Abstract
Spatiotemporal control of leukocyte dynamics within tissues is critical for successful innate and adaptive immune responses. Homeostatic trafficking and coordinated infiltration into and within sites of inflammation and infection rely on signaling in response to extracellular cues that in turn controls a variety of intracellular protein networks regulating leukocyte motility, migration, chemotaxis, positioning, and cell–cell interaction. In contrast to mesenchymal cells, leukocytes migrate in an amoeboid fashion by rapid cycles of actin polymerization and actomyosin contraction, and their migration in tissues is generally referred to as low adhesive and nonproteolytic. The interplay of actin network expansion, contraction, and adhesion shapes the exact mode of amoeboid migration, and in this review, we explore how leukocyte subsets potentially harness the same basic biomechanical mechanisms in a cell-type-specific manner. Most of our detailed understanding of these processes derives from in vitro migration studies in three-dimensional gels and confined spaces that mimic geometrical aspects of physiological tissues. We summarize these in vitro results and then critically compare them to data from intravital imaging of leukocyte interstitial migration in mouse tissues. We outline the technical challenges of obtaining conclusive mechanistic results from intravital studies, discuss leukocyte migration strategies in vivo, and present examples of mode switching during physiological interstitial migration. These findings are also placed in the context of leukocyte migration defects in primary immunodeficiencies. This overview of both in vitro and in vivo studies highlights recent progress in understanding the molecular and biophysical mechanisms that shape robust leukocyte migration responses in physiologically complex and heterogeneous environments.
Keywords: Leukocyte, Interstitial migration, Plasticity, Cytoskeleton, Tissue architecture, Imaging
Introduction
Several billion immune cells together form the immune system that plays the dominant role in host defense against pathogens. Both the innate (rapid, antigen-independent) and adaptive (slower, antigen-specific) immune responses providing such protection require a variety of leukocytes with diverse effector functions to exhibit coordinated cell migration, tissue positioning, and intercellular interactions. Aside from some sessile immune cell types that seed tissues during embryonic development, most leukocytes traffic through the body by exiting the bone marrow or thymus, circulating through the blood and lymphatic system, and entering peripheral organs for surveillance or exertion of specific effector functions [1, 2]. Over the last decade, much progress has been made in understanding the cell biology of leukocyte migration. Not only have we learned that this process differs mechanistically from the movement of mesenchymal cells such as fibroblasts or endothelial cells, but also that leukocytes are very flexible and adopt distinct migration modes depending on their environmental context [3–5]. For example, leukocyte motility on two-dimensional (2D) surfaces requires adhesive forces, whereas migration in three-dimensional (3D) environments is low adhesive and largely depends on cytoskeletal deformability. Moreover, immune cell movement is coordinated differently in artificial 3D porous networks with small or large channels, indicating that the specific 3D geometry of an environment can influence the mode of leukocyte migration.
Given the enormous flexibility of leukocytes in adapting their locomotion to the organization of such defined in vitro environments, it is obvious to ask about the natural 3D geometries of physiological tissues and how they influence in situ immune cell motility, potentially favoring one migration mode over the other. Initial studies in this area involved video rate imaging of inflamed vascular endothelium that elicits signals in interacting leukocytes to undergo a multistep adhesion cascade and 2D migration along the endothelial lining before undergoing trans- or paracellular emigration from the vessel [6, 7]. Outside the vasculature, leukocytes migrate in the tissue interstitium via fast locomotion that is low adhesive, largely independent of the molecular composition of the environment, preserves tissue structure rather than degrading it, and follows physiological paths of least resistance [3, 8]. Additional intravital imaging studies based on confocal and especially two-photon microscopy have revealed a broad range of interstitial leukocyte motility and migration patterns: largely stationary macrophage and dendritic cell networks, moderate speed dendritic cells crawling into lymphatic vessels, and rapidly moving T cells, to name just a few [9]. While we know that cells of the interstitium can provide pro-migratory activation factors or guidance cues, we are only beginning to understand how the architecture of this tissue compartment impacts leukocyte motility. Physiological interstitial environments are organ specific, very heterogeneous, and dynamically changing, and current efforts are directed toward characterizing tissue composition, geometries, and structural properties in diverse organs and relating these features to leukocyte dynamics in those sites.
In this review, we will critically summarize reductionist studies of leukocyte migration in vitro and then review our current knowledge of the functional relationship between tissue structure and leukocyte migration strategies in lymphoid and nonlymphoid organs. We outline the technical challenges for functionally dissecting interstitial leukocyte migration in vivo and provide recommendations for improved experimental design to obtain more insightful results. Given the heterogeneous environment of most tissues and the adaptability of leukocytes, we discuss whether leukocytes optimize their interstitial movement by switching between migration modes in vivo and speculate on potential differences between leukocyte subsets in this regard. Finally, we briefly touch on lessons learned from primary immunodeficiencies with leukocyte migration phenotypes. While important contributions to the analysis of interstitial migration have also been derived from studies with cancer cells [4, 10, 11] or leukocytes in other model systems such as zebrafish and Drosophila [12–15], we limit our discussion primarily to findings involving mammalian primary leukocytes.
Basic principles of leukocyte motility and migration
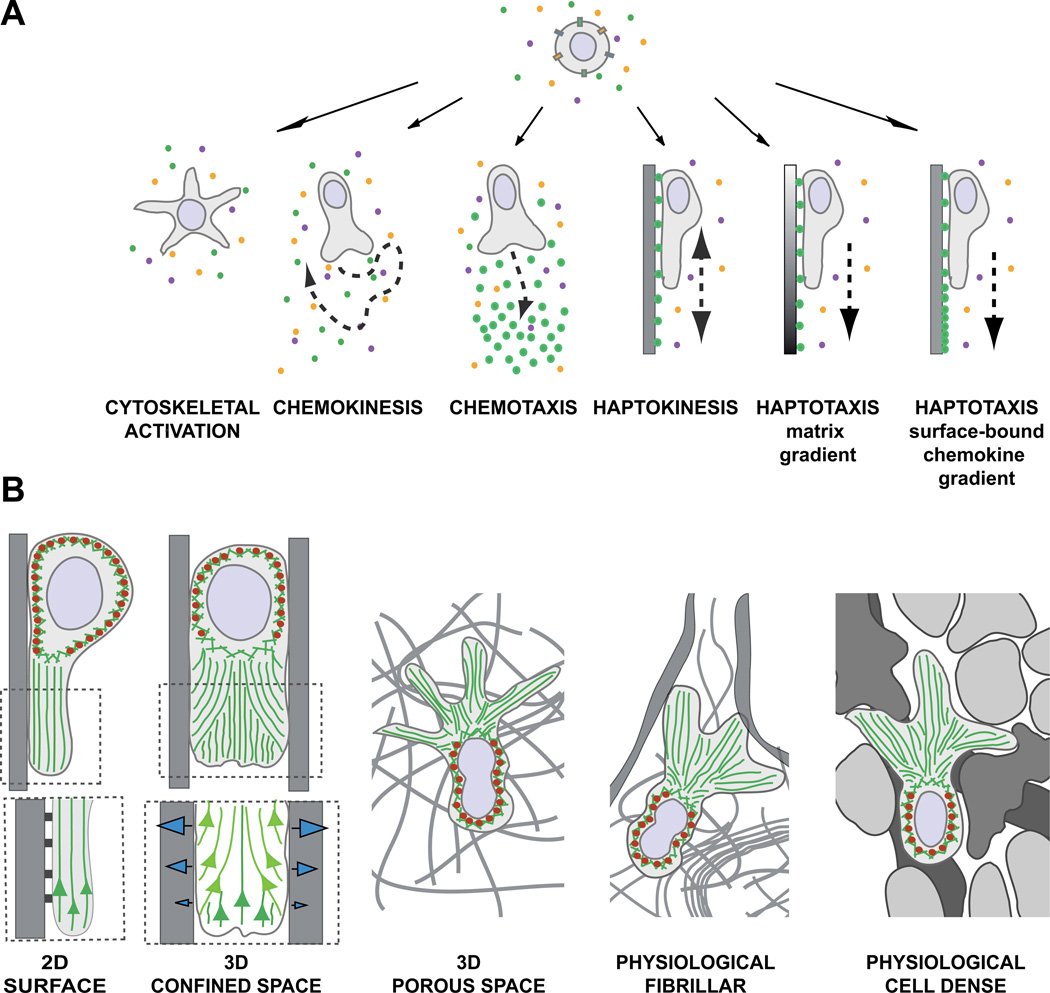
Many environmental signals can influence the activation state of leukocytes, regulate their cytoskeleton, and induce adhesiveness, which together contribute to cell shape and motility changes. These factors include cytokines, chemokines, growth factors, lipids, alarmins, purine and pyrimidine nucleotides and their triphosphates, formyl peptides, complement factors, extracellular matrix fragments, adhesive ligands, and even more unconventional stimuli such as hydrogen peroxide or electrical fields [16–19]. Leukocytes perceive such signals and translate them into intracellular signals that lead to morphological changes such as cell flattening, multidirectional development of protrusive veils and dendrites, or establishment of cell polarity (Fig. 1a).
Fig. 1.
(A) Environmental signals induce shape changes, motility patterns, and migration modes. In physiological environments, leukocytes perceive multiple signals and integrate them into intracellular signaling cascades to induce shape changes, cell polarization, or cell migration. In a homogeneous field of soluble ligands, leukocytes can (1) increase cytoskeletal activity, leading to morphological shape changes without cell movement or (2) induce self-polarization as prerequisite for nondirected cell migration (chemokinesis). (3) Soluble gradients of external ligands can polarize cells along the gradient and stimulate directed migration (chemotaxis). (4) Upon perceiving homogeneous surface-bound chemokines, leukocytes can increase cell adhesion, self-polarize, and confine their migration in a nondirected manner along the surface (haptokinesis). For inherently adhesive immune cell types, haptokinesis might not require the presence of an activating chemokine (not shown). Leukocytes can migrate by two forms of haptotaxis: (5) along a gradient of extracellular matrix and cellular adhesion sites and (6) along a gradient of substrate-bound chemoattractants. (B) The environmental geometry supports different leukocyte migration strategies. (1) Movement along 2D surfaces requires actin polymerization to push the leading edge membrane forward and surface anchorage to the substrate. Myosin II (red ellipses)-based contractions behind the leading edge detach very adhesive cells from the substrate and are not necessarily required when cells migrate on low adhesive substrates. (Insert below) Integrin-mediated adhesions not only confine migration to the surface, but also inhibit the retrograde actin flow (green), converting actin polymerization into forward protrusion at the leading edge. (2) In confined environments, the leukocyte body can exert lateral pushing forces on the substrate, which is not possible on 2D surfaces. Intracellular pressure gradients increase friction against the walls and potentially compensate for adhesion-dependent anchorage. Friction against the walls can be generated by actin polymerization or increase in hydrostatic pressure by actomyosin contractions. (Insert below) Model of leading edge actin flow during adhesive migration in microchannels (adapted from [20]): Two F-actin networks form at the leading edge and interact with each other. The “free” network (dark green) polymerizes from the membrane at the cell front. The “adherent” network (light green) polymerizes perpendicular to the walls and compresses the retrograde flow of the “free” network, converting new polymerization against the membrane and enabling forward protrusions (see text for molecular details). (3) In 3D porous environments, leukocytes can potentially exert the same lateral forces as in confined spaces, but require additional mechanisms to navigate through meshes, which are not necessary when moving in the open space of a microchannel. Depending on the leukocyte subtype, multiple frontal protrusions require coordinated cell shape changes for efficient movement. (4, 5) In physiological tissues, leukocytes navigate in a very heterogeneous environment with confined spaces, fibrillar 3D meshes, and 2D surfaces in close proximity, most likely adapting to the tissue geometry by constant switching of migration modes. Fibrillar (4) and cell-rich (5) tissues differ in their structure, composition, and texture, potentially favoring distinct migration strategies.
Establishing front–back polarity
The development of a front–back axis with an actin-rich leading edge at the cell front and a contractile trailing edge (also called “uropod”) at the cell rear precedes cell movement and is best appreciated in the context of directed migration toward an external soluble gradient of chemoattractant (chemotaxis). However, most leukocytes can also self-polarize in homogeneous fields of soluble chemokines, before they increase motility and migrate in a nondirected fashion (chemokinesis) (Fig. 1a). Apart from soluble molecular cues, leukocytes can perceive cell surface-bound chemoattractants as gradients or as homogeneous fields and undergo migration along surfaces in a directed (haptotaxis) or nondirected manner (haptokinesis), respectively (Fig. 1a).
Chemoattractants are classical inducers of cell polarity that act through G-protein-coupled receptors (GPCR) on intracellular signaling pathways that establish biochemical asymmetry in the cell [16]. Even in the absence of GPCR stimulation, cell adhesion alone can induce signaling cascades that polarize leukocytes [21]. Key intracellular biochemical components involved in establishing functional polarity downstream of GPCR signaling are phosphoinositide 3-kinases (PI3K) [22, 23], but PI3K-independent pathways can also mediate leukocyte polarity [24]. The polarized morphology of migrating leukocytes results from an intracellular biochemical asymmetry of key cytoskeletal regulators including the best studied members of the Rho family of small GTPases: Rac (1 and 2), Cdc42, and Rho A. Rac isoforms and Cdc42 localize to the front of the cell where Rac mediates F-actin polymerization and Cdc42 coordinates the persistence of the leading edge [23, 25, 26]. In contrast, RhoA localizes to the myosin II-rich cell rear where it regulates the cortical rigidity and contractile activity of the migrating leukocyte. Rho family small GTPases act as molecular switches and cycle between active (GTP-bound) and inactive (GDP-bound) states, which are regulated by various guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs) [25].
Three migration modules that define leukocyte migration modes
Leukocytes migrate in a fashion resembling crawling amoeba and lower eukaryotes (“amoeboid” motion) due to rapid cell shape changes that are almost exclusively driven by the actomyosin cytoskeleton [5]. The interplay of three migration modules largely defines the cell shape and migration mode of each locomoting leukocyte: (1) Actin polymerization (“P”) is considered essential for leukocyte movement and contributes to formation of protrusive cell structures such as dendrites, pseudopods, and leading edges at the front of migrating leukocytes. (2) Myosin II-dependent contraction (“C”) of the actin network can support cell movement by retracting the back of the cell, which is particularly important for adherent leukocytes. (3) While actin polymerization and actomyosin contraction are the major intracellular forces to deform the cell, migrating leukocytes can optionally increase adhesion (“A”) through activating receptors of the integrin family, causing them to stop, spread, or confine migration to an adhesive substrate (Figs. 1b and 2). The Rho family small GTPases discussed above represent major signal hubs that act through several effector molecules to regulate these three migration modules. Other cytoskeletal components (microtubules, intermediate filaments, septin cytoskeleton) have regulatory or supportive functions [27–29].
