Abstract
Effects of cold plasma treatment on soybean (Glycine max L. Merr cv. Zhongdou 40) seed germination and seedling growth were studied. Seeds were pre-treated with 0, 60, 80, 100 and 120 W of cold plasma for 15 s. Results showed that plasma treatments had positive effects on seed germination and seedling growth, and treatment of 80 W had the highest stimulatory effect. Germination and vigor indices significantly increased by 14.66% and 63.33%, respectively. Seed's water uptake improved by 14.03%, and apparent contact angle decreased by 26.19%. Characteristics of seedling growth, including shoot length, shoot dry weight, root length and root dry weight, significantly increased by 13.77%, 21.95%, 21.42% and 27.51%, respectively, compared with control. The seed reserve utilization, including weight of the mobilized seed reserve, seed reserve depletion percentage and seed reserve utilization efficiency significantly improved by cold plasma treatment. In addition, soluble sugar and protein contents were 16.51% and 25.08% higher than those of the control. Compared to a 21.95% increase in shoot weight, the root weight increased by 27.51% after treatment, indicating that plasma treatment had a greater stimulatory effect on plant roots. These results indicated that cold plasma treatment might promote the growth even yield of soybean.
Soybean (Glycine max (L.) Merr) is a major worldwide oilseed crop. Although soybean production reached 26 million metric tons, with an average yield of 1.76 million metric tons in 2005 in China1, it was insufficient to meet the increasing consumption demands. Soybean seeds have thick and impermeable testae, resulting in poor germination, long germination time and low germination uniformity, which subsequently leads to poor establishment and yield reduction2. Promoting seed germination is the most direct way to improve soybean production2. Methods for promoting seed germination are physical methods (magnetic treatment, sunlight, ultraviolet light and hot water soaking) and chemical methods (chemicals, fungicides and hormones)3,4,5,6. Although these methods can promote germination to a certain extent, they are time consuming, labor-intensive and produce chemical residues.
Cold plasma treatment is a fast, economic and pollution-free method to improve seed performance and crop yield7,8. It has essential roles in a broad spectrum of developmental and physiological processes in plants, including reducing the bacterial bearing rate of seeds, changing seed coat structures, increasing the permeability of seed coats, and stimulating seed germination and seedling growth9,10,11. This phenomenon has been demonstrated in several plants such as Chenopodium album11, Oryza sativa12, Triticum aestivum13, Lycopersicon esculentum14 and Solanum melongena L15. In addition, plasma treatment also could improve the physiological metabolism of the plant, such as dehydrogenase activity, superoxide dismutase14 and peroxidase activities16, photosynthetic pigments, photosynthetic efficiency and nitrate reductase activity17. Plasma treatment could significantly increase crop yields. Jiang et al. reported that an 80 W cold plasma treatment significantly increased wheat yields18, and Selcuk et al. have reported that grain and legume yields were significantly increased by cold plasma10.
However, reports about the effects of cold plasma on soybean are limited. The aims of the study were (1) to investigate the effects of cold plasma treatment on seed germination, seedling growth and seed reserve utilization of soybean, and (2) to explore the mechanisms of the effects of plasma on promoting seed germination and seedling growth of soybean.
Results
Seed germination
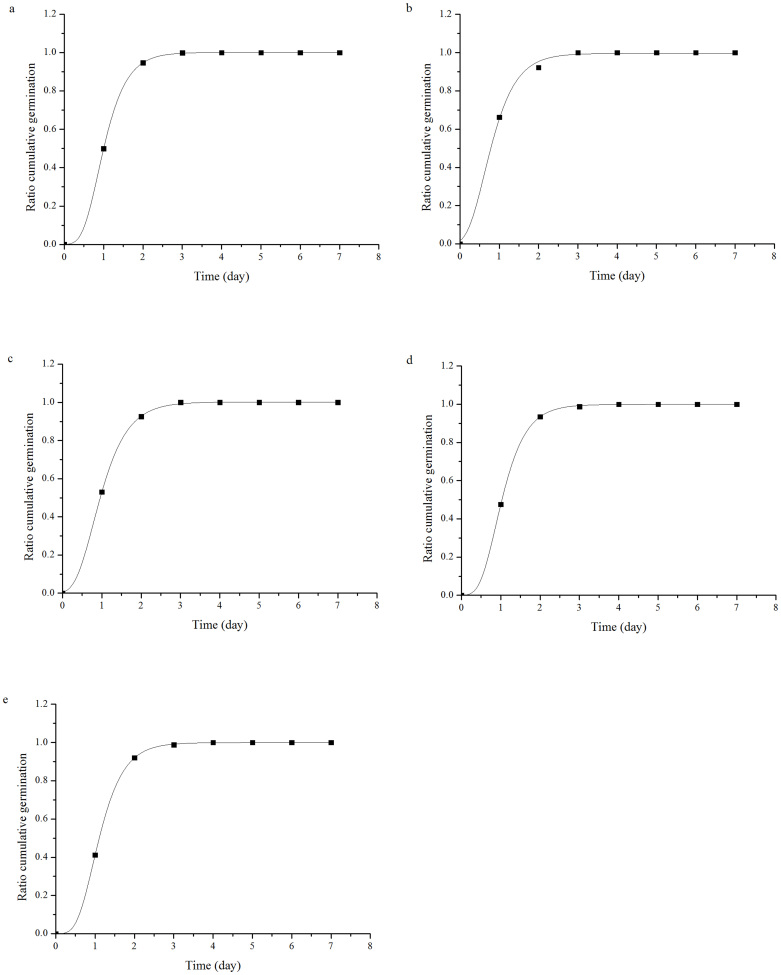
The effect of cold plasma on seed germination of soybean varied with different treatment power levels (Table 1). No treatment had a significant effect on the germination potential or rate. The T1 and T2 treatments significantly increased the germination index by 13.69% (p = 0.045) and 14.66% (p = 0.036), respectively, compared with the control, but there was no significant difference between T3 or T4 and the control. The vigor index was significantly increased 47.23% (p = 0.002), 63.33% (p < 0.001) and 37.66% (p = 0.009), compared to the control, by the T1, T2 and T3 treatments, respectively. The maximum germination potential, germination rate, germination index and vigor index were obtained from the T2 treatment, which indicated that the T2 plasma treatment could improve the germination of soybean seeds. The germination data was well fitted by the Richards' function (Fig. 1). Significant difference in the Vi (p = 0.017) and Me (p = 0.002) parameters were found by cold plasma treatments. But no differences were found among Qu and Sk parameters by cold plasma treatments (Table 2).
