmol/L) for 10 min (Dako, Glostrup, Denmark), quenched in 3% hydrogen peroxide/methanol solution and non-specific binding blocked in avidin–biotin solution and in 5% normal horse serum with 1% bovine serum albumin. Sections were incubated overnight at 4°C with affinity purified polyclonal goat anti-human FGF23 (225–244) (Immutopics, San Clemente, CA, USA), incubated with biotinylated anti-goat secondary antibody (Vector, Burlingame, CA, USA), developed and counterstained with dilute Mayer hematoxylin (Sigma-Aldrich, St. Louis, MO, USA). The primary antibody was omitted in negative controls; trabecular bone was used as a positive control [17]. Incubation with secondary antibody alone was used to control for non-specific binding to mineralized tissue. The presence of FGF23 was assessed in tissue by two separate observers (R.C.P. and M.C.F.), blinded to patient identity and renal function. The staining procedure was performed simultaneously on all specimens and controls.
Immunohistochemistry and von Kossa staining
A subset of coronary artery sections from eight separate individuals (four positive and four negative for FGF23 immunoreactivity) were selected for calcium deposition as well as for the immunoreactivity to Klotho, DMP1, osteopontin (OPN), fibroblast growth factor receptor (FGFR) 1, FGFR3 and cluster of differentiation (CD) 68 protein. Immunohistochemistry was performed as described for FGF23 using monoclonal mouse anti-human DMP1 (LFMb31; Dr Larry Fisher, National Institutes of Health), monoclonal mouse anti-human CD68 (M0876; Dako) polyclonal rabbit anti-human OPN (LF-123; Dr Larry Fisher, National Institutes of Health), polyclonal goat anti-human Klotho (Immutopics, San Clemente, CA, USA) (Supplementary Figure S1), FGFR1 (Sc-121) or FGFR3 (Sc-123, Santa Cruz Biotechnology, Santa Cruz, CA, USA). For co-localization, samples were incubated in secondary antibody for at least 2 h after incubation with primary antibodies. For all co-localization studies, with the exception of the FGF23 and Klotho co-localization, the primary antibodies were as above for immunohistochemical analysis. Since both the Immutopics anti-human FGF23 and the Immutopics anti-human Klotho were raised in goat, an additional anti-human Klotho (anti-human Klotho 603, Genprice) was obtained for co-localization. Since no immunohistochemical or immunofluorescence staining was observed for Klotho in either the positive control (kidney) or in coronary arteries when this antibody was tested using our standard protocol (listed above), the protocol was modified (but only for assays using this antibody) by omitting the blocking of non-specific binding. Secondary antibodies for all immunofluorescence assays were as follows: Alexa 598 anti-goat for FGF23 and Klotho (Immutopics) and Alexa 488 anti-mouse for DMP1, CD68 and Klotho (Genprice) (Invitrogen, Carlsbad, CA, USA). DAPI was used as a counterstain.
Von Kossa staining was performed by fixing deparaffinized sections in 0.1% glutaraldehyde, incubating them with 5% silver nitrate for 30 min at room temperature in the dark, exposing them to sunlight and counterstaining with hematoxylin. Reproducibility was ensured by repeating the immunohistochemical analysis on all specimens. The presence of calcium and protein expression were evaluated on a subjective scale ranging from 0 (no expression) to 3 (maximal expression noted throughout tissue). The readers (R.C.P. and L.L.D.) were blinded to the results of the FGF23 immunoreactivity.