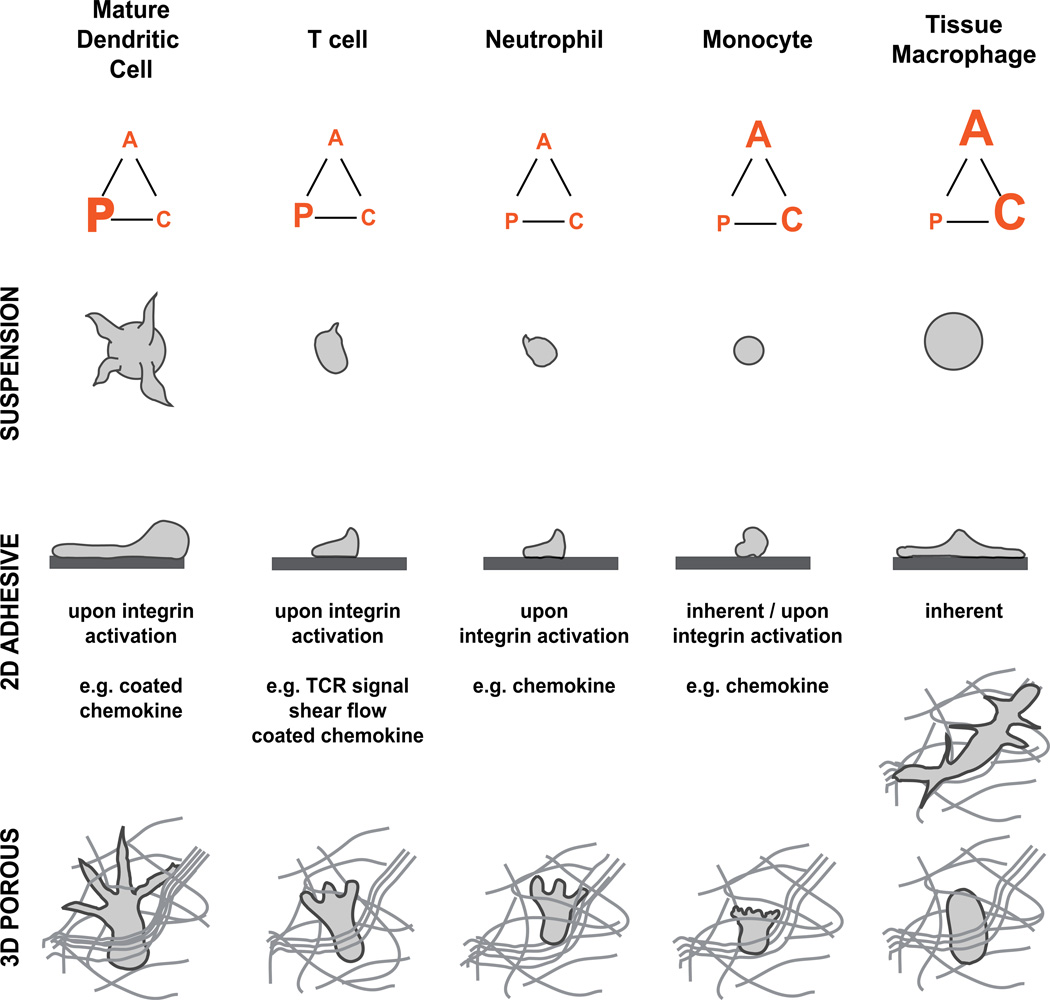
Fig. 2. Leukocyte subtypes cover a broad spectrum of morphological amoeboid phenotypes.
The force-relationship between the three migration modules—actin polymerization (“P”), contraction (“C”) and cell adhesion (“A”)—determines the shape of leukocytes and the exact mode of migration. This overview of selected leukocyte subtypes presents their presumptive force balances (displayed as force triangle) based on cell morphologies in suspension (upper row), 2D adhesive surfaces (middle row), and 3D porous scaffolds (lower row). Tissue macrophages are illustrated in an adhesive 3D porous network (e.g., fibronectin) (top) and nonadhesive 3D porous network (below). Assuming slight differences in the balanced force interplay between leukocyte subsets, pharmacological or genetic interference with actin flow, contraction or cell adhesion might affect leukocyte interstitial migration in a cell-type-specific manner.
Actin polymerization is the major protrusive force at the leading edge of migrating leukocytes. Actomyosin contraction also contributes to protrusion formation by generating intracellular hydrostatic pressure gradients that can lead to ruptures in the actin cortex, expand membrane, and morphologically manifest in blebs at the cell front. The intracellular balance of both actin polymerization and contraction shapes the exact mode of leukocyte migration [5]. For immune cells, this results in a morphological spectrum ranging from dendritic cells with pronounced, multibranched protrusions to some monocyte subtypes with small filopodia and occasional blebs (Fig. 2). In migrating leukocytes, polymerizing actin protrudes the membrane at the cell front, while actin filaments move backward into the cell (retrograde actin flow) [30]. To allow cell movement, the intracellular forces of actin polymerization and actomyosin contraction need to be transduced to the surrounding extracellular environment. Integrin adhesion receptors are transmembrane receptors that can provide surface anchorage and couple the retrograde forces of the actin cytoskeleton to the underlying extracellular substrate [31, 32]. On 2D surfaces, integrin-mediated adhesion is required for effective leukocyte migration (Fig. 1b). When dendritic cells and macrophages were depleted of all integrin receptors or could not induce high-affinity integrins, they floated in the medium of ligand-coated 2D cell culture dishes [33]. Some leukocyte subsets only activate high-affinity integrins when sensing chemokine-bound surfaces (e.g., dendritic cells), soluble attractants (e.g., neutrophils), or fluid shear forces (e.g., naïve T cells), which leads to stopping, cell spreading, or surface- confined migration. Whereas neutrophils and T cells can crawl just by actin network expansion with minimal adhesion to an underlying substrate [5], strongly adherent macrophages require myosin II-dependent contractions at the cell rear to detach from an adhesive substrate and retract the cell body during migration (Fig. 2).
In contrast to 2D migration, it is now widely accepted that interstitial leukocyte motility in vivo and in 3D environments does not depend on integrin receptors [3, 5]. Early studies in 3D in vitro gels and artificially confined spaces suggested a leukocyte migration mode independent of strong adhesive coupling to the environment, but this concept was initially received with skepticism [34–36]. However, a decade later, a number of in vivo studies have confirmed integrin-independent leukocyte migration [8, 33, 37]. Dendritic cells genetically depleted of all integrin heterodimers did not show altered migration in skin explants and lymph nodes [33]. Consistent with these findings, dendritic cells lacking the essential integrin activator talin also showed normal movement in vivo [33] and this behavior has been recently reported for talin-deficient neutrophils migrating in intact mouse skin [8]. How leukocytes moving in a 3D environment transduce their intercellular cytoskeletal forces in an integrin-independent manner has still not been resolved [38]. Ongoing studies are using 3D gels and artificially confined spaces in which leukocyte migration is also independent of integrins to gain insight into this question.
Learning from 3D in vitro systems: collagen gels and confined spaces
While commonly subsumed as “3D migration systems”, 3D gels and confined spaces are not totally equivalent (Fig. 1b). Collagen gels are the most widely used 3D gels and the best mimics of collagen-rich connective tissues. Leukocytes that are completely embedded in these fibrillar scaffolds move through a porous network of fine collagenous fibers. Confined environments are less complex, can be formed by two planar surfaces, and comprise any space smaller than the diameter of a leukocyte. In a classical study by Malawista et al. [39], human neutrophils with an average diameter of 8 µm performed integrin-independent “chimneying” between two adjacent glass surfaces with a gap size of 5.7 µm, but not 11.4 µm. Recent developments in microfabrication have led to a multitude of new custom-made devices that allow precise experimental control of both the geometry and chemistry of the environment in which leukocyte migration can be examined. Most studies of leukocyte dynamics in confined spaces are now performed in microchannels of varying width (usually in the range of 2–8 µm) [40–43]. While straight microchannels impose directional migration, they can be modified to guide leukocytes through complex maze-like geometries [44], constriction rings [45], or decision-requiring bifurcations [44]. In combination with microfluidics, these microchannels now also allow dissecting how chemokine gradient sensing, physical constraints, and fluid flow together influence leukocyte guidance [46–48]. Since the stiffness and texture of the environment are additional parameters affecting cell migration, researchers have begun to develop confined environments with elastic walls [49] and asymmetric features [50], in the form of micropillars [51] or structured nanopatterns [52]. While almost any structure, texture, and geometry seems technically feasible, such reductionist microfabricated devices will be most useful when wisely chosen to mimic a particular physiological aspect of leukocyte tissue migration.
Collagen gels
Collagen gels are predominantly made of type I collagen that polymerizes to form fibrillar networks. Several experimental protocols are available for investigating leukocyte random migration, chemotaxis, or cell–cell interactions [53–56]. Unlike material-dense 3D systems (e.g., Matrigel, hydrogel) [57], collagen gels form a porous 3D network that allows nonproteolytic migration of leukocytes [58]. The exact matrix structure and properties depend on the species and source of the solubilized collagen (mostly from bovine hides or rat tail) and specific gelling conditions (time, temperature, pH) [59–62]. While collagen species and concentration are indicated in most protocols, the exact matrix rigidity, evenness, elasticity, density, pore sizes, and collagen bundling often remain undefined [63]. Since these external parameters can heavily influence leukocyte dynamics, experimental outcomes can vary and better reporting of these values would aid the field going forward.
When leukocytes are embedded in 3D collagen gels and completely surrounded by this scaffold, they can migrate in a nonadhesive mode as shown by randomly migrating T cells upon blockade of integrin function [36] or by integrin-depleted dendritic cells, neutrophils, and B cells moving in a chemotactic gradient [33]. In contrast, when leukocytes are layered on top of collagen gels (e.g., in a Transwell), they require integrin-mediated adhesion to invade the matrix. Once in the collagenous mesh, leukocyte movement is almost exclusively driven by actin polymerization [33, 64]. Unless actin flow is completely stalled, leukocyte migration is very robust, meaning that complete interference with the contraction and adhesion module or partial block of actin polymerization still allows residual movement [33]. When facing narrow pores in denser collagen gels, locomoting leukocytes partially depend on RhoA activation, Rho-associated protein kinase (ROCK), and myosin II-dependent contraction [33, 65]. Upon chemical blockade of ROCK or myosin II, the rigid nucleus of dendritic cells becomes stuck at small pores in gels of 1.5 mg/ml collagen concentration. While the trailing edge immobilizes, the leading edge continues protruding and elongates the cell. Similarly treated neutrophils of smaller size do not show impaired motility in 1.5 mg/ml collagen gels, but only in gels of >3 mg/ml collagen concentration [33]. Together with recent data, this strongly suggests that the restricting pore size depends on the cellular diameter and nuclear size and rigidity [66, 67].
Unlike in 2D where crawling leukocytes form flat adhesive lamellipodia, immune cells form protrusions in the form of pseudopodia, blebs, or filopodia that can extend through matrix pores and branch in the 3D space of the collagen gel (Figs. 1b and 2). The complexity of the collagenous 3D environment requires tight coordination of the multiple frontal protrusions for efficient migration. Indeed, dendritic cells lacking such coordination mechanisms due to depletion of the Rho family small GTPase Cdc42 [68] or the Cdc42 effector DOCK8 [69] get entangled in 3D collagen gels, but can migrate with unaltered speed in the open space of uniformly structured, nonmesh-like confined environments of the type described below. However, Cdc42-deficient neutrophils that do not form such extensive cell protrusions as dendritic cells were not entangled and migrated with normal speeds in collagen networks (T. Lämmermann, unpublished observation), further confirming that the same 3D matrix structure has different effects on distinct immune cell types (Fig. 2).
Changing the 3D matrix geometry rather than the cell type can also reveal distinct modes of leukocyte migration, as exemplified with human macrophages. In discussions on classical leukocyte migration, macrophages are often left aside since their adhesive and elongated morphology suggests closer relationships to fibroblasts and tumor cells than other leukocytes. Indeed, macrophages in nonporous, dense 3D gels (Matrigel, very dense collagen gels) develop adhesion-rich podosomes and adopt a protease-dependent mesenchymal migration mode. However, once these cells are embedded in the porous matrix of loose fibrillar collagen, they switch to a nonproteolytic, contraction-dependent amoeboid migration mode reminiscent of classical leukocyte 3D migration [57]. Some macrophage subtypes are better in switching between migration modes than others, arguing for subset-specific requirements for adapting to the external environment [70].
Confined spaces
Microfabricated channels have precisely controlled geometry and structural properties superior to complex nonhomogeneous collagen meshes that provide advantages for some types of analyses. These reductionist channels can be reconstructed to address specific aspects of leukocyte migration difficult to assess in 3D collagen gels [49]. Similar to movement in 3D collagen gels, leukocytes do not require integrin adhesive forces when migrating in highly confined environments [39], and leukocytes that are squeezed between two surfaces migrate without a strict integrin requirement in a number of experimental settings such as under-agarose assays, EZ-Taxiscan devices, or microfabricated channels (2–8 µm in diameter). Immature dendritic cells crawling in 4-µm-diameter, fibronectin-coated microchannels require myosin II-dependent contractions [40, 41]. Myosin IIA-deficient dendritic cells migrate with half the median speed (2.5 µm/min) of control cells (5 µm/min) [49]. Studies with T cells in microchannels of various sizes demonstrated the existence of a confinement optimum for leukocyte motility [42]. With widths that approximated their girth (7–9 µm), T cells migrated at peak velocities, but their speed was significantly lower in channels of smaller (4–5 µm) or larger (12–20 µm) dimensions. Fast cell motility at the optimal confinement was largely independent of the specific channel coating (integrin ligands fibronectin or ICAM-1, 20–25 µm/min; nonintegrin ligands such as casein, 15–20 µm/min). Upon inhibition of myosin IIA, activated T cells dropped their speeds (7–12 µm/min) at all microchannel sizes, indicating that myosin IIA optimizes fast T cell crawling in many environments. When migrating in ligand-coated microchannels of larger width (20 µm, fibronectin-coated) that do not provide confinement, T cells can adopt two distinct migration modes depending on their balance between cortical tension and adhesiveness: normal T cells have sufficient contractile tension that counteracts and limits adhesion to channel walls. Consequently, T cells migrate for short distances along one wall before de-adhering and “walking” over to the opposing wall (“walking” mode). In contrast, T cells with decreased cortical tension (e.g., upon interference with myosin IIA function) rarely detach from one microchannel wall and instead “slide” along with prolonged adhesive contacts (“sliding” mode) [42, 71].
While these studies revealed an optimizing role for contractile forces during leukocyte migration in confined spaces, blockade of myosin II typically decreased cell speed by about half and still allowed residual movement. A very recent study investigated leading edge dynamics during the chemotactic migration of neutrophil-like HL60 cells in microfluidic channels (5µm × 5µm) that were coated with integrin ligand [20]. By visualizing F-actin dynamics in combination with FRAP experiments, this work revealed two distinct F-actin networks at the leading edge: (1) a dense network at the interface between the cell and channel walls (the “adherent” network) and (2) a less dense network in the center of the leading edge (the “free” network) (Fig. 1b). Similar to classical 2D lamellipodia, the free F-actin network was polymerized against the cell membrane at the cell front, had faster F-actin assembly, and required the actin-nucleating complex Arp2/3 for polymerization. The adherent network polymerized perpendicular to this leading edge network against the cell-to-wall interface and did not require the Arp2/3 complex for polymerization, but rather depended on actin-nucleating formins. Since the adherent network grows medially from the channel walls, it compresses the free network polymerizing from the membrane at the cell front. This prevents the retrograde movement of the free F-actin network and converts its actin polymerization into forward protrusions. When the free actin network was selectively diminished by chemical blockade of Arp2/3, cells formed blebs at the cell front and accelerated in the channel. This switch to a faster mode of migration now entirely depended on the contractility of the cell and the adherent F-actin network. Upon chemical inhibition of formins, the leading edge destabilized and cells halted migration, in agreement with an essential function of actin polymerization for leukocyte migration [20]. Since we have mentioned earlier that leukocytes can migrate in an integrin-independent manner in confined environments, it will be interesting to perform similar experiments under nonadhesive conditions and investigate cell migration when the adherent network will most likely not form. This is of particular interest since nonadherent dendritic cells migrating in confinement have been suggested to upregulate actin polymerization as a compensation mechanism [72].