Table 1. Effects of cold plasma on the germination of soybean seed.
| Treatment | Germination potential (%) | Germination rate (%) | Germination index | Vigor index |
|---|---|---|---|---|
| CK | 82.20 ± 2.93 a | 82.20 ± 2.20 a | 31.11 ± 1.81 b | 495.70 ± 28.74 c |
| T1 | 85.60 ± 2.94 a | 85.60 ± 2.94 a | 35.37 ± 1.21 a | 729.81 ± 24.96 ab |
| T2 | 90.00 ± 1.58 a | 90.00 ± 1.58 a | 35.67 ± 0.44 a | 809.63 ± 20.54 a |
| T3 | 82.20 ± 2.23 a | 83.30 ± 2.23 a | 32.56 ± 1.68 b | 682.41 ± 8.10 b |
| T4 | 81.10 ± 4.02 a | 82.10 ± 4.02 a | 29.26 ± 1.26 b | 471.54 ± 51.02 c |
Different lowercase letters denote statistical differences between treatments groups at the 5% level according to Duncan's test.
Figure 1. Richards' equation fitted to the germination of CK (a) and treatments of 60 W (b), 80 W (c), 100 W (d), 120 W(e) of soybean seeds.
Table 2. The parameters Vi (viability), Me (median germination time), Qu (dispersion) and Sk (skewness) of Richards' equation for germination of soybean seed under cold plasma treatments.
| Treatment | Vi (%) | Me (d) | Qu (d) | Sk (%) |
|---|---|---|---|---|
| Ck | 1.001 ± 0.001 | 1.006 ± 0.044 | 0.312 ± 0.023 | 0.437 ± 0.017 |
| T1 | 0.996 ± 0.001 | 0.780 ± 0.007 | 0.349 ± 0.004 | 0.466 ± 0.001 |
| T2 | 1.001 ± 0.001 | 0.957 ± 0.031 | 0.357 ± 0.076 | 0.441 ± 0.025 |
| T3 | 0.998 ± 0.002 | 1.019 ± 0.066 | 0.330 ± 0.032 | 0.428 ± 0.039 |
| T4 | 1.000 ± 0.001 | 0.773 ± 0.031 | 0.340 ± 0.041 | 0.408 ± 0.034 |
| P value | 0.017 * | 0.002** | 0.873 | 0.649 |
*, ** Differences at 5% and 1% levels according to Duncan's test.
Seedling growth
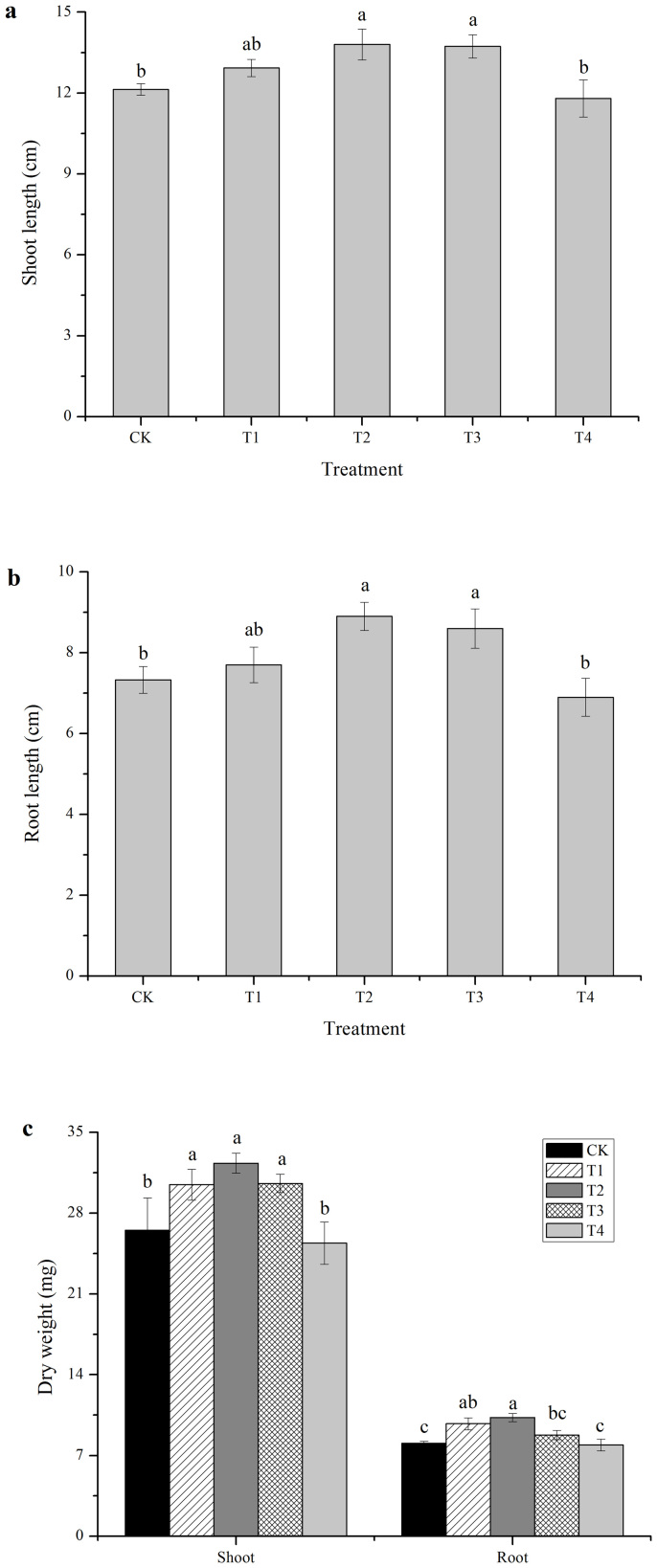
