Abstract
Human activities can create noise pollution and there is increasing international concern about how this may impact wildlife. There is evidence that anthropogenic noise may have detrimental effects on behaviour and physiology in many species but there are few examples of experiments showing how fitness may be directly affected. Here we use a split-brood, counterbalanced, field experiment to investigate the effect of repeated boat-noise playback during early life on the development and survival of a marine invertebrate, the sea hare Stylocheilus striatus at Moorea Island (French Polynesia). We found that exposure to boat-noise playback, compared to ambient-noise playback, reduced successful development of embryos by 21% and additionally increased mortality of recently hatched larvae by 22%. Our work, on an understudied but ecologically and socio-economically important taxon, demonstrates that anthropogenic noise can affect individual fitness. Fitness costs early in life have a fundamental influence on population dynamics and resilience, with potential implications for community structure and function.
Some anthropogenic (man-made) activities create noise. National and international legislation (e.g. US National Environment Policy Act and European Commission Marine Strategy Framework Directive) now recognises that some anthropogenic noise can be considered pollution. Yet such policies are in the early stages of being able to regulate noise and better information could improve their effectiveness. Noise from activities such as urban development, transportation and resource extraction has been shown to affect the behaviour and physiology of animals from a range of taxonomic groups (see1,2,3). However, if we are to assess the ultimate consequences (impacts on individual fitness and population viability), studies must include three key elements. First, measurements of survival or reproductive success are needed; if these are not feasible, biologically meaningful response parameters that translate directly to fitness consequences should be considered2,3. Second, experimental manipulations with suitable controls and replicates are required if the influence of noise is to be isolated; correlative and pseudo-replicated studies do not allow confounding factors to be ruled out or strong conclusions to be drawn3,4. Third, experiments need to consider repeated or chronic noise exposure because changes across time and cumulative effects may affect animal responses5.
Here, we use a field-based experiment to investigate how repeated exposure to anthropogenic noise might impact early-life development and survival. Embryos are adapted to tolerate normal environmental fluctuations and challenges6,7, but accelerating anthropogenic change can push conditions beyond the bounds of normal variability and/or create conditions that did not previously exist. Extremes of temperature and pH, heavy metals, and estrogen-mimicking chemicals, for instance, have been shown to impair development in a wide range of taxa (e.g.8,9). Noise stress affects various aspects of development in rats (reviewed in1), suggesting anthropogenic noise is likely to have detrimental influences on development. However, strong experimental evidence is currently lacking (for some preliminary work, see10).
Specifically, we investigated how repeated exposure to playback of boat-traffic noise (a widespread source of anthropogenic noise) affects early-life survival and development in a marine mollusc, the sea hare Stylocheilus striatus (Figure 1) at Moorea Island. Very little is known about the effect of any source of noise on aquatic invertebrates (for exceptions, see10,11,12,13). However, aquatic invertebrates are very diverse and important to the functioning of ecosystems, as well as often having the ability to hear and using sound for a variety of purposes (see11,12). Sea hares in particular play an important role in benthic reef ecology throughout their circumtropical distribution as a specialist grazer on the toxic cyanobacterium, Lyngbya majuscula14. We examined whether developmental success (eggs completing organogenesis within five days of incubation), hatching (embryos hatching after five days of incubation), and survival after hatching were affected by repeated boat-noise exposure during early development in S. striatus. We predicted that development of embryos would be compromised by increased noise and that those that survived would take longer to hatch and have higher mortality after hatching.
Figure 1. Stylocheilus striatus, photograph courtesy of Fabien Michenet.