Quantitative real-time PCR
Messenger RNA was extracted from the paraffin embedded tissue using the Paradise®Plus Reagent System (MDS Analytical Technologies, Sunnyvale, CA, USA). Real-time reverse transcriptase PCR (qRT–PCR) was performed using TaqMan primers for FGF23 (Hs00221003_m1), DMP1 (Hs00189368_m1), Klotho (Hs00183100_m1) and MGP (Hs00179899_m1). Expression levels were normalized to a control gene, GAPDH, using the 2−ΔΔC(T) method [18]. In brief, the number of cycles required for target gene copy number to cross SYBR® absorbance threshold (Ct) in each patient sample was subtracted from the number of cycles required to cross Ct in a normal control sample (ΔCt target). Similarly, the number of cycles required for GAPDH copy number to cross SYBR® absorbance threshold (Ct) in each patient sample was subtracted from the number of cycles required to cross Ct in a normal control sample (ΔCt GAPDH). Assuming a 100% efficiency, the overall increase in gene expression from normal control was calculated as 2(ΔCt target)/2(ΔCt GAPDH). All samples were run in triplicate and cycle numbers represented the average of the three.
Statistical analyses
Measurements for normally distributed variables are reported as mean ± standard error; median values and interquartile range are used to describe non-normally distributed variables. Differences between the FGF23 positive and negative groups were assessed by Student's t-test or the Mann–Whitney U-test. All statistical analyses were performed using the SAS software (SAS Institute, Inc., Cary, NC, USA) and all tests were two-sided. A probability of type I error of <5% was considered statistically significant and ordinary P-values are reported.
RESULTS
Subjects
Of 51 potential subjects who underwent heart transplantation during the study period at this institution, tissue was available and was analyzed from 50. Overall, subjects were predominantly male and Caucasian, with a minority of Hispanics, Asians and Blacks. The majority required heart transplantation for ischemic cardiomyopathy, although over one-third had non-ischemic cardiomyopathy or were requiring a second heart transplant. Thirty-seven percent were well-controlled diabetics, 36% were former smokers and 49% had a history of hypertension. Serum markers of mineral metabolism, including calcium, phosphorus and alkaline phosphatase, were within the normal range. Levels of C-reactive protein (CRP) were elevated and the cohort displayed a wide range of glomerular filtration rate (GFR) measurements as assessed by creatinine clearance.
FGF23 expression and renal function
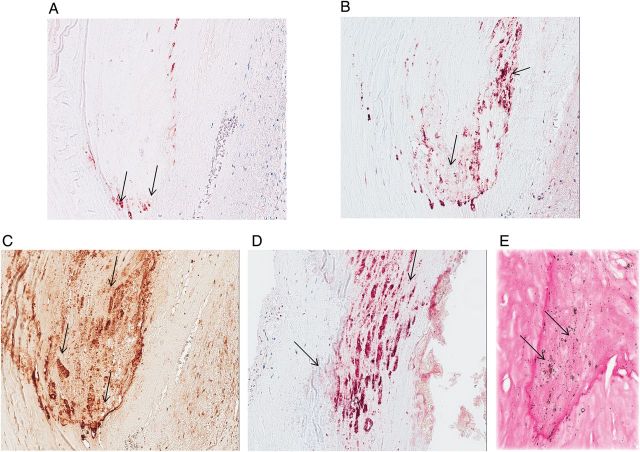
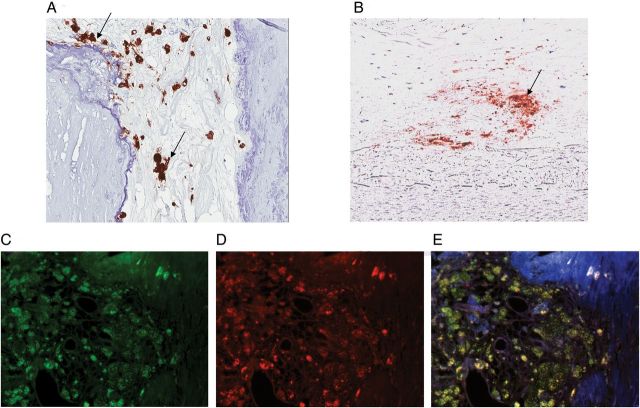
FGF23 immunoreactivity was present in at least one section of coronary artery tissue of 28 of the 50 study subjects; no FGF23 staining was observed in any sections of tissue in the remaining 22. Table 1 shows the demographic and biochemical characteristics of the two subject groups according to FGF23 immunoreactivity in the coronary arteries. Although both groups had a similar preponderance of male subjects, the FGF23 positive group had lower CRP values than the FGF23 negative group. Creatinine clearance was lower in patients with coronary arterial FGF23 positivity; similarly, more patients with positive FGF23 staining had stages 3 and 4 CKD. In tissues with positive staining, FGF23 was observed in areas of calcification (Figure 1a) in both the tunica intima and tunica media. Interestingly, foamy macrophages, as identified by CD68 immunostaining, were also present in calcified lesions. Immunostaining for FGF23 was observed in these inflammatory cells and co-localized with immunostaining for CD68 (Figure 2).