The effects of cold plasma on soybean seedling growth are shown in Figure 2. The shoot length and dry weight of shoots from T2 treated seeds were 13.80 cm and 32.33 mg, respectively, which were 13.77% and 21.95%, respectively, higher than those of the control. However, the T1 and T4 treatments had no significant effects on shoot growth (Fig. 2a and c). The lengths of root from T2 and T3 treated seeds were 8.9 cm and 8.6 cm, respectively, which were 21.42% and 17.33%, respectively, longer than that of the control. No significant differences in root length were found between T1 or T4 and the control (Fig. 2b). The dry weights of root from T1 and T2 treated seeds were significantly higher than that of the control by 20.82% (p = 0.003) and 27.51% (p = 0.015), respectively, but there were no significant differences between those of T3 or T4 and the control (Fig. 2c). Over all, the lengths and dry weights of the roots increased more than the lengths and dry weights of the shoots when subjected to the T2 treatment compared with the control.
Figure 2. Effects of cold plasma on shoot length (a), root length (b) and dry weight (c) of soybean.
Error bars indicate standard error (n = 3). Different lowercase letters denote statistical differences between treatment groups at the 5% level according to Duncan's test.
Seed water uptake
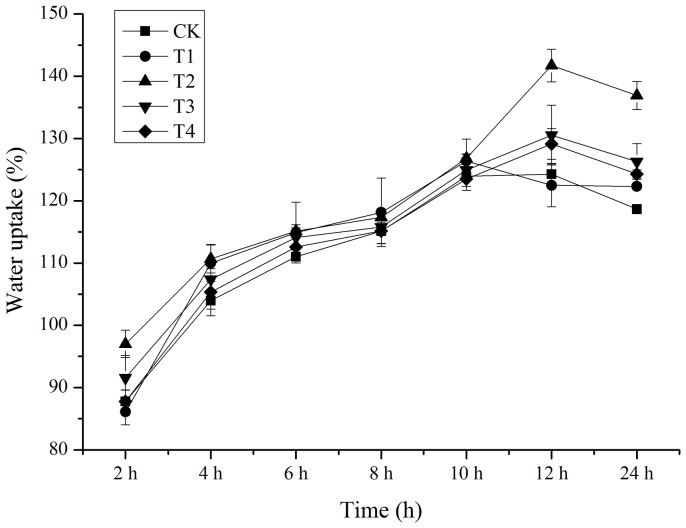
The water uptake of soybean seed is shown in Figure 3. In the first 10 h, the water uptake of the seeds slowly increased, and there was no significant difference between any treatment and the control. Between 10 and 12 h, the treated water uptake of seeds continued to rise toward its peak, while the control maintained and then began to decline. The T2 treatment had the best water uptake promoting effect, which was 14.03% higher than the control at 12 h.
Figure 3. Effect of cold plasma on the water uptake of soybean seeds.
Error bars indicate standard error (n = 3).
