Abstract
Photon absorption by rhodopsin triggers the phototransduction signaling pathway that culminates in degradation of cGMP, closure of cGMP-gated ion channels and hyperpolarization of the photoreceptor membrane. This process is accompanied by a decrease in free Ca2+ concentration in the photoreceptor cytosol sensed by Ca2+-binding proteins that modulate phototransduction and activate the recovery phase to reestablish the photoreceptor dark potential. Guanylate cyclase-activating proteins (GCAPs) belong to the neuronal calcium sensor (NCS) family and are responsible for activating retinal guanylate cyclases (retGCs) at low Ca2+ concentrations triggering synthesis of cGMP and recovery of the dark potential. Here we review recent structural insight into the role of the N-terminal myristoylation in GCAPs and compare it to other NCS family members. We discuss previous studies identifying regions of GCAPs important for retGC1 regulation in the context of the new structural data available for myristoylated GCAP1. In addition, we present a hypothetical model for the Ca2+-triggered conformational change in GCAPs and retGC1 regulation. Finally, we briefly discuss the involvement of mutant GCAP1 proteins in the etiology of retinal degeneration as well as the importance of other Ca2+ sensors in the modulation of phototransduction.
The phototransduction pathway links absorption of light to a decrease in cytosolic cGMP. Depletion of the cGMP pool induces closure of cGMP-gated cation channels and hyperpolarization of photoreceptor cells with a consequent decrease in glutamate neurotransmitter release detected by secondary neurons (1). Briefly, phototransduction is initiated with absorption of a photon by the chromophore 11-cis-retinal that is covalently linked to G-protein-coupled receptors known as opsins. Isomerization of 11-cis-retinal to all-trans-retinal and dissociation of the chromophore produces a conformational change in the opsin and consequent activation of the coupled heterotrimeric G-protein transducin (2). Activated transducin then activates a retina-specific phosphodiesterase (PDE6) that cleaves cGMP, depleting cytoplasmic cGMP and closing cGMP-gated cation channels. The level of cGMP is then restored to dark levels by activation of retina-specific guanylate cyclases (retGCs: retGC1 or GC-E; and retGC2 or GC-F) (3,4). retGC1 appears to be critical for cone function (5). Both disruption of normal phototransduction and recovery of the dark state by photoreceptors exposed to light are associated with a variety of cone-rod retinopathies (6).
The plasma membrane potential in photoreceptors is determined, in part, by open cGMP-gated cation channels. The fraction of open channels depends on the level of cGMP in the cytoplasm, which is controlled by the opposing effects of PDE6 and retGCs. RetGCs are transmembrane proteins located in the disk membranes of photoreceptor cells. An extracellular domain lies within the lumen of the disk membranes, but unlike homologous GCs in other tissues that are activated by ligand binding to the extracellular domain, no ligand has been identified for retGC1. In fact, they can be activated even when the extracellular domain has been truncated (7–9). Instead, retGCs are regulated by Ca2+, which plays a crucial role in the regulation of phototransduction in photoreceptors. The concentration of free Ca2+ in the cytoplasm drops from ~550 to ~50 nM during activation due to the closing of cGMP-gated cation channels that block the influx of Ca2+, as well as to the action of Na+/Ca2+-K+ exchangers which export Ca2+ from the cell (10). Retina-specific neuronal calcium sensor (NCS) proteins bind Ca2+ at the higher concentrations characteristic of the dark state but not at the lower concentrations achieved after prolonged activation and link phototransduction to Ca2+ signaling (3,4).
NCS proteins belong to the EF-hand superfamily of Ca2+-binding proteins. They are expressed in neurons and have four EF-hands but only two or three of them are able to bind Ca2+ (11). Most NCS proteins are myristoylated at the N-terminus. Two classes of myristoylated NCS proteins are expressed in photoreceptors and are active in phototransduction—(1) guanylate cyclase-activating proteins (GCAPs) that regulate retGCs in response to Ca2+ and mediate the restoration dark levels of cGMP; and (2) recoverin, which plays a role in prolonging the photoresponse.
GUANYLATE CYCLASE-ACTIVATING PROTEINS
GCAPs modulate cGMP production by retGCs, inhibiting the cyclase at high Ca2+ concentrations typical of the dark state, and activating the cyclase when the Ca2+ concentration drops after phototransduction. Two GCAPs, GCAP1 and GCAP2, are conserved among most vertebrates, while a third isoform, GCAP3, has been identified in humans and fish (12). Additional GCAPs (GCAP4–8) are present in teleosts and likely arise from gene duplication (13). All GCAPs are approximately 23 kDa proteins with four EF-hands. However, only the last three EF-hands are capable of binding Ca2+. Each GCAP can activate either of the two retGCs identified in rod and cone cells. Therefore, the specific roles of individual GCAP isoforms are unclear (14,15). GCAP isoforms have ~40% identity to each other with the greatest variation noted at the N- and C-termini; these polypeptide segments might play an important role in GCAP function as suggested by a related protein, guanylate cyclase-inhibitory protein (GCIP), which diverges from GCAPs in these regions. Identified in frogs, GCIP possesses the inhibitory function of GCAPs at high Ca2+ concentrations (~500 nM) but does not activate retGC1 (16).