Results
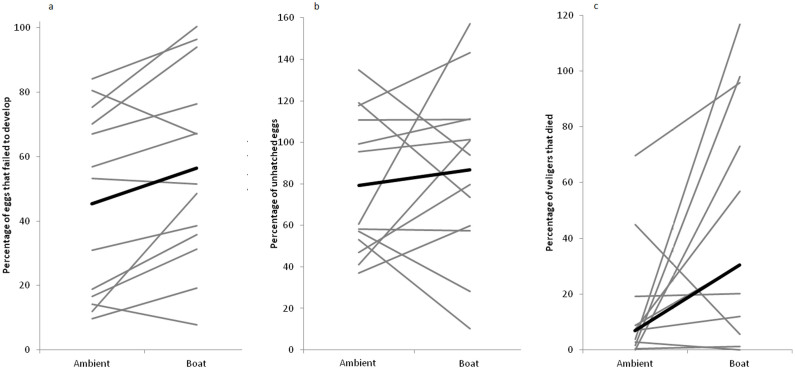
Of the 29,416 eggs counted, 7,497 failed to develop. The percentage failing to develop was significantly affected by sound treatment (paired t-test: t12 = −2.99, p = 0.011; Figure 2a), with 21.3 ± 10.8% (mean ± se) fewer eggs per mother developing when exposed to boat-noise playback compared to playback of ambient noise.
Figure 2.
Arcsin square root transformed percentage of egg capsules that (a) failed to develop and (b) were unhatched in each treatment. The thick black line represents the overall effect (mean for each treatment), whereas the grey lines connect values for the two treatments for each mother. N = 13 mothers. (c) Number of veligers that died as a percentage of egg capsules that hatched per treatment. The thick black line represents the overall effect (median for each treatment), whereas the grey lines connect values for the two treatments for each mother. N = 11 mothers.
Of the 21,919 eggs that developed successfully, 13,257 had not hatched after 5 days. The percentage of developing eggs that remained un-hatched was not significantly affected by sound treatment (paired t-test: t12 = −0.63, p = 0.538; Figure 2b).
From 8,662 eggs that successfully hatched, 3,514 veligers died before they were counted. The percentage of dead veligers was significantly affected by sound treatment (Wilcoxon signed ranks test: V = 10, N = 11, p = 0.045; Figure 2c), with 21.6 ± 24.4% (median ± median absolute deviation) more veligers dying after exposure to boat-noise playback than ambient-noise playback.
Discussion
Boat-noise playback significantly increased the likelihood of sea hares suffering developmental failure at the embryonic stage (see also15) and mortality at the free-swimming veliger stage, but had no discernible impact on the rate of embryo development (cf.10). Our controlled and replicated experimental manipulation in a field setting therefore indicates that anthropogenic noise has the potential to impact gastropod molluscs; although marine invertebrates have significant ecological and economic value11, the majority of studies have only considered how vertebrates are affected by noise3. Our results also suggest, more generally, the potential for anthropogenic noise to have detrimental fitness consequences early in life.
Care must be taken when interpreting our findings because we used underwater loudspeakers rather than real boats and enclosed sea hare embryos in Eppendorfs supported by iron bars, whereas eggs of this species are naturally found attached to a substrate such as rock or algae. While we ensured that the sound pressure and particle acceleration exposure in the water column matched that of a real boat as closely as possible, it is possible that vibrations of different substrates may affect sound transmission differently, and this will be a very interesting avenue for future research. If anthropogenic noise does indeed impact early-life survival, then there are implications for population dynamics and resilience, and for community structures due to shifts in selective pressures6,7. Herbivores, such as sea hares, associated with coral reefs play a key role in the equilibrium between corals and algae, while populations of S. striatus are particularly important as they are a specialist grazer on blooms of the toxic cyanobacterium, L. majuscula14.
The more prevalent developmental failure of sea hare embryos exposed to boat-noise playback might be the result of stronger molecular vibrations, caused by mechanical energy in the sound waves produced by the loudspeakers, compared with the ambient-noise treatment. Low frequency sound in combination with whole body vibrations induces sister chromatid exchanges and delays cell cycle progression in mice and humans16. Although specific frequencies that cause damage in water may be different to those in air due to the physical properties of sound, sine sound waves (900, 1000 and 1100 Hz) caused mechanical damage to unhatched red flour beetle (Tribolium castaneum) larvae17. Altered gene expression due to environmental (heat) stress during development affects the penetrance (the extent to which a particular gene or set of genes is expressed in the phenotypes of individuals carrying it) of genetic mutations, leading to disease in humans, Drosophila and mice9. Thus, the effect on sea hare development might be manifested through disrupted tissue formation, tissue damage or altered gene expression.