Seed apparent contact angle
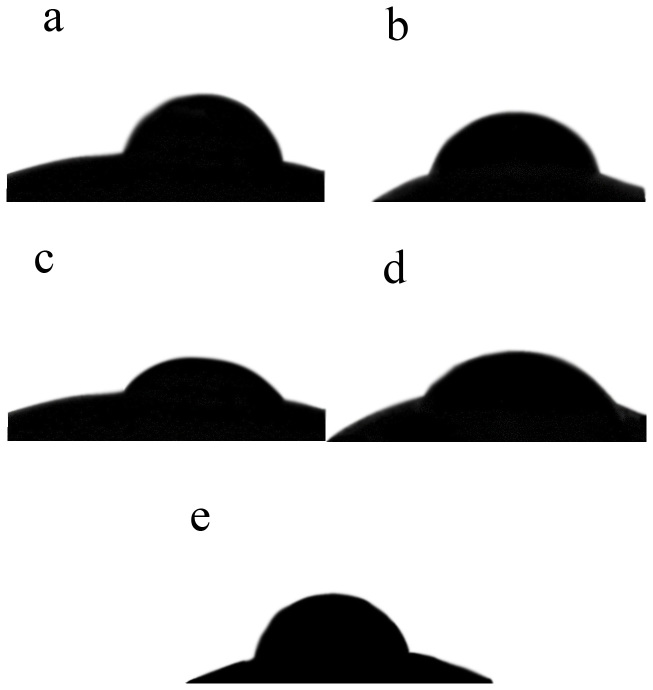
The apparent contact angle is shown in Figure 4. The apparent contact angle of the control was 74.14°. The T2 and T3 treatments dramatically decreased the apparent contact angle by 26.19% (p = 0.004) and 20.94% (p = 0.007), respectively, compared with the control. No significant differences in apparent contact angle were found between T1 or T4 and the control. The lowest apparent contact angle occurred using the T2 treatment.
Figure 4. Water droplet deposited on CK (a) and treatments of 60 W (b), 80 W (c), 100 W (d), 120 W(e) of soybean seeds.
Seed reserve utilization
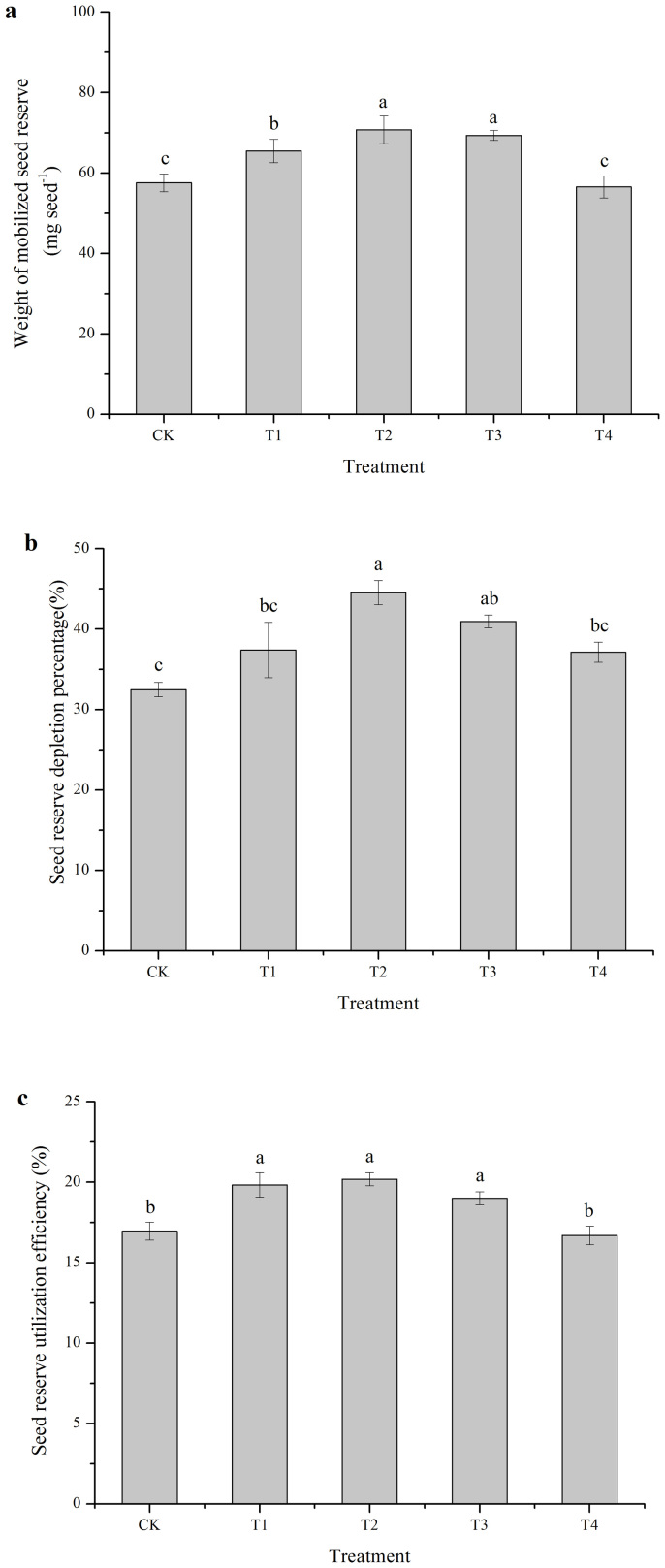
The weight of the mobilized seed reserves of the control was 57.55 mg.seed−1, and there was no significant difference between T4 and the control. T1, T2 and T3 treatments could significantly increase the weight of the mobilized seed reserves by 13.72% (p = 0.044), 22.92% (p < 0.001) and 20.47% (p = 0.002), respectively, compared with that of the control (Fig. 5a). Positive effect of the cold plasma treatment on the seed reserve depletion percentage was similar to that of the weight of the mobilized seed reserve. Compared with the control, the seed reserve depletion percentage was significantly increased by 37.14% (p = 0.001) and 26.12% (p = 0.008) by the T2 and T3 treatments, respectively. There were no significant differences between T1 or T4 treatments and the control (Fig. 5b). Seed reserve utilization efficiencies of T1, T2 and T3 treatments were significantly higher than the control by 16.96% (p = 0.001), 19.08% (p < 0.001) and 12.09% (p = 0.009), respectively (Fig. 5c). The best effect of cold plasma on the seed reserve utilization was obtained using the T2 treatment.