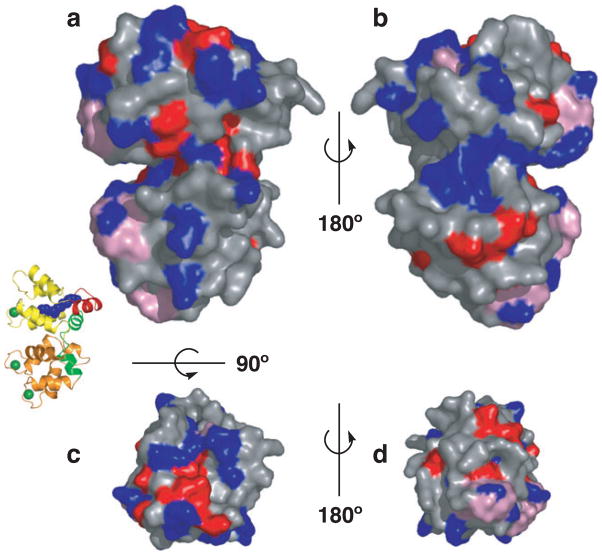
Structures of unmyristoylated GCAP2 and GCAP3 have been determined by NMR and X-ray crystallography respectively (17,18). Very recently, the first structure of a myristoylated GCAP (myrGCAP1) was solved crystallographically (Fig. 1) (19). The structures show that GCAPs are compact α-helical proteins with two domains, each with two EF-hands, similar to other NCS proteins. The members of the NCS family share substantial sequence identity (30–50%) and a similar overall fold. Several structures of NCS proteins with Ca2+ bound have been determined and a superposition of representative NCS proteins onto GCAP1 reveals that the overall structure is well conserved in this family (Fig. 2). The root-mean-square deviations for the structurally conserved α-carbons between myrGCAP1 and the NCS proteins with known structures oscillate between 3.3 and 3.9 Å (summarized in Table 1). In spite of the sequence and overall structural similarity among NCS family members, their functions are generally not interchangeable. The main differences arise from the length and conformation of the N- and C-terminal helices and, to a lesser extent, the relative orientation of the N-terminal domain with respect to the C-terminal domain. These structural differences and amino acids conserved only within each class of the NCS family determine the target specificity of these proteins. For GCAPs, conserved residues that do not participate in Ca2+-binding are highlighted in the surface representation of GCAP1 in Fig. 3 as possible sites of interaction with retGC1.
Figure 1.
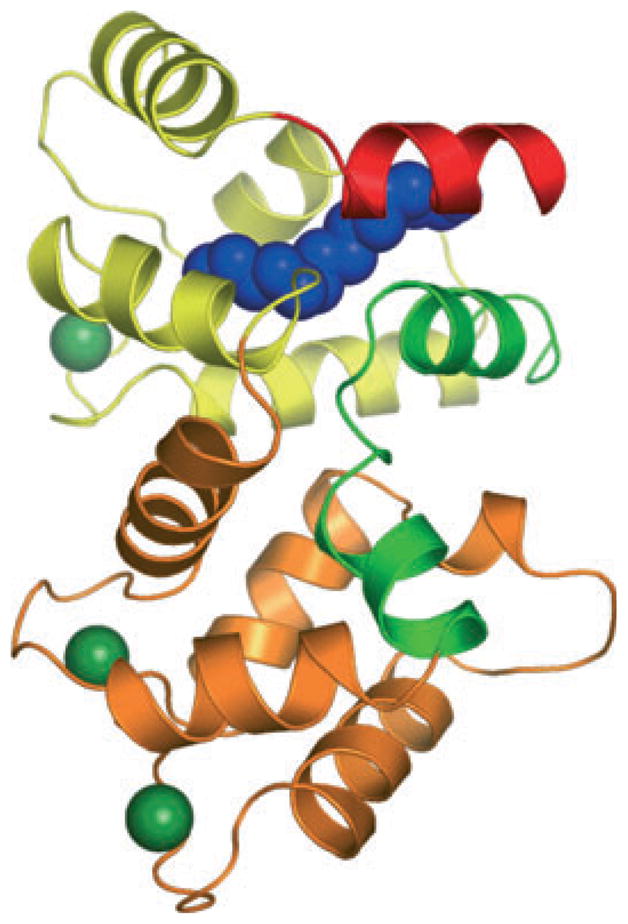
Structure of myristoylated chicken GCAP1 with Ca2+ bound. The cartoon representation is colored yellow for EF-hands 1 and 2 and orange for EF-hands 3 and 4. The N-terminal helix is colored red, the kinked C-terminal helix is green and the myristoyl group is shown as a space-filling model in blue. Bound Ca2+ ions are shown in dark green.
Figure 2.
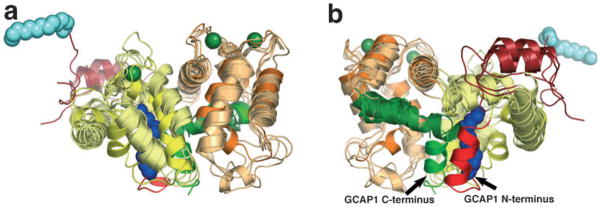
Superposition of representative NCS protein structures in the Ca2+-bound form. Front (a) and back (b) cartoon representations of myrGCAP1 superimposed onto the NCS family members myr-recoverin, neurocalcin, NCS-1 and KChIP1. The coloring of myrGCAP1 is as in Fig. 1 with the N- and C-terminal helices in bright red and green, respectively. The other NCS proteins are colored light yellow for EF-hands 1 and 2 and light orange for EF-hands 3 and 4, dark red for the N-terminal helix and dark green for the C-terminal segment. The recoverin myristoyl group is shown as a space-filling model in light blue. Ca2+ ions bound to GCAP1 are shown in dark green for reference but are omitted for the other NCS proteins for clarity.
Table 1.