Physiological leukocyte migration
Studies such as those described above have already taught us much about the plasticity of leukocytes and their capacity to adopt several migration strategies in defined 3D environments and switch between these when external geometries change or leukocytes lack one of the three migration modules (denoted “P,” “C,” and “A” above). Future developments in microfabrication and material sciences in combination with higher resolution live cell microscopy will provide even more insights into how leukocyte migration is mediated and controlled. Ultimately, however, we need to understand interstitial movement within physiological tissue environments that are very heterogeneous and dynamically changing. In situ migrating leukocytes often have options when facing multiple extracellular ligands in the forms of 2D surfaces, confined spaces, and 3D networks in close proximity to one another (Fig. 1b). For these reasons, direct analysis of leukocyte migration in vivo, including the organization of the tissue environment in which such movement takes place, is critical.
Animal organs are complex and differentially composed of epithelial, connective, muscle, and nervous tissues according to their function. Primary (thymus, bone marrow) and secondary lymphoid organs (lymph nodes, spleen white pulp, tonsils, Peyer’s patches, follicles of the mucosa-associated lymphoid tissues, BALT) have evolved tissue organizations that support blood cell development, instruct homeostatic immune cell trafficking, and regulate the tightly controlled steps involved in initiation of adaptive immune responses. All these tissues and organs have in common that they are densely packed with leukocytes embedded in a fine-structured, connective tissue-type reticulum that functionally compartmentalizes leukocyte development and immune responses [73]. In contrast to the cell-rich lymphoid organs, most nonlymphoid organs predominantly consist of collagenous connective tissue interspersed with cells of different origins. This tissue organization is functionally optimized to preserve vital functions (e.g., nutrient uptake, gas exchange, information flow, fluid balance, metabolism, movement, structural support), and leukocyte trafficking in the interstitium of nonlymphoid tissue is rather minimal during tissue homoeostasis. However, upon inflammation or infection, these tissues dynamically change in their cellular content: Immune cell trafficking in nonlymphoid tissues dramatically increases when blood-recruited inflammatory leukocytes invade and scan the tissue [2] or when tissue-resident dendritic cells start crawling into lymphatic vessels [74]. Depending on the type of inflammation, the structure of the extracellular environment might also undergo changes and form blisters (nonviscous serous fluid), abscesses (pus), ulcers (necrotic epithelial cells), and depositions of fibrin. In situations of wound healing, long-term remodeling of the extracellular matrix occurs when granulation tissues develop.
Many studies relying on static observations of tissue sections and flow cytometric as well as ELISA analysis of cells and material extracted from dispersed tissues have provided valuable insights about the cell types and soluble factors that contribute to immune homeostasis and inflammatory events. But to truly relate tissue organization to leukocyte migration, direct dynamic imaging is required and intravital techniques have markedly improved our understanding of the choreography of immune cell motion, interaction, and function by visualizing living leukocytes in their natural in vivo environment.
Intravital imaging and its (current) limitations
Intravital microscopy using single-photon (1P-IVM) or two- photon (2P-IVM) instruments has made important contributions to live immune cell imaging in vivo [9]. By exciting fluorescent dyes and proteins with pulses of near-infrared laser light, 2P-IVM is the preferred choice for deep tissue imaging (penetration depth −200–300 µm) with low tissue damage and has been applied to many organs. Imaging leukocyte dynamics in live anesthetized mice preserves close to physiological conditions (nutrient, gas, fluid exchange) and allows long-term imaging over several hours. Deep tissue imaging is technically challenging since the heterogeneous nature of the tissue causes light scattering and absorption that attenuate the exciting and emitted light and degrade optical resolution. This is why most published 2P-IVM studies have so far imaged primarily at the gross cellular level and focused the analysis on movement, positioning, and interactions. Recent novel tools and techniques are beginning to permit visualization of intracellular events in vivo [75–79] and progress is being made in terms of intravital imaging of multiple cell types and tissue structures in deeper regions, at faster imaging rates, and higher resolution [80]. When tissue movement due to respiration is problematic (e.g., lung) or very distant tissue regions need to be examined, immune imaging is sometimes performed in whole organ explants or tissue slices, respectively [9]. These ex vivo models require very tight control of temperature and tissue oxygenation [81], are less physiological due to additional cell death and tissue destruction during preparation, and are usually only used for short-term studies.
Unlike in vitro experiments where cell numbers, the composition of the medium, and the geometry of the environment can be well-controlled, each intravital imaging study observes events more or less within a “tissue black box”: without control of the diversity and cellular composition in a particular imaging field of view, without knowledge of the exact oxygen levels and cocktail of pro- and antimigratory factors in that specific tissue region, and in the absence of a complete picture of the exact tissue architecture. To maximize the information gained from such in vivo studies and to limit subjectivity/ uncertainty in analysis and interpretation, several “best practices” have been developed over the past several years for these types of experiments:
Imaging gene-deficient and control cells side-by-side in the same tissue volume in each experiment reduces the number of unmeasured parameters that could influence interpretation of motility data (Fig. 3). This experimental setup eliminates problematic quantitative comparisons based on measurements of cells imaged in different experiments and animals that can be influenced by the heterogeneous composition of the specific portion of the tissue imaged, by varying nutrient availability or oxygenation as a consequence of harmful imaging conditions, or by observations made in subtly different states of tissue inflammation. Functional perturbations based on systemic or local administration of blocking antibodies or chemical inhibitors are of pharmacological interest, but remain inconclusive regarding cell-specific effects. These reagents globally interfere with protein function of all cells in the body or treated tissue, potentially leading to indirect effects that influence motility of the cells of interest and preventing cause–effect conclusions about migration control with respect to the leukocytes themselves. Moreover, monitoring the exact local concentration of blocking reagents at a certain tissue site is often difficult and results can be inconclusive when effects are not seen with the applied material.
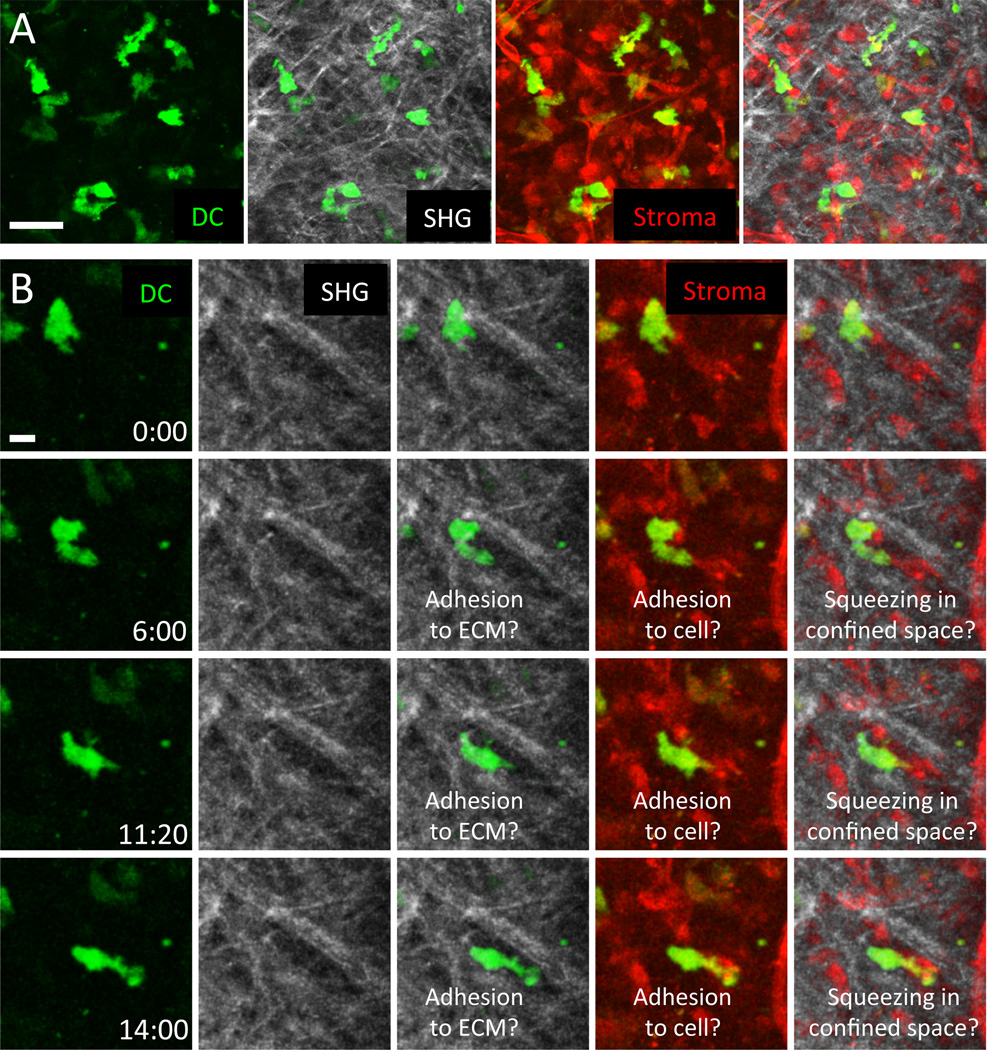
Multiplex imaging of leukocyte dynamics together with visualization of other tissue contents and stromal elements provides direct insight into how tissue geometry impacts leukocyte migration modes. Tracking migrating immune cells in situ can retrieve information about their cell shape, plasticity, speeds, velocity fluctuations, directionality, and meandering, but these tracking data alone are insufficient when considering the issue of cell-intrinsic behavior. Leukocytes can be guided by tissue geometry, stopped by interactions with other cells or tissue elements, or directed by chemokines in the tissue. As we have learned before from collagen gels and microchannels, the external environment heavily influences the mode of migration and provides contact guidance for immune cells. To address leukocyte migration modes in correlation to tissue architecture, we require as much structural information as possible. In Figs. 4 and 5, we suggest an intravital imaging setup in which immune cell tracking occurs together with simultaneous analysis of stromal elements and collagenous fiber bundles, detected by second harmonic generation (SHG), to provide the best available representation of physiological 3D geometry. While this experimental approach still does not provide information about all aspects of the tissue environment, such simultaneous multichannel visualization provides a more accurate picture of how channel-like structures, dense networks, and surfaces in vivo relate to cell movement than does collecting information separately on each element by itself.
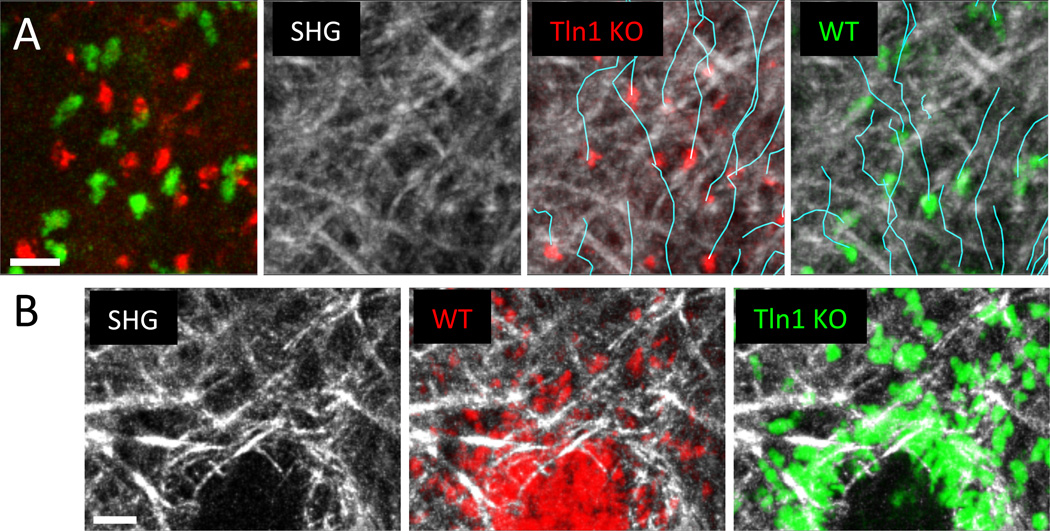
Fig. 3. Nonadhesive neutrophil migration in the dermal interstitium and mode switching to adherent crawling at wound centers.
(A) Subcutaneously injected talin-deficient (red) and control neutrophils (green) were imaged side-by-side in the same tissue volume to eliminate problematic quantitative comparisons based on measurements of cells imaged in different experiments and animals. This experimental design minimizes the influence of tissue heterogeneity between experiments and ensures the same imaging conditions and nutrient availability for both cell types. Collagen fibers are visualized by second harmonic generation (SHG) signals (white), cell tracks are indicated in turquoise. Neutrophils perform chemotaxis toward sites of sterile tissue damage and migrate in a nonadhesive mode through the dermal interstitium. (B) When neutrophils congregate at sites of local wounding, the developing cell aggregates remodel the interstitial fiber architecture and form a collagen-free wound center. At the transition zone between 3D fibrillar interstitium and the cell-rich, collagen-free wound zone, neutrophils switch to adhesion-dependent crawling. Unlike control cells (red), talin-deficient neutrophils (green) accumulate exactly at the transition zone. Scale bars = 20 µm.
With these considerations in mind, we summarize here leukocyte migration in tissue architectures at opposite sides of the spectrum, namely collagen-rich (fibrillar) versus cell-rich environments (Fig. 1b), and explore if (a) tissue structures impose motility modes, (b) certain locomotion strategies optimize trafficking paths or positioning, (c) leukocytes adopt tissue-specific migration strategies, and (d) immune cell subsets acquire different degrees of plasticity in physiological tissues.
Fibrillar environments
Most nonlymphoid organs have pronounced connective tissue components. Type I collagen is the major constituent of most connective tissues and assembles collagen fibrils and bundled fibers in the form of interstitial meshes. Together with other extracellular matrix components such as elastic fibers and basement membranes, collagenous networks act as a cellular backbone of most organs and tightly integrate blood vessels, lymphatics, fat cells, nerves, muscle strands, and glands, shaping a physiological maze of confined spaces, fibrillar 3D meshes, and perhaps even 2D surfaces (Fig. 1b). Leukocytes appear to navigate in a nonproteolytic, low adhesive manner in such tissue geometries, passively guided by paths of least resistance or barriers of dense matrix [3]. Within each tissue, the fibrillar collagen composition is very heterogeneous, with regions of densely packed thick collagen bundles often alternately with areas of loose matrix where leukocytes could penetrate through pores, clefts, and gaps of various sizes [59].