Figure 5. Effects of cold plasma on the weight of mobilized seed reserves (a), seed reserve depletion percentage (b) and seed reserve utilization efficiency (c) of soybean.
Error bars indicate standard error (n = 3); Different lowercase letters denote statistical differences between treatment groups at the 5% level according to Duncan's test.
Soluble sugar content
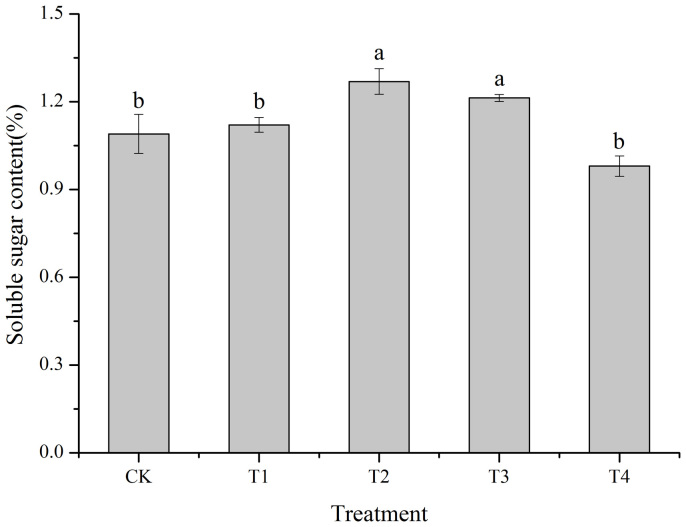
The soluble sugar content of the control was 1.09% (Fig. 6). T2 and T3 treatments significantly increased the soluble sugar contents by 16.51% (p = 0.001) and 11.01% (p = 0.015), respectively, compared with the control. The T1 and T4 treatments had no significant effects on the soluble sugar content. The maximum soluble sugar content was recorded using the T2 treatment.
Figure 6. Effect of cold plasma on the soluble sugar content of soybean.
Error bars indicate standard error (n = 3); Different lowercase letters denote statistical differences between treatment groups at the 5% level according to Duncan's test.
Soluble protein content
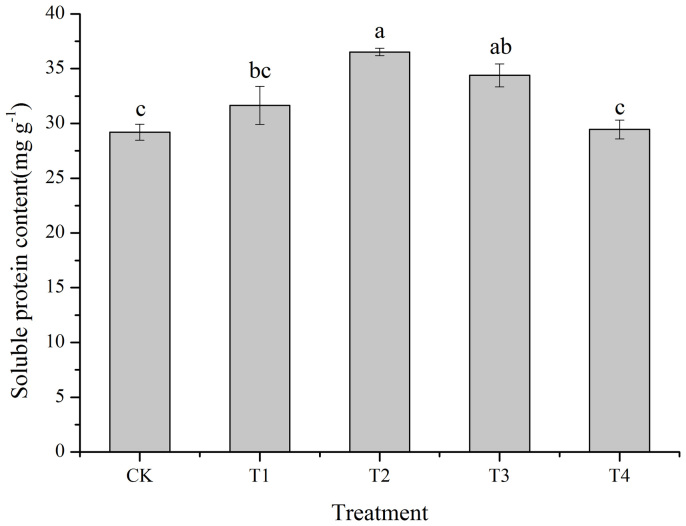
The influence of cold plasma on the soluble protein content of soybean seed is shown in Figure 7. Compared with the control, the T2 and T3 treatment significantly increased the soluble protein content by 25.08% (p = 0.001) and 17.78% (p = 0.006), respectively. The T1 treatment had a stimulatory but not significant effect (8.35%) on the soluble protein content of soybean seed. The T4 treatment had no significant effect on the soluble sugar content. The highest soluble protein content occurred using the T2 treatment.
Figure 7. Effect of cold plasma on the soluble protein content of soybean.
Error bars indicate standard error (n = 3); Different lowercase letters denote statistical differences between treatment groups at the 5% level according to Duncan's test.
Discussion
The present study showed that cold plasma had an active effect on soybean seed germination. The germination and vigor indices were increased by cold plasma treatments. The T2 cold plasma treatment produced the highest stimulatory effect among the different treatments; however, a cold plasma treatment with lower or higher energy levels had no significant influence on seed germination (Table 1 and Fig. 1). Numerous studies found that cold plasma significantly increased seed germination8,9,14. For example, Selcuk et al. found that a plasma treatment significantly increased tomato seed germination10. Šerá et al. also concluded that seed treated by plasma significantly improved the wheat germination rate13. Dhayala et al. indicated that the effects of a short low-pressure plasma treatment on safflower seed germination were much more effective than a long high-pressure plasma treatment8. However, these results were not consistent with the findings of Volin et al. who reported that fluorocarbon plasma treatments inhibited corn and bean seed germination19. Because they used fluorocarbon plasma, the characteristics of seed coats were modified via plasma-deposition of hydrophobic materials, which would reduce water uptake, leading to delayed seed germination. Furthermore, the Richards' function was used to fit the germination process of soybean seed treated by cold plasma. Our study showed that the germination of soybean seed was well followed to the Richards' equation (Figure 1), the germination was effectively promoted and the germination time was shortened by the cold plasma treatment (Table 2), which was in consistent with Šerá et al. who reported that the germination process of lamb's quarters influenced by the cold plasma was well fitted to the Richards' equation20.