Table 1.
Demographic and biochemical data according to FGF23 staining positivity
| Variable | FGF23 positive (n = 28) | FGF23 negative (n = 22) |
|---|---|---|
| Age (years) | 62.0 ± 1.3 | 56.9 ± 0.3 |
| Gender (male) | 89% | 77% |
| Ethnicity | ||
| Caucasian | 67% | 57% |
| Black | 11% | 14% |
| Hispanic | 22% | 14% |
| Asian | 14% | |
| Pre-transplant diagnosis | ||
| Ischemic cardiomyopathy | 65% | 55% |
| Non-ischemic cardiomyopathy | 21% | 35% |
| Second heart transplant | 14% | 10% |
| Diabetes | 36% | 36% |
| BMI > 25 | 25% | 50% |
| Prior smokers | 46% | 55% |
| History of hypertension | 56% | 63% |
| Normalized creatinine clearance (mL/min/1.73 m2) | 51 (38, 78)* | 61 (41, 90) |
| Creatinine clearance by CKD stage (%) | ||
| Normal kidney function ( > 90 mL/min/1.73 m2) | 18% | 32% |
| Decreased GFR (60–90 mL/min/1.73 m2) | 18% | 36% |
| CKD stage 3 (30–60 mL/min/1.73 m2) | 57%* | 32% |
| CKD stage 4 (15–30 mL/min/1.73 m2) | 7%* | |
| Serum calcium (ionized) (mmol/L) | 1.13 ± 0.01 | 1.13 ± 0.01 |
| Serum phosphorus (mg/dL) | 3.7 ± 0.2 | 3.9 ± 0.2 |
| Alkaline phosphatase (IU/L) | 78 (62, 101) | 80 (66, 100) |
| HS-CRP (mg/dL) | 7 (2, 24)* | 31 (9, 87) |
| HgA1c (%) | 6.2 ± 0.2 | 6.0 ± 0.3 |
| HDL (mg/dL) | 30 (22, 41) | 32 (27, 38) |
| LDL (mg/dL) | 70 (57, 91) | 78 (58, 119) |
| Triglycerides (mg/dL) | 126 (93, 147) | 104 (75, 177) |
The data are expressed as mean ± standard error or median (interquartile range).
BMI, body mass index; CKD, chronic kidney disease.
*P < 0.05 between groups.
FIGURE 1:
Immunohistochemical staining for (A) FGF23, (B) DMP1, (C) OPN and (D) Klotho and (E) calcium staining in sequential sections of calcified coronary artery (magnification: ×200). Red/brown staining (arrows) indicates immunoreactivity for each protein and/or calcium, respectively. FGF23, fibroblast growth factor 23; DMP1, dentin matrix protein 1; OPN, osteopontin.
FIGURE 2:
Immunohistochemical staining for (A) CD68 and (B) FGF23 in foamy macrophages. Red staining (arrows) indicates immunoreactivity. Immunofluorescence staining for (C) CD68 (green) and (D) FGF23 (red) and in calcified coronary artery. (E) Co-localization of the two proteins is demonstrated by the yellow color on merging of the two images. Blue color indicates DAPI staining. CD68, cluster of differentiation 68 (monocyte/macrophage lineage marker); FGF23, fibroblast growth factor 23.