Neutrophils
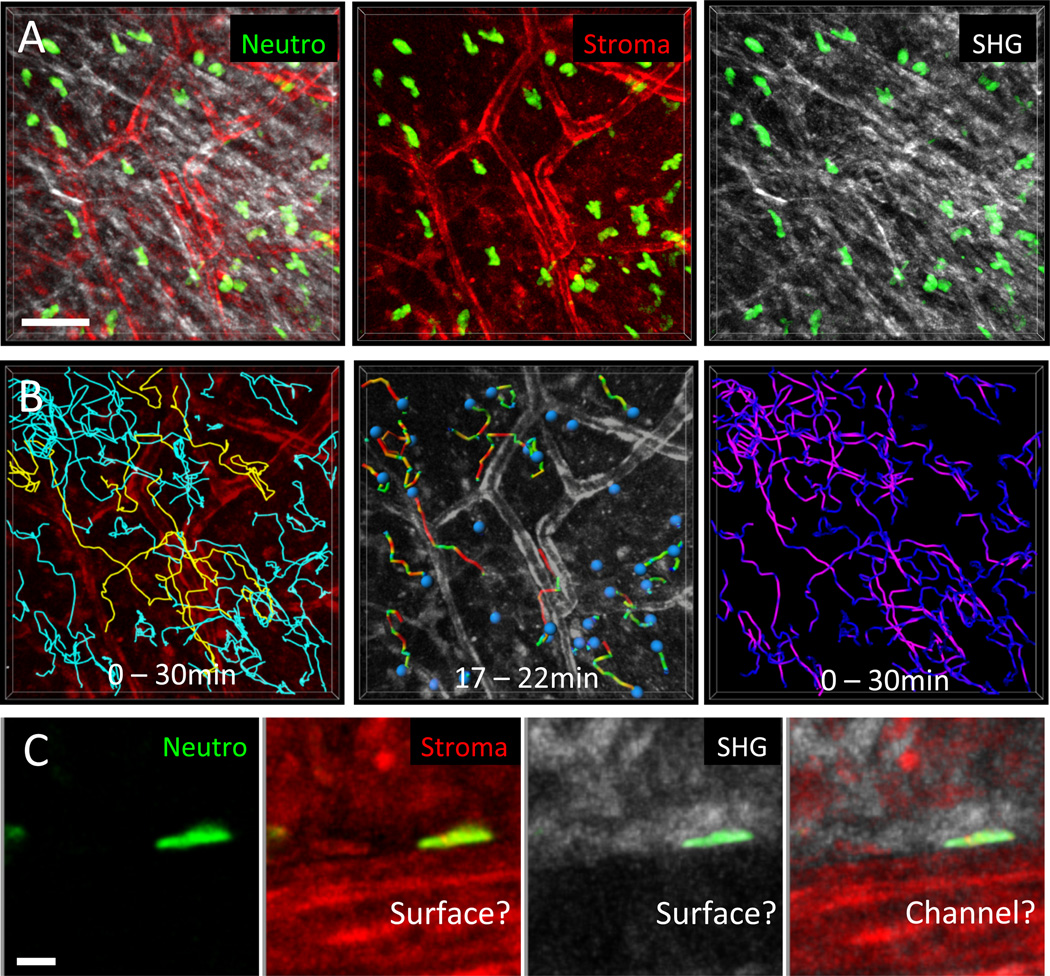
Neutrophils that are recruited from blood vessels upon tissue injury, inflammation, or infection need to flexibly reorient themselves in the tissue and migrate with high speeds to sites of tissue insult. Consistent with this notion, talin-deficient neutrophils that lack high-affinity integrins have similar speeds and directionality when migrating side-by-side with wild-type neutrophils to sites of sterile injury in the ear skin dermis (Fig. 3a) [8]. Instead of following a haptokinetic principle, neutrophils performed chemotactic migration to sites of tissue damage and amplified local cell death signals by secreting the soluble lipid leukotriene B4 to enhance the radius of interstitial cell recruitment. In agreement with previous work in collagen gels [33] and integrin-blocking studies in intact mouse skin [82], the interstitial geometry of the dermis appears to provide sufficient 3D guidance structure to allow adhesion-independent neutrophil migration based only on forces generated by the actomyosin cytoskeleton. A recent study challenged this concept by proposing adhesive perivascular tracks as “preferential” routes for interstitially migrating innate phagocytes [83]. A fraction of neutrophils and monocytes moved at higher speeds when close to pericytes along arterioles and capillaries in the ear skin. Based on human pericytes upregulating the integrin ligand ICAM-1 and the chemoattractant MIF upon exposure to inflammatory mediators and the outcome of integrin blockade of interactions between innate leukocytes and pericytes in vitro, it was concluded that pericytes could instruct haptokinetic phagocyte migration along vascular highways [83]. While chemical interference with MIF function in vivo seemed to shift phagocyte migration away from pericytes, these experiments were not conclusive. Since they did not include internal control cells and the blocking agents were administered throughout the tissue, the possible confounding effects of tissue heterogeneity on the one hand and secondary effects involving alteration of the behavior of other cells in the tissue on the other cannot be ruled out. We have confirmed that randomly migrating neutrophils that transiently crawl along vascular structures can reach higher speeds (Fig. 5), but in imaging fields of view with large cell numbers, only 10–15 % of the cells moved along vessels, while all other neutrophils migrate in the collagenous interstitial space (Fig. 5b). Multiplex imaging of neutrophils, vessels, and tissue collagen revealed that velocities were equally high for neutrophils migrating in perivascular versus areas of low collagen density, while only neutrophils in dense collagen regions were slower (Fig. 5b). Moreover, the interfaces between vessels and surrounding collagen network seem to form microchannel-like confined spaces that, once neutrophils enter them randomly, optimize rapid leukocyte movement without strict requirement for cell adhesion (Fig. 5c).
Fig. 5. Correlation of neutrophil migration paths and velocities with connective tissue structure.
(A) 2P-IVM was applied to murine ear skin to visualize the dermis of a transgenic DsRed+/− Lyz2-GFP+/− B6.Albino mouse. Ubiquitously expressed DsRed illuminates all stromal elements (red), GFP-bright cells represent endogenous neutrophils (green), and SHG signals reflect collagen bundles. Neutrophil motility was recorded simultaneously with stromal elements over 30 min. Scale bar = 50 µm. (B) Cell tracking analysis in relation to tissue structures. (Left) Tracks over the entire imaging session were plotted for all cells in the tissue volume and related to the vascular network; 15 % of migrating neutrophils crawled along vessels (yellow tracks), whereas the remaining cells migrated in the interstitial spaces between vessels (blue tracks). (Middle) Dragontails indicate the migration paths covered by individual cells in the last 5 min. The colors of the dragontails indicate instantaneous velocities (red: fast, green: slow). This particular short time frame suggests that neutrophils have high instantaneous velocities preferentially when migrating close to vessels. (Right) In contrast to the 5-min interval analysis, plotting the velocity distributions over the entire imaging session of 30 min (purple: fast, dark blue: slow) revealed that most neutrophils migrated fastest in interstitial spaces with a loose local collagen network. (C) Depending on the graphic representation, neutrophils close to vessels appear to migrate along the vascular surface (middle left) or along a 2D surface of collagen matrix (middle right), which might actually be a channel-like confined space (outer right). Scale bar = 50 µm. Lyz2-GFP mice were a kind gift of Thomas Graf.
In contrast, an unexpected essential role for integrin-mediated adhesion mechanisms has recently been discovered when neutrophils congregate at sites of tissue wounding. These dense cellular clusters rearrange the collagenous network of the dermis by physically excluding fibers from the core of the cell infiltrate. Integrin-mediated adhesion is required to promote strong local neutrophil interactions and localized migration. When talin-deficient or β2 integrin-deficient neutrophils were co-injected with control cells, they migrated unimpaired and with high speeds in the fibrillar interstitium toward the cellular aggregates (Fig. 3a), but accumulated at the transition zone from collagenous network to the cell-rich, collagen-free wound center (Fig. 3b). These observations represent important in situ examples where leukocytes maintain interstitial motility at the transition zone from fibrillar to nonfibrillar tissue geometry by switching from one migration mode (nonadhesive) to another (adhesive). Deficiency in either of LFA-1 or Mac-1, both members of the β2 integrin family, had a measurable effect on central accumulation, suggesting that both were independently involved in cell adhesion and movement within the cell cluster [8]. Formation of these cellular clusters further depended on lipids and chemoattractants that were locally secreted by neutrophils, presumably activating high-affinity integrins and inducing the switch to an adhesive motility mode.
While various results have been obtained about the roles of integrins [84, 85], actin polymerization [86], and myosin II function [86, 87] in neutrophil interstitial migration in vivo, the nature of the experiments leaves open questions about whether the manipulations employed acted directly on the studied leukocytes or indirectly via other tissue cells. On the other hand, numerous mouse knockout models for regulators of actin polymerization and actomyosin contraction have studied neutrophil function [88–96], but rarely addressed interstitial migration by direct in vivo imaging. One of the few examples that allowed conclusions about cis-effects comes from studies with neutrophils lacking Vav GEFs, important activators of Rac, the major Rho family GTPase to mediate actin polymerization. When Vav1/2/3-triple-deficient neutrophils were co-injected with wild-type cells and their movement analyzed in the dermis of mouse footpads and phalanges, they migrated with 35 % reduced median speeds compared to controls [97].
T cells
With the exception of some tissue-resident T cell lineages, most T cell subtypes are recruited from the blood to peripheral sites of inflammation or infection. In the connective tissue of the skin, the dynamics of γδ T cells, regulatory T cells, and memory and effector T cells have been visualized with 2P- IVM in the context of adaptive immune responses [98–106]. Only recently, though, was T cell interstitial motility investigated with simultaneous acquisition of data on tissue composition and architecture of the inflamed mouse ear skin [107]. In vitro generated CD4+ T cells were injected at the peak of ear inflammation, initially induced by local injection of antigenic peptide with complete Freund’s adjuvant (water-in-mineral oil emulsion). In this experimental model, inflammation seemed to have changed the connective tissue in two ways: (1) the collagen network seemed looser than uninflamed ear skin, and (2) the matrix glycoprotein fibronectin was widely deposited throughout the dermis. Based on computational image analysis of T cell tracks recorded together with collagen second harmonic generation (SHG) signals, T cells seemed to preferentially migrate along the collagen scaffold in inflamed ears. This study proposed a haptokinetic model of motility where T cells interact through integrin receptors of the αv family with fibronectin along the matrix scaffold and raised the interesting point that structural and contextual changes in inflammatory tissues might affect leukocyte motility. However, two major aspects of the experimental designs limit the conclusions that can be drawn from the data: (1) off-target tissue effects of systemically administered blocking agents and (2) absence of internal control cells in the same imaging field of view to control for tissue heterogeneity and varying imaging conditions. A few other studies have also observed effector T cell migration along collagen fibers in cell-rich tumor tissues and infected brains, but these associations were only occasional [108–110]. As we have outlined before (Figs. 4 and 5), visualization of cell migration in relation to collagen SHG signals alone provides a very limited picture of the physiological 3D geometry and leaves open questions if collagen fibers truly act as adhesive guidance structure or if they align along a path of least resistance where T cells preferentially migrate in a cell-rich inflammatory environment [106].
Dendritic cells
Unlike neutrophils that are recruited to connective tissues from the blood stream, many dendritic cells (DCs) are already tissue-resident and currently serve as the best studied model for interstitial migration. In homeostatic conditions, DCs form a cellular network that constantly samples the tissue for potential microbial entry. Immature DCs have adherent and proteolytic properties that they largely lose upon uptake of foreign material and sensing of danger signals. During this maturation process, they upregulate antigen-presenting properties, cytoskeletal activity, and chemokine receptors, most importantly the chemokine receptor CCR7, with the latter guiding them via lymphatic vessels to the draining lymph nodes [74]. There are several established techniques for following the sequential steps of DC migration from the body periphery to the lymph node, in particular for the subtypes of the skin: epidermal Langerhans cells and dermal DCs. While “crawl-out” assays of mouse ear skin explants analyze interstitial migration into lymphatics, contact sensitization of the ear skin using the chemical FITC monitors physiological movement of these endogenous dendritic cells into the lymph node. More commonly used are DCs differentiated in vitro from mouse bone marrow hematopoietic stem cells (BM-DCs) [53]. After subcutaneous (s.c.) injection into mouse footpads, BM-DCs migrate to skin-draining lymph nodes where their arrival can be quantified. In vitro matured BM-DCs were also applied to ear explants where they migrated within 30–90 min through the dermal interstitium into lymphatics, a process that can be followed with fluorescence confocal laser microscopy. These “crawl-in” assays preserve some aspects of dermal geometry and have revealed some details about how DCs enter lymphatic vessels [33, 111, 112]. However, they do not account for physiological flow of tissue fluids or provide insight into the dynamic behavior of immature dendritic cells. Alternatively, ear skin dermis can be surgically exposed in anesthetized mice preserving fluid flow, but causing tissue damage and inflammation [113]. Other than limited experiments observing endogenous Langerhans cell and dermal DC motility in intact murine skin of anesthetized mice [114–117], very few of these studies have addressed DC migration in relation to the interstitial architecture (Fig. 3) [118, 119]. Hence, most of our knowledge on interstitial DC mechanics comes from nonimaging studies with gene-deficient cells.
In contrast to neutrophils and other leukocytes with scanning behavior and persistent reorientation in the tissue, once skin DCs undergo activation, they move in a highly directed manner to the nearest lymphatic vessel. DC crawling into lymphatics and the lymph node does not require integrin receptors [33], ruling out an adhesive pre-formed path that leads into lymphatic vessels. Instead, DCs are guided in an integrin-independent manner into lymphatics by tissue-immobilized gradients of the CCR7 ligand CCL21 [112]. As mirrored morphologically in their extremely pronounced cell protrusions, migration of matured DCs is largely based on the forces of actin polymerization [33]. Accordingly, DCs depleted in genes that regulate actin dynamics or the architecture of the actin network show impaired physiological migration patterns. Double deficiency in Rac1 and Rac2, the major Rho GTPase isoforms controlling polymerization, yield completely round BM-DCs that cannot migrate to lymph nodes upon injection [120]. When DCs lack the Wiskott– Aldrich syndrome protein (WASp) or the formin mammalian Diaphanous-related-1 (mDia), both important catalysts for actin nucleation and polymerization, their migration to the lymph node is impaired [121–124]. DCs also express actin regulators that are rarely found in other immune cells. As one example, Eps8 acts downstream of Rac and is essential for actin capping and formation of dense actin networks in DCs [125]. As another example, the actin-bundling protein fascin-1 is specifically induced upon DC maturation [126]. In agreement with an important role for regulating actin-rich protrusions, DCs depleted of Eps8 or fascin-1 show impaired migration to lymph nodes [125, 126].
The role of actomyosin contractions during physiological DC migration has so far been addressed indirectly through studies of gene-deficient DCs with altered RhoA or myosin II activation levels that all lead to reduced DC migration to lymph nodes [40, 127, 128]. Actomyosin contractions might not be required all along the DC route to the lymph node, but only at specific sites where cell squeezing is required. In agreement with this concept, regulation of myosin II activity is spatially controlled when DCs need to pass narrow pores upon entering terminal lymphatics. Here, the semaphorin Sema3A is produced in lymphatics and signals through the plexin A1–Nrp1 receptor complex on DCs to induce myosin II-dependent contraction at local sites of DC entry [129]. A second receptor–ligand pair regulates lymphatic entry by controlling intracellular GTPase activities: DCs express the C-type lectin receptor CLEC-2 that can bind to the glycoprotein podoplanin (PDPN) on lymphatic vessels. CLEC-2 engagement by PDPN leads to upregulation of Rac1 activity and stimulation of actin polymerization, while RhoA activity is downregulated and myosin II function decreased. This DC spreading response seems to support entry into lymph vessels, since CLEC-2 knockout DCs have 30 % impaired lymphatic transmigration [130].
In agreement with an essential role for coordinating the cytoskeleton during physiological interstitial movement, DC migration is heavily perturbed when cells cannot maintain their spatial and temporal asymmetries. An important molecular regulatory axis for interstitial motility is DOCK8→ Cdc42→WASp. Cdc42-deficient BM-DCs, generated from mice where Cdc42 depletion was restricted to the hematopoietic cell lineage, largely maintained normal cell motility on 2D surfaces and in confined spaces in vitro. However, these knockout cells were markedly impaired in their migration through porous 3D meshes, including movement through the dermal interstitial space toward lymphatics [68, 131]. A similarly profound, but slightly milder, DC phenotype has been observed in mice deficient in DOCK8, a member of the DOCK (Dedicator of cytokinesis) family of proteins that function as GEFs for RhoGTPases. While motility on 2D surfaces was not significantly affected, DOCK8 knockout cells failed to migrate in 3D interstitial spaces and their arrival in lymph nodes was reduced to less than 25 % that of control cells. Although DOCK8 deficiency did not affect global Cdc42 activation, DOCK8 acted as Cdc42-specific GEF and specifically activated Cdc42 activity at the leading edge [69]. Recent descriptions of loss-of-function mutations in the human gene DOCK8 have revealed a new autosomal recessive primary immune deficiency and sparked interest into DOCK8 functions [132, 133]. Patients with DOCK8 deficiency present multiple abnormalities of the immune system, including defective T cell function and elevated IgE levels, leading to susceptibility to viral, fungal, and bacterial infections [134]. While studies in DOCK8-deficient mouse models revealed important roles for DOCK8 in long-lasting humoral immunity [135] and survival of T cell subsets [136–138], it is currently unknown if defects in interstitial motility contribute to the phenotypes observed in humans and mice.