A number of studies have suggested that plasma treatment promote seedling growth and the development of plants9,10,14. Šerá et al. found that the seedling growth of poppy was significantly increased by cold plasma treatments21. Zhou et al. also reported that tomato seedling growth was improved by atmospheric pressure plasma treatment9. The present study demonstrated that a cold plasma treatment promoted soybean seedling growth, especially the T2 treatment. However, if the cold plasma treatment used a lower or higher energy level then no significant effect was observed on seedling growth (Fig. 2). Effect of cold plasma was more dramatic on root growth than shoot growth. These results are in agreement with Šerá et al. who found that plasma had a larger effect on the dry weights of roots than shoots13.
There may be an indirect association between seed germination and water uptake. Many studies have suggested that a plasma-induced elevation in the seed germination and seedling growth rates might be associated with the water uptake of seeds11,22. The present study found that the cold plasma treatment improved the water uptake of soybean seed (Fig. 3), which indirectly increased the seed germination rate. This result agreed with the findings of Bormashenko et al. who reported that cold radiofrequency air plasma treatment plasma could improve the water uptake of oat and wheat seeds23. Seeds immersed into cold plasma are subjected to an attack by oxygen radicals and are bombarded by ions resulting in seed coat erosion and etched/eroded surfaces24. The altered seed coat could increase the hydrophilic ability of the seed, and eventually improve the water uptake of the seed.
The wettability of seed can be reflected by the apparent contact angle. The present study found that the cold plasma treatment significantly decreased the apparent contact angle (Fig. 4), which was in consistent with Bormashenko et al. who found that cold radiofrequency air plasma treatment dramatically decreased the apparent contact angle of lentil and wheat seeds23. The plasma treatment could change the chemical structure and the roughness of the surface, and finally lead to a dramatic change of wetting behavior of the seed, which may account for the increase in water uptake of seed25.
The increased absorptive ability is accompanied by the increased ability to absorb nutrients, which promotes the growth of plant seedlings. Our study showed that the cold plasma treatment significantly increased the weight of the mobilized seed reserves, seed reserve depletion percentage and seed reserve utilization efficiency of soybean (Fig. 5). Seed reserves are major factors that determine the success of early growth during the development of young seedlings2. It has been proposed that seedling dry weight was significantly related to seed reserve mobilization and the seed reserve depletion percentage2. According to Dobrynin et al., the interaction of cells with plasma might improve the activities of seedling germination enzymes and accelerate the decomposition of the seed's inner nutrients, which might contribute to the increased seed reserve utilization and seedling growth26.
Soluble sugar, the photosynthetic product of plants and the main form of carbohydrate, plays a dramatic role in plant metabolism. Many studies showed that the soluble sugar content was closely related to photosynthesis and yield27,28. Soluble protein plays an important role in the growth of the plants and is a very important component of numerous plant enzymes, which can reflect the overall metabolism of the plant29. In the present study, cold plasma pre-treatment significantly increased the soluble sugar and protein contents compared with those of the control (Fig. 6 and 7), which was similar to results of Wu et al. who reported that a plasma treatment significantly increased the soluble sugar and protein contents of corn seedlings compared with the control17. Yin et al. found that plasma treatment could promote the accumulation of soluble sugar and protein by increasing the activities of enzymes, such as α- amylase and proteases that were related to their metabolism14. It has been proposed that the degradation and conversion of fat during germination may be related to the increase in soluble sugar and protein contents30. It is likely that the degradation and conversion of fat is partly responsible for the increase in the soluble sugar and protein contents found using a cold plasma treatment. Our study indicated that a cold plasma treatment might promote the growth of soybean. The plasma treatment increases soluble sugar and protein contents, which may account for the increase in soybean seedling growth.
In summary, cold plasma treatments have the potential to enhance the germination and seedling growth rates of soybean. The improvement in seed germination and seedling growth of soybean in response to cold plasma treatment appears to be a consequence of the increases in water uptake, seed reserve utilization, and soluble sugar and protein contents. Cold plasma treatments may have positive effects on soybean yield, and could be used in future soybean production. More studies are needed to investigate the influence of cold plasma on soybean yield, as well as to elucidate the mechanisms that promote the effects of cold plasma treatment on soybean seed germination and seedling growth rates.
Methods
Experimental apparatus
Experiment was performed in the commercial computer-controlled plasma treatment apparatus HD-2N, which was consisted of a vacuum device, cold plasma generator and transmission device and inlet/outlet hopper. The core of the cold plasma processing system is the cold plasma generator. The device is composed of two parallel plates and has a metal suspension shell. The area between the plates and the metal shell is filled with insulating materials. Seeds receive the non-ionizing radiation treatment when they are in the cavity between the two polar plates.