Vascular calcification and osteocytic protein expression
To assess the association between FGF23 expression, vascular calcification and markers of osteocytic lineage, specimens from eight subjects were chosen for further evaluation. To determine whether machinery for FGF23 signaling was present in vascular tissue, immunohistochemical reactivity of FGF23 receptors 1 and 3 were assessed. Both FGFR1 and FGFR3 protein were detected by immunostaining throughout the tunica intima, tunica media, and in calcified plaques of all specimens evaluated (Figure 3). DMP1, OPN and Klotho immunoreactivity (Figure 1B–D), as well as calcification by von Kossa (Figure 1E), were subsequently assessed in sequential sections of calcified aorta. Using a subjective scale of the area of tissue with positive immunoreactivity, the scores for Klotho correlated with the scores for FGF23 staining (Tables 2 and 3). Similarly, vascular DMP1 and calcium scores also correlated directly with FGF23 scores (Tables 2 and 3). Next, to test for co-localization of these proteins, simultaneous immunofluorescence staining was performed for FGF23 and DMP1 as well as for FGF23 and Klotho proteins (Figure 4). The co-localization of FGF23 with both DMP1 and Klotho suggests that all three proteins are expressed together in calcified vascular tissue. Quantitative real-time polymerase chain reaction (qRT–PCR) was used to further confirm that the FGF23, Klotho and DMP1 protein identified by immunostaining was expressed in the coronary artery tissue itself and not merely trapped from circulating values. Using this technique, RNA expression of FGF23, Klotho and DMP1 were confirmed in five out of eight samples (Table 4).
FIGURE 3:
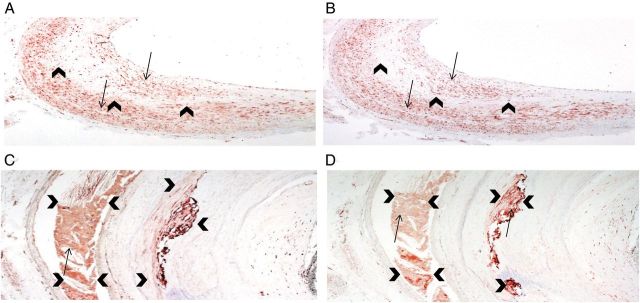
Immunoreactivity for fibroblast growth factor receptor 3 (FGFR3) and fibroblast growth factor receptor 1 (FGFR1) was present diffusely throughout the tunica intima and media as well as in calcified areas of coronary artery. (A) Immunohistochemical staining for FGFR3 and (B) immunohistochemical staining for FGFR1 in the tunica intima and media (×40 magnification). Red staining (arrows) indicates immunoreactivity. Arrowheads demarcate the border between the tunica intima and media. (C) Immunoreactivity for FGFR3 and (D) immunoreactivity for FGFR1 in calcified plaque (×200 magnification). Red staining (arrows) indicates immunoreactivity. Arrowheads demarcate the edge of the calcified lesion.
Table 2.
Intensity of FGF23, Klotho, DMP1, OPN and calcium deposition in coronary arteries. Expression was evaluated on a subjective scale: 0 (no expression) to 3 (maximal expression)
| Specimen # | FGF23 | Klotho | DMP1 | OPN | Calcium |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 1 |
| 3 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 1 | 3 | 0 |
| 8 | 0 | 0 | 0 | 1 | 0 |
| 6 | 1 | 1 | 2 | 2 | 2 |
| 7 | 1 | 1 | 1 | 1 | 2 |
| 2 | 2 | 2 | 3 | 3 | 3 |
| 5 | 2 | 1 | 2 | 3 | 1 |
FGF23, fibroblast growth factor 23; DMP1, dentin matrix protein 1; OPN, osteopontin
Bold values indicate samples with detectable FGF23 immunoreactivity.
Table 3.