In some ways similar to DOCK8 deficiency in regard to predilection for viral infections, elevated IgE and cutaneous eczema, Wiskott–Aldrich syndrome (WAS) represents another cytoskeletal disorder with various leukocyte migration defects [139, 140]. WAS is a rare X-linked primary immunodeficiency characterized by congenital microthrombocytopenia, moderate-to-severe eczema, recurrent or severe bacterial infections, and an increased incidence of autoimmunity and malignancies [140]. It is caused by mutations in the WAS gene that lead to the absence of the Wiskott–Aldrich syndrome protein (WASp) or loss of its functional activity. Classical WAS is related to three other Wiskott–Aldrich syndrome protein (WASP) associated diseases caused by reduced WASp function or expression levels: X-linked thrombocytopenia, X-linked neutropenia, and WIP deficiency [141]. Signaling downstream of Cdc42, WASp acts as key effector for Cdc42 in hematopoietic cells, primarily through activating the actin-nucleating complex Arp2/3 and promoting actin polymerization. As seen upon deletion of DOCK8 or Cdc42, WASp-deficient DCs showed impaired migration to lymphatics and lymph nodes in several experimental models [122–124], implicating an essential regulatory role of the DOCK8–Cdc42–WASp axis in interstitial DC motility.
From the gene knockout studies summarized here, we have gained valuable insights into the molecular regulators of actin network formation, contraction, and cytoskeletal control and their contribution to interstitial DC migration to lymph nodes. However, in most of these studies, DC migration was not prevented and residual motility might have occurred by switching migration modes. Combining the current gene defect models with imaging of DC movement in relation to extracellular components will provide a more detailed insight into how tissue geometry impacts DC motility.
Keeping in mind that nonlymphoid organs primarily evolved to maintain structural integrity and protect organs, there is at present little evidence that these interstitial tissue geometries have been optimized for leukocyte migration.
Cell-rich environments
In contrast to the fibrillar architecture in connective tissues, other tissues are mainly composed of cellular networks and lack pronounced matrix structures. Instead of moving within a stiff collagenous mesh, leukocytes navigate in cell-rich tissues through a more elastic interstitium in contact with other cell types. This includes movement through interconnected cell sheets (e.g., epidermis, intestinal and bronchial epithelia) and densely packed cells in the form of static networks (e.g., central nervous system) or with highly dynamic characteristics (e.g., secondary lymphoid organs). Trafficking of innate and adaptive immune cells has been imaged in numerous cell-rich environments, including epithelial linings [98, 99, 106, 116, 142–144], the parenchyma of the brain [79, 145–149], lymphoid organs [1, 9, 150–154], as well as cellular aggregates such as bacteria-induced granulomas [155–157]. Since most insights into interstitial leukocyte dynamics in cell-rich environments have come from 2P-IVM studies on immune cell trafficking in lymph nodes, the major anatomical sites for initiating an adaptive immune response, we specifically focus on migration strategies of dendritic cells entering the lymph node and intranodal steady-state trafficking of T cells.
Lymph nodes are encapsulated lymphoid organs with two entry sites for leukocytes: (a) afferent lymphatics that guide cells, in particular dendritic cells, from peripheral tissues through the subcapsular sinus into the functional regions of the lymph node, and (b) high endothelial venules that enable blood-circulating leukocytes, in particular lymphocytes, to directly enter the lymph node. The lymph node parenchyma is functionally compartmentalized into B cell follicles containing a fibroblastic dendritic cell (FDC) network and a T cell zone (paracortex) that is supported by fibroblastic reticular cells (FRCs). FRCs produce and almost completely cover a fine 3D network of reticular fibers, forming a stromal scaffold that serves as the structural backbone of the paracortex [158, 159]. Resident DCs and macrophages align along the FRC stroma forming a sessile phagocyte network, while T cells populate the spaces between the strands of this DC–FRC network [160–163]. Initial 2P-IVM work revealed high motility of T and B cells within lymph nodes and subsequently concentrated on interactions among T cells, B cells, and DCs, studies that have greatly shaped our understanding of the dynamic events at the onset of adaptive immune responses [9]. Later, it became apparent that T cell tracks were along the FRC network, suggesting an instructive role of the tissue stroma [161]. While there are strong indications supporting a haptokinetic model of lymphocyte trafficking in the lymph node, we still do not fully understand the underlying mechanisms.
T cells
Naïve T cells show highly dynamic migration patterns in the lymph node paracortex. The movement of T cells sometimes involves “stop-and-go” behavior, most likely due to collisions with other cells or environmental obstacles, but perhaps also due to internal clocks regulating motility persistence under steady-state conditions [101]. After discovering that T cells migrated under steady-state conditions in association with the FRC network, the former concept of “random” lymphocyte motility changed to “guided randomness” where FRCs act as scaffold for T cell trafficking. Scanning electron microscopy images identified numerous T cell microvilli that interacted with the FRC network, supporting a haptokinetic model of T cell crawling along FRC surfaces. Several lines of evidence further strengthened this concept: (1) chemokine signaling through Gαi with a major contribution of the receptor CCR7 promoted intranodal T cell motility [81, 164–166]; (2) FRCs express the CCR7 ligands, CCL19 and CCL21, of which the latter can directly bind to FRCs [167]; (3) FRCs express the integrin counterligands ICAM-1, ICAM-2, and VCAM-1 [37, 162, 168, 169]; and (4) T cells adhered in an integrin-dependent fashion to adhesive, chemokine-coated surfaces under shear flow [37]. Since naïve T cells express the integrins LFA-1 and α4β1, CCL21-binding FRCs might serve as excellent adhesive substrate. It was therefore surprising when several groups observed prominent T cell motility with only minimal changes in speed (10–30 % reduction) when integrin function was perturbed by gene deletions or functional antibody blockade [37, 169–171]. Based on these experiments, it was concluded that LFA-1 is only minimally engaged during T cell migration in lymph nodes, probably because of missing shear flow that could activate integrins. However, functional interference with LFA-1 and ICAM-1 led to slightly lower velocities and slightly less directed T cell movement, but it was unclear if this related to T cell crawling along FRCs. In a recent study, Katakai et al. [162] combined functional blocking of T cell adhesion with the visualization of the lymph node environment and revealed that LFA-1/ICAM-1 interactions promoted a very fast, highly directed T cell migration mode. Most T cells migrated in close association with the dense DC network, but where DCs were sparse, T cells were still able to migrate. In a series of bone marrow chimeric experiments, ICAM-1 expressed mainly by dendritic cells was found to promote the high-speed motility mode [162], in contrast to earlier studies [169]. In conclusion, basal T cell motility in lymph nodes does not require integrin-mediated forces, but LFA-1 engagement promotes a 10–40 % faster motility mode along two superposed ICAM-1- and CCL21-decorated cellular networks (DCs and FRCs). This might allow T cells to increase their scanning area and efficiency, but also to mediate prolonged residency in the lymph node paracortex, where LFA-1/ICAM-1 interactions have been shown to counteract early lymph node egress into medullary sinuses [171].
The basal nonadhesive migration mode of T cells argues for cytoskeletal forces promoting lymph node interstitial movement, and current work suggests a contributing role of both actin polymerization and actomyosin contraction. Since myosin II-dependent contractions are often required at sites of anatomical constriction, it was unclear how important contractile forces were in the cell-dense, nonfibrillar lymph node environment. Adoptively transferred myosin IIA-deficient CD8+ T cells showed 26 % lower velocities during intranodal migration and 49 % decrease in their motility coefficient, used as a measure of the volume a cell can survey over time, compared to control cells. Due to the loss of contractility, myosin IIA-deficient T cells showed overadhesive properties in vitro, which did not lead to significantly increased interactions with FRCs in vivo. In vitro cell–cell attachment assays of overadhesive myosin IIA-deficient T cells with FRCs, DCs, lymphatic endothelial cells, or T cells revealed statistically significant coupling only between T cells. Besides suggesting that myosin II provides contractile force to limit adhesion to other cells and optimize migration in a “confined” cell-dense environment, this work also implicated contact between T cells as potential interaction surface to generate traction [42]. Upon chemical interference with ROCK function (Y27632) and contractility, intranodal T cell speeds were minimally decreased, but motility coefficients reduced to almost the same extent as myosin IIA knockout T cells. These differences between control and Y27632-treated T cells were lost when interstitial migration was analyzed in lymph nodes of ICAM-1-deficient mice, arguing that ROCK–myosin II contractile forces mediate specific detachment from adhesive LFA-1– ICAM-1 interactions rather than squeezing forces through anatomically constricted regions [172]. An importance for cell shape changes during intranodal migration has been shown for T cells with increased membrane tensions. These were generated from transgenic mice with constitutive active ezrin, one of three members of the ezrin–moesin–radixin (ERM) family of proteins that link cortical actin to the plasma membrane. Transgenic T cells over-expressing this molecule had a 47 % reduction in motility as compared to internal controls in the structurally complex cortical ridges, while in other lymph node areas, the decrease was only 20 %, indicating structural heterogeneity within the T cell cortex that requires different degrees of cell shape change for optimal movement [173].
Maintaining a protrusive actin network is essential for T cell migration, as evidenced from interference with key molecules controlling F-actin polymerization. Upon genetic depletion of two isoforms of the Rho family GTPase Rac, Rac1 and Rac2, interstitial T cell velocities were severely reduced by 65 % compared to control cells [174]. T cells deficient in DOCK2, an important Rac-GEF in T cells, showed also strongly reduced interstitial motility and intranodal displacement [175]. Dysregulation of the actin cytoskeleton impairs T cell internodal motility, as seen in T cells that carry a point mutation in coronin-1A, causing irregular shape and larger size of leading edges due to increased inhibition of Arp2/3 [176]. Mice mutants for coronin1A showed defective T cell egress and peripheral T cell deficiencies, which led to the discovery of coronin1A mutations in a patient with T cell-deficient, B cell- and NK cell-sufficient severe combined immunodeficiency (T−B+NK+ SCID) [176].
Dendritic cells
Antigen-laden, activated DCs from peripheral tissues enter the lymph node through afferent lymphatic vessels ending in the subcapsular sinus (SCS) [74, 159]. Here, incoming DCs encounter a cell-rich lining of subcapsular sinus macrophages, lymphatic endothelial cells, other DC subsets, and passaging lymphocytes with a discontinuous basement membrane at the sinus floor. 2P-IVM studies revealed a prominent elongated morphology of DCs transmigrating through the SCS into the interfollicular regions of the lymph node cortex [177]. Cells elongate when they cannot detach adhesions at the trailing edge or need to coordinate migration through anatomical restrictions. The latter seems to apply for DC movement through the SCS, because adhesion-deficient, integrin-null BM-DCs transit with similar speeds through SCS and interfollicular regions [33]. On the other hand, BM-DCs lacking DOCK8 or WASp seemed to migrate poorly through SCS floors, suggesting that coordination of the actomyosin cytoskeleton is required for transit into deeper lymph node regions [69, 123]. Here, formation of dense actin networks seems dispensable, because Eps8-deficient BM-DCs were not retained anywhere in the lymph node [125]. As discussed above for T cells, DC migration in lymph nodes is also assumed to follow a haptokinetic model. This view is largely based on in vitro experiments showing that BM-DCs can activate integrin-mediated adhesion when sensing surface-bound chemokine CCL21, leading to preferred migration along chemokine-coated artificial microfibers [168]. As described before, FRC stromal cells express both ICAM-1 and CCL21, which might support adhesion-dependent haptokinesis in vivo. Based on immunohistochemical staining of CCL21 in lymph node sections showing a tissue-bound gradient in cortical regions, we can even assume a haptotaxis concept of DC migration in the interfollicular regions [178]. However, alternative scenarios cannot be ruled out in which a soluble gradient of cleaved CCL21 that was proteolytically “freed” by DCs might support directed movement in the tissue [168]. The findings on integrin-null BM-DCs efficiently traversing interfollicular regions do not rule out the existence of an adhesion-driven mode of migration [33], but strongly argue that BM-DC movement in lymph nodes depends largely on an integrin-independent strategy of cell deformation. DC migration into the lymph node parenchyma depends on the receptor CLEC-2 interacting with its ligand PDPN on FRCs. CLEC-2-deficient DCs had impaired access to the paracortex and stayed close to the subcapsular regions, indicating a tissue instructive role for controlling intracellular GTPase activation, DC shape changes, and motility [130]. Since almost all of these studies were based on s.c. injection of BM-DCs, we currently do not know, however, if the same mechanisms apply to endogenous and resident DC subsets.
Conclusion
We have summarized here the multiple faces that leukocytes show during interstitial migration. As we have learned from in vitro studies, leukocytes are very flexible and can switch between motility modes. Depending on the environmental context, their migration can largely depend on cell adhesion (2D surfaces), actomyosin contraction (confined spaces, dense fibrillar 3D networks), or actin polymerization alone (loose fibrillar 3D networks) (Fig. 1b). Moreover, we also know that these forces can compensate for each other, as evidenced by HL60 cells that switch their migration within minutes from protrusive to contractile blebbing mode in microchannels after losing their free actin network [20]. Based on such experiments in defined 3D geometries, we have come to the notion that leukocytes can adapt quickly to changing external conditions. There is currently much less known about the exact migration modes that leukocytes employ to navigate in tissues. The roles of chemokines and other external factors, acting upstream of cytoskeletal regulation, are understood to work together and control leukocyte positioning in lymphoid and nonlymphoid tissues. But only a few intravital dynamic imaging studies have provided clear insights into how physiological and pathological tissue geometry impacts leukocyte migration modes. Neutrophils can indeed switch from nonadhesive to adhesive migration at a sterile wound transition zone (Fig. 3) [8], but we do not know if optimal migration by other leukocyte subtypes also involves switching between adhesive, nonadhesive–protrusive, and nonadhesive–contractile motility modes. Several other important questions are still unanswered: Are there preferential tissue paths or geometries that would favor one migration strategy over the other? How do pathological changes in the tissue geometry and composition affect leukocyte migration strategies? If one interferes with only one migration mode, can cells compensate by switching to another mode? If mode switching occurs, is it a quick event or does it require gene transcriptional control? And would such mode switching alter the trafficking path in the tissue and impact the outcome of an immune response? For all these questions, we might expect different answers for each leukocyte subtype (Fig. 2, Table 1). While most of our current knowledge on interstitial migration stems from some few model cell types, there is more to explore and variety to be expected within the realm of endogenous immune cells and newly discovered subtypes. Our lessons from mouse models and primary immunodeficiencies already show that mutations of cytoskeletal regulators affect some immune cell types more than others. Since cell biologists, material scientists, and immunologists will learn from each other, we can soon expect in vitro devices that mimic physiological interstitium even better than those available today. This would allow the study of human leukocytes where intravital microscopy in living tissue is not an option. It is quite clear that we have not yet seen all the faces of leukocyte migration.
Table 1.