One potential mechanism for the death of veligers after hatching is barotrauma (tissue damage due to pressure changes in gas filled chambers, such as when fish are brought to the surface too quickly and their swim bladders rupture; see18). Sea hare embryos may have gas bubbles in their circulatory system that could cause barotrauma if they were to fluctuate in size in response to pressure changes. A second potential mechanism relates to stress if sea hare veligers are capable of detecting the noise via their statocysts. Statocysts are organs used for hearing in other molluscs19, are commonly found in various opisthobranchs (e.g.20) and were seen in S. striatus embryos in this study (pers. obs.). Noise is known to cause stress in a wide variety of taxa, and corticosterone levels are negatively associated with immune responses, survival and recruitment1 as well as affecting development via calcium transport disruption21.
Outboard motor boats are found all over the world wherever humans inhabit coastal areas and our results suggest that noise from them should be considered in the management of fisheries and protected areas. Other sources of anthropogenic noise, such as ships, seismic surveys and pile driving, may also overlap with developmental stages of vulnerable species raising concern for many populations. Findings from studies directly assessing fitness consequences, as ours has done, are vital to parameterise population-level models that can be used to develop international policy and thus regulate this issue of global concern.
Methods
Study species, husbandry and egg manipulation
We collected 50 sea hares from the lagoon of Moorea, French Polynesia. Sea hares were kept in aquaria at the CRIOBE research station, with oxygenated running seawater and at ambient temperature and light regimes. They grazed on cyanobacteria colonizing the aquaria and turf algae collected from the lagoon. We paired similarly sized individuals in 15 × 5 × 5 cm plastic breeding containers that allowed water flow but prevented sea hares mixing with the main population. We monitored pairs hourly overnight and separated them after they were observed copulating; maternity was thus known. We collected eggs the following morning; sea hare mothers lay a string of eggs in gelatinous material (a ribbon) with each egg containing 1–6 embryos. Preliminary observations revealed that most eggs hatched within five days and this determined the length of noise exposure eggs received in the field.
We took egg ribbons from 13 mothers over the course of the experiment (from January to March 2013) and cut each into 8–16 equally sized pieces with a scalpel. Ribbon pieces were transferred to individually labelled 1.5 ml Eppendorf tubes filled with fresh sea water that had been collected from outside the lagoon and sterilised. Eppendorfs from the same mother were split randomly between ‘Boat' and ‘Ambient' playback treatments (see below) in a balanced design to control for potential genetic or epigenetic effects on egg development. Eppendorfs are sealed, air- and water-tight containers, thus controlling for any differences in water chemistry. Also, no developmental differences were found between egg ribbons in closed eppendorfs versus those in open petri dishes in the laboratory (S. C. Mills pers. obs.).
Sound recordings and playback design
We made boat recordings during the day (on 4/11/2010 and 5/11/2010) at 2 m depth in a deep bay in the lagoon on the east coast of Moorea using a hydrophone (HiTech HTI-96-MIN with inbuilt preamplifier; sensitivity −165 dB re 1 V/μPa; frequency range 2 Hz–30 kHz; High Tech Inc., Gulfport MS) and a solid-state recorder (Edirol R-09HR 16-bit recorder; sampling rate 44.1 kHz; Roland Systems Group, Bellingham WA). The recorder was fully calibrated using pure sine wave signals generated in SAS Lab (Avisoft, Germany), played on an mp3 player, measured in line with an oscilloscope. We made 36 recordings of passes made by two typical outboard motor boats with 25 horse power Yamaha engines; one boat was used per recording. Boats started 50 m from the hydrophone and drove past in a straight line for 100 m; passing the hydrophone at a closest distance of 10 m. We also made 12 ambient-noise recordings (without boats) on location each day.