Structures of Ca2+-bound NCS family members.
| Protein (species) | Resolution (Å) | RSMD–GCAP1 (Å) | Myr | PDB ID (Ref.) |
|---|---|---|---|---|
| GCAP1 (chicken) | 2.00 | – | Yes | 2r2i (19) |
| Recoverin (bovine) | NMR | 3.7* | Yes | 1jsa (27) |
| Recoverin (bovine) | 1.9 | 3.4 | No | 1rec (58) |
| Recoverin (human) | 2.2 | 3.6 | No | 2d8n (S. Kamo, S. Kishishita, K. Murayama, M. Shirouzu and S. Yokohama, unpublished) |
| GCAP2 (bovine) | NMR | 3.6* | No | 1jba (17) |
| GCAP3 (human) | 3.00 | 3.9 | No | 2ggz (18) |
| Neurocalcin (bovine) | 2.40 | 3.8 (chain A) 3.5 (chain B) |
No | 1bjf (59) |
| NCS-1 (human) | 1.90 | 3.9 (chain A) 3.7 (chain B) |
No | 1g8i (60) |
| Frequenin (yeast) | NMR | 3.7* | No | 1fpw (61) |
| KChIP (human) | 2.3 | 3.3 | No | 1s1e (62) |
For NMR structures the root-mean-square deviations with GCAP1 was calculated using the “central” structure of the NMR family.
Figure 3.
Surface residue conservation in GCAPs. Surface representations of myrGCAP1 in front (a), back (b), top (c) and bottom (d) orientations (a cartoon model oriented as in [a] is shown for reference). Residues conserved in GCAP1 across species, but not GCAP2, are colored blue. Residues conserved in GCAP1 and GCAP2, but not across the NCS family, are in red. Residues required for myristoylation and Ca2+ coordination across NCS family are shown in pink.
GCAPS AND RETINAL DISEASE
GCAP1 is of particular interest due to its association with autosomal-dominant cone-rod dystrophies. Point mutations in five separate codons give rise to amino acid changes observed in families with cone degeneration including P50L, Y99C, I143NT, L150F and E155G. The mutations Y99C, I143NT, L150F and E155G each alter the Ca2+ sensitivity of GCAP1 (20–22). The E155G mutation eliminates the glutamate at position 12 of the Ca2+-binding loop of EF-hand 4 (23). The glutamate side chain at this position of the EF-hand-binding loop helps coordinate Ca2+ and is conserved in all EF-hands. Elimination of the corresponding glutamate in EF-hand 3 produces a similar phenotype to E155G, i.e. a constitutively active GCAP1 (24). Y99 stabilizes a kink in the C-terminal helix of GCAP1 that enables the C-terminal helix to contact the N-terminal domain and the myristoyl group (19). The side chain of Y99 hydrogen bonds with a main chain carbonyl within the C-terminal helix forcing a kink that is further stabilized by other hydrogen bonds and a salt bridge. Also, Y99 has hydrophobic interactions with surrounding residues and is the last residue of the entering helix to EF-hand 3. Of particular note, I107 within the EF-hand 3-binding loop interacts with Y99. Thus the Y99C mutation likely has two effects—(1) it destabilizes the C-terminal helix kink by removing a hydrogen bond and (2) it introduces a void volume in the hydrophobic core as a result of the smaller Cys side chain (19).
The effect of mutations in residues I143 and L150 is not entirely clear. I143 is located just before the Ca2+-binding loop of EF-hand 4 and substitution of a residue capable of hydrogen bonding might alter the geometry of the EF-hand, preventing Ca2+ binding. Likewise, substitution of L150 by the larger phenylalanine residue might disrupt the conformation of EF-hand 4 enough to prevent proper Ca2+ binding (18). All these GCAP1 mutations share a similar phenotype wherein retGC1 is inadequately inhibited. Finally, the P50L mutation appears to affect GCAP function by a different mechanism. This mutation has been shown to increase the susceptibility of GCAP1 to trypsin proteolysis, suggesting that a shorter half-life of GCAP1 might reduce GCAP1 levels in cones to the point that retGC activity is insufficiently inhibited in the dark state (25).
All mutations in GCAP1 are linked to increased levels of cGMP in photoreceptors leading to degeneration of the retina. One speculative possibility for the mechanism of degeneration is that the increased levels of cGMP in cones open a higher percentage of cGMP-gated cation channels leading to a prolonged increase in intracellular Ca2+ sufficient to trigger apoptosis (26).
N-TERMINAL ACYLATION OF NCS PROTEINS
The structures of myristoylated recoverin in both Ca2+-bound and Ca2+-free forms have been determined by NMR (27,28). Like other NCS proteins, recoverin is a compact α-helical protein with each pair of EF-hands connected by a central loop. The Ca2+-bound structure has the myristoyl group exposed whereas the Ca2+-free structure has the myristoyl group bound in a hydrophobic cleft on the surface of the protein. The exposure of the myristoyl group in Ca2+ is essential for anchoring the protein to the membrane. Upon Ca2+ release, the myristoyl group is sequestered in a deep hydrophobic cleft of the protein allowing free movement of recoverin through the cytoplasm. This behavior has been termed the “Ca2+-myristoyl switch” (27,29,30). Other NCS proteins including hippocalcin and the visinin-like proteins (ViLiPs) show myristoyl switch behavior (31–33). However, some NCS proteins including GCAPs do not appear to have a canonical myristoyl switch (34,35). Therefore, the role of the N-terminal acylation in these proteins has remained obscure (11).
The recently determined structure of Ca2+-bound myrGCAP1 shows that, in sharp contrast to Ca2+-bound recoverin, the myristoyl group is completely buried within the N-terminal domain of GCAP1 (Fig. 1) (19). Moreover, fluorescence quenching experiments using a 16-NBD-palmitoyl group attached to GCAP1 indicate that the myristoyl group remains buried in both the Ca2+-bound and Ca2+-free forms of the protein (19). Therefore, the myristoyl group is “nonswitching” in GCAP1 and instead has a stabilizing, structural role in this protein. The published structures of GCAP2 and GCAP3 are from unmyristoylated proteins and it is likely that these structures are affected by the absence of the natural myristoyl group. In particular, the N- and C-terminal regions of both GCAP2 and GCAP3 are poorly defined or extremely flexible in the unmyristoylated structures. In myrGCAP1 however, the N- and C-terminal helices are clustered together with the buried myristoyl group, which stabilizes their conformation. Importantly, removal of the C-terminal helix residues contacting the myristoyl group prevents GCAP1 activation of retGC1 (36). The contacts between the C-terminal helix with the N-terminal helix and myristoyl group stabilize the orientation of the N- and C-terminal domains relative to each other. Whereas myristoylation has been shown to be necessary for proper Ca2+ sensitivity of GCAP1, it appears to be less important for Ca2+ sensitivity of GCAP2 (35).