Spearman correlation coefficients between intensity of Klotho, DMP1, osteopontin (OPN), FGF23 and calcium deposition in coronary arteries
| FGF23 | Klotho | DMP1 | OPN | |
|---|---|---|---|---|
| FGF23 | r = 1 | |||
| Klotho | r = 0.96, P < 0.001 | r = 1 | ||
| DMP1 | r = 0.89, P < 0.001 | r = 0.90, P < 0.001 | r = 1 | |
| OPN | r = 0.61, NS | r = 0.60, NS | r = 0.84, P < 0.01 | r = 1 |
| Calcium | r = 0.79, P < 0.05 | r = 0.90, P < 0.001 | r = 0.74, P < 0.05 | r = 0.29, NS |
NS, not significant; FGF23, fibroblast growth factor 23; DMP1, dentin matrix protein 1; OPN, osteopontin.
FIGURE 4:
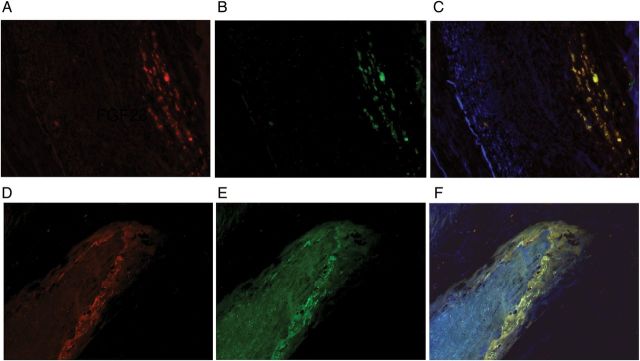
Immunofluorescence staining for FGF23, DMP1 and Klotho in calcified coronary artery. (A) FGF23 (red) and (B) DMP1 (green) in coronary artery. (C) Yellow color on merging (A) and (B) indicates co-localization FGF23 and DMP1. (D) Immunofluorescence staining for FGF23 (red) and (E) Klotho (green) in coronary artery. (F) Yellow color on merging (D) and (E) indicates co-localization Klotho and FGF23. Blue color indicates DAPI staining. FGF23, fibroblast growth factor 23; DMP1, dentin matrix protein 1.
Table 4.
FGF23, DMP1 and Klotho RNA expression in eight sections of coronary artery
| Specimen # | FGF23 | DMP1 | Klotho |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 |
| 3 | 0 | 0 | 29.65 |
| 4 | 0.74 | 1.61 | 30.71 |
| 5 | 16.64 | 0 | 0 |
| 6 | 0.48 | 1.48 | 13.63 |
| 7 | 0.62 | 0 | 34.01 |
| 8 | 1.09 | 2.04 | 32.50 |
FGF23, fibroblast growth factor 23; DMP1, dentin matrix protein 1.
DISCUSSION
The results of the current study demonstrate that FGF23 immunoreactivity was present in the calcified lesions of coronary arteries in 56% of subjects presenting for cardiac transplantation at our institution during the study period. Creatinine clearance measurements were lower in patients with coronary FGF23 immunoreactivity; similarly, a higher prevalence of stages 3 and 4 CKD were identified in these individuals. Although FGF23 immunoreactivity was observed in foamy macrophages, overall levels of inflammation, as assessed by high-sensitivity CRP, were lower in patients with positive FGF23 staining in coronary arteries. Vascular FGF23 expression, as determined by immunostaining, correlated directly with calcium deposition score and also with Klotho and DMP1 immunoreactivity. FGF23 co-localized with both DMP1 and Klotho in coronary artery tissue, suggesting that all three proteins are expressed in close proximity.