We clipped boat recordings to 45 s samples that each contained one pass; ambient-noise recordings were clipped into ~64 s samples. We then constructed two 12-h replicate playback tracks for each sound treatment using different mixtures of boat and ambient-noise samples selected at random. Boat-noise playback tracks (‘Boat' treatment) included one boat and four ambient-noise samples each 5 min, to give a regular rate of boat passes; ambient-noise tracks (‘Ambient' treatment) included no boat-noise samples. A chosen 12-h track was played to sea hare egg ribbon pieces every day for five days between the hours of 06:00 and 18:00 (during daylight hours when boats normally moved around the island). All eggs therefore received ambient sound from the environment (e.g. from the nearby reef), in addition to that included in the playback of recordings taken from another location. Only eggs in the Boat treatment received the added effect of boat noise for 45 s every 5 min, totalling 144 boat passes per day.
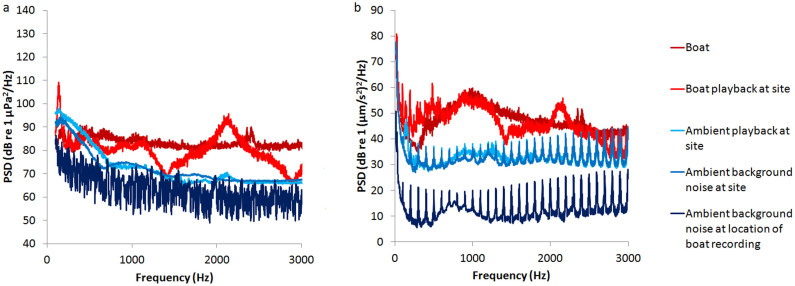
Playbacks were from underwater loudspeakers (UW-30, frequency response 0.1–10 kHz, University Sound, Columbus, USA) connected to mp3 players (Sansa Clip+, SanDisk, Milpitas, CA, USA). Loudspeakers were fixed facing upwards to the sandy bottom of experimental sites. We measured both sound pressure and particle acceleration (using the hydrophone described above and an M30 accelerometer, sensitivity 0–3 kHz, manufactured and calibrated by GeoSpectrum Technologies, Dartmouth, Canada; recorded on a laptop via a USB soundcard, MAYA44, ESI Audiotechnik GmbH, Leonberg, Germany) to compare playbacks of boat passes with real boat passes. Playbacks were recorded at 1 m from the speaker, at the location of the experiment with experimental apparatus in place; the recording sensors were the same distance from the speaker and the sandy bottom as the embryos and were within 30 cm of the embryos. Real boat passes for the comparison were recorded with the same hydrophone and accelerometer using the same boats making passes at 10 m at a nearby lagoon location (Figure 3). Acoustic analyses were performed in MATLAB v2010a: Fast-Fourier Transforms transformed time domain recordings into the frequency domain before power spectral density was calculated to allow comparison of sound levels for each treatment across the frequency range 100–3000 Hz.
Figure 3.
Power spectral densities (PSD) of (a) sound pressure in decibels relative to 1 micropascal per second squared per hertz (above 100 Hz due to the capability of the recorder used) and (b) monoaxial particle acceleration in decibels relative to 1 (nanometre per second squared) squared per hertz of original recordings of boats and boat playbacks at experimental site along with ambient noise and ambient noise playbacks.
Experimental sites and design
We used two sites, one for each sound treatment (Boat and Ambient) in each of four replicate trials. Treatment allocation to sites was alternated between temporal replicates to control for unknown site differences; sites were similar in depth (1.3–1.8 m), water turbidity, prevailing currents, proximity to reef (>10 m) and nearest boat channel (>60 m). We allocated replicate 12-h playbacks to sites and temporal replicates in a Latin-square design. Sites were 100 m apart and playbacks at one site could not be heard above local ambient noise levels from the other (verified with sound pressure and particle acceleration recordings – the recordings shown in Figure 3 of playbacks at each site were taken while playbacks were ongoing at the alternate site. Sound travel between the two sites may have been limited due to the sandy bottom with occasional coral bommies and coral rubble and the fact that between the two sites there is a large area where the depth reduces to ~40 cm, cutting off low frequencies.)