List of gene mutations tested for leukocyte interstitial migration
| How was interstitial migration addressed? | |||||||
|---|---|---|---|---|---|---|---|
| Mod. | Protein | Gene mutation |
Gene function |
In vivo/Ex vivo imaging |
In vivo nonimaging |
In vitro collagengel |
In vitro confinement |
| Neutrophils | |||||||
| A | Integrins | Itgav/Itgb1/Itgb2/Itgb7 | Integrin adhesion receptors | - | - | Yes [32] | - |
| A | Talin-1 | Tln1 | Cytoplasmic integrin activator, links integrins to the actin cytoskeleton | Skin (IC) [8] | - | - | - |
| A | β1 integrins | Itgb1 | Integrin adhesion receptors | Skin (IC) [8] | - | - | - |
| A | β2 integrins | Itgb2 | Integrin adhesion receptors | Skin (IC) [8] | - | - | - |
| A | Mac-1 | Itgam | Integrin adhesion receptor | Skin (IC) [8] | - | - | - |
| A | LFA-1 | Itgal | Integrin adhesion receptor | Skin (IC) [8] | - | - | - |
| A | Integrin α3β1 | Itga3 | Integrin adhesion receptor | Skin (no IC) [82] | - | - | - |
| P | Vav | Vav1/Vav2/Vav3 | Rac-GEF, stimulates the active GTP-bound conformation of Rac | Skin (IC) [97] | - | - | - |
| T cells | |||||||
| A | β2 integrins | Itgb2 | Integrin adhesion receptors | LN (IC) [36] | - | - | - |
| A | β1 integrins | Itgb1 | Integrin adhesion receptors | Skin (no IC) [107] | - | - | - |
| A | αV integrins | Itgav | Integrin adhesion receptors | Skin (no IC) [107] | - | - | - |
| A | LFA-1 | Itgal | Integrin adhesion receptor | LN (IC) [171] | - | - | - |
| A | LFA-1 | Itgal-I306A | Point mutation in LFA-1 that upregulates constitutive affinity of LFA-1 | LN (IC) [170] | - | - | - |
| A | Integrin α1β1 | Itga1 | Integrin adhesion receptor | - | Skin (no IC) [181] | - | - |
| A | Kindlin-3 | Fermt3 | Cytoplasmic regulator of integrin activation | LN (no IC) [179] | - | - | - |
| P | Rac | Rac1/Rac2 | Small Rho GTPase, regulates F-actin polymerization |
LN (IC) [174] | - | - | - |
| P | DOCK2 | Dock2 | Rac-GEF, stimulates the active GTP-bound conformation of Rac | LN (IC) [175] | - | - | - |
| P | Coronin 1A | Ptcd | Inhibitor of F-actin formation mediated by the actin-nucleating complex Arp2/3. Ptcd mice have a point mutation in the Coro1a that increases Arp2/3 inhibition. | LN (IC) [176] | - | - | - |
| C | Myosin II | MyoIIA | Myosin II consists of 2 heavy chains (MHC) and 4 light chains (MLC). The small GTPase RhoA activates ROCK, which phosphorylates MLC, leading to contraction of actin fibers. | LN (IC) [41] | - | - | Yes [41] |
| Dendritic cells | |||||||
| A | Integrins | Itgav/Itgb1/Itgb2/Itgb7 | Integrin adhesion receptors | LN (IC), Skin explant (no IC) [32] | BMDC (IC) [32] | BMDC [32] | BMDC [72] |
| A | Talin-1 | Tln1 | Cytoplasmic integrin activator, links integrins to the actin cytoskeleton | - | BMDC (IC) [32] | BMDC [32] | BMDC [72] |
| A | β2 integrins | Itgb2 | Integrin adhesion receptors | - | BMDC (no IC), LC/dDC [180] | - | BMDC [72] |
| P | Rac | Rac1/Rac2 | Small Rho GTPase, regulates F-actin polymerization |
- | BMDC (no IC) [120] | - | - |
| P | WASp | Was | WASp is activated by Cdc42, binds and activates the actin-nucleating complex Arp2/3 | - | BMDC (IC) [123], LC/dDC [122, 124] | - | - |
| P | mDia formin | mDia1 | Actin-nucleating protein, mDia1 is activated by Rho GTPases | LN (IC) [121] | BMDC (IC), LC/dDC [121] | - | - |
| P | Eps8 | Eps8 | Actin-capping protein | - | BMDC (IC), LC/dDC [125] | BMDC [125] | BMDC [125] |
| P | Fascin-1 | Fscn1 | Actin-bundling protein | - | LC/dDC [126] | - | - |
| P | CLEC-2 | Clec1b | C-type lectin receptor; binding of CLEC-2 to its ligand podoplanin increases Rac activation and downregulates RhoA activity | LN (IC), Skin explant (no IC) [130] | BMDC (IC), LC/dDC [130] | - | - |
| P/C | Cdc42 | Cdc42 | Small Rho GTPase, regulates the temporal and spatial coordination of the cytoskeleton | - | BMDC (IC) [67], LC/dDC [131] | BMDC [67] | BMDC [67] |
| P/C | DOCK8 | Dock8 | Cdc42-specific GEF in DC, stimulates the active GTP-bound conformation of Cdc42 at the leading edge of DC | LN (IC), Skin explant (no IC) [68] | BMDC (IC), LC/dDC [68] | BMDC [68] | - |
| C | Myosin II | MyoIIA | Myosin II consists of 2 heavy chains (MHC) and 4 light chains (MLC). The small GTPase RhoA activates ROCK, which phosphorylates MLC, leading to contraction of actin fibers. | - | - | - | iBMDC [44] |
| C | Arhgef5 | Arhgef5 | RhoA-GEF, stimulates the active GTP-bound conformation of RhoA in immature DC | - | LC/dDC [127] | - | - |
| C | SWAP-70 | Swap70 | Swap70−/− DCs fail to activate RhoA upon sphingosine-1-phosphate stimulation | Skin explant (no IC) [128] | LC/dDC [128] | BMDC [128] | - |
| C | Cathepsin S | Ctss | Ctts−/− DCs show altered subcellular localization and decreased activity of myosinII | - | BMDC (IC) [39] | - | iBMDC [39] |
| C | CD74 (CLIP, Ii) | Cd74 | Ii regulates MHCII trafficking and peptide loading in DC. Lack of CD74 enhances DC contractility. | - | BMDC (IC), LC/dDC [39] | - | iBMDC [39] |
| C | Plexin A1 | Plxna1 | Receptor component that activates actomyosin contraction upon binding to its ligand semaphorin Sema3A | - | BMDC (no IC), LC/dDC [129] | BMDC [129] | - |
Abbreviations: Mod. module, A adhesion, P (actin) polymerization, C (actomyosin) contraction, IC with internal control cells, no IC without internal control cells, LN lymph node, BMDC mature bone marrow-derived dendritic cells, iBMDC immature bone marrow-derived dendritic cells, LC Langerhans cells, dDC dermal dendritic cells
Fig. 4. Optimized analysis of interstitial leukocyte migration in physiological settings.
To dissect cell-intrinsic aspects of leukocyte motility from external guidance, simultaneous imaging of cell dynamics and tissue structures is recommended. (A) 2P-IVM was applied to murine ear skin to visualize the dermis of a transgenic DsRed+/+ CD11c-YFP+/− B6.Albino mouse. Ubiquitously expressed DsRed illuminates all stromal elements (red), YFP-signals highlight endogenous dendritic cells/macrophages (yellow), and SHG signals reflect collagen bundles (white). The mouse was crossed to a B6.Albino background to avoid cell death of light-sensitive melanophages, avoiding laser light-induced artifactual effect in the tissue. Scale bar = 40 µm. (B) The movement of a wild-type dendritic cell, presumably not activated, is followed over 14 min in relation to tissue structures. Simultaneous visualization of cells, stroma, and collagen gives a closer representation of the 3D tissue geometry and reveals channel-like structures and dense networks and surfaces. Still, several other tissue determinants that might influence leukocyte behavior are unknown, e.g., the potential presence of cytokines and chemokines that can induce cell shape changes and upregulation of cell adhesion. Scale bar = 10 µm. CD11c-YFP mice were a kind gift of Michel Nussenzweig.
Acknowledgments
We thank Dr. Angelika Rambold for carefully preparing figure illustrations and critically reviewing the manuscript. This work was supported by the Intramural Research Program of NIAID, NIH. T.L. was supported by a long-term fellowship of the Human Frontier Science Program Organization.
References
- 1.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 2.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6(12):1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 3.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9(9):960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 4.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188(1):11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lämmermann T, Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol. 2009;21(5):636–644. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 7.Hickey MJ, Kubes P. Intravascular immunity: the host-pathogen encounter in blood vessels. Nat Rev Immunol. 2009;9(5):364–375. doi: 10.1038/nri2532. [DOI] [PubMed] [Google Scholar]
- 8.Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498(7454):371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germain RN, Robey EA, Cahalan MD. A decade of imaging cellular motility and interaction dynamics in the immune system. Science. 2012;336(6089):1676–1681. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen CD, Sahai E. Cancer dissemination—lessons from leukocytes. Dev Cell. 2010;19(1):13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Tozluoglu M, Tournier AL, Jenkins RP, Hooper S, Bates PA, Sahai E. Matrix geometry determines optimal cancer cell migration strategy and modulates response to interventions. Nat Cell Biol. 2013;15(7):751–762. doi: 10.1038/ncb2775. [DOI] [PubMed] [Google Scholar]
- 12.Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18(2):226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shelef MA, Tauzin S, Huttenlocher A. Neutrophil migration: moving from zebrafish models to human autoimmunity. Immunol Rev. 2013;256(1):269–281. doi: 10.1111/imr.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peri F. Breaking ranks: how leukocytes react to developmental cues and tissue injury. Curr Opin Genet Dev. 2010;20(4):416–419. doi: 10.1016/j.gde.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Evans IR, Wood W. Drosophila embryonic hemocytes. Curr Biol CB. 2011;21(5):R173–R174. doi: 10.1016/j.cub.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 16.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 17.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 18.Lin F, Baldessari F, Gyenge CC, Sato T, Chambers RD, Santiago JG, Butcher EC. Lymphocyte electrotaxis in vitro and in vivo. J Immunol. 2008;181(4):2465–2471. doi: 10.4049/jimmunol.181.4.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480(7375):109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson K, Lewalle A, Fritzsche M, Thorogate R, Duke T, Charras G. Mechanisms of leading edge protrusion in interstitial migration. Nat Commun. 2013;4:2896. doi: 10.1038/ncomms3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu W, Wang P, Petri B, Zhang Y, Tang W, Sun L, Kress H, Mann T, Shi Y, Kubes P, Wu D. Integrin-induced PIP5K1C kinase polarization regulates neutrophil polarization, directionality, and in vivo infiltration. Immunity. 2010;33(3):340–350. doi: 10.1016/j.immuni.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18(3):501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Germena G, Hirsch E. PI3Ks and small GTPases in neutrophil migration: two sides of the same coin. Mol Immunol. 2013;55(1):83–86. doi: 10.1016/j.molimm.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Heit B, Liu L, Colarusso P, Puri KD, Kubes P. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J Cell Sci. 2008;121(Pt 2):205–214. doi: 10.1242/jcs.020412. [DOI] [PubMed] [Google Scholar]
- 25.Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9(9):630–644. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vicente-Manzanares M, Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 2004;4(2):110–122. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- 27.Tooley AJ, Gilden J, Jacobelli J, Beemiller P, Trimble WS, Kinoshita M, Krummel MF. Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nat Cell Biol. 2009;11(1):17–26. doi: 10.1038/ncb1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8(2):156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 29.Niggli V. Microtubule-disruption-induced and chemotactic- peptide-induced migration of human neutrophils: implications for differential sets of signalling pathways. J Cell Sci. 2003;116(Pt 5):813–822. doi: 10.1242/jcs.00306. [DOI] [PubMed] [Google Scholar]
- 30.Renkawitz J, Sixt M. Mechanisms of force generation and force transmission during interstitial leukocyte migration. EMBO Rep. 2010;11(10):744–750. doi: 10.1038/embor.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11(6):416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 32.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lämmermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453(7191):51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 34.Brown AF. Neutrophil granulocytes: adhesion and locomotion on collagen substrata and in collagen matrices. J Cell Sci. 1982;58:455–467. doi: 10.1242/jcs.58.1.455. [DOI] [PubMed] [Google Scholar]
- 35.Haston WS, Shields JM, Wilkinson PC. Lymphocyte locomotion and attachment on two-dimensional surfaces and in three-dimensional matrices. J Cell Biol. 1982;92(3):747–752. doi: 10.1083/jcb.92.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28(8):2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Hartmann T, Sixt M, Cyster JG, Alon R. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8(10):1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 38.Barry NP, Bretscher MS. Dictyostelium amoebae and neutrophils can swim. Proc Natl Acad Sci U S A. 2010;107(25):11376–11380. doi: 10.1073/pnas.1006327107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malawista SE, de Boisfleury Chevance A. Random locomotion and chemotaxis of human blood polymorphonuclear leukocytes (PMN) in the presence of EDTA: PMN in close quarters require neither leukocyte integrins nor external divalent cations. Proc Natl Acad Sci U S A. 1997;94(21):11577–11582. doi: 10.1073/pnas.94.21.11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faure-Andre G, Vargas P, Yuseff MI, Heuze M, Diaz J, Lankar D, Steri V, Manry J, Hugues S, Vascotto F, Boulanger J, Raposo G, Bono MR, Rosemblatt M, Piel M, Lennon-Dumenil AM. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science. 2008;322(5908):1705–1710. doi: 10.1126/science.1159894. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez MI, Heuze ML, Martinez-Cingolani C, Volpe E, Donnadieu MH, Piel M, Homey B, Lennon-Dumenil AM, Soumelis V. The human cytokine TSLP triggers a cell-autonomous dendritic cell migration in confined environments. Blood. 2011;118(14):3862–3869. doi: 10.1182/blood-2010-12-323089. [DOI] [PubMed] [Google Scholar]
- 42.Jacobelli J, Friedman RS, Conti MA, Lennon-Dumenil AM, Piel M, Sorensen CM, Adelstein RS, Krummel MF. Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat Immunol. 2010;11(10):953–961. doi: 10.1038/ni.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hung WC, Chen SH, Paul CD, Stroka KM, Lo YC, Yang JT, Konstantopoulos K. Distinct signaling mechanisms regulate migration in unconfined versus confined spaces. J Cell Biol. 2013;202(5):807–824. doi: 10.1083/jcb.201302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambravaneswaran V, Wong IY, Aranyosi AJ, Toner M, Irimia D. Directional decisions during neutrophil chemotaxis inside bifurcating channels. Integr Biol Quant Biosci Nano Macro. 2010;2(11–12):639–647. doi: 10.1039/c0ib00011f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heuze ML, Collin O, Terriac E, Lennon-Dumenil AM, Piel M. Cell migration in confinement: a micro-channel-based assay. Methods Mol Biol. 2011;769:415–434. doi: 10.1007/978-1-61779-207-6_28. [DOI] [PubMed] [Google Scholar]
- 46.Irimia D, Charras G, Agrawal N, Mitchison T, Toner M. Polar stimulation and constrained cell migration in microfluidic channels. Lab Chip. 2007;7(12):1783–1790. doi: 10.1039/b710524j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abhyankar VV, Toepke MW, Cortesio CL, Lokuta MA, Huttenlocher A, Beebe DJ. A platform for assessing chemotactic migration within a spatiotemporally defined 3D microenvironment. Lab Chip. 2008;8(9):1507–1515. doi: 10.1039/b803533d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sackmann EK, Berthier E, Young EW, Shelef MA, Wernimont SA, Huttenlocher A, Beebe DJ. Microfluidic kit-on-a-lid: a versatile platform for neutrophil chemotaxis assays. Blood. 2012;120(14):e45–e53. doi: 10.1182/blood-2012-03-416453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heuze ML, Vargas P, Chabaud M, Le Berre M, Liu YJ, Collin O, Solanes P, Voituriez R, Piel M, Lennon-Dumenil AM. Migration of dendritic cells: physical principles, molecular mechanisms, and functional implications. Immunol Rev. 2013;256(1):240–254. doi: 10.1111/imr.12108. [DOI] [PubMed] [Google Scholar]
