Abstract
In unfolded RNase A there is an interconversion between slow-folding and fast-folding forms (US ⇌ UF) that is known to show properties characteristic of proline isomerization in model peptides. Here, we accept the evidence that US molecules contain nonnative proline isomers and we ask about the isomerization of these proline residues during folding. The US ⇌ UF reaction in unfolded RNase A is used both to provide data on the kinetics of proline isomerization in the unfolded protein and as the basis of an assay for measuring proline isomerization during folding.
The tyrosine-detected folding kinetics at low temperatures have been compared to those of proline isomerization in unfolded RNase A. The comparison is based on the recent observation that the US ⇌ UF kinetics are independent of guanidinium chloride concentration, so that they can be extrapolated to low guanidinium chloride concentrations, at which folding takes place. At 0°C the tyrosine-detected folding reaction is 100-fold faster than the conversion of US to UF in unfolded RNase A. Consequently, the folding reaction is not rate-limited by proline isomerization as it occurs in unfolded RNase A.
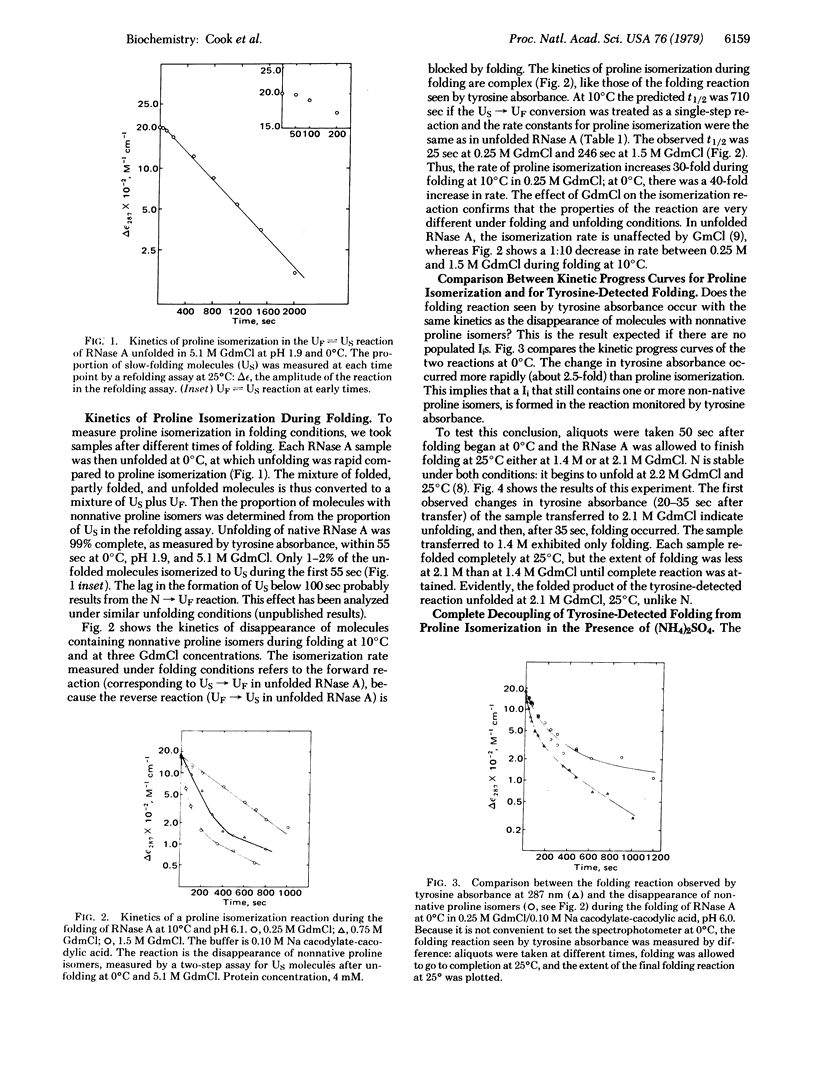
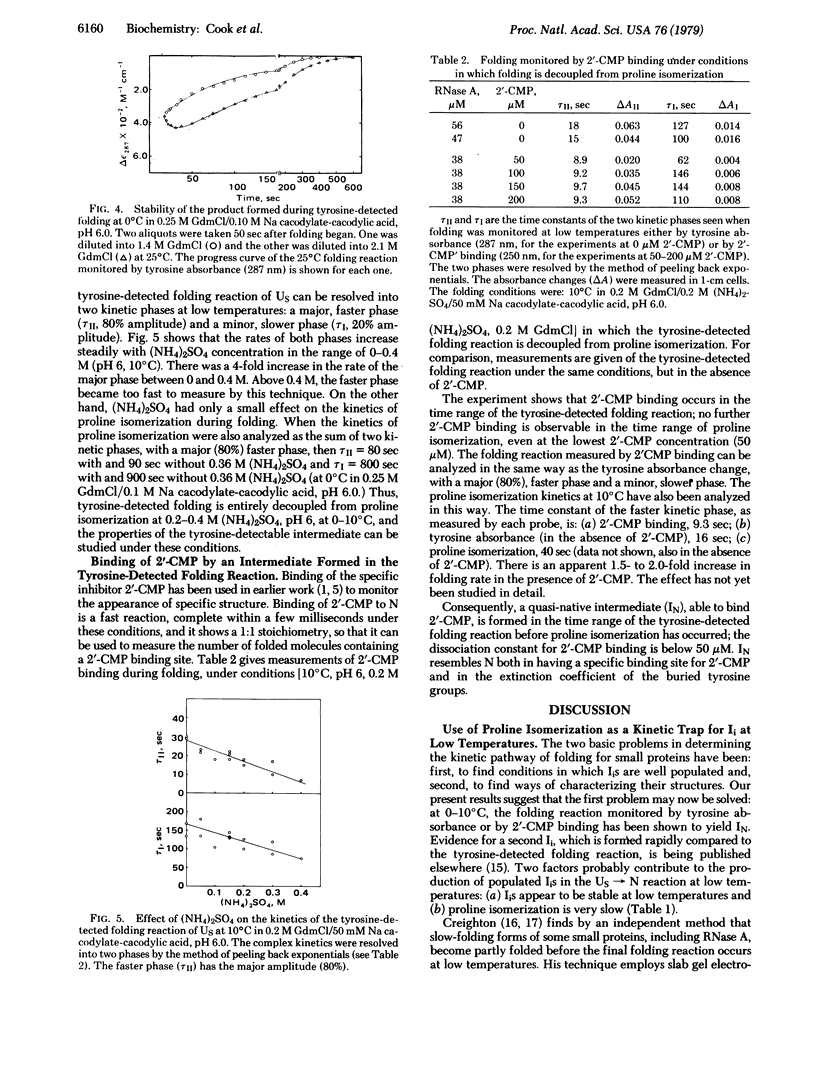
An assay is given for proline isomerization during folding. The principle is that native RNase A yields UF on unfolding, whereas protein molecules that still contain nonnative proline isomers yield US. Unfolding takes place at 0°C, at which proline isomerization is slow compared to unfolding. This assay yields two important results: (i) The kinetics of proline isomerization during folding are substantially faster than in unfolded RNase A—e.g., 40-fold at 0°C. The mechanism of the rate enhancement is unknown. (ii) At low temperatures (0-10°C), and also in the presence of (NH4)2SO4, the tyrosine-detected folding reaction occurs before proline isomerization and yields a folded intermediate IN that is able to bind the specific inhibitor 2′-CMP. The results demonstrate that a folding intermediate is spectrally detectable when folding occurs at low temperatures. They suggest that low temperatures provide suitable conditions for determining the kinetic pathway of folding by characterizing folding intermediates.