GCAP REQUIREMENTS FOR RETGC REGULATION
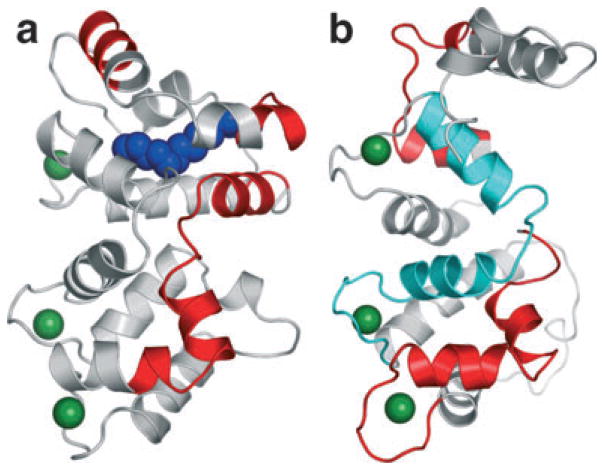
The interface(s) of interaction between retGCs and GCAPs have not been determined with certainty. GCAP1 and GCAP2 are bound to the intracellular part of retGC1 in both the Ca2+-free (activating) and Ca2+-bound (inhibiting) forms (8). Several experiments have been performed to identify the regions of GCAP important for the interaction with retGC1 (Fig. 4). Chimeras of bovine GCAP1 and GCIP have shown that the N-terminal region of GCAP1 is crucial for retGC regulation. A chimera composed of the N-terminal 20 residues from GCIP and the remaining residues from GCAP1 did not activate retGC1 while a chimera of the N-terminal 43 residues of GCAP1 along with the remaining residues from GCIP activated retGC1 in a Ca2+-dependent manner (37). Additionally, chimeric proteins containing sequences from the NCS family protein neurocalcin (~35% identity to GCAPs) were substituted into bovine GCAP1; these experiments identified the polypeptides M157-R182 and W21-T27 in bovine GCAP1 as necessary for activation of retGC1 (36). Also, N-terminal deletions of the myristoyl group and the first 25 residues of GCAP1 showed that this region is essential for proper Ca2+-dependent retGC1 activation (38).
Figure 4.
Regions of GCAP1 (a) and GCAP2 (b) identified in chimera studies as important for retGC regulation. Regions affecting retGC regulation are shown in red. The region of GCAP2 that reverses Ca2+ dependency is shown in cyan. The myristoyl group in GCAP1 is shown as a space-filling model in blue.
Point mutations in the EF-hand 1 region of GCAP2 prevent activation of retGC1, confirming the importance of this region for GCAP activity (39). Moreover, experiments with bovine GCAP2 identified residues K29 through F48 as required for both activation and inhibition of retGC1 whereas V171-N189 was only required for activation. Curiously, the region between EF-hand 2 and EF-hand 3 (F78-D113) in GCAP2 determined the direction of Ca2+ regulation of retGC1. Substituting this region for the corresponding sequence of neurocalcin created a chimera that activated retGC1 at high Ca2+ and inhibited retGC1 at low Ca2+ (40).
Alternative approaches to pinpoint GCAP residues important for retGC1 regulation have provided additional information. Peptide competition experiments suggest that the region between F73 and K87 in GCAP1 is important for interaction with retGC1 (41). Moreover, experiments in which GCAP and retGC1 were crosslinked, trypsin digested and the fragments analyzed by mass spectrometry, revealed that the regions around C17 and C105 on GCAP1 were in close proximity to the kinase homology domain on retGC1 (42).
MODELS OF THE CONFORMATIONAL CHANGE IN GCAPS AND RETGC REGULATION
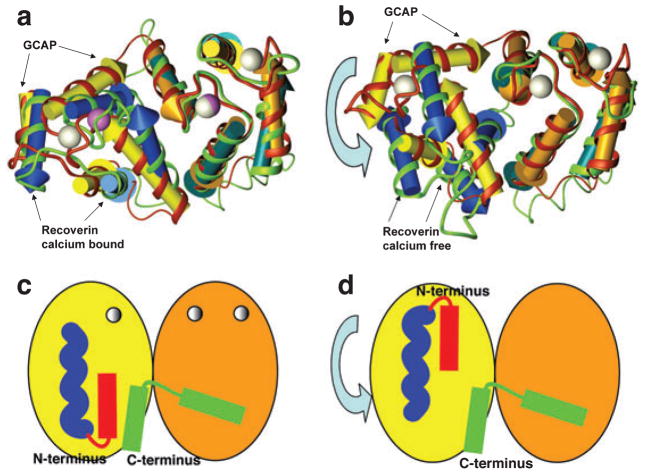
The structure of the Ca2+ free, activating form of a GCAP is not yet available. However, it is thought that the conformational change induced by Ca2+ release involves relatively little change in the domain structure but a significant rotation of the N-terminal domain with respect to the C-terminal domain. Such a conformational change has been experimentally observed in recoverin (Fig. 5a,b) (27–29). Furthermore, tryptophan fluorescence experiments with GCAP1 support a conformational change involving reorientation of the N- and C-terminal domains because W91, located on the central helix, is exposed only under low Ca2+ conditions (43).
Figure 5.