We taped Eppendorfs in random groups of three or four around an iron bar (5 mm diameter, 1 m long) using electrical tape. The bar was fixed horizontally at 50 cm height from the sandy bottom in the lagoon, 1 m from an underwater loudspeaker; sound recordings were taken next to the structures that supported the iron bars, with iron bars in place. We placed eggs into experimental noise treatments within 4–8 h of laying (i.e. around the time of their first division or cleavage). We never approached the sites by a motor boat apart from at the beginning and end of the experiment. Boating activity in the nearest boat channel was present in the vicinity of both sites, but this was adequately controlled for by running multiple temporal replicates of the experiment and balancing allocation of treatments between the sites.
After five days of noise exposure, Eppendorfs were returned to the laboratory where we examined the contents under 20× magnification using a light microscope (Leitz diavert). The following categories were counted: (a) eggs that were dead or had failed to undergo organogenesis (i.e. failed to develop); (b) unhatched eggs with mature developed embryos; (c) empty eggs (indicating successful hatching); and (d) dead veligers (post-hatching larvae). Counters were blind to the sound treatment, and the order in which Eppendorfs from the same mother were counted was balanced between treatments by an additional observer. We used counts to calculate three response measures for analysis. First, the percentage of eggs that failed to develop (category a/categories a + b + c). Second, the percentage of successfully developing eggs that had slowed development (category b/categories b + c). Third, the number of veligers that died as a percentage of eggs that hatched (category d/category c); as there was more than one veliger per egg, the percentage in the third response measure may exceed 100.
Statistical analyses
Analyses were conducted at the level of the mother. The percentages of eggs that failed to develop and that had slowed development were normally distributed and heteroskedastic after arcsine square root transformations. Thus, we used paired t-tests to examine differences between means (n = 13 mothers); percentages that are arcsine square root transformed may exceed 100 (see Figure 2). The percentage of hatched individuals that died did not meet the assumptions of parametric testing and thus we used a Wilcoxon signed ranks test to examine differences between medians. No hatching was observed in ribbons from two mothers in the Boat treatment, thus they were excluded from the analysis of hatched individuals that died (n = 11).
Author Contributions
S.M., S.N. and A.R. designed the study, S.N. and B.N. conducted fieldwork with help from S.M., S.M. conducted labwork with help from S.N. and B.N., S.N. analysed the data with help from A.R., S.N. wrote the first draft of the manuscript and S.M., A.R., S.S. and D.L. all contributed to the manuscript. S.M., A.R., S.S. and D.L. provided funding.
Acknowledgments
We thank Ricardo Beldade, Daniel Holley, Stephen Murray and Hin Ano for field and laboratory assistance, and the CRIOBE research station (www.criobe.pf) for providing the facilities to conduct this study. Very useful acoustics advice was provided by Nathan Merchant and Michael Ainslie and statistics advice by Innes Cuthill. Geospectrum provided us with an accelerometer. Funding was provided to Sophie Nedelec by a studentship from EPSRC, Subacoustech and an Eiffel grant – Egide program – from Campus France. Funding was provided to Suzanne Mills and David Lecchini by the Agence National de Recherche, ANR-11-JSV7-012-01 Live and Let Die and Partnership University Fund of the French American Cultural Exchange (Ocean Bridges Program, http://facecouncil.org/puf/). Funding was provided to Andrew Radford by a Defra grant (ME5207). Funding was provided to Stephen Simpson by a NERC Fellowship (NE/J500616/2).