The association between decreasing renal function and an increasing prevalence of vascular FGF23 expression mirrors the increase in both circulating FGF23 levels [19, 20] and bone FGF23 expression [17] observed with declining renal function. CKD is associated with both an increase in the prevalence of vascular calcification [11, 21] and with a transformation of smooth muscle cells to an osteoblastic lineage [22]; thus the increase in vascular FGF23 may result from either or both of these mechanisms. Interestingly, however, 18% of patients with positive FGF23 also had normal kidney function. This raises the possibility that vascular FGF23 expression has multiple causes and/or that it may be an earlier manifestation of renal dysfunction than is creatinine clearance. The overlap in GFR between FGF23 positive and negative samples may also be due to the patchy nature of vascular calcification. Recent data have also suggested that FGF23 expression in the myocardium of patients with heart failure may be induced by macrophage-derived oncostatin M expression [15], a cytokine that is expressed in atherosclerotic lesions. In the current study, FGF23 was detected in foamy macrophages, as well as in calcified arterial lesions. Although inflammation on a systemic level, as measured by CRP levels, was lower in subjects displaying coronary artery FGF23 immunoreactivity, local increases in inflammation may have contributed to inter-patient differences in coronary FGF23 expression. Indeed, FGF23 immunoreactivity was observed in foamy macrophages, suggesting that FGF23 is produced by macrophages and that differences in macrophage infiltration may contribute to differences in vascular FGF23 expression.
The implications of Klotho-FGF23-FGFR system in vascular physiology and pathophysiology remain unknown. The presence of both protein and mRNA expression of FGF23, Klotho and DMP1 in calcified plaques suggests that these factors are synthesized by the calcified plaques in coronary arteries and were not trapped from the circulation. The current study also demonstrates that FGFR1 and FGFR3 are present throughout the tunicae intima and media of all vessels and that their expression is particularly intense in areas of calcium deposition, demonstrating that the machinery for FGF23 signaling is intact in calcified vessels. Whether the presence of FGF23 and its cofactors and receptors represents a mechanism by which calcification is enhanced, whether it reflects a protective response to vascular injury or whether it reflects transition of calcified tissue from an osteoblastic to an osteocytic phenotype is not known. Recent data by Scialla et al. suggested that FGF23 does not induce vascular calcification [23] and Wang et al. have previously demonstrated that FGF23 overexpression during osteoblast development is associated with suppression of osteoblastic differentiation and matrix mineralization [24], suggesting that one potential role for FGF23 may be a feedback regulatory mechanism to limit vascular calcification.
Klotho expression has been demonstrated in human aortic tissue as well as in atherosclerotic thrombi [25]. Loss of Klotho expression has been associated with a destruction of nitric oxide mediated vascular relaxation, leading to vascular calcification and loss of smooth muscle differentiation [26], and some data suggest that vascular Klotho expression may decline in response to CKD [27]. However, other data have failed to confirm the presence of Klotho either in mouse vascular rings or in human vascular smooth muscle cells [23]. In the current study, Klotho expression was detected only in sections of calcified coronary arteries that were positive for FGF23 and DMP1, contrasting with previous data suggesting that Klotho is vital to normal vascular smooth muscle tissue function [25–27]. Moreover, the current findings contrast with previous data suggesting that vascular Klotho expression declines with impairment of renal function [27]. These differences between studies may reflect differences in the specificities of Klotho antibodies (see Supplementary Figure S1), secondary antibodies, preservation of tissues and conditions used for immunohistochemistry. It is interesting to note, however, that Klotho is expressed in osteocytes [28] and in smooth muscle [26]; thus, the previous results may have detected the decrease in vascular smooth muscle cell Klotho expression while the current one identified an increasing expression of the protein in areas of calcification, where smooth muscle cells may be undergoing osteoblastic differentiation.
In the current study, vascular FGF23 and Klotho expression were higher in patients with lower CRP levels. Thus, if higher Klotho expression in calcified vessels represents a response to inflammation, as suggested by a recent study linking circulating FGF23 concentrations to inflammation [29], the inflammatory stimulus is likely local rather than systemic. The presence of FGF23 protein in macrophages, along with the co-localization of FGF23 with CD68, suggests a crucial role for inflammatory cells in extra-osseous FGF23 expression.