- 50.Prentice-Mott HV, Chang CH, Mahadevan L, Mitchison TJ, Irimia D, Shah JV. Biased migration of confined neutrophil-like cells in asymmetric hydraulic environments. Proc Natl Acad Sci U S A. 2013;110(52):21006–21011. doi: 10.1073/pnas.1317441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Berre M, Liu YJ, Hu J, Maiuri P, Benichou O, Voituriez R, Chen Y, Piel M. Geometric friction directs cell migration. Phys Rev Lett. 2013;111(19):198101. doi: 10.1103/PhysRevLett.111.198101. [DOI] [PubMed] [Google Scholar]
- 52.Kwon KW, Park H, Song KH, Choi JC, Ahn H, Park MJ, Suh KY, Doh J. Nanotopography-guided migration of T cells. J Immunol. 2012;189(5):2266–2273. doi: 10.4049/jimmunol.1102273. [DOI] [PubMed] [Google Scholar]
- 53.Sixt M, Lämmermann T. In vitro analysis of chemotactic leukocyte migration in 3D environments. Methods Mol Biol. 2011;769:149–165. doi: 10.1007/978-1-61779-207-6_11. [DOI] [PubMed] [Google Scholar]
- 54.Reichardt P, Gunzer F, Gunzer M. Analyzing the physicodynamics of immune cells in a three-dimensional collagen matrix. Methods Mol Biol. 2007;380:253–269. doi: 10.1007/978-1-59745-395-0_15. [DOI] [PubMed] [Google Scholar]
- 55.Weigelin B, Friedl P. A three-dimensional organotypic assay to measure target cell killing by cytotoxic T lymphocytes. Biochem Pharmacol. 2010;80(12):2087–2091. doi: 10.1016/j.bcp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Friedl P, Brocker EB. Reconstructing leukocyte migration in 3D extracellular matrix by time-lapse videomicroscopy and computer-assisted tracking. Methods Mol Biol. 2004;239:77–90. doi: 10.1385/1-59259-435-2:77. [DOI] [PubMed] [Google Scholar]
- 57.Van Goethem E, Poincloux R, Gauffre F, Maridonneau-Parini I, Le Cabec V. Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol. 2010;184(2):1049–1061. doi: 10.4049/jimmunol.0902223. [DOI] [PubMed] [Google Scholar]
- 58.Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102(9):3262–3269. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- 59.Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Sem Cell Dev Biol. 2009;20(8):931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raub CB, Suresh V, Krasieva T, Lyubovitsky J, Mih JD, Putnam AJ, Tromberg BJ, George SC. Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy. Biophys J. 2007;92(6):2212–2222. doi: 10.1529/biophysj.106.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raub CB, Unruh J, Suresh V, Krasieva T, Lindmo T, Gratton E, Tromberg BJ, George SC. Image correlation spectroscopy of multiphoton images correlates with collagen mechanical properties. Biophys J. 2008;94(6):2361–2373. doi: 10.1529/biophysj.107.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gobeaux F, Mosser G, Anglo A, Panine P, Davidson P, Giraud-Guille MM, Belamie E. Fibrillogenesis in dense collagen solutions: a physicochemical study. J Mol Biol. 2008;376(5):1509–1522. doi: 10.1016/j.jmb.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 63.Doyle AD, Petrie RJ, Kutys ML, Yamada KM. Dimensions in cell migration. Curr Opin Cell Biol. 2013;25(5):642–649. doi: 10.1016/j.ceb.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klemke M, Kramer E, Konstandin MH, Wabnitz GH, Samstag Y. An MEK-cofilin signalling module controls migration of human T cells in 3D but not 2D environments. EMBO J. 2010;29(17):2915–2929. doi: 10.1038/emboj.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quast T, Tappertzhofen B, Schild C, Grell J, Czeloth N, Forster R, Alon R, Fraemohs L, Dreck K, Weber C, Lämmermann T, Sixt M, Kolanus W. Cytohesin-1 controls the activation of RhoA and modulates integrin-dependent adhesion and migration of dendritic cells. Blood. 2009;113(23):5801–5810. doi: 10.1182/blood-2008-08-176123. [DOI] [PubMed] [Google Scholar]
- 66.Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol. 2013;201(7):1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Berre M, Aubertin J, Piel M. Fine control of nuclear confinement identifies a threshold deformation leading to lamina rupture and induction of specific genes. Integr Biol Quant Biosci Nano Macro. 2012;4(11):1406–1414. doi: 10.1039/c2ib20056b. [DOI] [PubMed] [Google Scholar]
- 68.Lämmermann T, Renkawitz J, Wu X, Hirsch K, Brakebusch C, Sixt M. Cdc42-dependent leading edge coordination is essential for interstitial dendritic cell migration. Blood. 2009;113(23):5703–5710. doi: 10.1182/blood-2008-11-191882. [DOI] [PubMed] [Google Scholar]
- 69.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, Hanawa-Suetsugu K, Kukimoto-Niino M, Nishizaki T, Shirouzu M, Duan X, Uruno T, Nishikimi A, Sanematsu F, Yokoyama S, Stein JV, Kinashi T, Fukui Y. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;119(19):4451–4461. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cougoule C, Van Goethem E, Le Cabec V, Lafouresse F, Dupre L, Mehraj V, Mege JL, Lastrucci C, Maridonneau-Parini I. Blood leukocytes and macrophages of various phenotypes have distinct abilities to form podosomes and to migrate in 3D environments. Eur J Cell Biol. 2012;91(11–12):938–949. doi: 10.1016/j.ejcb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Jacobelli J, Bennett FC, Pandurangi P, Tooley AJ, Krummel MF. Myosin-IIA and ICAM-1 regulate the interchange between two distinct modes of T cell migration. J Immunol. 2009;182(4):2041–2050. doi: 10.4049/jimmunol.0803267. [DOI] [PubMed] [Google Scholar]
- 72.Renkawitz J, Schumann K, Weber M, Lammermann T, Pflicke H, Piel M, Polleux J, Spatz JP, Sixt M. Adaptive force transmission in amoeboid cell migration. Nat Cell Biol. 2009;11(12):1438–1443. doi: 10.1038/ncb1992. [DOI] [PubMed] [Google Scholar]
- 73.Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol. 2008;8(10):764–775. doi: 10.1038/nri2414. [DOI] [PubMed] [Google Scholar]
- 74.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5(8):617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 75.Lodygin D, Odoardi F, Schlager C, Korner H, Kitz A, Nosov M, van den Brandt J, Reichardt HM, Haberl M, Flugel A. A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity. Nat Med. 2013;19(6):784–790. doi: 10.1038/nm.3182. [DOI] [PubMed] [Google Scholar]
- 76.Marangoni F, Murooka TT, Manzo T, Kim EY, Carrizosa E, Elpek NM, Mempel TR. The transcription factor NFAT exhibits signal memory during serial T cell interactions with antigen-presenting cells. Immunity. 2013;38(2):237–249. doi: 10.1016/j.immuni.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Azar GA, Lemaitre F, Robey EA, Bousso P. Subcellular dynamics of T cell immunological synapses and kinapses in lymph nodes. Proc Natl Acad Sci U S A. 2010;107(8):3675–3680. doi: 10.1073/pnas.0905901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garrod KR, Moreau HD, Garcia Z, Lemaitre F, Bouvier I, Albert ML, Bousso P. Dissecting T cell contraction in vivo using a genetically encoded reporter of apoptosis. Cell Rep. 2012;2(5):1438–1447. doi: 10.1016/j.celrep.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 79.Mues M, Bartholomaus I, Thestrup T, Griesbeck O, Wekerle H, Kawakami N, Krishnamoorthy G. Real-time in vivo analysis of T cell activation in the central nervous system using a genetically encoded calcium indicator. Nat Med. 2013;19(6):778–783. doi: 10.1038/nm.3180. [DOI] [PubMed] [Google Scholar]
- 80.Tang J, van Panhuys N, Kastenmuller W, Germain RN. The future of immunoimaging—deeper, bigger, more precise, and definitively more colorful. Eur J Immunol. 2013;43(6):1407–1412. doi: 10.1002/eji.201243119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang JH, Cardenas-Navia LI, Caldwell CC, Plumb TJ, Radu CG, Rocha PN, Wilder T, Bromberg JS, Cronstein BN, Sitkovsky M, Dewhirst MW, Dustin ML. Requirements for T lymphocyte migration in explanted lymph nodes. J Immunol. 2007;178(12):7747–7755. doi: 10.4049/jimmunol.178.12.7747. [DOI] [PubMed] [Google Scholar]
- 82.Hyun YM, Sumagin R, Sarangi PP, Lomakina E, Overstreet MG, Baker CM, Fowell DJ, Waugh RE, Sarelius IH, Kim M. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med. 2012;209(7):1349–1362. doi: 10.1084/jem.20111426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14(1):41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 84.Werr J, Johansson J, Eriksson EE, Hedqvist P, Ruoslahti E, Lindbom L. Integrin alpha(2)beta(1) (VLA-2) is a principal receptor used by neutrophils for locomotion in extravascular tissue. Blood. 2000;95(5):1804–1809. [PubMed] [Google Scholar]
- 85.Werr J, Xie X, Hedqvist P, Ruoslahti E, Lindbom L. Beta1 integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J Exp Med. 1998;187(12):2091–2096. doi: 10.1084/jem.187.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lerchenberger M, Uhl B, Stark K, Zuchtriegel G, Eckart A, Miller M, Puhr-Westerheide D, Praetner M, Rehberg M, Khandoga AG, Lauber K, Massberg S, Krombach F, Reichel CA. Matrix metalloproteinases modulate ameboid-like migration of neutrophils through inflamed interstitial tissue. Blood. 2013;122(5):770–780. doi: 10.1182/blood-2012-12-472944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khandoga AG, Khandoga A, Reichel CA, Bihari P, Rehberg M, Krombach F. In vivo imaging and quantitative analysis of leukocyte directional migration and polarization in inflamed tissue. PloS ONE. 2009;4(3):e4693. doi: 10.1371/journal.pone.0004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vemula S, Shi J, Hanneman P, Wei L, Kapur R. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood. 2010;115(9):1785–1796. doi: 10.1182/blood-2009-08-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang H, Schaff UY, Green CE, Chen H, Sarantos MR, Hu Y, Wara D, Simon SI, Lowell CA. Impaired integrin-dependent function in Wiskott-Aldrich syndrome protein-deficient murine and human neutrophils. Immunity. 2006;25(2):285–295. doi: 10.1016/j.immuni.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun C, Forster C, Nakamura F, Glogauer M. Filamin-A regulates neutrophil uropod retraction through RhoA during chemotaxis. PloS ONE. 2013;8(10):e79009. doi: 10.1371/journal.pone.0079009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi Y, Zhang J, Mullin M, Dong B, Alberts AS, Siminovitch KA. The mDial formin is required for neutrophil polarization, migration, and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. J Immunol. 2009;182(6):3837–3845. doi: 10.4049/jimmunol.0803838. [DOI] [PubMed] [Google Scholar]
- 92.Sun CX, Magalhaes MA, Glogauer M. Rac1 and Rac2 differentially regulate actin free barbed end formation downstream of the fMLP receptor. J Cell Biol. 2007;179(2):239–245. doi: 10.1083/jcb.200705122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar S, Xu J, Perkins C, Guo F, Snapper S, Finkelman FD, Zheng Y, Filippi MD. Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood. 2012;120(17):3563–3574. doi: 10.1182/blood-2012-04-426981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schnoor M, Lai FP, Zarbock A, Klaver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D. Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J Exp Med. 2011;208(8):1721–1735. doi: 10.1084/jem.20101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park H, Staehling-Hampton K, Appleby MW, Brunkow ME, Habib T, Zhang Y, Ramsdell F, Liggitt HD, Freie B, Tsang M, Carlson G, Friend S, Frevert C, Iritani BM. A point mutation in the murine Hem1 gene reveals an essential role for hematopoietic protein 1 in lymphopoiesis and innate immunity. J Exp Med. 2008;205(12):2899–2913. doi: 10.1084/jem.20080340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, Tanaka Y, Shibasaki M, Kanaho Y, Sasaki T, Frohman MA, Fukui Y. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science. 2009;324(5925):384–387. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Graham DB, Zinselmeyer BH, Mascarenhas F, Delgado R, Miller MJ, Swat W. ITAM signaling by Vav family Rho guanine nucleotide exchange factors regulates interstitial transit rates of neutrophils in vivo. PloS ONE. 2009;4(2):e4652. doi: 10.1371/journal.pone.0004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gray EE, Suzuki K, Cyster JG. Cutting edge: identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186(11):6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477(7363):216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 100.Celli S, Albert ML, Bousso P. Visualizing the innate and adaptive immune responses underlying allograft rejection by two-photon microscopy. Nat Med. 2011;17(6):744–749. doi: 10.1038/nm.2376. [DOI] [PubMed] [Google Scholar]
- 101.Mrass P, Petravic J, Davenport MP, Weninger W. Cell-autonomous and environmental contributions to the interstitial migration of T cells. Semin Immunopathol. 2010;32(3):257–274. doi: 10.1007/s00281-010-0212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, de la Maza LM, Monuki E, Flugel A, Pennington MW, Parker I, Chandy KG, Cahalan MD. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29(4):602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chow Z, Mueller SN, Deane JA, Hickey MJ. Dermal regulatory T cells display distinct migratory behavior that is modulated during adaptive and innate inflammation. J Immunol. 2013;191(6):3049–3056. doi: 10.4049/jimmunol.1203205. [DOI] [PubMed] [Google Scholar]
- 104.Filipe-Santos O, Pescher P, Breart B, Lippuner C, Aebischer T, Glaichenhaus N, Spath GF, Bousso P. A dynamic map of antigen recognition by CD4 T cells at the site of Leishmania major infection. Cell Host Microbe. 2009;6(1):23–33. doi: 10.1016/j.chom.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 105.Egawa G, Honda T, Tanizaki H, Doi H, Miyachi Y, Kabashima K. In vivo imaging of T-cell motility in the elicitation phase of contact hypersensitivity using two-photon microscopy. J Invest Dermatol. 2011;131(4):977–979. doi: 10.1038/jid.2010.386. [DOI] [PubMed] [Google Scholar]
- 106.Honda T, Egen JG, Lämmermann T, Kastenmüller W, Torabi-Parizi P, Germain RN. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity. 2014 doi: 10.1016/j.immuni.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun YM, Lambert K, Acharya M, Billroth-Maclurg AC, Rosenberg AF, Topham DJ, Yagita H, Kim M, Lacy-Hulbert A, Meier-Schellersheim M, Fowell DJ. Inflammation-induced interstitial migration of effector CD4(+) T cells is dependent on integrin alphaV. Nat Immunol. 2013;14(9):949–958. doi: 10.1038/ni.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204(2):345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mrass P, Takano H, Ng LG, Daxini S, Lasaro MO, Iparraguirre A, Cavanagh LL, von Andrian UH, Ertl HC, Haydon PG, Weninger W. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203(12):2749–2761. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, Pepper M, Wherry EJ, Dzierzinski F, Roos D, Haydon PG, Laufer TM, Weninger W, Hunter CA. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30(2):300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009;206(13):2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, Luther SA, Bollenbach T, Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339(6117):328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 113.Kilarski WW, Guc E, Teo JC, Oliver SR, Lund AW, Swartz MA. Intravital immunofluorescence for visualizing the microcirculatory and immune microenvironments in the mouse ear dermis. PloS ONE. 2013;8(2):e57135. doi: 10.1371/journal.pone.0057135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol. 2006;126(4):787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- 115.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22(5):643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 116.Gaiser MR, Lämmermann T, Feng X, Igyarto BZ, Kaplan DH, Tessarollo L, Germain RN, Udey MC. Cancer-associated epithelial cell adhesion molecule (EpCAM; CD326) enables epidermal Langerhans cell motility and migration in vivo. Proc Natl Acad Sci U S A. 2012;109(15):E889–E897. doi: 10.1073/pnas.1117674109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ng LG, Hsu A, Mandell MA, Roediger B, Hoeller C, Mrass P, Iparraguirre A, Cavanagh LL, Triccas JA, Beverley SM, Scott P, Weninger W. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PloS Pathog. 2008;4(11):e1000222. doi: 10.1371/journal.ppat.1000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG, Angeli V, Shakhar G. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med. 2011;208(10):2141–2153. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nitschke M, Aebischer D, Abadier M, Haener S, Lucic M, Vigl B, Luche H, Fehling HJ, Biehlmaier O, Lyck R, Halin C. Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady-state and inflammation. Blood. 2012;120(11):2249–2258. doi: 10.1182/blood-2012-03-417923. [DOI] [PubMed] [Google Scholar]