References
- Kight C. R. & Swaddle J. P. How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol. Lett. 14, 1052–1061, 10.1111/j.1461-0248.2011.01664.x (2011). [DOI] [PubMed] [Google Scholar]
- Francis C. D. & Barber J. R. A framework for understanding noise impacts on wildlife: an urgent conservation priority. Front. Ecol. Environ. 11, 305–313, 10.1890/120183 (2013). [Google Scholar]
- Morley E. L., Jones G. & Radford A. N. The importance of invertebrates when considering the impacts of anthropogenic noise. Proc. R. Soc. Lond. [Biol] 281, 20132683, http://dx.doi.org/10.1098/rspb.2013.2683 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbekoorn H. Songs of the city: noise-dependent spectral plasticity in the acoustic phenotype of urban birds. Anim. Behav. 85, 1089–1099, http://dx.doi.org/10.1016/j.anbehav.2013.01.021 (2013). [Google Scholar]
- Bejder L., Samuels A., Whitehead H., Finn H. & Allen S. Impact assessment research: use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar. Ecol. Prog. Ser. 395, 177–185, 10.3354/meps07979 (2009). [Google Scholar]
- Gilbert S. F. Ecological developmental biology: developmental biology meets the real world. Dev. Biol. 233, 1–12, 10.1006/dbio.2001.0210 (2001). [DOI] [PubMed] [Google Scholar]
- Hamdoun A. & Epel D. Embryo stability and vulnerability in an always changing world. Proc. Natl. Acad. Sci. 104, 1745–1750, 10.1073/pnas.0610108104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey C. M., Luque E. H., de Toro M. M., Sonnenschein C. & Soto A. M. In utero exposure to bisphenol a alters the development and tissue organization of the mouse mammary gland. Biol. Reprod. 65, 1215–1223 (2001). [DOI] [PubMed] [Google Scholar]
- Baradaran-Heravi A. et al. Penetrance of biallelic SMARCAL1 mutations is associated with environmental and genetic disturbances of gene expression. Hum. Mol. Genet. 21, 2572–2586, 10.1093/hmg/dds083 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar de Soto N. et al. Anthropogenic noise causes body malformations and delays development in marine larvae. Sci. Rep. 3, 2831 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wale M. A., Simpson S. D. & Radford A. N. Size-dependent physiological responses of shore crabs to single and repeated playback of ship noise. Biol. Lett. 9, 20121194, 10.1098/rsbl.2012.1194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wale M. A., Simpson S. D. & Radford A. N. Noise negatively affects foraging and antipredator behaviour in shore crabs. Anim. Behav. 86, 111–118, http://dx.doi.org/10.1016/j.anbehav.2013.05.001 (2013). [Google Scholar]
- Chan A., Giraldo-Perez P., Smith S. & Blumstein D. T. Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biol. Lett. 6, 458–461 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul V. J. & Pennings S. C. Diet-derived chemical defenses in the sea hare Stylocheilus longicauda (Quoy et Gaimard 1824). J. Exp. Mar. Biol. Ecol. 151, 227–243, http://dx.doi.org/10.1016/0022-0981(91)90126-H (1991). [Google Scholar]
- Banner A. & Hyatt M. Effects of noise on eggs and larvae of two estuarine fishes. T. Am. Fish Soc. 102, 134–136, doi:10.1577/1548-8659(1973)102<134:EONOEA>2.0.CO;2 (1973). [Google Scholar]
- Silva M. J., Dias A., Barreta A., Nogueira P. J., Castelo-Branco N. A. A. & Boavida M. G. Low frequency noise and whole-body vibration cause increased levels of sister chromatid exchange in splenocytes of exposed mice. Teratogenesis, Carcinogenesis, and Mutagenesis 22, 195–203 (2002). [DOI] [PubMed] [Google Scholar]
- Jinham A., Kiruba S., Kumaran J. & Manohar Das S. Efficacy of audible sound waves in inflicting tissue damage and mortality in Trilobium castaneum (Coleoptera: Tenebrionidae) larvae. Agriculture Tropica et Subtropica 45, 32–36 (2012). [Google Scholar]
- Gross J. A., Irvine K. M., Wilmoth S., Wagner T. L., Shields P. A. & Fox J. R. The effects of pulse pressure from seismic water gun technology on northern pike. T. Am. Fish. Soc. 142, 1335–1346 (2013). [Google Scholar]
- Mooney T. A. et al. Sound detection by the longfin squid (Loligo pealeii) studied with auditory evoked potentials: sensitivity to low-frequency particle motion and not pressure. J. Exp. Biol. 213, 3748–3759, 10.1242/jeb.048348 (2010). [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E. & Richard E. A fine structural analysis of the statocyst in Aplysia californica. J. Morphol. 127, 113–131 (1969). [Google Scholar]
- Siegel M. I. & Mooney M. P. Perinatal stress and increased fluctuating asymmetry of dental calcium in the laboratory rat. Am. J. Phys. Anthropol. 73, 267–270, 10.1002/ajpa.1330730213 (1987). [DOI] [PubMed] [Google Scholar]