Model of the Ca2+-induced conformational change in GCAP1. (a) EF-hands of Ca2+-bound GCAP represented by yellow and orange arrowed cylinders, red ribbon and white calcium ions, superimposed on Ca2+-bound recoverin represented by blue and green-blue arrowed cylinders, green ribbon and violet Ca2+ ions. N- and C-terminal parts of the structure were removed for clarity. Superimposition was done on C-terminal EF-hand pairs (orange and green-blue cylinders). (b) EF-hands of Ca2+-bound GCAP superimposed on Ca2+-free recoverin. Superimposition was done on C-terminal EF-hand pairs. Coloring as in (a). (c, d) Schematic representation of a possible Ca2+-induced conformational change in GCAP1 where the N-terminal (red) and C-terminal (green) helices are clustered together in the Ca2+-bound state (c), but separated by domain rotation in the Ca2+-free form (d). The myristoyl group is represented in blue.
The chimera experiments described above suggest that the N- and C-terminal helices of GCAP1 are the most important for retGC1. The structure of myristoylated chicken GCAP1 shows these regions in close proximity to each other and clustered with the myristoyl group in the Ca2+ is bound, inhibitory conformation of the protein (Figs. 1 and 4 and schematically in Fig. 5c). Any rotation of the N- and C-terminal domains relative to each other would necessarily pull the terminal helices apart (schematically in Fig. 5d). We propose that this separation of the N- and C-terminal helices in GCAP1 is crucial to induce the activated conformation of retGC1.
A separate model of GCAP activation of retGC1 involving dimerization of the GCAP has been proposed based on the observation that GCAP2 migrates as a dimer in the absence of Ca2+ but entirely as a monomer in the presence of Ca2+ during gel chromatography (40,44,45). However, this behavior was not observed for GCAP1 (40). Structures of retGC and its complex with GCAPs will be necessary to unravel the mechanistic details of GCAP regulation of retGCs. These structures would also allow the mechanistic integration of other modulatory signals like retGC phosphorylation (46) as well as understanding the roles of the noncatalytic retGC domains such as the extracellular, kinase homology and dimerization domains (47).
OTHER CA2+ SENSORS IN PHOTOTRANSDUCTION
Recoverin is another member of the NCS family active in phototransduction. In mouse rods, recoverin prolongs the photoresponse, thereby increasing rod sensitivity in dim light. (48). One model of recoverin mechanism suggests that it functions as a Ca2+-sensitive regulator of rhodopsin kinase (RK) in the outer segment (49,50), a function that does not require recoverin myristoylation (51,52). However, inhibition of RK is not specific to recoverin as other NCS proteins are capable of Ca2+-sensitive regulation of RK as well (53). Also, recoverin is present in the rod inner segment and synaptic terminals and it has an effect on the photo-response that must be downstream of its outer segment action in addition to any outer segment effects it may have. Recoverin has also been proposed to act either as a Ca2+ buffer, a regulator of the conductance in the inner segment of photoreceptors, or by an interaction with the neurotransmitter release machinery at the synapse (48).
The ubiquitous Ca2+-binding protein calmodulin (CaM) might also have a role in recovery of the dark state after photoexcitation. It has been reported that Ca2+-bound CaM decreases the sensitivity of cGMP-gated cation channels to cGMP in rods (54). Moreover, the β subunit of rod CNG channels has a CaM-binding site (55). While CaM dramatically alters sensitivity of many types of cyclic-nucleotide channels, the magnitude of the effect in rods is small, arguing against this being a significant mechanism (56). Additionally, a study of catfish cone channels concluded that, unlike in rods, Ca2+/CaM did not alter cone CNG channel activity at all (57).
While progress has been made on the mode of Ca2+-dependent regulation of phototransduction, much remains unknown. The mechanism of activation of retGCs by GCAPs is still poorly understood. Work on the structure of Ca2+-free GCAPs and GCAPs in complex with retGCs is needed to assess the role of different GCAPs in photoreceptors and the mechanism of retGC regulation. Finally, the contributions of additional Ca2+ sensors, such as recoverin and CaM, to the regulation of phototransduction in vivo remain to be fully addressed.
Acknowledgments
We thank Dr. Leslie T. Webster Jr. for critical comments on the manuscript. This work was supported in part by NIH grant R01 EY008061. Structural biology research at the University of Colorado at Boulder is supported in part by the William M. Keck Foundation.
Footnotes
This invited paper is part of the Symposium-in-Print: Photoreceptors and Signal Transduction.