The process of seed treatment by cold plasma is:
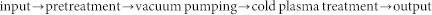 |
Plant material
Seeds (cv. Zhongdou 40) of soybean (G. max (L.) Merr.) were obtained from the Oil Crops Research Institute of the Chinese Academy of Agricultural Science, Wuhan, China.
Treatment conditions
Healthy uniform seeds were selected and were exposed to inductive helium plasma discharge under the following parameters: the plasma frequency was 13.56 MHz, the power was 60 (T1), 80 (T2), 100 (T3) and 120 W (T4), the pressure was 150 Pa and the volume of the discharge chamber was 1200 mm × 180 mm × 20 mm. The time span of irradiation was 15 s and the temperature of the discharge, measured by a thermistor, was about 25°C. Meanwhile, without plasma treatment, control seeds were also submitted to the same vacuum and helium flux as the treated seeds (CK). Experiments were carried out approximately one day after seeds were treated with cold plasma.
Experimental design
The experiment was carried out at the Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China (118°46′E, 32°03′N), from June to December 2012. Seeds were germinated in 9 cm petri dishes on two layers of filter paper. A total of 100 seeds were placed on each filter paper and 10 ml distilled water was added. During the experiment, 5 ml distilled water was added to the petri dishes every other day to maintain sufficient moisture for germination. Seeds were incubated in a 25°C light incubator. Germination was considered to have occurred when the radicals were half of the seed length. The germination percentage was recorded every 24 h for 7 days. Root length, bud length, and soluble sugar and protein contents were measured after the 7th day. The seedlings were separated into shoots and roots for dry weight measurements. The experiment was planned as a completely randomized design with three replications.
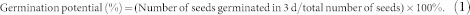 |
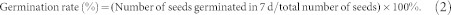 |
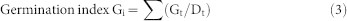 |
where Gt represents number of germinated seeds on the t day and Dt represents germination days.
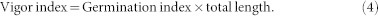 |
The ratio cumulative germination was calculated as in Šerá et al.20. The Richards' equation and population parameters Vi - final germination rate, index of viability; Me - median germination time, demonstrate the germination time of seed; Qu - dispersion, uniformity of seedling growth; and Sk - skewness, the composition of a population, were calculated according to Hara31.
Determination of seed water uptake
Determination of seed water uptake is based on the method given by Turk and Tawaha32.
Determination of seed apparent contact angle
The apparent contact angle was established using a Kino goniometer (model SL200B).
Determination of seed reserve utilization
The weight of mobilized seed reserves, seed reserve depletion percentage, and seed reserve utilization efficiency were determined according to Soltani et al.2.
Determination of soluble sugar and protein content
The soluble sugar content was determined according to Zheng et al.33, and the soluble protein content was assayed following Bradford's method34.
Statistical analysis
All data are presented as the mean value ± standard error (SE) of three replicates. Analyses were performed using the SPSS statistical software package (Version 16.0) and the variance (p < 0.05) of the data was analyzed by a one-way ANOVA test (Duncan's test).
Author Contributions
D.Y.H., L.L., J.J.F. and S.H.L. initiated and designed the research, L.L., J.J.F., S.M.C. and H.X. performed the experiments, L.L., J.J.F. and L.J.G. analyzed the data and wrote the paper, D.Y.H., S.M.C. and H.X. also revised and edited the manuscript.
Acknowledgments
We thank Professor Zhang Chunlei who provided us with soybean seeds. This project was funded by Jiangsu Province Science and Technology Support Program (NO. BE2013452) and Main Direction Program of Knowledge Innovation of Chinese Academy of Science (NO. KSCX2-EW-B-6).
References
- Liu Z. T. & Bi Y. L. Yield rise and potential of Heilongjiang soybean production in the perspective of science and technology progress. Soybean Bull. 80, 1–3 (2006). [Google Scholar]
- Soltania A., Gholipoor M. & Zeinalia E. Seed reserve utilization and seedling growth of wheat as affected by drought and salinity. Environ. Exp. Bot. 55, 195–200 (2006). [Google Scholar]
- Zhang S. J., Li L., Zhang C. L. & Li G. M. ALA altered ABA content of winter oilseed rape (Brassica napus L.) seedling. Agri. Sci. Tech. 12, 484–487 (2011). [Google Scholar]
- Błaszczak W., Doblado R., Frias J., Vidal-Valverde C., Jadwiga Sadowska J. & Fornal J. Microstructural and biochemical changes in raw and germinated cowpea seeds upon high-pressure treatment. Food Res. Int. 40, 415–423 (2007). [Google Scholar]
- Al-Bachir M. Effect of gamma irradiation on microbial load and sensory characteristics of aniseed (Pimpinella anisum). Bioresource Technol. 98, 1871–1876 (2007). [DOI] [PubMed] [Google Scholar]
- Yao Y., Li Y., Yang Y. & Li C. Effect of seed pretreatment by magnetic field on the sensitivity of cucumber (Cucumis sativus) seedlings to ultraviolet-B radiation. Environ. Exp. Bot. 54, 286–294 (2005). [Google Scholar]