Keywords: protein folding
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandts J. F., Brennan M., Lung-Nan Lin Unfolding and refolding occur much faster for a proline-free proteins than for most proline-containing proteins. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4178–4181. doi: 10.1073/pnas.74.10.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandts J. F., Halvorson H. R., Brennan M. Consideration of the Possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry. 1975 Nov 4;14(22):4953–4963. doi: 10.1021/bi00693a026. [DOI] [PubMed] [Google Scholar]
- Brown R. D., 3rd, Brewer C. F., Koenig S. H. Conformation states of concanavalin A: kinetics of transitions induced by interaction with Mn2+ and Ca2+ ions. Biochemistry. 1977 Aug 23;16(17):3883–3896. doi: 10.1021/bi00636a026. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Bächinger H. P., Timpl R., Engel J. Three conformationally distinct domains in the amino-terminal segment of type III procollagen and its rapid triple helix leads to and comes from coil transition. Eur J Biochem. 1978 Oct 16;90(3):595–603. doi: 10.1111/j.1432-1033.1978.tb12640.x. [DOI] [PubMed] [Google Scholar]
- Creighton E. T. Possible implications of many proline residues for the kinetics of protein unfolding and refolding. J Mol Biol. 1978 Nov 5;125(3):401–406. doi: 10.1016/0022-2836(78)90411-4. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Electrophoretic analysis of the unfolding of proteins by urea. J Mol Biol. 1979 Apr 5;129(2):235–264. doi: 10.1016/0022-2836(79)90279-1. [DOI] [PubMed] [Google Scholar]
- Garel J. R., Baldwin R. L. A physical difference between the fast- and slow-refolding forms of nitrotyrosyl ribonuclease A: the pK values of the nitrotyrosyl groups. J Mol Biol. 1975 Jun 5;94(4):621–632. doi: 10.1016/0022-2836(75)90326-5. [DOI] [PubMed] [Google Scholar]
- Garel J. R., Baldwin R. L. Both the fast and slow refolding reactions of ribonuclease A yield native enzyme. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3347–3351. doi: 10.1073/pnas.70.12.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel J. R., Baldwin R. L. The heat-unfolded state of ribonuclease A is an equilibrium mixture of fast and slow refolding species. J Mol Biol. 1975 Jun 5;94(4):611–620. doi: 10.1016/0022-2836(75)90325-3. [DOI] [PubMed] [Google Scholar]
- Garel J. R., Nall B. T., Baldwin R. L. Guanidine-unfolded state of ribonuclease A contains both fast- and slow-refolding species. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1853–1857. doi: 10.1073/pnas.73.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel J. R. pK changes of ionizable reporter groups as an index of conformational changes in proteins. A study of fluorescein-labelled ribonuclease A. Eur J Biochem. 1976 Nov 1;70(1):179–189. doi: 10.1111/j.1432-1033.1976.tb10968.x. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J., Baldwin R. L. A quantitative treatment of the kinetics of the folding transition of ribonuclease A. Biochemistry. 1976 Apr 6;15(7):1462–1473. doi: 10.1021/bi00652a017. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Further evidence suggesting that the slow phase in protein unfolding and refolding is due to proline isomerization: a kinetic study of carp parvalbumins. Biochemistry. 1978 Sep 19;17(19):4102–4110. doi: 10.1021/bi00612a036. [DOI] [PubMed] [Google Scholar]
- Nall B. T., Garel J. R., Baldwin R. L. Test of the extended two-state model for the kinetic intermediates observed in the folding transition of ribonuclease A. J Mol Biol. 1978 Jan 25;118(3):317–330. doi: 10.1016/0022-2836(78)90231-0. [DOI] [PubMed] [Google Scholar]
- SELA M., ANFINSEN C. B. Some spectrophotometric and polarimetric experiments with ribonuclease. Biochim Biophys Acta. 1957 May;24(2):229–235. doi: 10.1016/0006-3002(57)90186-5. [DOI] [PubMed] [Google Scholar]
- Schmid F. X., Baldwin R. L. Acid catalysis of the formation of the slow-folding species of RNase A: evidence that the reaction is proline isomerization. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4764–4768. doi: 10.1073/pnas.75.10.4764. [DOI] [PMC free article] [PubMed] [Google Scholar]