References
- 1.Belgum JH, Copenhagen DR. Synaptic transfer of rod signals to horizontal and bipolar cells in the retina of the toad (Bufo marinus) J Physiol. 1988;396:225–245. doi: 10.1113/jphysiol.1988.sp016960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palczewski K, Polans AS, Baehr W, Ames JB. Ca2+-binding proteins in the retina: Structure, function, and the etiology of human visual diseases. Bioessays. 2000;22:337–350. doi: 10.1002/(SICI)1521-1878(200004)22:4<337::AID-BIES4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 4.Polans A, Baehr W, Palczewski K. Turned on by Ca2+! The physiology and pathology of Ca2+-binding proteins in the retina. Trends Neurosci. 1996;19:547–554. doi: 10.1016/s0166-2236(96)10059-x. [DOI] [PubMed] [Google Scholar]
- 5.Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–8847. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duda T, Koch KW. Retinal diseases linked with photoreceptor guanylate cyclase. Mol Cell Biochem. 2002;230:129–138. [PubMed] [Google Scholar]
- 7.Duda T, Goraczniak R, Surgucheva I, Rudnicka-Nawrot M, Gorczyca WA, Palczewski K, Sitaramayya A, Baehr W, Sharma RK. Calcium modulation of bovine photoreceptor guanylate cyclase. Biochemistry. 1996;35:8478–8482. doi: 10.1021/bi960752z. [DOI] [PubMed] [Google Scholar]
- 8.Laura RP, Dizhoor AM, Hurley JB. The membrane guanylyl cyclase, retinal guanylyl cyclase-1, is activated through its intracellular domain. J Biol Chem. 1996;271:11646– 11651. doi: 10.1074/jbc.271.20.11646. [DOI] [PubMed] [Google Scholar]
- 9.Sokal I, Alekseev A, Baehr W, Haeseleer F, Palczewski K. Soluble fusion proteins between single transmembrane photoreceptor guanylyl cyclases and their activators. Biochemistry. 2002;41:251–257. doi: 10.1021/bi015606u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray-Keller MP, Detwiler PB. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994;13:849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 11.Burgoyne RD. Neuronal calcium sensor proteins: Generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci. 2007;8:182–193. doi: 10.1038/nrn2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeseleer F, Sokal I, Li N, Pettenati M, Rao N, Bronson D, Wechter R, Baehr W, Palczewski K. Molecular characterization of a third member of the guanylyl cyclase-activating protein subfamily. J Biol Chem. 1999;274:6526–6535. doi: 10.1074/jbc.274.10.6526. [DOI] [PubMed] [Google Scholar]
- 13.Imanishi Y, Yang L, Sokal I, Filipek S, Palczewski K, Baehr W. Diversity of guanylate cyclase-activating proteins (GCAPs) in teleost fish: Characterization of three novel GCAPs (GCAP4, GCAP5, GCAP7) from zebrafish (Danio rerio) and prediction of eight GCAPs (GCAP1–8) in pufferfish (Fugu rubripes) J Mol Evol. 2004;59:204–217. doi: 10.1007/s00239-004-2614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imanishi Y, Li N, Sokal I, Sowa ME, Lichtarge O, Wensel TG, Saperstein DA, Baehr W, Palczewski K. Characterization of retinal guanylate cyclase-activating protein 3 (GCAP3) from zebrafish to man. Eur J Neurosci. 2002;15:63–78. doi: 10.1046/j.0953-816x.2001.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorczyca WA, Gray-Keller MP, Detwiler PB, Palczewski K. Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc Natl Acad Sci USA. 1994;91:4014–4018. doi: 10.1073/pnas.91.9.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Fariss RN, Zhang K, Otto-Bruc A, Haeseleer F, Bronson D, Qin N, Yamazaki A, Subbaraya I, Milam AH, Palczewski K, Baehr W. Guanylate-cyclase-inhibitory protein is a frog retinal Ca2+-binding protein related to mammalian guanylate-cyclase-activating proteins. Eur J Biochem. 1998;252:591–599. doi: 10.1046/j.1432-1327.1998.2520591.x. [DOI] [PubMed] [Google Scholar]
- 17.Ames JB, Dizhoor AM, Ikura M, Palczewski K, Stryer L. Three-dimensional structure of guanylyl cyclase activating protein-2, a calcium-sensitive modulator of photoreceptor guanylyl cyclases. J Biol Chem. 1999;274:19329–19337. doi: 10.1074/jbc.274.27.19329. [DOI] [PubMed] [Google Scholar]
- 18.Stephen R, Palczewski K, Sousa MC. The crystal structure of GCAP3 suggests molecular mechanism of GCAP-linked cone dystrophies. J Mol Biol. 2006;359:266–275. doi: 10.1016/j.jmb.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephen R, Bereta G, Golczak M, Palczewski K, Sousa MC. Stabilizing function for myristoyl group revealed by the crystal structure of a neuronal calcium sensor, guanylate cyclase-activating protein 1. Structure. 2007;15:1392–1402. doi: 10.1016/j.str.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dizhoor AM, Boikov SG, Olshevskaya EV. Constitutive activation of photoreceptor guanylate cyclase by Y99C mutant of GCAP-1. Possible role in causing human autosomal dominant cone degeneration. J Biol Chem. 1998;273:17311–17314. doi: 10.1074/jbc.273.28.17311. [DOI] [PubMed] [Google Scholar]