- Tong J. Y., He R., Zhang X. L., Zhan R. T., Chen W. W. & Yang S. Z. Effects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of Andrographis paniculata. Plasma Sci. Technol. 16, 260 (2014). [Google Scholar]
- Dhayal M., Lee S. Y. & Park S. U. Using low-pressure plasma for Carthamus tinctorium L. seed surface modification. Vacuum 80, 499–506 (2006). [Google Scholar]
- Zhou Z. W., Huang Y. F., Yang S. Z. & Chen W. Introduction of a new atmospheric pressure plasma device and application on tomato seeds. Agri. Sci. 2, 23–27 (2011). [Google Scholar]
- Selcuk M., Oksuz L. & Basaran P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresource Technol. 99, 5104–5109 (2008). [DOI] [PubMed] [Google Scholar]
- Šerá B., Stranák V., Šerý M., Tichy M. & Špatenka P. Germination of Chenopodium Album in response to microwave plasma treatment. Plasma Sci. Technol. 10, 506–511 (2008). [Google Scholar]
- Chen H. H., Chen Y. K. & Chang H. C. Evaluation of physicochemical properties of plasma treated brown rice. Food Chem. 135, 74–79 (2012). [Google Scholar]
- Šerá B., Špatenka P., Šerý M., Vrchotová N. & Hrušková I. Influence of plasma treatment on wheat and oat germination and early growth. IEEE Transactions on Plasma Science 38, 2963–2968 (2010). [Google Scholar]
- Yin M. Q., Huang M. J., Ma B. Z. & Ma T. C. Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci. Techno. 7, 3143–3147 (2005). [Google Scholar]
- Zhou Z. W., Huang Y. F., Yang S. Z. & Xiong D. Y. Progress in electromagnetics research symposium proceedings. Kuala Lumpur, Malaysia. 1577 (2012).
- Jiang J. F., Lu Y. F., Li J. G., Li L., He X., Shao H. L. & Dong Y. H. Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (bacterial wilt). Plos one 9, 1–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. H., Chi L. H., Bian S. F. & Xu K. Z. Effects of plasma treatment on maize seeding resistance. J Maize Sci. 15, 111–113 (2007). [Google Scholar]
- Jiang J. F., He X., Li L., Li J. G., Shao H. L., Xu Q. L., Ye H. R. & Dong Y. H. Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Sci. Technol. 16, 54–58 (2014). [Google Scholar]
- Volin J. C., Denes F. S., Young R. A. & Park S. M. T. Modification of seed germination performance through cold plasma chemistry technology. Crop Sci. 40, 1706–1718 (2000). [Google Scholar]
- Šerá B., Šerý M., Stranák V., Špatenka P. & Tichý M. Does cold plasma affect breaking dormancy and seed germination? A study on seeds of Lambs quarters (Chenopodium album agg.). Plasma Sci. Technol. 11, 749–754 (2009). [Google Scholar]
- Šerá B., Gajdvá I., Šerý M. & Špatenka P. New physicochemical treatment method of poppy seeds for agriculture and food industries. Plasma Sci. Technol. 15, 935–938 (2013). [Google Scholar]
- Filatova I., Azharonok V., Kadyrov M., Beljavsky V., Gvozdov A., Shik A. & Antonuk A. The effect of plasma treatment of seeds of some grains and legumes on their sowing quality and productivity. Rom. J. Phys. 56, 139–143 (2011). [Google Scholar]
- Bormashenko E., Grynyov R., Bormashenko Y. & Drori E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Scientific reports 2, 741–748 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffels E., Sakiyama Y. & Graves D. B. Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Transactions on Plasma Science 36, 1441–1451 (2008). [Google Scholar]
- Wild S. & Kesmodel L. L. High resolution electron energy loss spectroscopy investigation of plasma-modified polystyrene surfaces. J. Vac. Sci. Technol. 19, 856–860 (2001). [Google Scholar]
- Dobrynin D., Fridman G., Friedman G. & Fridman A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 11, 2–26 (2009). [Google Scholar]
- Tian B. Q., Xie B. J., Shi J., Wu J., Cai Y., Xu T. M., Xue S. & Deng Q. C. Physicochemical changes of oat seeds during germination. Food Chem. 119, 1195–1200 (2010). [Google Scholar]
- Saratha K., Hume D. J. & Godfrey C. Genetic improvement in short season soybeans:IV. Dry matter accumulation, partitioning, and leaf area duration. Crop Sci. 41, 391–398 (2001). [Google Scholar]
- Berry J. A. & Downton W. J. S. Environmental Regulation of Photosynthesis. Academic-Press, New York, 294–306 (1982).
- Goyoagaa C., Burbano C., Cuadrado C., Romero C., Guillaḿon E., Varela A., Pedrosa M. M. & Muzquiz. M. Content and distribution of protein, sugars and inositol phosphates during the germination and seedling growth of two cultivars of Vicia faba. J Food Compos. Anal. 24, 391–397 (2011). [Google Scholar]
- Hara Y. Calculation of population parameters using Richards function and application of indices of growth and seed vigor to rice plants. Plant Prod. Sci. 2, 129–135 (1999). [Google Scholar]
- Turk M. A. & Tawaha A. M. Allelopathic effect of black mustard (Brassica nigra L.) on germination and growth of wild oat (Avena fatua L.). Crop Prot. 22, 673–677 (2003). [Google Scholar]
- Zheng Y. H., Jia A. J. & Ning T. Y. Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J. Plant Physiol. 165, 1455–1465 (2008). [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]