- 120.Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VL, Amigorena S. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305(5687):1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 121.Tanizaki H, Egawa G, Inaba K, Honda T, Nakajima S, Moniaga CS, Otsuka A, Ishizaki T, Tomura M, Watanabe T, Miyachi Y, Narumiya S, Okada T, Kabashima K. Rho-mDia1 pathway is required for adhesion, migration, and T-cell stimulation in dendritic cells. Blood. 2010;116(26):5875–5884. doi: 10.1182/blood-2010-01-264150. [DOI] [PubMed] [Google Scholar]
- 122.Snapper SB, Meelu P, Nguyen D, Stockton BM, Bozza P, Alt FW, Rosen FS, von Andrian UH, Klein C. WASP deficiency leads to global defects of directed leukocyte migration in vitro and in vivo. J Leukoc Biol. 2005;77(6):993–998. doi: 10.1189/jlb.0804444. [DOI] [PubMed] [Google Scholar]
- 123.Bouma G, Burns S, Thrasher AJ. Impaired T-cell priming in vivo resulting from dysfunction of WASp-deficient dendritic cells. Blood. 2007;110(13):4278–4284. doi: 10.1182/blood-2007-06-096875. [DOI] [PubMed] [Google Scholar]
- 124.de Noronha S, Hardy S, Sinclair J, Blundell MP, Strid J, Schulz O, Zwirner J, Jones GE, Katz DR, Kinnon C, Thrasher AJ. Impaired dendritic-cell homing in vivo in the absence of Wiskott-Aldrich syndrome protein. Blood. 2005;105(4):1590–1597. doi: 10.1182/blood-2004-06-2332. [DOI] [PubMed] [Google Scholar]
- 125.Frittoli E, Matteoli G, Palamidessi A, Mazzini E, Maddaluno L, Disanza A, Yang C, Svitkina T, Rescigno M, Scita G. The signaling adaptor Eps8 is an essential actin capping protein for dendritic cell migration. Immunity. 2011;35(3):388–399. doi: 10.1016/j.immuni.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamakita Y, Matsumura F, Lipscomb MW, Chou PC, Werlen G, Burkhardt JK, Yamashiro S. Fascin1 promotes cell migration of mature dendritic cells. J Immunol. 2011;186(5):2850–2859. doi: 10.4049/jimmunol.1001667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Z, Kumamoto Y, Wang P, Gan X, Lehmann D, Smrcka AV, Cohn L, Iwasaki A, Li L, Wu D. Regulation of immature dendritic cell migration by RhoA guanine nucleotide exchange factor Arhgef5. J Biol Chem. 2009;284(42):28599–28606. doi: 10.1074/jbc.M109.047282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ocana-Morgner C, Reichardt P, Chopin M, Braungart S, Wahren C, Gunzer M, Jessberger R. Sphingosine 1-phosphate-induced motility and endocytosis of dendritic cells is regulated by SWAP-70 through RhoA. J Immunol. 2011;186(9):5345–5355. doi: 10.4049/jimmunol.1003461. [DOI] [PubMed] [Google Scholar]
- 129.Takamatsu H, Takegahara N, Nakagawa Y, Tomura M, Taniguchi M, Friedel RH, Rayburn H, Tessier-Lavigne M, Yoshida Y, Okuno T, Mizui M, Kang S, Nojima S, Tsujimura T, Nakatsuji Y, Katayama I, Toyofuku T, Kikutani H, Kumanogoh A. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol. 2010;11(7):594–600. doi: 10.1038/ni.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, Jakus Z, Kuligowski M, Fletcher AL, Elpek KG, Bellemare-Pelletier A, Sceats L, Reynoso ED, Gonzalez SF, Graham DB, Chang J, Peters A, Woodruff M, Kim YA, Swat W, Morita T, Kuchroo V, Carroll MC, Kahn ML, Wucherpfennig KW, Turley SJ. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity. 2012;37(2):276–289. doi: 10.1016/j.immuni.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luckashenak N, Wahe A, Breit K, Brakebusch C, Brocker T. Rho-family GTPase Cdc42 controls migration of Langerhans cells in vivo. J Immunol. 2013;190(1):27–35. doi: 10.4049/jimmunol.1201082. [DOI] [PubMed] [Google Scholar]
- 132.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, Matthews HF, Davis J, Turner ML, Uzel G, Holland SM, Su HC. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361(21):2046–2055. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez- Herrera G, Chen A, Kim HS, Lloret MG, Schulze I, Ehl S, Thiel J, Pfeifer D, Veelken H, Niehues T, Siepermann K, Weinspach S, Reisli I, Keles S, Genel F, Kutukculer N, Camcioglu Y, Somer A, Karakoc-Aydiner E, Barlan I, Gennery A, Metin A, Degerliyurt A, Pietrogrande MC, Yeganeh M, Baz Z, Al-Tamemi S, Klein C, Puck JM, Holland SM, McCabe ER, Grimbacher B, Chatila TA. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124(6):1302, e1284. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Su HC, Jing H, Zhang Q. DOCK8 deficiency. Ann N Y Acad Sci. 2011;1246:26–33. doi: 10.1111/j.1749-6632.2011.06295.x. [DOI] [PubMed] [Google Scholar]
- 135.Randall KL, Lambe T, Johnson AL, Treanor B, Kucharska E, Domaschenz H, Whittle B, Tze LE, Enders A, Crockford TL, Bouriez-Jones T, Alston D, Cyster JG, Lenardo MJ, Mackay F, Deenick EK, Tangye SG, Chan TD, Camidge T, Brink R, Vinuesa CG, Batista FD, Cornall RJ, Goodnow CC. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10(12):1283–1291. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, Tan A, Arkwright PD, Al Suwairi W, Lugo Reyes SO, Yamazaki- Nakashimada MA, Garcia-Cruz Mde L, Smart JM, Picard C, Okada S, Jouanguy E, Casanova JL, Lambe T, Cornall RJ, Russell S, Oliaro J, Tangye SG, Bertram EM, Goodnow CC. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208(11):2305–2320. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lambe T, Crawford G, Johnson AL, Crockford TL, Bouriez-Jones T, Smyth AM, Pham TH, Zhang Q, Freeman AF, Cyster JG, Su HC, Cornall RJ. DOCK8 is essential for T-cell survival and the maintenance of CD8+ T-cell memory. Eur J Immunol. 2011;41(12):3423–3435. doi: 10.1002/eji.201141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crawford G, Enders A, Gileadi U, Stankovic S, Zhang Q, Lambe T, Crockford TL, Lockstone HE, Freeman A, Arkwright PD, Smart JM, Ma CS, Tangye SG, Goodnow CC, Cerundolo V, Godfrey DI, Su HC, Randall KL, Cornall RJ. DOCK8 is critical for the survival and function of NKT cells. Blood. 2013;122(12):2052–2061. doi: 10.1182/blood-2013-02-482331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McGhee SA, Chatila TA. DOCK8 immune deficiency as a model for primary cytoskeletal dysfunction. Dis Markers. 2010;29(3–4):151–156. doi: 10.3233/DMA-2010-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Massaad MJ, Ramesh N, Geha RS. Wiskott-Aldrich syndrome: a comprehensive review. Ann N Y Acad Sci. 2013;1285:26–43. doi: 10.1111/nyas.12049. [DOI] [PubMed] [Google Scholar]
- 141.Badolato R. Defects of leukocyte migration in primary immunodeficiencies. Eur J Immunol. 2013;43(6):1436–1440. doi: 10.1002/eji.201243155. [DOI] [PubMed] [Google Scholar]
- 142.Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Maree AF, Zal T, de Boer RJ, Haanen JB, Schumacher TN. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A. 2012;109(48):19739–19744. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal gammadelta TCRs. Nat Immunol. 2012;13(3):272–282. doi: 10.1038/ni.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Edelblum KL, Shen L, Weber CR, Marchiando AM, Clay BS, Wang Y, Prinz I, Malissen B, Sperling AI, Turner JR. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U S A. 2012;109(18):7097–7102. doi: 10.1073/pnas.1112519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kawakami N, Flugel A. Knocking at the brain’s door: intravital two-photon imaging of autoreactive T cell interactions with CNS structures. Semin Immunopathol. 2010;32(3):275–287. doi: 10.1007/s00281-010-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120(5):1368–1379. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2013 doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 149.Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, Flugel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462(7269):94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 150.Yin X, Chtanova T, Ladi E, Robey EA. Thymocyte motility: mutants, movies and migration patterns. Curr Opin Immunol. 2006;18(2):191–197. doi: 10.1016/j.coi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 151.Arnon TI, Horton RM, Grigorova IL, Cyster JG. Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress. Nature. 2013;493(7434):684–688. doi: 10.1038/nature11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11(11):989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 153.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143(4):592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristan C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, Germain RN. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity. 2011;34(5):807–819. doi: 10.1016/j.immuni.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28(2):271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Torabi-Parizi P, Vrisekoop N, Kastenmuller W, Gerner MY, Egen JG, Germain RN. Pathogen-related differences in the abundance of presented antigen are reflected in CD4+ T cell dynamic behavior and effector function in the lung. J Immunol. 2014 doi: 10.4049/jimmunol.1301743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9(9):618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lammermann T, Sixt M. The microanatomy of T-cell responses. Immunol Rev. 2008;221:26–43. doi: 10.1111/j.1600-065X.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- 160.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5(12):1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 161.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25(6):989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Katakai T, Habiro K, Kinashi T. Dendritic cells regulate high-speed interstitial T cell migration in the lymph node via LFA-1/ ICAM-1. J Immunol. 2013;191(3):1188–1199. doi: 10.4049/jimmunol.1300739. [DOI] [PubMed] [Google Scholar]
- 163.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22(1):19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 164.Asperti-Boursin F, Real E, Bismuth G, Trautmann A, Donnadieu E. CCR7 ligands control basal T cell motility within lymph node slices in a phosphoinositide 3-kinase-independent manner. J Exp Med. 2007;204(5):1167–1179. doi: 10.1084/jem.20062079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med. 2007;204(3):489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Okada T, Cyster JG. CC chemokine receptor 7 contributes to Gi-dependent T cell motility in the lymph node. J Immunol. 2007;178(5):2973–2978. doi: 10.4049/jimmunol.178.5.2973. [DOI] [PubMed] [Google Scholar]
- 167.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97(23):12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schumann K, Lammermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Forster R, Lutz MB, Sorokin L, Sixt M. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32(5):703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 169.Boscacci RT, Pfeiffer F, Gollmer K, Sevilla AI, Martin AM, Soriano SF, Natale D, Henrickson S, von Andrian UH, Fukui Y, Mellado M, Deutsch U, Engelhardt B, Stein JV. Comprehensive analysis of lymph node stroma-expressed Ig superfamily members reveals redundant and nonredundant roles for ICAM-1, ICAM-2, and VCAM-1 in lymphocyte homing. Blood. 2010;116(6):915–925. doi: 10.1182/blood-2009-11-254334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Park EJ, Peixoto A, Imai Y, Goodarzi A, Cheng G, Carman CV, von Andrian UH, Shimaoka M. Distinct roles for LFA-1 affinity regulation during T-cell adhesion, diapedesis, and interstitial migration in lymph nodes. Blood. 2010;115(8):1572–1581. doi: 10.1182/blood-2009-08-237917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Reichardt P, Patzak I, Jones K, Etemire E, Gunzer M, Hogg N. A role for LFA-1 in delaying T-lymphocyte egress from lymph nodes. EMBO J. 2013;32(6):829–843. doi: 10.1038/emboj.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Soriano SF, Hons M, Schumann K, Kumar V, Dennier TJ, Lyck R, Sixt M, Stein JV. In vivo analysis of uropod function during physiological T cell trafficking. J Immunol. 2011;187(5):2356–2364. doi: 10.4049/jimmunol.1100935. [DOI] [PubMed] [Google Scholar]
- 173.Liu Y, Belkina NV, Park C, Nambiar R, Loughhead SM, Patino- Lopez G, Ben-Aissa K, Hao JJ, Kruhlak MJ, Qi H, von Andrian UH, Kehrl JH, Tyska MJ, Shaw S. Constitutively active ezrin increases membrane tension, slows migration, and impedes endothelial transmigration of lymphocytes in vivo in mice. Blood. 2012;119(2):445–453. doi: 10.1182/blood-2011-07-368860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Faroudi M, Hons M, Zachacz A, Dumont C, Lyck R, Stein JV, Tybulewicz VL. Critical roles for Rac GTPases in T-cell migration to and within lymph nodes. Blood. 2010;116(25):5536–5547. doi: 10.1182/blood-2010-08-299438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nombela-Arrieta C, Mempel TR, Soriano SF, Mazo I, Wymann MP, Hirsch E, Martinez AC, Fukui Y, von Andrian UH, Stein JV. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J Exp Med. 2007;204(3):497–510. doi: 10.1084/jem.20061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, An J, Xu Y, Jenne CN, Foger N, Sorensen RU, Goodnow CC, Bear JE, Puck JM, Cyster JG. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat Immunol. 2008;9(11):1307–1315. doi: 10.1038/ni.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, Munk A, Forster R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol. 2011;12(9):879–887. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- 178.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3(6):e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Andreasen SO, Thomsen AR, Koteliansky VE, Novobrantseva TI, Sprague AG, de Fougerolles AR, Christensen JP. Expression and functional importance of collagen-binding integrins, alpha 1 beta 1 and alpha 2 beta 1, on virus-activated T cells. J Immunol. 2003;171(6):2804–2811. doi: 10.4049/jimmunol.171.6.2804. [DOI] [PubMed] [Google Scholar]
- 180.Cohen SJ, Gurevich I, Feigelson SW, Petrovich E, Moser M, Shakhar G, Fassler R, Alon R. The integrin coactivator Kindlin-3 is not required for lymphocyte diapedesis. Blood. 2013;122(15):2609–2617. doi: 10.1182/blood-2013-04-495036. [DOI] [PubMed] [Google Scholar]
- 181.Grabbe S, Varga G, Beissert S, Steinert M, Pendl G, Seeliger S, Bloch W, Peters T, Schwarz T, Sunderkotter C, Scharffetter-Kochanek K. Beta2 integrins are required for skin homing of primed T cells but not for priming naive T cells. J Clin Invest. 2002;109(2):183–192. doi: 10.1172/JCI11703. [DOI] [PMC free article] [PubMed] [Google Scholar]