- 21.Nishiguchi KM, Sokal I, Yang L, Roychowdhury N, Palczewski K, Berson EL, Dryja TP, Baehr W. A novel mutation (I143NT) in guanylate cyclase-activating protein 1 (GCAP1) associated with autosomal dominant cone degeneration. Invest Ophthalmol Vis Sci. 2004;45:3863–3870. doi: 10.1167/iovs.04-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokal I, Dupps WJ, Grassi MA, Brown J, Jr, Affatigato LM, Roychowdhury N, Yang L, Filipek S, Palczewski K, Stone EM, Baehr W. A novel GCAP1 missense mutation (L151F) in a large family with autosomal dominant cone-rod dystrophy (adCORD) Invest Ophthalmol Vis Sci. 2005;46:1124–1132. doi: 10.1167/iovs.04-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkie SE, Li Y, Deery EC, Newbold RJ, Garibaldi D, Bateman JB, Zhang H, Lin W, Zack DJ, Bhattacharya SS, Warren MJ, Hunt DM, Zhang K. Identification and functional consequences of a new mutation (E155G) in the gene for GCAP1 that causes autosomal dominant cone dystrophy. Am J Hum Genet. 2001;69:471–480. doi: 10.1086/323265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudnicka-Nawrot M, Surgucheva I, Hulmes JD, Haeseleer F, Sokal I, Crabb JW, Baehr W, Palczewski K. Changes in biological activity and folding of guanylate cyclase-activating protein 1 as a function of calcium. Biochemistry. 1998;37:248–257. doi: 10.1021/bi972306x. [DOI] [PubMed] [Google Scholar]
- 25.Newbold RJ, Deery EC, Walker CE, Wilkie SE, Srinivasan N, Hunt DM, Bhattacharya SS, Warren MJ. The destabilization of human GCAP1 by a proline to leucine mutation might cause cone-rod dystrophy. Hum Mol Genet. 2001;10:47–54. doi: 10.1093/hmg/10.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Newbold RJ, Deery EC, Payne AM, Wilkie SE, Hunt DM, Warren MJ. Guanylate cyclase activating proteins, guanylate cyclase and disease. Adv Exp Med Biol. 2002;514:411–438. doi: 10.1007/978-1-4615-0121-3_25. [DOI] [PubMed] [Google Scholar]
- 27.Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. doi: 10.1038/38310. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T, Ames JB, Harvey TS, Stryer L, Ikura M. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature. 1995;376:444–447. doi: 10.1038/376444a0. [DOI] [PubMed] [Google Scholar]
- 29.Ames JB, Hamasaki N, Molchanova T. Structure and calcium-binding studies of a recoverin mutant (E85Q) in an allosteric intermediate state. Biochemistry. 2002;41:5776–5787. doi: 10.1021/bi012153k. [DOI] [PubMed] [Google Scholar]
- 30.Ames JB, Ikura M. Structure and membrane-targeting mechanism of retinal Ca2+-binding proteins, recoverin and GCAP-2. Adv Exp Med Biol. 2002;514:333–348. doi: 10.1007/978-1-4615-0121-3_20. [DOI] [PubMed] [Google Scholar]
- 31.Burgoyne RD, Weiss JL. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J. 2001;353:1–12. [PMC free article] [PubMed] [Google Scholar]
- 32.O’Callaghan DW, Ivings L, Weiss JL, Ashby MC, Tepikin AV, Burgoyne RD. Differential use of myristoyl groups on neuronal calcium sensor proteins as a determinant of spatio-temporal aspects of Ca2+ signal transduction. J Biol Chem. 2002;277:14227–14237. doi: 10.1074/jbc.M111750200. [DOI] [PubMed] [Google Scholar]
- 33.Spilker C, Richter K, Smalla KH, Manahan-Vaughan D, Gundelfinger ED, Braunewell KH. The neuronal EF-hand calcium-binding protein visinin-like protein-3 is expressed in cerebellar Purkinje cells and shows a calcium-dependent membrane association. Neuroscience. 2000;96:121–129. doi: 10.1016/s0306-4522(99)00536-9. [DOI] [PubMed] [Google Scholar]
- 34.Hwang JY, Koch KW. Calcium- and myristoyl-dependent properties of guanylate cyclase-activating protein-1 and protein-2. Biochemistry. 2002;41:13021–13028. doi: 10.1021/bi026618y. [DOI] [PubMed] [Google Scholar]
- 35.Olshevskaya EV, Hughes RE, Hurley JB, Dizhoor AM. Calcium binding, but not a calcium-myristoyl switch, controls the ability of guanylyl cyclase-activating protein GCAP-2 to regulate photoreceptor guanylyl cyclase. J Biol Chem. 1997;272:14327–14333. doi: 10.1074/jbc.272.22.14327. [DOI] [PubMed] [Google Scholar]
- 36.Krylov DM, Niemi GA, Dizhoor AM, Hurley JB. Mapping sites in guanylyl cyclase activating protein-1 required for regulation of photoreceptor membrane guanylyl cyclases. J Biol Chem. 1999;274:10833–10839. doi: 10.1074/jbc.274.16.10833. [DOI] [PubMed] [Google Scholar]
- 37.Li N, Sokal I, Bronson JD, Palczewski K, Baehr W. Identification of functional regions of guanylate cyclase-activating protein 1 (GCAP1) using GCAP1/GCIP chimeras. Biol Chem. 2001;382:1179–1188. doi: 10.1515/BC.2001.148. [DOI] [PubMed] [Google Scholar]
- 38.Otto-Bruc A, Buczylko J, Surgucheva I, Subbaraya I, Rudnicka-Nawrot M, Crabb JW, Arendt A, Hargrave PA, Baehr W, Palczewski K. Functional reconstitution of photoreceptor guanylate cyclase with native and mutant forms of guanylate cyclase-activating protein 1. Biochemistry. 1997;36:4295– 4302. doi: 10.1021/bi963000d. [DOI] [PubMed] [Google Scholar]
- 39.Ermilov AN, Olshevskaya EV, Dizhoor AM. Instead of binding calcium, one of the EF-hand structures in guanylyl cyclase activating protein-2 is required for targeting photoreceptor guanylyl cyclase. J Biol Chem. 2001;276:48143–48148. doi: 10.1074/jbc.M107539200. [DOI] [PubMed] [Google Scholar]
- 40.Olshevskaya EV, Ermilov AN, Dizhoor AM. Dimerization of guanylyl cyclase-activating protein and a mechanism of photoreceptor guanylyl cyclase activation. J Biol Chem. 1999;274:25583–25587. doi: 10.1074/jbc.274.36.25583. [DOI] [PubMed] [Google Scholar]
- 41.Schrem A, Lange C, Beyermann M, Koch KW. Identification of a domain in guanylyl cyclase-activating protein 1 that interacts with a complex of guanylyl cyclase and tubulin in photoreceptors. J Biol Chem. 1999;274:6244–6249. doi: 10.1074/jbc.274.10.6244. [DOI] [PubMed] [Google Scholar]