Limitations of the current study include the cross-sectional nature of the analysis, which precludes a causal relationship between FGF23, calcification and renal function. In addition, since true kidney function is difficult to assess in patients with heart failure due to altered hemodynamics, 24-h creatinine clearance measurements provided the best available measurement of kidney function in these individuals. Finally, complete evaluation of local synthesis of Klotho, FGF23, and the FGF receptors was limited by tissue availability. Nevertheless, these findings suggest that osteogenic transformation of vascular smooth muscle cells appears to occur in individuals as kidney function declines. However, whether FGF23 plays a role in the development of cardiovascular calcification or is solely a marker of the disease process requires further investigation.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared. (See related article by Messa. FGF23 and vascular calcifications: another piece of the puzzle? Nephrol Dial Transplant 2014; 29: 1447–1449.)
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by USPHS grants DK-67563, DK-35423, DK-51081, DK-073039, DK-080984, DK-081346, CTSI grant # UL1TR 000124 and funds from the Casey Lee Ball Foundation.
REFERENCES
- 1.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 2.Wesseling-Perry K, Pereira RC, Wang H, et al. Relationship between plasma FGF-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab. 2009;94:511–517. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christov M, Waikar SS, Pereira RC, et al. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int. 2013;84:639–641. doi: 10.1038/ki.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 5.Wan M, Smith C, Shah V, et al. Fibroblast growth factor 23 and soluble klotho in children with chronic kidney disease. Nephrol Dial Transplant. 2013;28:153–161. doi: 10.1093/ndt/gfs411. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22:956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson T, Nisbeth U, Ljunggren O, et al. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 10.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo D, Palmiero G, De Blasio AP, et al. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024–1030. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 13.Moe SM, Duan D, Doehle BP, et al. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003;63:1003–1011. doi: 10.1046/j.1523-1755.2003.00820.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshiko Y, Wang H, Minamizaki T, et al. Mineralized tissue cells are a principal source of FGF23. Bone. 2007;40:1565–1573. doi: 10.1016/j.bone.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Richter M, Polyakova V, Gajawada P, et al. Oncostatin M induces FGF23 expression in cardiomyocytes. J Clin Exp Cardiol. 2012;S:9. [Google Scholar]
- 16.Voigt M, Fischer DC, Rimpau M, et al. Fibroblast growth factor (FGF)-23 and fetuin-A in calcified carotid atheroma. Histopathology. 2010;56:775–788. doi: 10.1111/j.1365-2559.2010.03547.x. [DOI] [PubMed] [Google Scholar]
- 17.Pereira RC, Jüppner H, Azucena-Serrano CE, et al. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45:1161–1168. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1380. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesseling-Perry K, Pereira RC, Tseng CH, et al. Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:146–152. doi: 10.2215/CJN.05940611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo D, Battaglia Y, Buonanno E. Phosphorus and coronary calcification in predialysis patients. Kidney Int. 2010;78:818. doi: 10.1038/ki.2010.307. [DOI] [PubMed] [Google Scholar]
- 22.Shanahan CM. Mechanisms of vascular calcification in renal disease. Clin Nephrol. 2005;63:146–157. doi: 10.5414/cnp63146. [DOI] [PubMed] [Google Scholar]
- 23.Scialla JJ, Lau WL, Reilly MP, et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83:1159–1168. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Yoshiko Y, Yamamoto R, et al. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008;23:939–948. doi: 10.1359/jbmr.080220. [DOI] [PubMed] [Google Scholar]
- 25.Donate-Correa J, Mora-Fernandez C, Martinez-Sanz R, et al. Expression of FGF23/KLOTHO system in human vascular tissue. Int J Cardiol. 2013;165:179–183. doi: 10.1016/j.ijcard.2011.08.850. [DOI] [PubMed] [Google Scholar]
- 26.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim K, Lu TS, Molostvov G, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to FGF-23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 28.Rhee Y, Bivi N, Farrow E, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49:636–643. doi: 10.1016/j.bone.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendoza JM, Isakova T, Ricardo AC, et al. Fibroblast growth factor 23 and inflammation in CKD. Clin J Am Soc Nephrol. 2012;7:1155–1162. doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.