- 42.Krylov DM, Hurley JB. Identification of proximate regions in a complex of retinal guanylyl cyclase 1 and guanylyl cyclase-activating protein-1 by a novel mass spectrometry-based method. J Biol Chem. 2001;276:30648–30654. doi: 10.1074/jbc.M104121200. [DOI] [PubMed] [Google Scholar]
- 43.Sokal I, Otto-Bruc AE, Surgucheva I, Verlinde CL, Wang CK, Baehr W, Palczewski K. Conformational changes in guanylyl cyclase-activating protein 1 (GCAP1) and its tryptophan mutants as a function of calcium concentration. J Biol Chem. 1999;274:19829–19837. doi: 10.1074/jbc.274.28.19829. [DOI] [PubMed] [Google Scholar]
- 44.Peshenko IV, Olshevskaya EV, Dizhoor AM. Ca(2+)-dependent conformational changes in guanylyl cyclase-activating protein 2 (GCAP-2) revealed by site-specific phosphorylation and partial proteolysis. J Biol Chem. 2004;279:50342–50349. doi: 10.1074/jbc.M408683200. [DOI] [PubMed] [Google Scholar]
- 45.Yu H, Olshevskaya E, Duda T, Seno K, Hayashi F, Sharma RK, Dizhoor AM, Yamazaki A. Activation of retinal guanylyl cyclase-1 by Ca2+-binding proteins involves its dimerization. J Biol Chem. 1999;274:15547–15555. doi: 10.1074/jbc.274.22.15547. [DOI] [PubMed] [Google Scholar]
- 46.Johnston JP, Aparicio JG, Applebury ML. Purification and autophosphorylation of retinal guanylate cyclase. Methods Enzymol. 2000;315:673–689. doi: 10.1016/s0076-6879(00)15874-4. [DOI] [PubMed] [Google Scholar]
- 47.Tucker CL, Laura RP, Hurley JB. Domain-specific stabilization of photoreceptor membrane guanylyl cyclase by adenine nucleotides and guanylyl cyclase activating proteins (GCAPs) Biochemistry. 1997;36:11995–12000. doi: 10.1021/bi971212k. [DOI] [PubMed] [Google Scholar]
- 48.Sampath AP, Strissel KJ, Elias R, Arshavsky VY, McGinnis JF, Chen J, Kawamura S, Rieke F, Hurley JB. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 2005;46:413–420. doi: 10.1016/j.neuron.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. Ca(2+)-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270:18060–18066. doi: 10.1074/jbc.270.30.18060. [DOI] [PubMed] [Google Scholar]
- 50.Levay K, Satpaev DK, Pronin AN, Benovic JL, Slepak VZ. Localization of the sites for Ca2+-binding proteins on G-protein-coupled receptor kinases. Biochemistry. 1998;37:13650– 13659. doi: 10.1021/bi980998z. [DOI] [PubMed] [Google Scholar]
- 51.Calvert PD, V, Klenchin A, Bownds MD. Rhodopsin kinase inhibition by recoverin. Function of recoverin myristoylation. J Biol Chem. 1995;270:24127–24129. doi: 10.1074/jbc.270.41.24127. [DOI] [PubMed] [Google Scholar]
- 52.Kawamura S, Cox JA, Nef P. Inhibition of rhodopsin phosphorylation by non-myristoylated recombinant recoverin. Biochem Biophys Res Commun. 1994;203:121–127. doi: 10.1006/bbrc.1994.2157. [DOI] [PubMed] [Google Scholar]
- 53.Iacovelli L, Sallese M, Mariggio S, de Blasi A. Regulation of G-protein-coupled receptor kinase subtypes by calcium sensor proteins. FASEB J. 1999;13:1–8. doi: 10.1096/fasebj.13.1.1. [DOI] [PubMed] [Google Scholar]
- 54.Kramer RH, Molokanova E. Modulation of cyclic-nucleotide-gated channels and regulation of vertebrate phototransduction. J Exp Biol. 2001;204:2921–2931. doi: 10.1242/jeb.204.17.2921. [DOI] [PubMed] [Google Scholar]
- 55.Hsu YT, Molday RS. Interaction of calmodulin with the cyclic GMP-gated channel of rod photoreceptor cells. Modulation of activity, affinity purification, and localization. J Biol Chem. 1994;269:29765–29770. [PubMed] [Google Scholar]
- 56.Molday RS. Calmodulin regulation of cyclic-nucleotide-gated channels. Curr Opin Neurobiol. 1996;6:445–452. doi: 10.1016/s0959-4388(96)80048-1. [DOI] [PubMed] [Google Scholar]
- 57.Haynes LW, Stotz SC. Modulation of rod, but not cone, cGMP-gated photoreceptor channels by calcium-calmodulin. Vis Neurosci. 1997;14:233–239. doi: 10.1017/s0952523800011378. [DOI] [PubMed] [Google Scholar]
- 58.Flaherty KM, Zozulya S, Stryer L, McKay DB. Three-dimensional structure of recoverin, a calcium sensor in vision. Cell. 1993;75:709–716. doi: 10.1016/0092-8674(93)90491-8. [DOI] [PubMed] [Google Scholar]
- 59.Vijay-Kumar S, V, Kumar D. Crystal structure of recombinant bovine neurocalcin. Nat Struct Biol. 1999;6:80–88. doi: 10.1038/4956. [DOI] [PubMed] [Google Scholar]
- 60.Bourne Y, Dannenberg J, Pollmann V, Marchot P, Pongs O. Immunocytochemical localization and crystal structure of human frequenin (neuronal calcium sensor 1) J Biol Chem. 2001;276:11949–11955. doi: 10.1074/jbc.M009373200. [DOI] [PubMed] [Google Scholar]
- 61.Ames JB, Hendricks KB, Strahl T, Huttner IG, Hamasaki N, Thorner J. Structure and calcium-binding properties of Frq1, a novel calcium sensor in the yeast Saccharomyces cerevisiae. Biochemistry. 2000;39:12149–12161. doi: 10.1021/bi0012890. [DOI] [PubMed] [Google Scholar]
- 62.Scannevin RH, Wang K, Jow F, Megules J, Kopsco DC, Edris W, Carroll KC, Lu Q, Xu W, Xu Z, Katz AH, Olland S, Lin L, Taylor M, Stahl M, Malakian K, Somers W, Mosyak L, Bowlby MR, Chanda P, Rhodes KJ. Two N-terminal domains of Kv4 K(+) channels regulate binding to and modulation by KChIP1. Neuron. 2004;41:587–598. doi: 10.1016/s0896-6273(04)00049-2. [DOI] [PubMed] [Google Scholar]